Introduction
Spontaneous regression of malignant melanoma is defined by the disappearance of melanocytic neoplastic cells partially or completely. In contrast to the partial form, complete spontaneous regression of primary malignant melanoma is a rare-occurring phenomenon, with 76 cases reported in the literature since 1866.1 The criteria for the diagnosis of regression were established by Smith and Stehlin2 and modified later.3, 4 Overall, the impact of partial or complete regression on prognosis is anecdotal and controversial; however, we report a case with complete regression of a histologically diagnosed thick primary malignant melanoma with rapid development of metastatic disease and death.
Case report
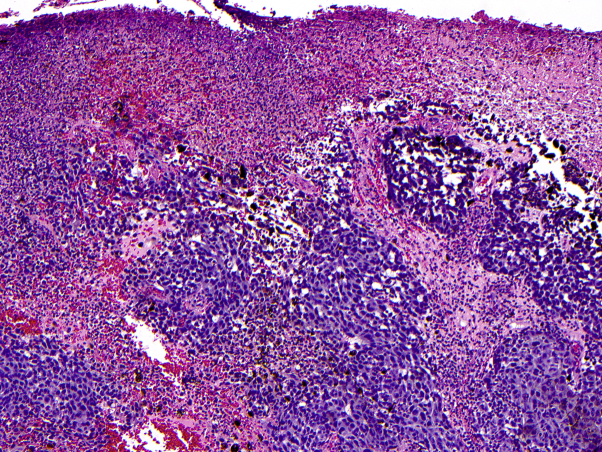
A 49-year-old man was referred to the surgical oncology department after biopsy of a lesion on the left chest found a newly diagnosed melanoma. The skin lesion was present for several years and had recently grown larger and darker without associated pain or pruritus. Pathologic examination found a nodular melanoma, broadly transected along the base, at least 8 mm in depth, level IV with extensive ulceration. There were 12 mitoses per square millimeter with positive immunohistochemical staining for Mart-1 and HMB-45 and an increased Ki-67 proliferation index of greater than 25% (Fig 1). Tumor-infiltrating lymphocytes were nonbrisk, and there was no evidence of tumor regression.
Fig 1.
Left side of the chest skin biopsy for primary malignant melanoma. Histopathologic examination found a thick primary melanoma characterized by atypical pigmented epithelioid cells with extensive ulceration. (Hematoxylin-eosin stain; original magnification: ×100.)
The patient did not have a family history of melanoma and did not exhibit signs of a new cough, melena, hematochezia, or headache at the time of initial presentation. His physical examination was notable for a palpable, mobile 1- to 2-cm lymph node in the left supraclavicular fossa and a 1- × 0.5-cm, intact, elliptical pigmented primary melanoma on his anterior chest. Thus, the patient was scheduled for staging scans, ultrasound scan with fine-needle aspiration of his left supraclavicular lymph node, and wide local excision of the melanoma on his chest. Computed tomography scans of the chest, abdomen, and pelvis along with magnetic resonance imaging (MRI) of the brain did not show evidence of metastatic disease; however, results of fine-needle aspiration of the palpable lymphadenopathy on the left side of the neck were positive for melanoma, placing the disease at stage III. At follow-up 4 weeks later, the patient had no residual pigmentation at the site of his original melanoma (Fig 2), a distinct clinical change since his initial presentation.
Fig 2.
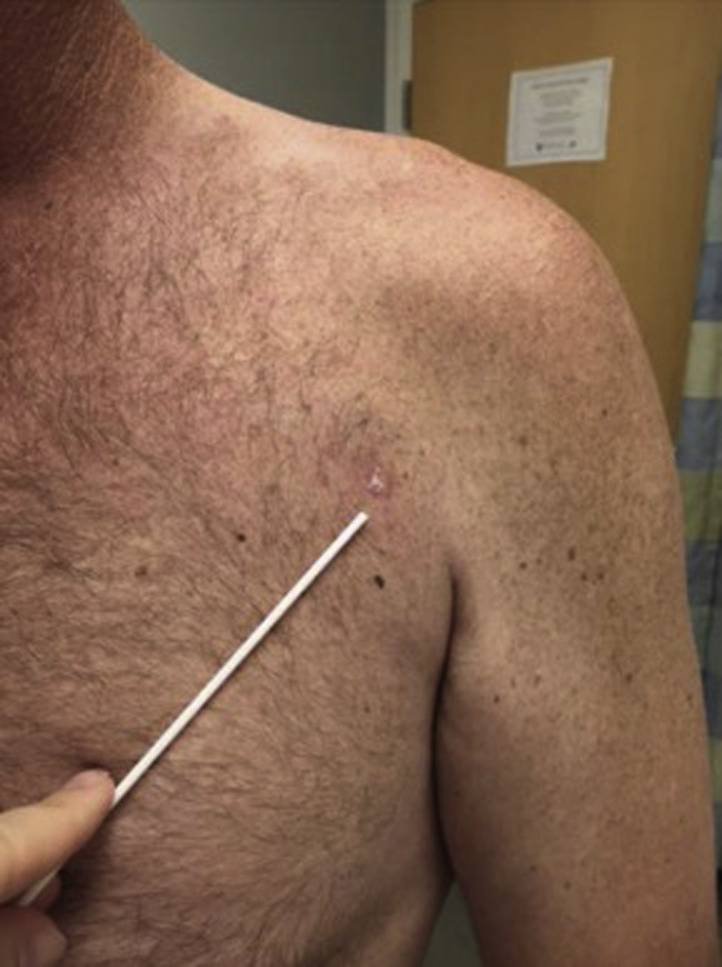
Clinical photograph of the primary melanoma on the left side of the chest 4 weeks after biopsy. The clinically notable pigmented lesion had regressed to a small scaled area of skin.
He then underwent wide local excision of the left anterior chest melanoma site with left cervical lymphadenectomy. Interestingly, pathology findings from the left anterior chest skin excision showed multifocal satellite melanoma metastases present in the dermis and subcutaneous fat with absence of the primary lesion (Fig 3). Snapshot polymerase chain reaction assay performed on the specimen was positive for variants in BRAF and STK11. In addition, the patient had 2 lymph nodes that were positive for melanoma with the largest macrometastasis entirely replacing the lymph node with extracapsular extension, placing the disease at stage IIIC (T4bN3M0). The patient was subsequently offered adjuvant interferon therapy, but he deferred treatment and was placed on active surveillance with a planned return date of 3 months.
Fig 3.
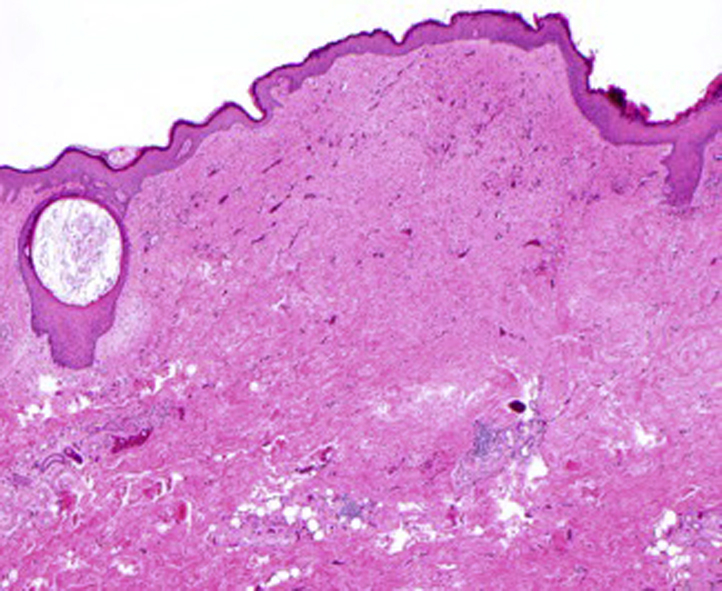
Histologic evaluation of the site of the primary lesion showed a scar with no residual melanoma identified. (Hematoxylin-eosin stain; original magnification: ×40.)
Three months later, as part of routine surveillance, the patient had an lactate dehydrogenase level of 1591 IU/L with multiple new foci of intra-abdominal disease; in addition, he had increasing pulmonary nodules and bilateral hilar, peribronchial, periesophageal, and left axillary lymphadenopathy suspicious for metastasis. Head MRI found numerous enhancing lesions scattered throughout both cerebral hemispheres, and computed tomography of the neck showed an enhancing nodule suspicious for tumor recurrence (Fig 4).
Fig 4.
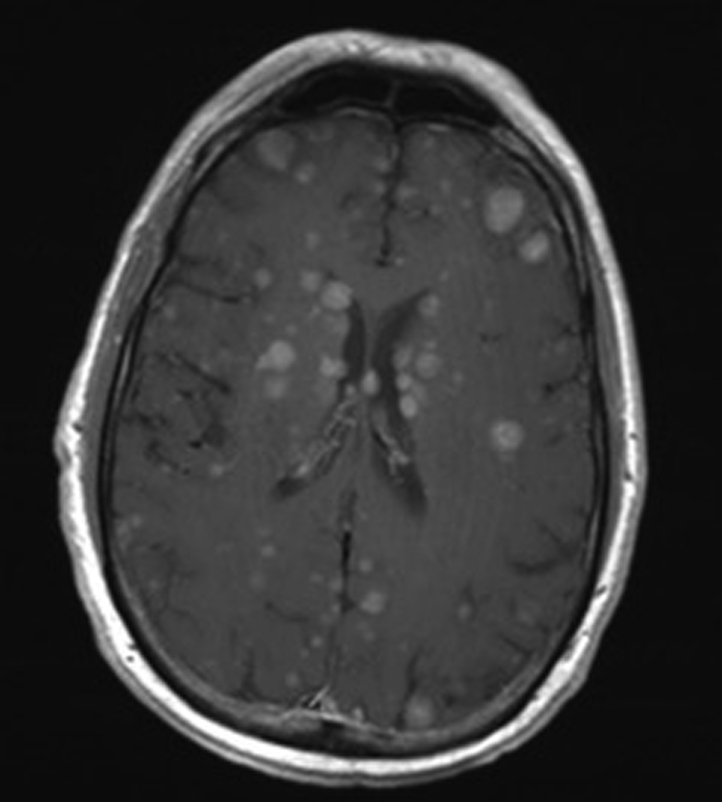
MRI T1 postcontrast axial image shows more than 100 new enhancing hemorrhagic intracranial metastases throughout the cerebral hemispheres.
The patient was started on vemurafenib and cobimetinib; after 3 months of therapy with continued brain metastases, the patient underwent whole-brain radiotherapy followed by treatment with ipilimumab, nivolumab, and dabrafenib. Unfortunately, the patient died 1 month later of cerebral edema.
Discussion
We present a patient who had rapid development of metastatic disease from a thick, at least 8-mm primary lesion detected with clinically positive lymph node disease. Between the patient's first and second preoperative visits, his primary lesion completely regressed, and aggressive dermal disease was found at resection. This finding is of clinical interest given the occasional presentation of melanoma with unknown primary tumor thought to be caused by spontaneous complete regression. In addition, this case shows the rapid changes in tumor presentation, behavior, and metastatic potential associated with this process.
Available literature on the complete regression of primary malignant melanoma shows that it is a rare phenomenon, with an incidence of 0.22% to 0.27%.5 The significance of primary regression on the prognosis of melanoma patients is controversial; however, the incidence of metastatic disease in the setting of complete regression of the primary lesion is estimated to range from 4% to 10%.3 These data have led clinicians to believe that complete regression of primary malignant melanoma may have an association with progressive metastatic disease. However, one must keep in mind that the regression of a primary melanoma usually comes to attention when associated with metastasis, leading to underreporting of nonmetastatic cases.4, 6 Interestingly, metastatic disease was also significantly increased among cases of thin melanoma with extensive (>50%) regression, suggesting that these lesions are either understaged or exhibit unfavorable biology associated with spontaneous regression.6
Although the mechanism of spontaneous regression is not fully understood, immunologic factors are considered a key aspect of this process. One potential explanation for spontaneous regression is via a natural tumor-specific cytolytic T-cell response not seen in subsequent melanoma metastases.7 Another theory posits that complete regression of a primary malignant melanoma is caused by the immune-stimulating presence of metastatic melanoma within a regional lymph node.8 Lastly, others propose that operative trauma or infection can act as mediating factors that may increase an individual's immune response against a tumor.9 These theories all suggest that innate immune cells may recognize distinct structures on cancer cells, triggering complete regression of the primary malignancy. The continued presence of metastatic lesions, however, may indicate immune escape through such possible mechanisms as mutation or down-regulation of human leukocyte antigen, myeloid-derived suppressor cells, or the production of suppressive cytokines.
Although the mechanism and prognosis of spontaneous regression of primary malignant melanoma is uncertain, it is interesting to note the aggressive and rapid progression from a cutaneous primary melanoma to widely metastatic disease in a few short months. Our case suggests that a completely regressing, thick primary melanoma in the setting of metastatic disease may portend a poor prognosis, showing a need for vigilant clinical and radiologic observation.
Footnotes
Funding sources: None.
Conflicts of interst: None declared.
References
- 1.Ong S.F., Harden M., Irandoust S., Lee R.W. Spontaneous regression of pulmonary metastatic melanoma. Respirol Case Rep. 2016;4(1):7–9. doi: 10.1002/rcr2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith J.L., Stehlin J.S. Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965;18(11):1399–1415. doi: 10.1002/1097-0142(196511)18:11<1399::aid-cncr2820181104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.High W.A., Stewart D., Wilbers C.R., Cockerell C.J., Hoang M.P., Fitzpatrick J.E. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53(1):89–100. doi: 10.1016/j.jaad.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Shai A., Avinoach I., Sagi A. Metastatic malignant melanoma with spontaneous and complete regression of the primary lesion. Case report and review of the literature. J Dermatol Surg Oncol. 1994;20(5):342–345. doi: 10.1111/j.1524-4725.1994.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 5.Emanuel P.O., Mannion M., Phelps R.G. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30(2):178–181. doi: 10.1097/DAD.0b013e318165641a. [DOI] [PubMed] [Google Scholar]
- 6.Fontaine D., Parkhill W., Greer W., Walsh N. Partial Regression of Primary Cutaneous Melanoma: is there an Association with Sub- Clinical Sentinel Lymph Node Metastasis? Am J Dermatopathol. 2003;25(5):371–376. doi: 10.1097/00000372-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Zorn E., Hercend T. A natural cytotoxic T cell response in a spontaneously regressing human melanoma targets a neoantigen resulting from a somatic point mutation. Eur J Immunol. 1999;29(2):592–601. doi: 10.1002/(SICI)1521-4141(199902)29:02<592::AID-IMMU592>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Shaw H.M., Mccarthy S.W., Mccarthy W.H., Thompson J.F., Milton G.W. Thin regressing malignant melanoma: significance of concurrent regional lymph node metastases. Histopathology. 1989;15(3):257–265. doi: 10.1111/j.1365-2559.1989.tb03076.x. [DOI] [PubMed] [Google Scholar]
- 9.Kalialis L.V., Drzewiecki K.T., Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19(5):275–282. doi: 10.1097/CMR.0b013e32832eabd5. [DOI] [PubMed] [Google Scholar]