Introduction
Angina bullosa hemorrhagica (ABH) is a rare benign disorder characterized by sudden onset of painless, blood-filled, blisters of the oral cavity that quickly expand and rupture spontaneously within 24 to 48 hours. First reported in 1967,1 ABH has been noted under other diagnostic terms including recurrent or traumatic oral hemophlyctenosis and benign hemorrhagic bullous stomatitis.2 Localization of blisters is usually restricted to the soft palate, yet the buccal mucosa, tongue, and lips may also be involved.3 Associated symptoms have been noted in some cases, including hoarseness and blood-tinged sialorrhea. Blisters tend to heal without scarring. Most cases occur in adults, ages 50 to 70, with no differentiation in incidence between men or women.2, 4 Because it is infrequently encountered in the clinical setting and sparsely reported in the dermatologic literature, its recognition and diagnosis make it challenging for providers. We report a recent case of ABH in a middle-aged man with idiopathic thrombocytopenia, including its emergent presentation and relevant histopathology.
Case
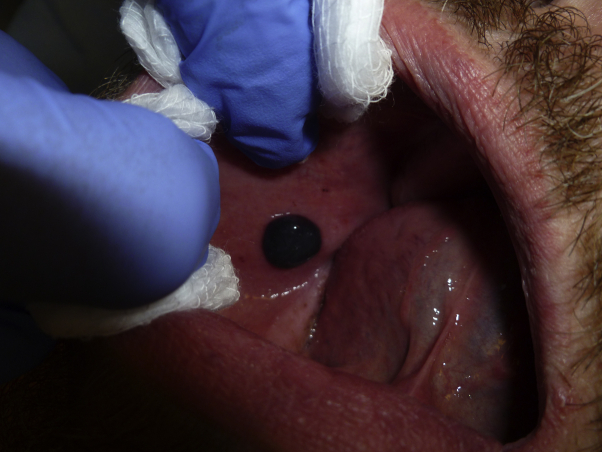
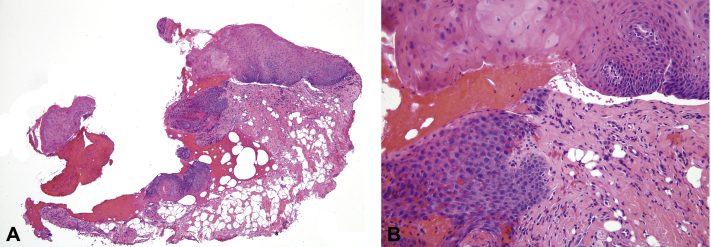
A 45-year-old man presented to clinic with a 4-day history of multiple, tender hemorrhagic bullae of the oral cavity. He reported the first lesion appeared on the left buccal mucosa after he consumed an apple, followed by acute rupture and subsequent bleeding. Two more lesions appeared successively leading to a hospital admission where he was treated empirically with oral clindamycin at a dose of 300 mg 3 times daily and discharged. An additional bulla appeared on the right buccal mucosa the evening before his visit, which was present at the time of the examination. Examination found a 1-cm hemorrhagic tense bulla overlying edematous mucosa and a shallow, atrophic erosion with ragged border at the site of the previous bullae on the left buccal mucosa (Fig 1). No other papules, plaques, or ulcers were appreciated in the oropharynx. His medical records showed a recent history of thrombocytopenia, congestive heart failure, and shortness of breath and a medication regimen including aspirin (81 mg/d), losartan, levothyroxine, gabapentin, furosemide, esomeprazole, docusate sodium, and amiodarone. Complete review of systems was otherwise unremarkable. Laboratory test results highlighted a platelet count of 98,000/L (normal range, 150–440 10*9/L) and a lymphocyte count of 1.4/L (normal range, 1.5–5.0 10*9/L). Four-millimeter punch biopsy specimens were taken from the edge of the bulla for hematoxylin-eosin (H&E) staining and from perilesional skin for direct immunofluorescence Before biopsy, the bullae was aspirated with an 18-gauge needle. Hemostasis was obtained with manual pressure and electrodessication. The clinical differential diagnosis included autoimmune blistering disorders, bullous lichen planus, and ABH. H&E staining of a punch biopsy from the oral mucosa found a pauci-inflammatory subepithelial cleft with hemorrhage (Fig 2, A and B). Direct immunofluorescence mapping of the mucosa was negative, showing no immunoreactants detected using specific antihuman IgG, IgM, IgA, C3, and fibrinogen conjugates. Indirect immunofluorescence of the serum did not detect any autoantibodies. Both the clinical history and histopathologic findings were directly in line with classic reports of ABH.5 The patient's blisters healed with no scarring. His idiopathic thrombocytopenia remained stable. The patient died of unrelated causes without any recurrences 1 year after his initial presentation.
Fig 1.
A tense hemorrhagic bulla on the buccal mucosa.
Fig 2.
A, Punch biopsy from the edge of the bulla. B, Punch biopsy specimen at greater specification. (A and B, H&E stain; original magnifications: A, 40×; B, ×200.)
Discussion
Diagnosis of ABH highly depends on recognition of clinical features and is often delayed because of its resemblance to other similar-appearing conditions involving oral mucosa. Mucosal biopsies are inconclusive, usually yielding nonspecific findings such as hemorrhagic subepithelial bulla, chronic inflammatory cell infiltrate within the lamina propria,6, 7 and a decrease in elastic network leading to fragility of vessel walls.6, 7, 8 Direct and indirect immunofluorescence consistently show negative results.3, 4
The cause of ABH is still poorly understood.9 However, several etiologic factors are identified as potential precipitating conditions, such as arterial hypertension, diabetes mellitus, long-term inhaled steroid use, and masticatory trauma.2, 8, 10 Several isolated cases of drug-induced thrombocytopenia associated with ABH-like lesions have been reported,11 but it seems unlikely that our patient's stable idiopathic thrombocytopenia contributed to the development of his ABH given that it is typical for patients with ABH to have platelet counts and coagulation test results within normal range. A few reports of ABH showed a significant decrease in elastic fibers in microscopic sections of affected mucosa, thus, proposing that poor anchorage of small blood vessels could explain the appearance of hemorrhagic lesions in response to minor trauma.12 Spontaneous ABH with no notable predispositions was noted to occur in up to 47% of patients reporting related clinical signs, including bullous blisters appearing most often on the soft palate and some in the buccal and lingual regions.3
The differential diagnosis is broad when assessing ABH because of its nonspecific symptoms and clinical presentation. It includes mucous membrane pemphigoid, epidermolysis bullosa acquisita, linear IgA dermatosis, erythema multiforme, oral amyloidosis, pemphigus, dermatitis herpetiformis, and bullous lichen planus.2, 3, 5, 13 Many of these conditions differ histologically from ABH and tend to be associated with immunologic and autoimmune abnormalities. Hemorrhagic blisters can also arise in hematologic disorders, such as leukemia, vasculitis,14 and thrombocytopenia,2 but normal blood count and hemostatic function help distinguish between them. No treatment is necessary, although active blisters can be gently ruptured to prevent further separation of the epithelium, followed by the use of antibacterial mouthwash, chlorhexidine gluconate, to prevent infection.3 Ascorbic acid/citroflavonoid (200 mg twice daily) has been reported to possibly prevent recurrences.2 In one atypical case, intubation was required because of excessive bleeding from a large bulla that resulted in airway compromise.15
ABH is a rare yet benign condition affecting middle-age to elderly adults, both men and women equally. Although several case series describing ABH do exist within dental and oral surgery research, relatively few reports exist within dermatologic literature. Our case emphasizes the need for dermatologists specifically to consider ABH in the differential diagnosis involving oral cavity blisters, as previous reports suggest knowledge of the condition is limited in the field.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Badham N.J. Blood blisters and the oesophageal cast. J Laryngol Otol. 1967;81(7):791–803. doi: 10.1017/s0022215100067700. [DOI] [PubMed] [Google Scholar]
- 2.Grinspan D., Abulafia J., Lanfranchi H. Angina bullosa hemorrhagica. Int J Dermatol. 1999;38(7):525–528. doi: 10.1046/j.1365-4362.1999.00682.x. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson P., Lamey P.J., Scully C., Prime S.S. Angina bullosa haemorrhagica: clinical and laboratory features in 30 patients. Oral Surg Oral Med Oral Pathol. 1987;63(5):560–565. doi: 10.1016/0030-4220(87)90228-3. [DOI] [PubMed] [Google Scholar]
- 4.Deblauwe B.M. Van der waal I. Blood blisters of the oral mucosa (angina bullosa haemorrhagica) J Am Acad Dermatol. 1994;31(2 Pt 2):341–344. doi: 10.1016/s0190-9622(94)70168-7. [DOI] [PubMed] [Google Scholar]
- 5.Beguerie J.R., Gonzalez S. Angina bullosa hemorrhagica: report of 11 cases. Dermatol Reports. 2014;6(1):5282. doi: 10.4081/dr.2014.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins R., Walker D.M. Oral blood blisters: angina bullosa haemorrhagica. Br J Oral Maxillofac Surg. 1985;23(1):9–16. doi: 10.1016/0266-4356(85)90073-7. [DOI] [PubMed] [Google Scholar]
- 7.Edwards S., Wilkinson J.D., Wojnarowska F. Angina bullosa haemorrhagica–a report of three cases and review of the literature. Clin Exp Dermatol. 1990;15(6):422–424. doi: 10.1111/j.1365-2230.1990.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 8.Guillot B. Skin reactions to inhaled corticosteroids. Clinical aspects, incidence, avoidance, and management. Am J Clin Dermatol. 2000;1(2):107–111. doi: 10.2165/00128071-200001020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto K., Fujimoto M., Inoue M., Maeda M., Yamakawa N., Kirita T. Angina bullosa hemorrhagica of the soft palate: report of 11 cases and literature review. J Oral Maxillofac Surg. 2006;64(9):1433–1436. doi: 10.1016/j.joms.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 10.High A.S., Main D.M. ‘Angina bullosa haemorrhagica: a complication of long-term steroid inhaler use’. Br Dent J. 1988;165(10):357. doi: 10.1038/sj.bdj.4806645. [DOI] [PubMed] [Google Scholar]
- 11.Abhinav C., Mahajan V.K., Mehta K.S., Chauhan P.S. Angina bullosa hemorrhagica-like lesions: a rare presentation of drug-induced thrombocytopenia. Int J Dermatol. 2015;54(7):819–822. doi: 10.1111/ijd.12143. [DOI] [PubMed] [Google Scholar]
- 12.Higgins E.M., Du vivier A.W. Angina bullosa haemorrhagica–a possible relation to steroid inhalers. Clin Exp Dermatol. 1991;16(4):244–246. doi: 10.1111/j.1365-2230.1991.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez J.D., Rodríguez-peralto J.L., Iglesias L. Recurrent oral blood blisters. Arch Dermatol. 1999;135(5):593–594. doi: 10.1001/archderm.135.5.593-a. 596-597. [DOI] [PubMed] [Google Scholar]
- 14.Vaillant L., Fontès V. [Bullous diseases of the oral mucosa] Rev Prat. 2002;52(4):385–388. [PubMed] [Google Scholar]
- 15.Pahl C., Yarrow S., Steventon N., Saeed N.R., Dyar O. Angina bullosa haemorrhagica presenting as acute upper airway obstruction. Br J Anaesth. 2004;92(2):283–286. doi: 10.1093/bja/aeh029. [DOI] [PubMed] [Google Scholar]