Abstract
After sudden traumatic brain injuries, secondary injuries may occur during the following days or weeks, which leads to the accumulation of reactive oxygen species (ROS). Since ROS exacerbate brain damage, it is important to protect neurons against their activity. Zinc finger protein 179 (Znf179) was shown to act as a neuroprotective factor, but the regulation of gene expression under oxidative stress remains unknown. In this study, we demonstrated an increase in Znf179 protein levels in both in vitro model of hydrogen peroxide (H2O2)-induced ROS accumulation and animal models of traumatic brain injury. Additionally, we examined the sub-cellular localization of Znf179, and demonstrated that oxidative stress increases Znf179 nuclear shuttling and its interaction with specificity protein 1 (Sp1). Subsequently, the positive autoregulation of Znf179 expression, which is Sp1-dependent, was further demonstrated using luciferase reporter assay and green fluorescent protein (GFP)-Znf179-expressing cells and transgenic mice. The upregulation of Sp1 transcriptional activity induced by the treatment with nerve growth factor (NGF) led to an increase in Znf179 levels, which further protected cells against H2O2-induced damage. However, Sp1 inhibitor, mithramycin A, was shown to inhibit NGF effects, leading to a decrease in Znf179 expression and lower cellular protection. In conclusion, the results obtained in this study show that Znf179 autoregulation through Sp1-dependent mechanism plays an important role in neuroprotection, and NGF-induced Sp1 signaling may help attenuate more extensive (ROS-induced) damage following brain injury.
Keywords: Zinc finger protein 179, Specificity protein 1, Reactive oxygen species, Traumatic brain injury, Nerve growth factor
Graphical abstract
Highlights
-
•
Znf179 levels increase in vitro after hydrogen peroxide treatment.
-
•
Znf179 levels increase in vivo in traumatic brain injury mouse model.
-
•
Oxidative stress increases Znf179 translocation to nucleus.
-
•
Znf179 autoregulates its expression through Sp1-dependent mechanism.
-
•
Sp1-Znf179 pathway plays an important role in neuroprotection.
1. Introduction
Traumatic brain injury (TBI) is a serious public health problem resulting in death or disability [1], due to damages caused by both immediate and secondary injuries. Secondary injuries generate the most damage because they develop and progress over many hours and months after the immediate injury. Environmental stressors include ischemic sugar/oxygen deprivation, inflammatory cytokine release, the release of excitatory neurotransmitters, and metabolic depression. This leads to the accumulation of reactive oxygen species (ROS) [2], [3], which plays a major role in the pathophysiology of various kinds of brain damage. The accumulated ROS can damage cellular organelles and induce apoptosis of brain cells if untreated. Therefore, preventing ROS-mediated cellular damage is important in the therapy of neurological disorders.
Zinc finger protein 179 (Znf179, also known as Zfp179/RNF112/Bfp) is predominantly expressed in human nervous system [4], and it is necessary for the embryonic nervous system development [5]. When retinoic acid (RA) is used to induce P19 EC cells to differentiate into neural cells, Znf179 expression was shown to be upregulated, which results in the increase of p35 and p27 protein levels, leading to the cell cycle arrest at the G0/G1 phase and the initiation of cell differentiation. The inhibition of Znf179 expression was shown to significantly suppress neuronal differentiation [5]. Recently, the results of our study indicated that Znf179 acts as a novel neuroprotector mitigating cell death after hydrogen peroxide (H2O2)-induced oxidative stress, and its accumulation correlates with H2O2 exposure time [6]. However, the mechanism of H2O2-induced elevation of Znf179 expression remains unclear.
Specificity protein 1 (Sp1) is a transcription factor, which is essential for the regulation of the expression of many genes involved in cell growth, angiogenesis, and survival [7]. Previous studies indicated that Sp1 in neurons acts as a pleiotropic oxidative stress response protein [8], [9], [10]. It was shown that the exposure of primary cortical neurons to ischemia-like oxygen-glucose deprivation, Sp1 expression increased, which led to the accumulation of this protein, and the protection of neurons against ischemic damage [9]. Additionally, Na+/Ca2+ exchanger 1 (NCX1), was shown to represent a Sp1 target protein [11], and it is important for the reduction of brain damage following cerebral ischemia [12]. Therefore, Sp1 activation may provide neuroprotection and neurorestoration to cells in vitro and in the animal models of brain ischemia.
In this study, we investigated the mechanisms of Znf179 upregulation during the exposure to stressful conditions. Our results demonstrated that Znf179 positively autoregulates its own expression through Sp1-dependent activation of transcription, and that the increase in nerve growth factor (NGF)-induced Sp1 activity significantly increases Znf179 levels and improves cell survival after H2O2 treatment. These findings may have potential therapeutic value in the treatment of ROS-induced damage in neurotraumatic diseases.
2. Materials and methods
2.1. Experimental animals
We used 10–12 weeks old male wild-type mice (C57BL/6: n =24 and FVB/NJ: n =12, National Laboratory Animal Center, Taipei, Taiwan) and 12 weeks old male Znf179-expressing transgenic mice (n =8) on the C57BL/6 genetic background (Table 1), housed five per cage in an air-conditioned vivarium with free access to food and water. Throughout the study, a 12-h light/dark cycle was maintained with lights on at 8 AM. Each mouse was used for one experiment only. All procedures adhered to the Guidelines for Care and Use of Experimental Animals of the Taipei Medical University (Taipei, Taiwan). Ten C57BL/6 mice were excluded from weight-drop TBI because they: (1) had missed target areas (wild-type: n =4) or (2) died during the experimental procedures (wild-type: n =5; znf179 transgenic: n =1) and within 24 h after the impact (wild-type: n =1).
Table 1.
The number of animals used in each group of weight-drop TBI and CCI.
|
Weight-drop TBI model (C57BL/6) |
CCI model (FVB/NJ) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Wild-type |
znf179 transgenic |
Wild-type |
|||||||
| sham | TBI | Exclusion of TBI | Sham | TBI | Exclusion of TBI | Sham | TBI | Exclusion of TBI | |
| n | 8 | 7 | 9 | 3 | 4 | 1 | 3 | 9 | 0 |
2.2. Bacterial artificial chromosome (BAC) transgene construction and transgenic mice generation
By the assistance of National Laboratory Animal Center, the transgenic mouse overexpressing green fluorescent protein (GFP)-Znf179 under control of znf179 gene promoter presented in a BAC expression vector were generated. Mouse znf179 gene fused to GFP was inserted into the BAC DNA (RP23-354C18) using homologous recombination in Escherichia coli. Subsequently, the recombined BAC clone was injected into fertilized mouse oocytes from C57BL/6 mice, and the oocytes were implanted into the uterus of pseudopregnant foster mothers. After birth, potential founders were screened for the presence of the transgene using PCR with primers: 5′-CGCACCATCTTCTTCAAGGACG-3′ and 5′-TTCTCGTTGGGGTCTTTGCTC-3′. Animal positive for the transgene mated to wild-type (C57BL/6) mice to stabilize the line and for further characterization.
2.3. Weight-drop TBI model
Mice (C57BL/6) weighing 25–30 g were anesthetized lightly by inhalation of isoflurane (3%) in a closed glass chamber for 2 min. The left side of the head, between the eye and ear, was positioned under the guide tube of a weight-drop device and held in place by a sponge. In the device, a cylindrical iron weight (50 g) with a spherical tip was dropped from the full height of the vertical, graduated guide tube (100 cm long). The effect of the injury on the brain was studied at 4 days following the trauma.
2.4. Controlled cortical impact (CCI) model
Mice (FVB/NJ) weighing 25–30 g were anaesthetized and placed in a Kopf stereotaxic head frame (David Kopf Instruments). By using a dental drill, a 5-mm craniotomy was performed over the left parietal cortex between the bregma and lambda. The bone flap was removed and injury was made using a Precision Systems and Instrumentation TBI-0310 (Fairfax Station, VA) that administered a 1 mm cortical compression (3 mm impactor diameter, 2.5 m/s velocity, 150 ms duration dwell time) [13]. Sham animals were anesthetized but no CCI. Body temperature was monitored throughout the surgery by a rectal probe; temperature was maintained at 37.0±0.5 °C using a heated pad.
2.5. Cell culture and transfection
Mouse neuroblastoma Neuro-2a (N2a) cells (ATCC) were cultured in minimum essential medium Eagle (MEM, Invitrogen) containing 10% (vol/vol) fetal bovine serum (FBS), and 1% penicillin/streptomycin in an incubator set at 37 °C with 5% CO2. Cellular differentiation was induced by serum deprivation in MEM/BSA medium (MEM supplemented with 0.1% bovine serum albumin) for 24 h [14], and differentiating N2a cells were used for all experiments. The 80% confluent cells were treated with H2O2 (Sigma-Aldrich), NGF (Invitrogen), and/or mithramycin A (Sigma-Aldrich). Transfection of a reporter plasmid (pGL2-Basic-znf179), protein-expressing vectors (pEGFP, pEGFP-Sp1, and pEGFP-Znf179) or shRNA plasmids (pLKO-shLuc and pLKO-shSp1) was performed by using Lipofectamine 2000 according to the manufacturer's protocol (Invitrogen). Each transfection experiment was performed more than three times as indicated, and each sample in each experiment was prepared in duplicate.
2.6. Western blot analysis
Protein samples from cells (1×106) were separated by electrophoresis on a polyacrylamide gel in the presence of sodium dodecyl sulfate, and then transferred onto a PVDF membrane (Bio-Rad Laboratories). The membrane after transfer was blocked with 5% skim milk in TBST for 1 h and incubated with primary antibodies: Anti-Sp1 (0.5 µg/ml), anti-GAPDH (0.1 µg/ml), anti-actin (1 µg/ml) antibodies from Millipore, anti-p53 (1 µg/ml), anti-phospho-p53 (Ser15) (1 µg/ml), anti-p38 (1 µg/ml), anti-phospho-p38 (Thr180/Tyr182) (1 µg/ml) antibodies from Cell Signaling Technology, and anti-Znf179 (0.5 µg/ml) antibody [5], for 2 h at room temperature. After primary antibody incubation and washing, the membrane was then incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies (0.15 µg/ml, Santa Cruz Biotechnology) for 1 h at room temperature. Finally, the membranes were washed three times with TBST buffer, and the peroxidase was developed by chemiluminescent substrates (GE Healthcare).
2.7. Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA of cells (1×106) was isolated using a TRIzol RNA extraction kit (Invitrogen) and subjected to RT-PCR using SuperScript II reverse transcriptase (Invitrogen). Znf179 primers for PCR were 5′-GAAGGTGGCTGTGTTCTTAGTG-3′ and 5′-GATCGGTATCCTTCAGCTCTTG-3′. Actin primers for PCR were 5′-ACTGGGACGACATGGAGAAG-3′ and 5′-GGTACGACCAGAGGCATACAG-3′. The PCR products were separated using agarose gel electrophoresis and visualized with ethidium bromide staining.
2.8. Luciferase reporter assay
The promoter fragment of mouse znf179 (−533 to −5) was constructed into the pGL2-Basic vector (Promega). Twenty-four hours after transfection and reagent treatments, cells (5×105) were harvested, and the luciferase activities from these cell lysates were measured using the Dual-Luciferase Reporter (DLR) Assay System as per the manufacturer's instructions (Promega).
2.9. DNA affinity precipitation assay (DAPA)
The Sp1-binding oligonucleotide 5′-GCTCTCCCCCTCCCCTCCCCCTCCCTGTCCTT-3′, localized –372 to −341 bp within the promoter of znf179, and the Sp1 mutant oligonucleotide 5′-GCTCTCaCaaTCaaCTCaCaaTCaCTGTCCTT-3′ were biotinylated at 5′ termini and then annealed with their complementary strands. Cell extract (300 µg) was incubated with the biotin-labeled oligonucleotide (1 µg) in 500 µl of binding buffer (60 mM KCl, 12 mM HEPES, pH 7.9, 4 mM Tris–HCl, 5% glycerol, 0.5 mM EDTA, 1 mM dithiothreitol) for 2 h at 4 °C. Then the streptavidin-agarose beads (30 µl, Sigma-Aldrich) were added, and the mixture was incubated for 1 h at 4 °C, in order to pull down the DNA-protein complexes.
2.10. Immunoprecipitation
Cells (1×107) were washed with PBS, and the cellular lysates were prepared by using modified RIPA buffer (50 mM Tris, pH 7.8, 150 mM NaCl, 5 mM EDTA, 0.5% Triton-X100, 0.1% Nonidet P-40, and protease inhibitors). Polyclonal anti-Sp1 antibodies or control immunoglobulin G (IgG) were then added (2 µg/ml) to the lysates and incubated at 4 °C with rotation. After 2 h, protein-A/G agarose beads (30 µl, Santa Cruz Biotechnology) were also added to the mixture and further incubated for 1 h. The protein beads were washed three times with modified RIPA buffer. Bound proteins were eluted by using electrophoresis sample buffer.
2.11. Immunofluorescence
The cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) in PBS according to a method described previously [15]. Immunostaining was conducted with anti-Sp1 (Millipore) and anti-Flag M2 (Sigma-Aldrich) antibodies. The cells were then treated with Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 568-conjugated goat anti-mouse IgG polyclonal antibodies (Invitrogen). Finally, the cells were mounted in 90% glycerol containing 4′-6-diamidino-2-phenylindole (DAPI) (Invitrogen), and examined using a fluorescence microscope (Leica STP6000).
2.12. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay
Cells were plated into 24-well plates at a concentration of 10,000 cells/well. After treatment with reagents as indicated, cells were wash and incubated with MTT (300 µl of 0.5 mg/ml, Sigma Aldrich) in PBS for 1 h at 37 °C. Following incubation, the MTT solution in each well was then carefully removed, and 300 µl of 100% DMSO was added to each well. The amount of resultant formazan crystals was determined by measuring the absorbance at 570 nm using an iMark Microplate Absorbance Reader (Bio-Rad).
2.13. Statistical analysis
Each assay was run in duplicates and similar results were obtained from more than three independent experiments. The statistical analyses for data from the Western blots, reporter assays, and MTT assays were calculated using the unpaired, two-tailed Student's t-test. The significance level was set less than 0.05 (p<0.05).
3. Results
3.1. Peroxide insult increases znf179 promoter activity
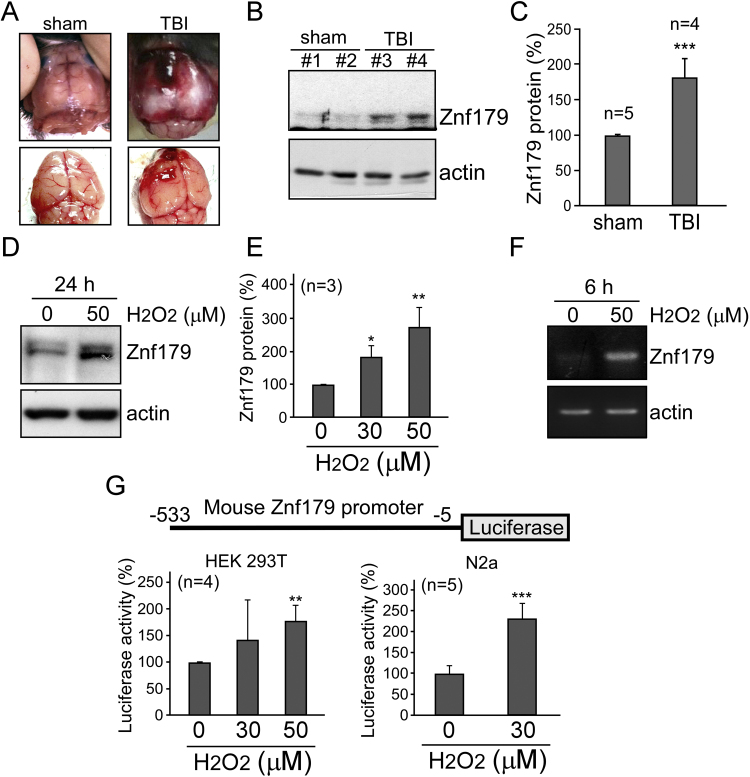
Closed head injuries were successfully induced using the weight-drop device, as demonstrated by the hematomas that formed on top of the brain lesions (Fig. 1A). The levels of Znf179 protein after TBI were analyzed by the western blot analyses of cortical brain tissue homogenates. A statistically significant increase in Znf179 protein levels was detected in the injured brain tissues compared to those in the sham-operated controls (Fig. 1B and C). ROS accumulation is a major cause of neuronal death in the secondary injury following TBI [16], and therefore, we induced ROS generation in differentiated N2a cells using H2O2, which demonstrated that the activation of cell death signaling pathways following H2O2 treatment was similar to that seen in the animal models of TBI (data not shown). Furthermore, to examine the correlation between Znf179 expression and intracellular ROS production, differentiated N2a cells were treated with H2O2 for 24 h, and the obtained results showed that Znf179 expression is induced by H2O2 in a dose-dependent manner (Fig. 1D and E). We further investigated znf179 expression and the activity of its promoter using RT-PCR and luciferase reporter assay, respectively, and demonstrated that peroxide insult leads to the upregulation of znf179 expression, and enhanced transcriptional activity (Fig. 1F and G).
Fig. 1.
Oxidative stress and traumatic brain injury (TBI) induce Znf179 expression. (A) Weight-drop TBIs; A clot in the left mouse hemisphere was generated in order to induce a cerebral injury. (B) Mouse brain (cortex) homogenates from sham-operated and TBI mice were analyzed using western blot. (C) Znf179 levels, normalized to actin levels, were quantified. Results from five independent experiments, described in B, are presented (t-test: ***p<0.001). (D and E) Differentiated neuron-like N2a cells were treated with different H2O2 concentrations for 24 h, and Znf179 levels were analyzed. (E) Bars represent mean±standard error of mean (s.e.m.) obtained in three independent experiments (t-test: *p<0.05, **p<0.01). (F) Znf179 mRNA levels were analyzed in N2a cells treated with 50 µM H2O2 for 6 h. Actin was used as an internal control. (F) Luciferase assay results, using HEK 293 T and N2a cells overexpressing znf179, which were treated with H2O2 for 24 h (**p<0.01, ***p<0.001).
3.2. Sp1 is involved in the regulation of znf179 gene expression
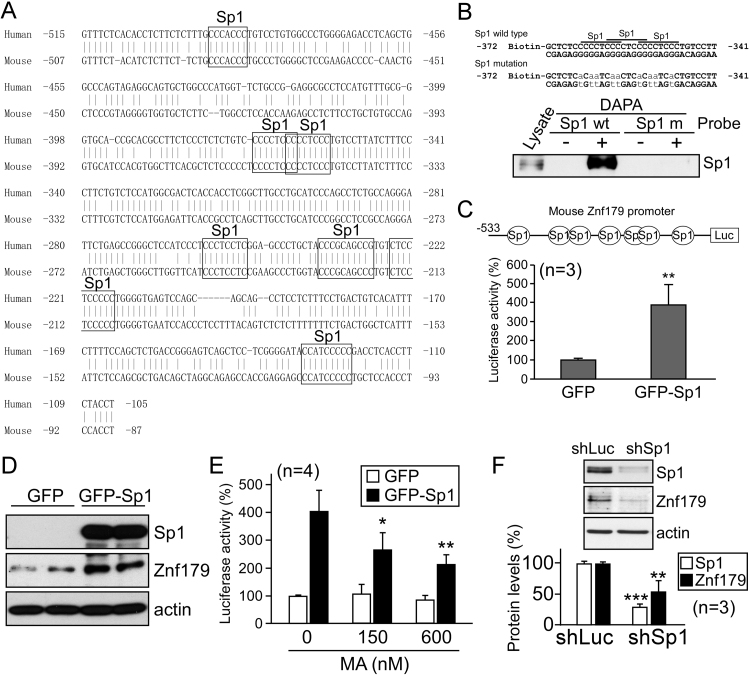
To identify transcription factors regulating znf179 gene expression, we searched for the potential transcription factor-binding sites in the promoter‐proximal region of human and mouse znf179 genes, spanning from position −500 to position −100, relative to the translation start codon, and found at least seven conserved Sp1-binding elements in this promoter region (Fig. 2A). Therefore, we examined Sp1 binding to these Sp1-binding elements, and DAPA results confirmed that Sp1 binds to znf179 promoter, but its binding was significantly lower when Sp1 mutant oligonucleotide was used (Fig. 2B). To confirm the roles of Sp1 in the regulation of znf179 expression, we overexpressed Sp1 in the differentiated N2a cells. Luciferase reporter assay and western blot results showed that both the activity of znf179 promoter and the expression of this protein were increased following the overexpression of Sp1 (Fig. 2C and D). However, a considerable reduction in Znf179 mRNA and protein levels was detected by using the cells treated with Sp1 antagonist mithramycin A (MA) and short hairpin RNA (shRNA) Sp1 knockdown cells (Fig. 2E and F).
Fig. 2.
Sp1 induces znf179 promoter activity. (A) The promoter‐proximal region of human and mouse znf179. (B) DNA affinity precipitation assay (DAPA) results, using N2a cell extracts without (-) or with (+) a DNA probe, containing Sp1 binding sites in the znf179 promoter, or the mutant sites. (C and D) Luciferase assay, using N2a cells co-transfected with the empty vectors or pEGFP-Sp1 together with pGL2-Basic-znf179 (**p<0.01, C). These samples were analyzed using western blot as well (D). (E) Plasmid-transfected cells, as in (C), were treated with mithramycin A (MA) for 24 h. Bars represent luciferase activity (mean±s.e.m.) obtained in three independent experiments (t-test: *p<0.05, **p<0.01). (E) Cells were transfected with pLKO-shSp1 (shSp1), and Sp1 and Znf179 protein levels were analyzed using western blot (**p<0.01, ***p<0.001). pLKO-shLuc (encoding luciferase shRNA, shLuc) was used as a control.
3.3. Znf179 positively autoregulates its own expression
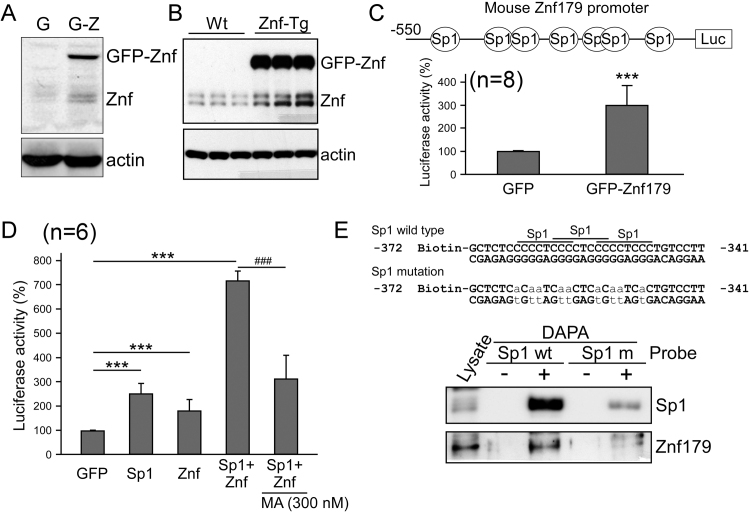
Previously, we had generated a stable cell line, with cells expressing GFP-Znf179 fusion protein [6]. The endogenous Znf179 levels were shown to be upregulated in these cells as well, in comparison with those in the GFP-expressing control cells (Fig. 3A). In order to further elucidate this, znf179 transgenic mice (Znf-Tg) were generated, with constitutive GFP-Znf179 expression in neurons. A significant increase in endogenous Znf179 levels was observed in Znf-Tg mice as well (Fig. 3B), strongly indicating that Znf179 positively autoregulates its expression. We investigated the transcriptional activity of znf179, and confirmed that Znf179 can induce the activity of its own promoter (Fig. 3C). By using Sp1-antagonist treatment combined with DAPA analysis, Sp1 was identified as a critical mediator in Znf179 autoregulation (Fig. 3D and E).
Fig. 3.
Znf179 autoregulates its promoter through Sp1 activity. (A) Znf179 levels were analyzed in GFP (G) and GFP-Znf179 (G-Z) expressing N2a cells. (B) Znf179 levels were analyzed in brain cortex homogenates obtained from the wild-type (Wt) and GFP-Znf179-expressing transgenic mice (Znf-Tg). (C) N2a cells were transfected with reporter plasmid pGL2-Basic-znf179 and co-transfected with pEGFP-Znf179 (GFP-Znf179) or empty pEGFP vector (GFP) for 1 day. The luciferase activity was then analyzed (***p<0.001). (D) pGL2-Basic-znf179-transfected cells were co-transfected with pEGFP, pEGFP-Sp1, and/or pEGFP-Znf179, or treated with MA as indicated for 24 h. Bars represent luciferase activity (mean±s.e.m.) obtained in three independent experiments (The effects of Sp1 and Znf179 on znf179 promoter activity, t-test: ***p<0.001; The effects of MA on znf179 promoter activity, t-test: ###p<0.001). (E) DAPA analysis was performed using a biotinylated DNA probe (+), containing the wild-type or mutated Sp1 binding sequence, which was incubated with cellular lysate harvested from N2a cells, but no probe (-) was used as a control.
3.4. H2O2 stimulates the protein shuttling of cytoplasmic Znf179 into the nucleus
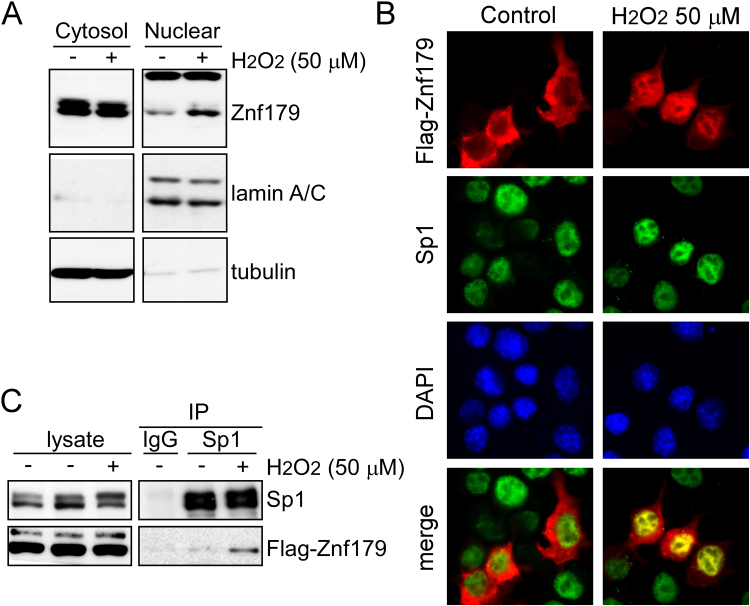
Znf179 was shown to localize to both nucleus and cytoplasm in the primary cultures of mouse cerebellar cells [5]. However, whether stress induces the sub-cellular Znf179 localization changes remains unclear. We performed cellular fractionation to obtain nuclear and cytoplasmic fractions, and Znf179 was shown to localize predominantly in the cytoplasm in untreated cells, but nuclear Znf179 levels increased following a 30-min exposure to oxidative insults (Fig. 4A). Immunofluorescence staining and image analyses demonstrated nuclear import of Znf179 and the co-localization of this protein with Sp1 in nucleus after H2O2 treatment (Fig. 4B). To examine the interactions between Sp1 and Znf179 further, Flag-Znf179 overexpressing cells were used for co-immunoprecipitation analysis, and the result showed an obvious association between Znf179 and Sp1 following H2O2 treatment (Fig. 4C).
Fig. 4.
Hydrogen peroxide treatment induces Znf179 shuttling into the nucleus. (A) Znf179 levels in the nuclear and cytoplasmic fractions of N2a cells treated or not with H2O2 were determined using western blot. The relative purity of the nuclear and cytoplasmic fractions was confirmed using nuclear marker lamin A/C and the cytoplasmic marker tubulin. (B and C) Flag-Znf179-expressing cells were treated with (+) or without (-) H2O2, and analyzed using immunoblotting (B) or fluorescence microscopy (C).
3.5. TBI and H2O2 treatment induce both Sp1 and Znf179 expression
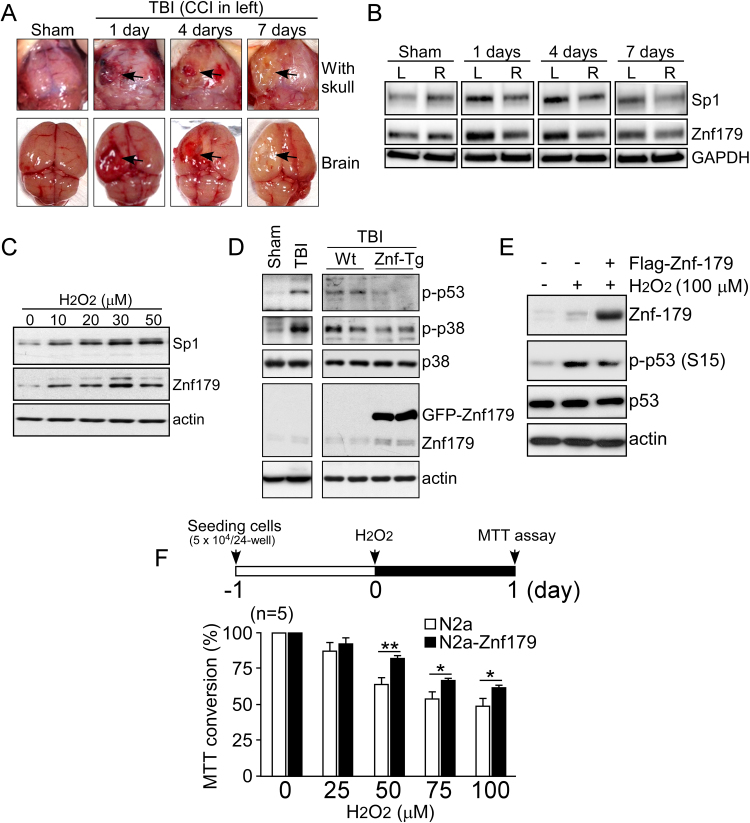
TBI was induced using the CCI model, as demonstrated by hematomas (at day 1–4) and swelling (at day 7) that formed on left side of the brain (Fig. 5A). We examined Sp1 and Znf179 protein levels after TBI. Western blot analyses of cortical brain tissue homogenates demonstrated concordant upregulation of Sp1 and Znf179 levels in the injured tissues compared with those measured in the right hemisphere controls and sham-operated controls (Fig. 5B). Additionally, we analyzed protein levels of Sp1 and Znf179 following H2O2 treatment, and found that the upregulation of Znf179 correlated with the increase in Sp1 levels (Fig. 5C). These results suggested that the Sp1-mediated upregulation of Znf179 expression play an important role during brain injury, particularly in oxidative insults. Therefore, we overexpressed Znf179 in Znf-Tg mice that underwent TBI, and Flag-Znf179 in cells that underwent oxidative stress. Western blot results showed that cell death signaling pathways, such as the phosphorylation of p53 and p38, were induced by TBI and H2O2 treatment, but Znf179 upregulation significantly reduced the activation of these pathways activation (Fig. 5D and E). Furthermore, cell viability was measured using the MTT assay, which showed that Znf179 overexpression can prevent cell death induced by oxidative stress (Fig. 5F).
Fig. 5.
Sp1 and Znf179 display similar expression patterns in stressful conditions, and Znf179 upregulation protects cell from stress-induced damage. (A) Twelve mice were subjected to the controlled cortical impact (CCI). At day 1 (n =3), 4 (n =3), and 7 (n =3) post-injury, the injured mice as well as the sham-operated mice (n =3) were sacrificed and the cerebral injuries in the left parietal lobes were visualized (arrows). (B) The expression of Sp1 and Znf179 in these tissues was analyzed. GAPDH was used as a loading control. (C) Wild-type (Wt, sham: n =3; weight-drop TBI: n =3) and transgenic mice expressing GFP-Znf179 (Znf-Tg, sham: n =3; weight-drop TBI: n =4) were subjected to TBI, and the brain tissues were analyzed using the indicated antibodies. (D) N2a cells were treated with different doses of H2O2 for 24 h, and the levels of the indicated proteins were analyzed. (E) N2a cells were transfected with empty pCMV-Tag 2A vector or Flag-Znf179 expressing vector. After 1 day, cells were treated with or without H2O2 for 30 min and the obtained lysates were analyzed using western blot. (F) N2a cells were treated with different concentrations of H2O2 for 1 day, and cell viability was assessed using the MTT assay (*p<0.05, **p<0.01).
3.6. NGF stimulates Znf179 expression which protects cells against oxidative injury
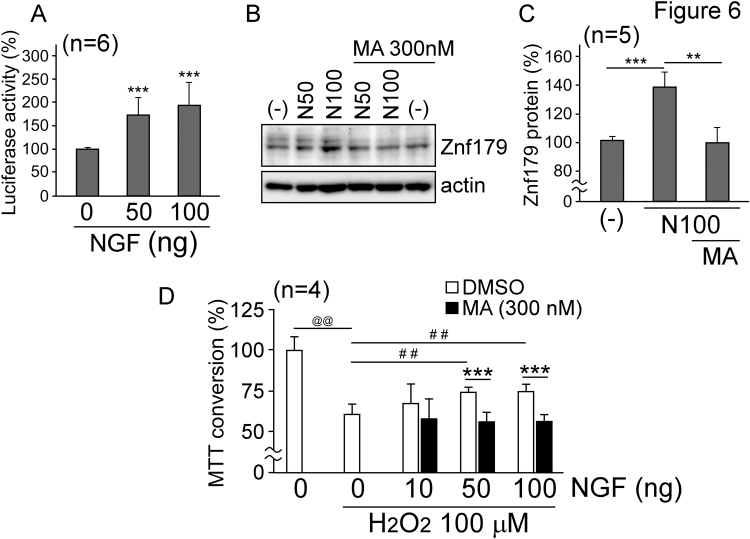
Previous studies indicated that NGF levels in the cerebrospinal fluid and serum of TBI patients are significantly increased [17], [18], and Lin et al. recently demonstrated by using a pseudo lentiviral-delivery method that the long-term expression of NGF can rescue the hippocampus function following the TBI [19]. However, the precise role of NGF in the recovery after head injury remains unclear. As Sp1 is a main downstream effector of NGF [20], which affects znf179 gene expression, we further investigated whether NGF mitigates H2O2-induced cytotoxicity through the activation of Sp1-Znf179 pathway. N2a cells were treated with different doses of NGF for 1 day, and the reporter assay and western blot results confirmed that NGF increases both mRNA and protein levels of Znf179 (Fig. 6A and B), but the pre-treatment with Sp1 antagonist nullified the protective effects of NGF (Fig. 6B and C). Following this, the viability of cells treated with H2O2 was measured using MTT assay, and we showed that NGF prevents H2O2-induced cell death (Fig. 6D). However, Sp1 antagonist treatment reduced cell survival.
Fig. 6.
Znf179 is a target of nerve growth factor (NGF), which decreases cell death rate after H2O2treatment. (A) pGL2-Basic-znf179-transfected N2a cells were treated with NGF for 1 day, and the luciferase activity was analyzed (***p<0.001). (B and C) N2a cells were treated with different concentrations of NGF or co-treated with 300 nM MA for 1 day. Znf179 and actin levels were analyzed (B). (C) Znf179 levels, normalized to the loading control were quantified (t-test: **p<0.01, ***p<0.001). (D) N2a cells were treated with H2O2 or co-treated with different concentrations of NGF and/or 300 nM MA for 24 h. Following the treatment, cell viability was assessed using the MTT assay. Bars represent means±s.e.m. of the results obtained in three independent experiments (H2O2 effects on N2a cells, t-test: @@p<0.01; NGF effects on H2O2-treated N2a cells, t-test: ##p<0.01; MA effects on H2O2-/NGF-treated N2a cells, t-test: ***p<0.001).
4. Discussion
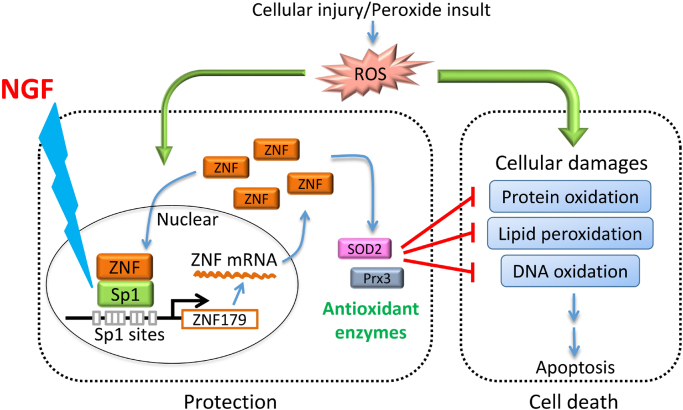
The data obtained in this study demonstrate that Sp1-mediated upregulation of Znf179 expression plays a role in the mitigation of cell death following the TBI. We showed that the expression of Sp1 and its downstream target, znf179, is upregulated after TBI and peroxide insult, that H2O2 induces positive Znf179 autoregulation by stimulating Sp1 activity, and that NGF-stimulated Sp1 activation leads to an increase in Znf179 expression, providing maximal protection against oxidative injury (Fig. 7). These findings indicate that NGF-induced increase in neuroprotective functions of Sp1-Znf179 pathway may allow the development of new therapeutic approaches for the prevention or reduction of extensive secondary damage caused by TBI.
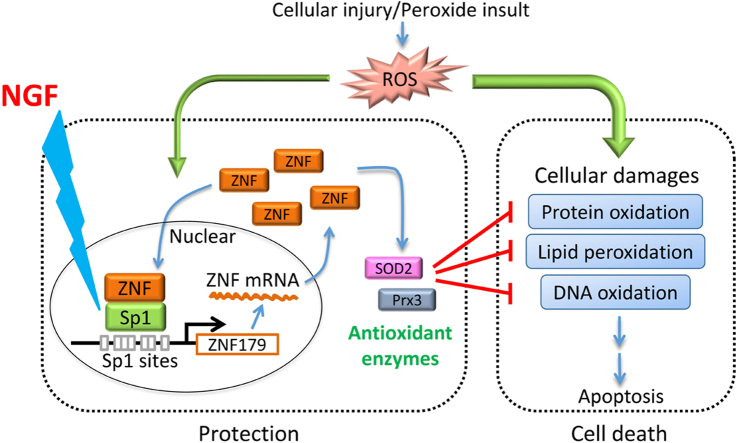
Fig. 7.
The illustration of the neuroprotective mechanisms of NGF. NGF regulates Sp1-Znf179 pathway, mitigating reactive oxygen species (ROS)-mediated cytotoxic effects. The expression of antioxidant enzymes, such as peroxiredoxin III (Prx3) and superoxide dismutase 2 (SOD2), is regulated by Znf179 [6].
Using tissue distribution analysis, Znf179 was shown to be primarily expressed in brain and upregulated during neuronal differentiation [5], [21]. Recently, several studies revealed that Znf179 is an important regulator of neural development, synaptic plasticity, and cellular survival [5], [6], [22], [23]. During the embryonic development, Znf179 induces cell cycle arrest at the G0/G1 phase, which initiates neuronal differentiation [5]. znf179-/- mouse embryos were shown to have smaller brains compared with those of the wild-type mice, and the knockout of this gene led to an early death in utero, indicating that Znf179 is necessary for the embryonic neural development [24]. In adult brain, Znf179 regulates excitatory synapses and spine density at the cytoplasm through endosomal membrane association [22]. The treatment of brain cells with pro-inflammatory cytokines or the exposure to oxidative stress were shown to lead to an increase in Znf179 levels, which further leads to the downregulation of pro-apoptotic genes and the upregulation of antioxidant gene expression [6], [23]. Therefore, high expression levels of Znf179 are crucial not only in the fetal period during the development of the nervous system, but also in the adult brain, where this protein is involved in the maintenance of neural functions and protection of the nervous tissue cells from stress-induced damage. A recent study demonstrated that in astrocytes, CCAAT/enhancer binding protein delta (CEBPD) represents a key factor leading to znf179 upregulation upon interleukin-1β treatment [23]. However, in neurons, the mechanisms regulating the expression of this gene remain unknown. Here, we identified Sp1 as an additional key factor leading to znf179 upregulation during the differentiation of neuron-like cells, and the increase in the levels of Sp1 protein coincided with Znf179 expression levels during TBI and H2O2-induced oxidative damage. Furthermore, we demonstrated a novel Sp1-mediated mechanism of positive Znf179 autoregulation. Sp1 binding to Znf179 may induce the activation of the Znf179-positive autoregulatory loop, since their interaction was detected within 30 min after the peroxide treatment. To further examine the protective effects of Sp1-Znf179 pathway, Znf179 overexpression and NGF-induced Sp1 activation were analyzed, and we conformed that the upregulation of Sp1-Znf179 pathway attenuates the damage after TBI and oxidative insults.
Previous studies showed that Znf179 is a bifunctional transcription regulator capable of activating and inhibiting gene expression [6], [23]. Znf179 overexpression in U373MG human glioma cells led to the significant changes in the expression of 437 genes (98 upregulated and 339 downregulated) [23]. Znf179 was shown to interact with transcription repressors, such as promyelocytic leukemia zinc finger (Plzf) [25], and this repression complex targets several pro-apoptotic genes, including RING1- and YY1-binding protein (RYBP), Bcl2-interacting killer (BIK), growth arrest and DNA-damage-inducible (GADD45B), and insulin-like growth factor binding protein 3 (IGFBP3), exerting anti-apoptotic roles in astrocytes [23]. Additionally, the expression of several genes, for instance CDK 5 regulatory subunit (CDK5R), peroxiredoxin III (Prx3), and superoxide dismutase 2 (SOD2), is upregulated by Znf179 [5], [6]. However, the mechanisms of Znf179-mediated gene upregulation are poorly understood, and the transcriptional activator inducing Znf179 expression has not been elucidated. Sp1 is a redox-regulated transcriptional activator [26]. Here, we demonstrated that Znf179 interacts with Sp1 in order to induce its own expression following the oxidative stress. As Sp1 regulates the levels of several neuroprotective proteins and antioxidants after brain damage [11], [27], [28], [29], [30], Znf179 may upregulate the expression of these genes through the activation of Sp1. However, the number of genes targeted by Znf179-Sp1 complex requires further investigation.
Although Znf179 resides mainly in the cytoplasm and regulates synaptic plasticity, including excitatory synapses and spine density [22], here we observed nuclear translocation of this protein following the oxidative stress. Previous study showed that the interaction between Plzf and Znf179 and the overexpression of Plzf lead to Znf179 translocation from cytoplasm to nucleus [25]. The increase in nuclear levels of Sp1 may play a similar role as Plzf, affecting sub-cellular localization of Znf179 after H2O2 treatment. In our previous studies, we demonstrated that protein-protein interactions, protein levels, and the transcriptional activity of Sp1 are regulated by post-translational modifications (PTMs), such as phosphorylation [31], [32], [33], [34]. Additionally, oxidative stress affects the phosphorylation state of Sp1 through the induction of various signaling pathways [35]. Therefore, it is possible that nerve injury-mediated ROS generation induces Sp1 phosphorylation, increasing the ability of this protein to interact with Znf179. Furthermore, NGF was shown to elevate Sp1 phosphorylation levels through the induction of phosphatidylinositol 3-kinase/PKC-ζ pathway [36]. Taken together, NGF-induced increase in Sp1 phosphorylation may protect the neurons against oxidative stress, through the induction of Sp1-Znf179 interactions, triggering the activation of positive Znf179 autoregulatory loop.
In conclusion, the results obtained in this study indicate that Znf179 represents a novel binding partner of Sp1, and the Sp1-Znf179 pathway plays an important role in the neuroprotection against oxidative stress. Notably, NGF induces Znf179 expression through a Sp1-dependent autoregulatory loop, exerting its protective effects. The obtained results may be further used for the development of novel treatment of TBI and injury-induced oxidative damage during the subacute posttraumatic period.
Acknowledgments
This work was supported by the Ministry of Science and Technology, Taiwan [grant numbers MOST 103-2320-B-038-046, MOST 105-2320-B-038-063, MOST 105-2911-I-038-503, and MOST 104-2923-B-038-002] and Taipei Medical University Hospital [grant numbers 104TMU-TMUH-01].
Contributor Information
Jian-Ying Chuang, Email: chuangcy@tmu.edu.tw.
Tzu-Jen Kao, Email: geokao@tmu.edu.tw.
Shu-Hui Lin, Email: linivy9@yahoo.com.tw.
An-Chih Wu, Email: anchinwu@gmail.com.
Pin-Tse Lee, Email: pin-tse.lee@nih.gov.
Tsung-Ping Su, Email: TSU@intra.nida.nih.gov.
Shiu-Hwa Yeh, Email: bau9763@nhri.org.tw.
Yi-Chao Lee, Email: yc590626@gmail.com.
Chung-Che Wu, Email: johnwu@tmu.edu.tw.
Wen-Chang Chang, Email: wcchang@tmu.edu.tw.
References
- 1.Chua K.S., Ng Y.S., Yap S.G., Bok C.W. A brief review of traumatic brain injury rehabilitation. Ann. Acad. Med. Singap. 2007;36:31–42. [PubMed] [Google Scholar]
- 2.Ugoya S.O., Tu J. Bench to bedside of neural stem cell in traumatic brain injury. Stem Cells Int. 2012;2012:141624. doi: 10.1155/2012/141624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong Y., Mahmood A., Chopp M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura T., Arakawa Y., Inoue S., Fukushima Y., Kondo I., Koyama K., Hosoi T., Orimo A., Muramatsu M., Nakamura Y., Abe T., Inazawa J. The brain finger protein gene (ZNF179), a member of the RING finger family, maps within the Smith-Magenis syndrome region at 17p11.2. Am. J. Med. Genet. 1997;69:320–324. [PubMed] [Google Scholar]
- 5.Pao P.C., Huang N.K., Liu Y.W., Yeh S.H., Lin S.T., Hsieh C.P., Huang A.M., Huang H.S., Tseng J.T., Chang W.C., Lee Y.C. A novel ring finger protein, Znf179, modulates cell cycle exit and neuronal differentiation of P19 embryonal carcinoma cells. Cell Death Differ. 2011;18:1791–1804. doi: 10.1038/cdd.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su T.C., Lin S.H., Lee P.T., Yeh S.H., Hsieh T.H., Chou S.Y., Su T.P., Hung J.J., Chang W.C., Lee Y.C., Chuang J.Y. The sigma-1 receptor-zinc finger protein 179 pathway protects against hydrogen peroxide-induced cell injury. Neuropharmacology. 2016;105:1–9. doi: 10.1016/j.neuropharm.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L., Davie J.R. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. 2010;192:275–283. doi: 10.1016/j.aanat.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Kiryu-Seo S., Kato R., Ogawa T., Nakagomi S., Nagata K., Kiyama H. Neuronal injury-inducible gene is synergistically regulated by ATF3, c-Jun, and STAT3 through the interaction with Sp1 in damaged neurons. J. Biol. Chem. 2008;283:6988–6996. doi: 10.1074/jbc.M707514200. [DOI] [PubMed] [Google Scholar]
- 9.Yeh S.H., Yang W.B., Gean P.W., Hsu C.Y., Tseng J.T., Su T.P., Chang W.C., Hung J.J. Translational and transcriptional control of Sp1 against ischaemia through a hydrogen peroxide-activated internal ribosomal entry site pathway. Nucleic Acids Res. 2011;39:5412–5423. doi: 10.1093/nar/gkr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J., Kosaras B., Aleyasin H., Han J.A., Park D.S., Ratan R.R., Kowall N.W., Ferrante R.J., Lee S.W., Ryu H. Role of cyclooxygenase-2 induction by transcription factor Sp1 and Sp3 in neuronal oxidative and DNA damage response. FASEB J. 2006;20:2375–2377. doi: 10.1096/fj.06-5957fje. [DOI] [PubMed] [Google Scholar]
- 11.Formisano L., Guida N., Valsecchi V., Cantile M., Cuomo O., Vinciguerra A., Laudati G., Pignataro G., Sirabella R., Di Renzo G., Annunziato L. Sp3/REST/HDAC1/HDAC2 complex represses and Sp1/HIF-1/p300 complex activates ncx1 gene transcription, in brain ischemia and in ischemic brain preconditioning, by epigenetic mechanism. J. Neurosci. 2015;35:7332–7348. doi: 10.1523/JNEUROSCI.2174-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valsecchi V., Pignataro G., Del Prete A., Sirabella R., Matrone C., Boscia F., Scorziello A., Sisalli M.J., Esposito E., Zambrano N., Di Renzo G., Annunziato L. NCX1 is a novel target gene for hypoxia-inducible factor-1 in ischemic brain preconditioning. Stroke. 2011;42:754–763. doi: 10.1161/STROKEAHA.110.597583. [DOI] [PubMed] [Google Scholar]
- 13.Boulet T., Kelso M.L., Othman S.F. Long-term in vivo imaging of viscoelastic properties of the mouse brain after controlled cortical impact. J. Neurotrauma. 2013;30:1512–1520. doi: 10.1089/neu.2012.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evangelopoulos M.E., Weis J., Kruttgen A. Signalling pathways leading to neuroblastoma differentiation after serum withdrawal: HDL blocks neuroblastoma differentiation by inhibition of EGFR. Oncogene. 2005;24:3309–3318. doi: 10.1038/sj.onc.1208494. [DOI] [PubMed] [Google Scholar]
- 15.Tsai S.Y., Chuang J.Y., Tsai M.S., Wang X.F., Xi Z.X., Hung J.J., Chang W.C., Bonci A., Su T.P. Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc. Natl. Acad. Sci. USA. 2015;112:E6562–E6570. doi: 10.1073/pnas.1518894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angeloni C., Prata C., Dalla Sega F.V., Piperno R., Hrelia S. Traumatic brain injury and NADPH oxidase: a deep relationship. Oxid. Med. Cell Longev. 2015;2015:370312. doi: 10.1155/2015/370312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiaretti A., Antonelli A., Riccardi R., Genovese O., Pezzotti P., Di Rocco C., Tortorolo L., Piedimonte G. Nerve growth factor expression correlates with severity and outcome of traumatic brain injury in children. Eur. J. Paediatr. Neurol. 2008;12:195–204. doi: 10.1016/j.ejpn.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuang Y.F., Li J. Serum EGF and NGF levels of patients with brain injury and limb fracture. Asian Pac. J. Trop. Med. 2013;6:383–386. doi: 10.1016/S1995-7645(13)60043-7. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y., Wan J.Q., Gao G.Y., Pan Y.H., Ding S.H., Fan Y.L., Wang Y., Jiang J.Y. Direct hippocampal injection of pseudo lentivirus-delivered nerve growth factor gene rescues the damaged cognitive function after traumatic brain injury in the rat. Biomaterials. 2015;69:148–157. doi: 10.1016/j.biomaterials.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Sobue S., Hagiwara K., Banno Y., Tamiya-Koizumi K., Suzuki M., Takagi A., Kojima T., Asano H., Nozawa Y., Murate T. Transcription factor specificity protein 1 (Sp1) is the main regulator of nerve growth factor-induced sphingosine kinase 1 gene expression of the rat pheochromocytoma cell line, PC12. J. Neurochem. 2005;95:940–949. doi: 10.1111/j.1471-4159.2005.03399.x. [DOI] [PubMed] [Google Scholar]
- 21.Seki N., Hattori A., Muramatsu M., Saito T. cDNA cloning of a human brain finger protein, BFP/ZNF179, a member of the RING finger protein family. DNA Res. 1999;6:353–356. doi: 10.1093/dnares/6.5.353. [DOI] [PubMed] [Google Scholar]
- 22.Lomash R.M., Gu X., Youle R.J., Lu W., Roche K.W. Neurolastin, a dynamin family GTPase, regulates excitatory synapses and spine density. Cell Rep. 2015;12:743–751. doi: 10.1016/j.celrep.2015.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S.M., Lee Y.C., Ko C.Y., Lai M.D., Lin D.Y., Pao P.C., Chi J.Y., Hsiao Y.W., Liu T.L., Wang J.M. Increase of zinc finger protein 179 in response to CCAAT/enhancer binding protein delta conferring an antiapoptotic effect in astrocytes of Alzheimer's disease. Mol. Neurobiol. 2015;51:370–382. doi: 10.1007/s12035-014-8714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsou J.H., Yang Y.C., Pao P.C., Lin H.C., Huang N.K., Lin S.T., Hsu K.S., Yeh C.M., Lee K.H., Kuo C.J., Yang D.M., Lin J.H., Chang W.C., Lee Y.C. Important roles of ring finger protein 112 in embryonic vascular development and brain functions. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-9812-7. [DOI] [PubMed] [Google Scholar]
- 25.Lin D.Y., Huang C.C., Hsieh Y.T., Lin H.C., Pao P.C., Tsou J.H., Lai C.Y., Hung L.Y., Wang J.M., Chang W.C., Lee Y.C. Analysis of the interaction between Zinc finger protein 179 (Znf179) and promyelocytic leukemia Zinc finger (Plzf) J. Biomed. Sci. 2013;20:98. doi: 10.1186/1423-0127-20-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu H., Lee J., Zaman K., Kubilis J., Ferrante R.J., Ross B.D., Neve R., Ratan R.R. Sp1 and Sp3 are oxidative stress-inducible, antideath transcription factors in cortical neurons. J. Neurosci. 2003;23:3597–3606. doi: 10.1523/JNEUROSCI.23-09-03597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo S.J., Kim H.T., Cho G., Rho H.M., Jung G. Sp1 and C/EBP-related factor regulate the transcription of human Cu/Zn SOD gene. Gene. 1996;178:177–185. doi: 10.1016/0378-1119(96)00383-6. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y., Porntadavity S., Clair D.K., St Transcriptional regulation of the human manganese superoxide dismutase gene: the role of specificity protein 1 (Sp1) and activating protein-2 (AP-2) Biochem. J. 2002;362:401–412. doi: 10.1042/0264-6021:3620401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan P.H. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann. N. Y. Acad. Sci. 2005;1042:203–209. doi: 10.1196/annals.1338.022. [DOI] [PubMed] [Google Scholar]
- 30.Mikawa S., Kinouchi H., Kamii H., Gobbel G.T., Chen S.F., Carlson E., Epstein C.J., Chan P.H. Attenuation of acute and chronic damage following traumatic brain injury in copper, zinc-superoxide dismutase transgenic mice. J. Neurosurg. 1996;85:885–891. doi: 10.3171/jns.1996.85.5.0885. [DOI] [PubMed] [Google Scholar]
- 31.Chuang J.Y., Chang W.C., Hung J.J. Hydrogen peroxide induces Sp1 methylation and thereby suppresses cyclin B1 via recruitment of Suv39H1 and HDAC1 in cancer cells. Free Radic. Biol. Med. 2011;51:2309–2318. doi: 10.1016/j.freeradbiomed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Chuang J.Y., Hung J.J. Overexpression of HDAC1 induces cellular senescence by Sp1/PP2A/pRb pathway. Biochem. Biophys. Res. Commun. 2011;407:587–592. doi: 10.1016/j.bbrc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 33.Chuang J.Y., Wang S.A., Yang W.B., Yang H.C., Hung C.Y., Su T.P., Chang W.C., Hung J.J. Sp1 phosphorylation by cyclin-dependent kinase 1/cyclin B1 represses its DNA-binding activity during mitosis in cancer cells. Oncogene. 2012;31:4946–4959. doi: 10.1038/onc.2011.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang W.C., Hung J.J. Functional role of post-translational modifications of Sp1 in tumorigenesis. J. Biomed. Sci. 2012;19:94. doi: 10.1186/1423-0127-19-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors, redox. Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rojo A.I., Salina M., Salazar M., Takahashi S., Suske G., Calvo V., de Sagarra M.R., Cuadrado A. Regulation of heme oxygenase-1 gene expression through the phosphatidylinositol 3-kinase/PKC-zeta pathway and Sp1. Free Radic. Biol. Med. 2006;41:247–261. doi: 10.1016/j.freeradbiomed.2006.04.002. [DOI] [PubMed] [Google Scholar]