Abstract
Complex behaviors, such as aggression, are comprised of distinct stereospecific behavioral patterns (modules). How such patterns get wired into nervous systems remains unknown. Recently, we reported on a quantitative analysis of fighting behavior in male flies of the common Canton-S strain of Drosophila melanogaster. Here, we report a similar analysis of fighting behavior in females of the same species. Fights were carried out between pairs of virgin and pairs of mated females in competition for a yeast resource. Each fight was videotaped and analyzed by using transition matrices and Markov chain analyses. We observe only small difference in fighting intensity between virgin and mated females. In contrast to what is seen in male fights, however, no clear hierarchical relationship is formed in the female fights. A further comparison of the behavioral patterns making up male and female fights reveals that some modules are shared by both sexes, whereas others are highly selective. Within the shared components, transitions between the modules also show gender-selective differences. By using the powerful genetic methods available for examining behavior in fruit flies, it should be possible to use the gender-selective differences in fighting behavior to address the question of how these behavioral patterns get established in the brains of fruit flies.
Survival in a complex world requires that organisms maintain a rich repertoire of context-dependent, malleable, and recognizable behaviors. To describe these behaviors, ethograms are used. Ethograms are word descriptions of all distinct patterns (modules, components) that are seen when organisms engage in behaviors like mating, foraging for food, or fighting. Although differences exist in the details of expression of the patterns and in when and for how long animals display them during behavioral rituals, behavioral modules remain as distinct and recognizable entities that can be shown by statistical analyses to have unique likelihoods of occurrence and of transitional linkages with each other. How such stereotyped modules become wired into nervous systems remains unknown. One might anticipate that just as combinatorial codes and sequences of gene expression are involved in specifying the identity, connectivity, and ultimate fate of neurons during development (1–3), so too might combinatorial codes of genes be involved in the initial establishment of patterns of behavior. In the studies reported here, we (i) report a quantitative analysis of agonistic behavior in female Drosophila melanogaster and (ii) compare the patterns of fighting behavior between pairs of male and between pairs of female fruit flies. The comparisons reveal that although many modules that make up fighting behavior are similar in male and female fights, some male-selective and some female-selective components exist. By using the powerful genetic methods that are available for studies in fruit flies, it should be possible to exploit these differences to attempt to identify genes important in characterizing male- and female-selective patterns of aggression in the fruit fly nervous system.
Although not as extensively studied or widely known as male aggression, there have been numerous studies of female aggression in both vertebrate and invertebrate species (4–15). Among vertebrates, rodents have been used extensively in studies of female aggression (4–8), but detailed studies also have been performed with other species (16, 17). Maternal aggression in defense of pups dominates this literature (4, 16, 17), but isolation-induced and territorial female aggression has been reported and characterized also (5–8). In laboratory-reared wild populations of female mice, isolation-induced spontaneous aggression was found to be a selectable trait (18). Careful comparative studies have revealed that similar amine, steroid, and peptide hormones modulate aggression in both males and females, and in some cases, parallel changes have been seen in hormone usage in males and females during aggressive interactions (4, 19). In nonsocial insects, aggression among females occurs but has been rarely reported (for e.g. in cockroaches, see ref. 20), whereas in social insects, females play dominant roles in the social hierarchy by frequently displaying aggression to maintain their position in the colony (11–15). In crustacean species like lobsters, aggression is also seen between females with both sexes reported to show identical patterns of behavior during fights (20). Males appear to show greater proportions of higher-intensity components during fights.
In D. melanogaster, male aggression has been characterized carefully by our laboratory (21) and others (22), but little is known of female aggression (10). As with male aggression, the first reports of female aggression in D. melanogaster begin with Sturtevant (23), who noticed that females occasionally would move quickly at males with their wings extended as a nonreceptive response to male courtship. Manning (24) mentions female D. melanogaster kicking, flicking their wings, and twisting their abdomens to escape courting males (see also ref. 25). Jacobs observed female–female aggression in ebony mutants as “brief charges at flies standing in their paths as they were feeding” (26). The first formal study of female aggression in D. melanogaster was carried out by Ueda and Kidokoro (10), who focused their studies on the following three observed behaviors: “approach,” “lunge,” and erection of wings. They reported that these behavioral patterns are similar to those seen in male flies, and they observed further that aggression was enhanced by isolation and the availability of fresh yeast. In this article, we extend our studies of fighting behavior in D. melanogaster with a detailed analysis of fighting between females. A simple dyadic experimental paradigm was established, similar to the one used to study male fighting behavior (21). Comparisons are drawn between mated and virgin female fights and in the patterns of fighting behavior seen in males and females.
Materials and Methods
Fly Stock and Rearing Conditions. Wild-type Canton-S D. melanogaster were obtained from the Bloomington Stock Center (Bloomington, IN). The stock was maintained on standard cornmeal medium and kept on a 12 h/12 h day/night cycle at 25°C with 50% relative humidity. To collect and maintain socially naïve adults, ferrate pupae were isolated in individual 16 × 100-mm glass vials containing 2 ml of food medium without yeast. For mated females, after 24 h of isolation, a socially naïve male was added to the isolation vial for a period of 3 days and then removed. After 4 days, isolated flies were anesthetized briefly with CO2 to paint an identifying mark on the dorsal side of the thorax by using acrylic paint and returned to isolation vials. Flies were maintained for 1 additional day to allow for recovery from anesthesia before testing.
Experimental Protocol. Fights between pairs of males and pairs of females were conducted by using conditions modified from those used previously in fights between males (4). In place of a decapitated female, ≈2 μl of yeast paste in H2O was applied to the center of the food surface. Same-sex pairs, raised under similar conditions and painted different colors, were introduced to the chamber by gentle aspiration. A digital video camera was used to videotape the food-cup surface for 1 h after introducing the females to the chamber. All interactions between the flies were analyzed for 0.5 h after both flies were on the food cup. If no interactions were seen during the first 0.5 h after introducing flies to a chamber, the trial was discarded. Experiments were run at 1–5 h after subjective dawn at 22–26°C at 19–46% relative humidity.
Behavioral Analysis. By using imovie software (Apple), video recordings of each trial were screened for agonistic meetings (called encounters) between the pairs of flies. For an example movie, see Movie 1, which is published as supporting information on the PNAS web site. Encounters are defined as when animals are within one body length of each other and show one or more of the behavioral patterns defined by the ethogram (Table 1). A period of >2 s without behavioral displays defines the end of an encounter. Each encounter was analyzed by scoring the behavioral patterns (interactions) shown by each fly. The position of each fly relative to the yeast at the beginning and end of each encounter, as well as the onset time and duration of each encounter, were recorded.
Table 1. Ethogram of female fighting.
| Designation | Behavioral pattern |
|---|---|
| Flying retreat* | Flying away from the opponent |
| Sidestep retreat* | Rapid sidesteping away from the opponent |
| Walking retreat* | Walking away from the opponent |
| Fencing threat† | Extending the middle legs without contacting the opponent |
| Turn towards† | Turning to face the opponent from an adjacent position |
| Approach† | Walking towards the opponent |
| Wing threat† | Brief (<1 s) lifting of one or both wings to a 45°-90° angle |
| Fencing and feeding‡ | Extending the middle or rear legs and contacting the opponent while feeding on the yeast |
| Side low-posture fencing‡ | Extending the middle or rear legs and contacting the opponent |
| Low-posture fencing‡ | Extending the forelegs and contacting the opponent in a normal standing posture |
| High-posture fencing‡ | Standing tall on the middle and rear legs and contacting the opponent with the forelegs |
| Head butt (thrust)‡ | Thrusting the torso towards the opponent and appearing to strike the opponent with the head: one of the forelegs may be elevated, usually followed by recoiling of the torso |
| Lunge (thrust)‡ | Thrusting the torso at an upward angle towards the opponent with both forelegs extended and usually collapsing on the opponent to end the thrust |
| Shove (thrust)‡ | Thrusting the torso towards the opponent with both forelegs extended without recoil |
| Thrust with a wing threat‡ | Thrusting and briefly (<1 s) lifting of one or both wings to a 45°-90° angle |
| High-posture fencing with a wing threat‡ | Standing tall on the middle and rear legs and contacting the opponent with the forelegs and briefly (<1 s) lifting of one or both wings to a 45°-90° angle |
Retreat behavioral pattern.
Noncontact behavioral pattern.
Contact behavioral pattern.
Statistical Analysis. The recorded behaviors were analyzed by using a first-order Markov chain analysis (25), which calculates the likelihood of all transitions between behavioral patterns. Behavioral transitions were identified as changes in the behavioral pattern displayed between the pair of flies. Behavioral patterns were chronologically distinguishable except for the “fencing” patterns, which are continuous and, thus, displayed with other behavioral patterns. When two behavioral patterns were reported at the same time, the higher-intensity pattern was selected according to the following order: “noncontact fencing,” “side low-posture fencing,” “low-posture fencing,” “high-posture fencing,” and then all other behavioral patterns. Grouping of similar behavioral patterns was done as described in Results. The probability of statistical similarity between two matrices with the same dimensions was calculated by using Mantel matrix procedures. All statistical analyses were performed by using programs that are in the public domain and freely available at http://casper.bgsu.edu/∼software/java.
Results
Selection of Experimental Conditions. To study aggressive interactions between female flies, we sought conditions that (i) would minimize the time between introduction of flies to the arena and the time when both were on the food cup (the fight latency), and (ii) would maximize the amount of agonistic behavior seen when both flies were on the food cup. Not surprisingly, females did not respond effectively to the following cues that worked well in earlier studies with males: a food cup with a headless mated female, a drop of apple juice on the food cup, and illumination from above (21). In this scenario, female flies pushed the headless female off of the food cup by using “head butt” and fencing.
To optimize conditions, we replaced the headless female and apple juice with a small drop of yeast paste in the center of the food cup (see ref. 10) in an attempt to force a confrontation between the flies over a limited resource. Our concern that losing flies would turn to the standard fly food covering the rest of the food cup was unfounded, because (i) flies continued to compete over the yeast paste throughout the fight even though the rest of the surface of the vial cap was food, and (ii) replacing the food with agar and adding a drop of yeast paste caused a significant decrease in fighting behavior (45.8 vs. 17 behavioral transitions per 15-min period, P = 0.016). Other variables that were explored in preliminary studies were the age of the flies, the effects of starvation, and the use of virgin and mated females. The 5-day-old nonstarved females gave optimal results, and a detailed comparison of fighting behavior in virgin and mated females is presented below.
The Ethogram of Female Fighting Behavior. Extensive observations of videotaped fights in female–female pairs of D. melanogaster produced a listing of 15 discernable behavioral patterns (Table 1). Leg extensions (fencing) were done either from a low-normal body posture or from an extended leg, stilt-walking, high posture. In low-posture fencing, any adjacent legs could be used, but in high-posture fencing only the forelegs were used. During low-posture fencing, females often extend the second leg toward the opponent without making contact (“fencing threat”). “Thrusts” were displayed in the following three patterns. (i) The body snapped forward with negligible change in leg position (“head butt”); (ii) the body snapped forward along with the forelegs in a level trajectory (“shove”); or (iii) the body snapped forward with the forelegs extended in an upward trajectory followed by a downward collapse (“lunge”). All thrusts were done with the head directed toward the opponent. Wing threats consisted of raising one or both wings upward at a 45–90° angle for <1 s, followed by simultaneous or staggered lowering of the wings. Wing threats were observed (i) while flies were standing still (usually facing the opponent), (ii) while flies were approaching the opponent, (iii) during high-posture fencing, and (iv) during all types of thrusts. Retreats usually involved losing flies walking away, sometimes flying away, and rarely rapidly side-stepping away.
Comparison of Fighting Behavior in Mated vs. Virgin Female Flies. Fights between 26 pairs of virgin females and 26 pairs of mated females were compared by constructing of transition matrices and using them to carry out first-order Markov chain analyses. Of the possible methods for constructing transition matrices (25), we chose a method in which the highest intensity level behavioral action seen during an encounter was scored (see Materials and Methods). Such a method gives valuable information about the dynamic of fights but ignores repeated sequences. Within the matrices, to simplify the schematic representation of the behavior and/or to allow for large enough numbers of transitions for analysis, certain patterns listed in the ethogram were grouped into single categories. Thus, we combined the following: turning toward opponent and walking approach were combined into “approach,” shove and lunge were combined into “shove/lunge,” and the various forms of retreat were grouped into “retreat.” In addition, “fencing and feeding” was scored as a pattern separate from other low-posture fencing patterns because feeding can affect the next behavioral pattern that is seen.
The differences in fighting behavior between virgin and mated females are small (Fig. 1). In general, they suggest that mated females are willing to fight for longer periods of time, that their fights escalate to higher intensity levels more readily, and that they retreat less from encounters. For example, mated females go through more behavioral transitions than virgin females in a 30-min fight period (94 mated vs. 71 virgin transitions per fight). The difference is not due to an increased number of encounters per 30-min period (17.1 ± 1.6 mated vs. 15.3 ± 1.1 virgins, P = 0.36) but is reflected in a 21% increase in encounter duration (11.6 ± 0.6 s mated vs. 9.6 ± 0.4 s virgins, P = 5.22E-03). The encounter duration was found to be independent of the interencounter interval or the total number of encounters per fight. In addition, the distribution of encounters over the fight period does not differ in fights between pairs of mated or virgin females. The number of encounters increased proportionately with time during a fight (average R2 = 0.89), allowing us to use either encounter number or time in comparing fights.
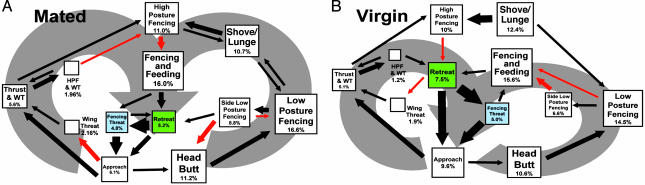
Fig. 1.
Schematic representations of average fights in mated (A) and virgin (B) female flies. The box sizes represent the numbers of transitions to and from the pattern, with the relative frequency given in percentages. Arrows between boxes represent the likelihood of transitions. Some transitions (red arrows) are unique to mated or virgin females. The large gray arrows outline two different behavioral loops. Note that fencing threat (blue box) and retreat (green box) are in opposite positions in the two schematics to prevent an overlap of transition arrows. The patterns are analyzed from first-order Markov chains (see Materials and Methods) by using 2,550 and 1,857 behavioral transitions from 26 mated and 26 virgin fights, respectively. HPF, high-posture fencing; WT, wing threat.
The Markov chain analyses illustrating behavioral transitions emphasize that only small differences are observed in the patterns of fighting behavior between pairs of mated (Fig. 1A) and pairs of virgin (Fig. 1B) females. Certain transitions rose to statistical likelihood in virgins compared with mated females, whereas others showed the reverse pattern. For example, approach to wing threat, and side-limb low-posture fencing to retreat, or head butt reached significance in the mated pairs but not in virgins (Fig. 1). In addition, some behavioral transitions were favored after insemination, such that 63% more transitions were seen involving high-posture fencing with wing threat, 20% more transitions were observed involving low-posture fencing, and 31% fewer transitions were seen to retreat along with 36% fewer transitions from approach. The net result, although not dramatic, suggests that insemination increases the likelihood of encounters escalating through high-posture fencing and shove/lunge behavioral patterns and decreases the likelihood of retreat.
The frequency of behavioral patterns during encounters shows only small differences between mated and virgin female fly fights. Inseminated females, on average, spend more time per encounter in low-posture fencing (5 s for virgin females vs. 7 s for mated females, P < 0.005) and high-posture fencing (0.5 s for virgin females vs. 0.7 s for mated females, P = 0.04) than do virgin females. Mated females show a higher frequency of head butt than virgins (0.32 head butt per encounter for mated females and 0.23 head butt per encounter for virgin females, P = 0.02), whereas virgin females show a 58% increase in the frequency of retreat per encounter, but there are no significant differences seen in the total number of behavioral patterns observed per encounter.
Markov Chain Analysis-Combined Mated and Virgin Matrices. Four distinct categories involving low-posture fencing (low-posture fencing, side low-posture fencing, fencing threat, and fencing while feeding) were included in the analyses in which we compared mated and virgin female fly fights, in part because we anticipated that certain of these behaviors might help to distinguish between the two groups. Despite the inclusion of all these patterns, however, only small differences in fighting behavior were found between the mated and virgin female flies. In addition, because three of these patterns (side low-posture fencing, fencing threat, and fencing while feeding) hardly ever escalate into other forms of aggression (and, instead, may be more closely related to behavioral patterns engaged in while females feed in groups; ref. 27), they were eliminated from the transition matrices of mated and virgin female fly fights. When this alteration was done, the resulting matrices were statistically homogeneous (Mantel's Z = 38,315; P < 0.005), allowing us to pool the data on mated and virgin females to construct a first-order Markov chain of aggression in female flies.
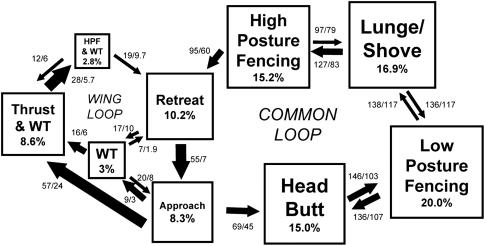
The resultant sequence analysis revealed that two major behavioral loops comprise female aggression, which begin with approach and return to retreat (Fig. 2). The “common loop” begins with head butt, transitions to low-posture fencing and then to a shove/lunge attack, and ends in high-posture fencing and retreat. The less common “wing loop” begins with a thrust combined with a wing threat, transitions to high-posture fencing with a wing threat, and also is likely to end in retreat. A small loop among approach, wing threat, and retreat is displayed between flies that are too far apart to make physical contact with each other. This sequence demonstrates that wing threats, like high-posture fencing, can cause an opponent to retreat.
Fig. 2.
Female fighting pattern after simplification of analysis by removal of three categories of fencing (see text). The total number of transitions are 2,597 and the box and arrow dimensions are calculated as described for Fig. 1. The observed and expected transitions are given adjacent to each transition arrow. HPF, high-posture fencing; WT, wing threat.
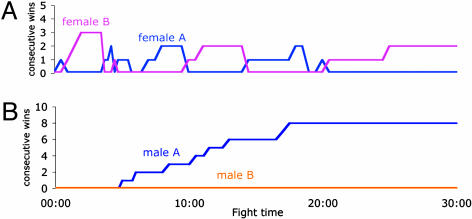
The Absence of Dominance Relationships in Female Fights. Unlike what was observed in fights between male flies, no strong dominance relationships were formed in fights between females. To illustrate this difference, the probability of winning an encounter during 10-min time bins for each opponent fly was calculated. The probability of winning begins and remains high for only one male in a fight (Table 2). In a typical male fight (see Fig. 3), the number of wins by one of the flies continually increases, demonstrating that a hierarchical relationship is formed and that, when formed, is maintained. The probability of a female scoring wins during a fight begins significantly higher for one of the females, but this probability is reversed later in the fight (Table 2). In the sample fight between virgin females shown on Fig. 3, the opponents alternately score wins. In fights between mated, compared with virgin, females, it takes longer for the initial winning pattern to reverse (Table 2). This difference may be because fights between pairs of mated females have fewer clearly decided encounters than fights between virgin females (mated females, 4.9; virgin females, 7.7 decisive encounters per fight, P = 0.02).
Table 2. Comparison of winner–loser status in male and female fights.
| Virgin males
|
Virgin females
|
Mated females
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fight time | A | B | N | A | B | N | A | B | N |
| 0-10 min | 0.36 | 0.003** | 0.64 | 0.23 | 0.06* | 0.71 | 0.16 | 0.03* | 0.81 |
| 10-20 min | 0.28 | 0.02** | 0.70 | 0.21 | 0.27 | 0.52 | 0.13 | 0.09 | 0.78 |
| 20-30 min | 0.26 | 0.00* | 0.74 | 0.29 | 0.24 | 0.47 | 0.19 | 0.12 | 0.69 |
A is the first fly to win an encounter. A, A wins; B, B wins; N, neither wins. *, P < 0.05. **, P < 0.005.
Fig. 3.
An example of a fight between a pair of virgin females (A) and a pair of males (B). In the male fight, the subordinate fly retreated from the food cup without returning after the last encounter. In the female fight, both females were on the food cup for the 30-min period, and there were five reversals in the pattern of consecutive wins.
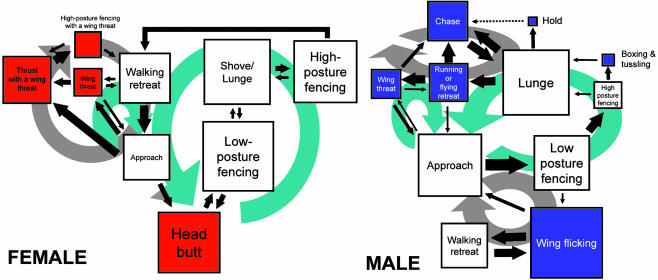
Comparison of Female and Male Patterns of Fighting Behavior. To compare the behavioral patterns seen in male and female fights, we analyzed 19 fights carried out between pairs of male flies by using yeast paste as a resource, instead of a headless mated female, as was done in our original studies (21). Some of the patterns seen are common to male and female fights (Fig. 4, white boxes), whereas others are seen selectively in female (red boxes) or male (purple boxes) fights. “Hold,” “boxing” and “tussling,” “running retreat,” and rapid “wing flicking” are selective for male aggressive behavior, whereas head butt and thrust with a wing threat are common in females and, on rare occasions, seen in males. Both sexes display wing threat while standing still or during interactions with an opponent, but the behavior differs in that it commonly lasts >1 s in males and is always <1 s in females. The male wing threat is accompanied by a lunge-like movement, similar to the female thrust with a wing threat, when advancing toward the opponent that seldom involves physical contact. The male wing-flicking pattern commonly is observed simultaneously with fencing threat or low-posture side-limb fencing, two patterns that are also seen in females. Females display short periods (1–2 s) of a boxing-like behavior by using only their forelegs while they engage in high-posture fencing. Male boxing, by contrast, can last several seconds and includes fencing with the forelegs and second legs. Females also can display a tussling-like exchange of brief rapid thrusts, which do not result in a loss of footing and do not include hold, as seen in male tussling.
Fig. 4.
Behavioral patterns and transitions seen in fights between pairs of male and pairs of female D. melanogaster. Females and males share five common behavioral patterns (white boxes), whereas several gender-selective behavioral patterns also are seen (male, purple boxes; female, red boxes). The transition arrows and box sizes are as defined for Fig. 1, with the addition that the dashed line between hold and chase in males indicating a transition that approaches statistical likelihood. The large light blue arrows indicate similar transition loops, and the gray arrows indicate gender-selective transition loops. The female data are a rearrangement of the data presented in Fig. 2, and the male data were collected from 2,526 transitions from 376 encounters in 19 trials.
The Markov chain diagram (Fig. 4) shows the common and selective behavioral loops seen in male and female fights. The common behavioral patterns (Fig. 4, light blue) follow similar transition loops in both sexes. They start at approach with both sexes, moving through low-posture fencing, high-posture fencing, lunge or shove/lunge, running or flying retreat or walking retreat, and return to approach. Within this loop, gender-selective behavioral patterns are introduced by females (head butt) and males (boxing and tussling and hold). The order within the loop of male lunge and female high-posture fencing is interchanged. Female walking retreat occupies an equivalent position in the Markov chain to male-selective running or flying retreat. Both sexes show a shared behavioral loop between retreat, approach, and wing threat. From wing threat, both sexes also transition to the following other gender-selective behavioral patterns (Fig. 4, large gray arrows): females (Fig. 4, red squares) display the wing-loop pattern; males (Fig. 4, purple squares) show two different behavioral loops, one between lunge, running or flying retreat, wing threat, and chase (the “chase sequence”), and the other between approach, low-posture fencing, wing-flicking, and walking retreat (the “low-aggression sequence”). The low-aggression sequence is commonly seen early in fights before a hierarchical relationship is formed, whereas the chase sequence is seen later in fights after dominance has been established.
The common behavioral patterns (white squares) are not used at the same frequency in each sex (indicated by box sizes in Fig. 4). Females use low-posture fencing and high-posture fencing more often than males, whereas males use approach more often than females.
Discussion
The results presented here demonstrate that pairs of female D. melanogaster, like males, show robust reproducible patterns of agonistic behavior under controlled experimental conditions. Pairs of males and females both compete for resources, which in these studies was a highly desired food source, yeast paste, and in our earlier studies (21) was a potential mate for males. An important difference in the male and female fights, however, is that no sustained hierarchical relationship develops in the female fights. It is not certain whether this difference is due to females pausing to eat yeast after winning a series of encounters and subsequent inhibitory effects of feeding on aggression, or whether it is due to other factors. Males rarely pause to eat during fights, whether they are competing over potential mates or fresh yeast paste. Therefore, males may be more territorial than females in protecting potential resources and in not sharing them with others. Moreover, recent unpublished studies reveal that male flies alter their fighting strategies because a hierarchical relationship is established and retain a memory of that altered strategy for at least 30 min when separated from an opponent (A. Yurkovic and E.A.K., unpublished data). The patterns that comprise fights between pairs of males and pairs of females also differ, although certain behavioral patterns in male and female fights are indistinguishable from each other. Thus, males and females show approach, fencing, lunge, and retreat, although differences are seen in the likelihood of transition between these behavioral patterns in the two genders. Females commonly show a unique form of lunging called head butt that is seldom seen in male fights. Although males and females can elevate both wings in a threatening posture, males hold this position for longer periods of time than females. Males engage in higher-intensity components of fights (boxing, tussling, and hold) that are not seen in females. Females show a high-posture fencing behavior that resembles boxing. The overall conclusions are that some behavioral patterns and transitions are shared by male and female flies during fights, whereas others are gender-selective. Addressing the issue of how the gender-selective patterns and the transitions between them get established in male and female nervous systems is a challenging problem but one that should be approachable by using genetic methods, particularly if a simplified scoring system is used that focuses only on the easily identified differences between male and female fighting behavior (i.e., using head butt to characterize female fighting and the chase sequence for male fighting).
The patterns that make up aggression in flies are likely to be innate because flies with no social experience after emerging as adults display the entire set of complex, highly stereotypical patterns seen during fights. We have evidence that experience molds the behavior, although it does so as flies develop effective strategies to compete for resources with other organisms in constantly changing environments (A. Yurkovic and E.A.K., unpublished data). Some experience with aggression is obtained during larval life in flies because competition for resources is seen during this developmental stage (28), but the absence of wings and limbs in larvae make it unlikely that the social experiences of larval life can translate readily into patterns of aggression in adults. More than likely, as already demonstrated for courtship behavior (29), the initial establishment of the behavioral patterns seen during aggression in adult flies will be governed by genes. Thus, aggression in flies, like most complex behaviors in most organisms, comprises both innate and learned components.
Much literature exists defining the roles of gene cascades during development in establishing neuronal lineages (1–3), determining neuronal identity (2), correctly targeting growing axonal and dendritic processes (30), and localizing the sites at which terminal arbors form within the CNS (31). These early developmental steps lead to the establishment of central circuitry, and some laboratories are beginning to address the question of how patterns of behavior are established within that circuitry (32). For example, Suster and Bate (33) are examining how central pattern generators for forward and reverse peristaltic movements seen in late Drosophila embryos and larval stages get established. These investigators demonstrated that sensory input is not necessary to establish the motor patterns that underlie the movements (33); instead, the patterns appear to be intrinsic to the involved neurons and the connections already established between those neurons. Without sensory input, however, abnormalities are seen in the movement patterns, suggesting that sensory input is required to refine the initially established patterns. A large gap in understanding exists, however, between the establishment of simple central pattern generators and the far more elaborate behavioral patterns that we observe during fly fights.
An approach toward exploring genetic cascades that might be important in establishing male- and female-selective patterns of aggression is to ask whether genes of the sex-determination pathway are involved (34, 35). It is reasonable to suspect that this pathway might be involved because these genes define gender in flies, including the establishment of secondary sex characteristics and the patterns of male and female mating behavior. One clue that this pathway might be involved comes from studies from the Hall laboratory (36) reporting on a unique aggression-like phenotype seen in male flies with homozygous mutations in the fruitless (fru) gene. The interactions were described as head-to-head confrontations in which the bodies of the flies form a single straight line. Although similar body positions of individual flies were reported toward the sides of opponents, these interactions were not scored by the authors. The reported description of these interactions closely matches what we call head butt, which is a major component of female-fly fights that is rarely seen in males. Thus, the interesting possibility is raised that by fru mutations, behavioral patterns specific for female aggression have appeared in the male flies. The characteristic aggressive behavior of male Drosophila silvestris is a head-to-head interaction, which is described in ref. 37, that closely resembles the head-butt pattern used by female D. melanogaster. Comparing the expression pattern of fru or other genes in these species may reveal clues as to how the gender selectivity of head butt is patterned. Further study along these lines, including a more detailed analysis of the behavior of gender mutants or other species of flies, may allow definition of the role of the sex-determination pathway of genes in establishing male- and female-selective patterns of aggressive behavior in fruit flies.
It remains a challenge to address the issue of how genes might be involved in establishing situation- and species-specific patterns of behavior in nervous systems. Many models have been proposed for how patterns of behavior are generated by nervous systems (38–45). These include, at one extreme, suggestions that large numbers of small prewired circuits of neurons (e.g., modules like the central-pattern generator for larval locomotion described above) exist in nervous systems that can be selected among by sensory input and assembled into appropriate patterns of behavior by the sensory input and by hormones and neurohormones released in response to the situation. At another extreme, it has been suggested that behavioral patterns are established de novo between individual neurons in a situation-specific manner, again dependent on sensory input and hormones. As in most cases when two extreme alternatives are presented, probably both will turn out ultimately to be correct. Still, the question of how genes or cascades of genes can specify circuitries involving hundreds or thousands of neurons and myriad synaptic connections to establish patterns of behavior remains a daunting one. It is probably not any more daunting, however, than the notion that genes define the formation of organisms. One exciting recent result demonstrates that one component of mating behavior in fruit flies is selectively inactivated and other components are sped up by interfering with male-specific fru transcripts in a subgroup of neurons in the suboesophageal ganglion (46). The challenge for the future is to find additional experimental paradigms that allow meaningful exploration and identification of the genes involved in establishing behavioral patterns and then to address the issue of how genes, hormones, and environmental factors combine to generate behavior.
Supplementary Material
Acknowledgments
We thank the members of the Kravitz laboratory for helpful discussions and the Bloomington Stock Center for providing us flies. This work was supported by National Institute of General Medical Sciences Grant R01-GM067645.
References
- 1.Skeath, J. B. & Thor, S. (2003) Curr. Opin. Neurobiol. 13, 8–15. [DOI] [PubMed] [Google Scholar]
- 2.Thor, S. & Thomas, J. (2002) Curr. Opin. Genet. Dev. 12, 558–564. [DOI] [PubMed] [Google Scholar]
- 3.Briscoe, J. & Ericson, J. (2001) Curr. Opin. Neurobiol. 11, 43–49. [DOI] [PubMed] [Google Scholar]
- 4.Lonstein, J. S. & Gammie, S. C. (2002) Neurosci. Biobehav. Rev. 26, 869–888. [DOI] [PubMed] [Google Scholar]
- 5.Huhman, K. L., Solomon, M. B., Janicki, M., Harmon, A. C., Lin, S. M., Israel, J. E. & Jasnow, A. M. (2003) Horm. Behav. 44, 293–299. [DOI] [PubMed] [Google Scholar]
- 6.Davis, E. S. & Marler, C. A. (2003) Horm. Behav. 44, 185–198. [DOI] [PubMed] [Google Scholar]
- 7.Bales, K. L. & Carter, C. S. (2003) Horm. Behav. 44, 178–184. [DOI] [PubMed] [Google Scholar]
- 8.Bowler, C. M., Cushing, B. S. & Carter, C. S. (2002) Physiol. Behav. 76, 559–566. [DOI] [PubMed] [Google Scholar]
- 9.Woodley, S. K., Matt, K. S. & Moore, M. C. (2000) Physiol. Behav. 71, 373–381. [DOI] [PubMed] [Google Scholar]
- 10.Ueda, A. & Kidokoro, Y. (2002) Physiol. Entemol. 27, 21–28. [Google Scholar]
- 11.Ruther, J., Sieben, S. & Schricke, B. (2002) Naturwissenschaften 89, 111–114. [DOI] [PubMed] [Google Scholar]
- 12.Gilley, D. C. (2001) Ethology 107, 601–622. [Google Scholar]
- 13.Bernasconi, G., Ratnieks, F. L. W. & Rand, E. (2000) Insectes Sociaux 47, 21–26. [Google Scholar]
- 14.Powell, S. & Tschinkel, W. R. (1999) Anim. Behav. 58, 965–972. [DOI] [PubMed] [Google Scholar]
- 15.Nowbahari, E., Fénéron, R. & Malherbe, M. C. (1999) Aggress. Behav. 25, 369–379. [Google Scholar]
- 16.Packer, C. & Pusey, A. E. (1979) Folia Primatol. (Basel) 31, 212–218. [DOI] [PubMed] [Google Scholar]
- 17.Jolly, A. (1998) Folia Primatol. (Basel) 69, 1–13. [DOI] [PubMed] [Google Scholar]
- 18.Ebert, P. D. & Hyde, J. S. (1976) Behav. Genet. 6, 291–304. [DOI] [PubMed] [Google Scholar]
- 19.Mong, J. A. & Pfaff, D. W. (2003) Neurobiol. Aging 24, S83–S92. [DOI] [PubMed] [Google Scholar]
- 20.Scrivner, J. (1971) Fish Res. Board Can. Tech. Rep. 235, 113. [Google Scholar]
- 21.Chen, S., Lee, A. Y., Bowens, N. M., Huber, R. & Kravitz, E. A. (2002) Proc. Natl. Acad. Sci. USA 99, 5664–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman, A. (1987) Anim. Behav. 35, 807–818. [Google Scholar]
- 23.Sturtevant, H. (1915) J. Anim. Behav. 5, 351–366. [Google Scholar]
- 24.Manning, A. (1959) Behavior 15, 123–145. [Google Scholar]
- 25.Slater, P. J. B. (1973) in Perspectives in Ethology, eds. Bateson, P. P. G. & Klopfer, P. H. (Plenum, New York).
- 26.Jacobs, M. E. (1960) Ecology 41, 182–188. [Google Scholar]
- 27.Sexton, O. J. & Stalker, H. D. (1960) Anim. Behav. 9, 77–81. [Google Scholar]
- 28.de Miranda, J. R. & Eggleston, P. (1989) Heredity 63, 221–229. [DOI] [PubMed] [Google Scholar]
- 29.Taylor, B. J., Villella, A., Ryner, L. C., Baker, B. S. & Hall, J. C. (1994) Dev. Genet. 15, 275–296. [DOI] [PubMed] [Google Scholar]
- 30.Landgraf, M., Roy, S., Prokop, A., VijayRaghavan, K. & Bate, M. (1999) Neuron 22, 43–52. [DOI] [PubMed] [Google Scholar]
- 31.Schrader, S. & Merritt, D. J. (2000) J. Comp. Neurol. 425, 34–44. [PubMed] [Google Scholar]
- 32.Bate, M. (1999) Curr. Opin. Neurobiol. 9, 670–675. [DOI] [PubMed] [Google Scholar]
- 33.Suster, M. L. & Bate, M. (2002) Nature 416, 174–178. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen, A. E., Keisman, E. L., Ahmad, S. M. & Baker, B. S. (2002) Trends Genet. 18, 510–516. [DOI] [PubMed] [Google Scholar]
- 35.Wolfner, M. F. (2003) Curr. Biol. 13, R101–R103. [DOI] [PubMed] [Google Scholar]
- 36.Lee, G. & Hall, J. C. (2000) Behav. Genet. 30, 263–275. [DOI] [PubMed] [Google Scholar]
- 37.Spieth, H. T. (1981) Evolution (Lawrence, Kans.) 35, 921–930. [Google Scholar]
- 38.Arnold, A. & Breedlove, S. (1985) Horm. Behav. 19, 469–498. [DOI] [PubMed] [Google Scholar]
- 39.Bicker, G. & Menzel, R. (1989) Nature 337, 33–39. [DOI] [PubMed] [Google Scholar]
- 40.Kow, L. M. & Pfaff, D. W. (1981) Exp. Brain Res., Suppl., 262–273. [DOI] [PubMed]
- 41.Wu, J. Y., Cohen, L. B. & Falk, C. X. (1994) Science 263, 820–823. [DOI] [PubMed] [Google Scholar]
- 42.Altman, J. S. & Kien, J. (1987) in Nervous Systems in Invertebrates, ed. Ali, M. A. (Plenum, New York), pp. 621–643.
- 43.Kravitz, E. A. (1988) Science 241, 1775–1781. [DOI] [PubMed] [Google Scholar]
- 44.Kravitz, E. A. (2000) J. Comp. Physiol. A 186, 221–238. [DOI] [PubMed] [Google Scholar]
- 45.Morton, D. W. & Chiel, H. J. (1994) Trends Neurosci. 17, 413–420. [DOI] [PubMed] [Google Scholar]
- 46.Manoli, D. S. & Baker, B. S. (2004) Nature 430, 564–569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.