Abstract
The membrane actions of estrogens can facilitate their genomic actions. To determine whether this facilitation bears on CNS mechanisms for estrogen-dependent behaviors, ovariectomized rats were subjected to a two-pulse treatment of estrogen directly in the hypothalamic ventromedial nucleus. Two days later, each rat was given progesterone and then tested for lordosis behavior, the induction of which requires the genomic actions of estrogen. When estrogen was given in both pulses (15 min to 2 h duration, and 5 h apart) lordosis was induced. Based on results from studies on neuroblastoma cells, we hypothesized that the membrane actions of estrogen in the first pulse would potentiate the genomic actions of estrogen in the second. This hypothesis was confirmed with the use of a membrane-impermeable estrogen. However, surprisingly, the order of the pulses could be reversed and still achieve lordosis behavior induction. Finally, activators of protein kinase A or PKC were effective substitutes for the membrane-limited pulse of estrogen. Thus, estrogen-induced membrane actions in the hypothalamus can potentiate its lordosis-inducing genomic actions on behavior and may be mediated by signaling pathways involving the activation of protein kinase A and PKC.
Keywords: hypothalamus, transcriptional potentiation, protein kinase A, PKC, sex behavior
Estrogens are known to have at least two kinds of actions: genomic and membrane (1–5). The former are mediated by nuclear estrogen receptors (ERs) and involve transcription and, hence, require long (hours to days) times to achieve. The latter, with rare exceptions, are mediated by membrane ERs, act rapidly (seconds to minutes), and do not require transcription. The membrane actions activate a wide variety of intracellular signaling pathways, including those involving the increase in intracellular Ca2+ and activation of mitogen-activated PK, protein kinase A (PKA), PKC, cAMP response element-binding protein, phosphatidylinositol 3-kinase, etc. (see ref. 6 and references therein). Because the pathways involving these signaling components can modulate transcriptional functions, we hypothesized that the membrane actions of estrogen can modulate the genomic actions of estrogen. Indeed, by using a two-pulse paradigm and a membrane-impermeable estrogen with cultured neuroblastoma cells transiently transfected with human ERα, we found that membrane actions can potentiate genomic actions (7). For this transcriptional potentiation, the order of hormonal actions was critical: The genomic actions must be preceded by membrane actions. The potentiation also was found to require the activation of PKA and PKC and increases in intracellular Ca2+. Similar results were found with our study on a breast cancer cell line, MCF-7, which possesses natural ERs (N. Devidze, D.W.P. and L.-M.K., unpublished data). Based on these findings, we further hypothesized that the potentiating membrane actions of estrogen are mediated by signaling pathways involving the activation of PKA and PKC.
Estradiol (E) conjugated to BSA (E-BSA) has been shown to act only on membrane ERs by several lines of evidence. First, by using a fluorescent form, E-BSA-FITC (E-BSA conjugated to FITC), E-BSA has been shown with confocal (8, 9) and regular microscopy (10) to bind only to the plasma membrane, even after a prolonged (40 min) incubation (N. Vasudevan, personal communication) that far exceeded the application time sufficient for achieving rapid estrogen actions. Second, this membrane binding is due specifically to E, because BSA-FITC alone devoid of E does not bind to the membrane (8). Third, E binds to membrane ERs, because (i) no binding was observed in cells devoid of ERs (10); (ii) the binding of E-BSA-FITC to plasma membrane was blocked by excess E (10) but not by BSA (9); and (iii) the binding is blocked by ER antagonist ICI 182,780 and the ER Ab H222, which is directed against the ligand-binding domain of ERα (10). There is, however, evidence that E-BSA does not bind to classical ER (11). Finally, E-BSA does not always work like E (12), indicating that direct genomic E/ER functions have been excluded.
In the present study, estrogen induction of lordosis behavior in rats was used as a model to investigate the question of whether the results gathered in vitro from cell lines are physiologically relevant. If so, are the activation of PKA and PKC involved? The induction of lordosis requires the genomic action of estrogen (ref. 13 and reviewed in ref. 14) and can be very effectively achieved by applying estrogen to the hypothalamic ventromedial nucleus (VMN) (15). To initiate membrane actions separate from genomic, the two-pulse paradigm was again used to apply estrogen in one pulse and a membrane-impermeable E-BSA or other test agents in the other pulse to the VMN of female rats, to see what combination could induce lordosis behavior. The two-pulse (or “discontinuous”) paradigm was first used to study estrogen induction of uterine cell division (16). It was later adapted to study the induction of lordosis behavior in rats (17–19). In our studies of cell lines (7), the two-pulse paradigm successfully separated membrane actions from genomic actions when combined with the use of E-BSA. Significantly, the order of application was important. The agents that induce or mimic the membrane actions of estrogen, such as E-BSA or PK activators, had to be applied in the first pulse to potentiate the genomic actions of estrogen in the second pulse. No potentiation was observed when the order was reversed. Therefore, the findings from the present study, that the membrane actions of estrogen can potentiate the genomic actions of estrogen in the induction of lordosis, but that either order of eliciting membrane and genomic actions could work, surprised us. In sum, these experiments on the behavior of whole animals show that the synergy discovered in transient transfection studies, between membrane and genomic steroid hormone action, is physiologically meaningful.
Methods
Adult female and male Sprague–Dawley rats were used. All animal procedures used in this study were approved by The Rockefeller University's Animal Care and Use Committee in accordance with the Animal Welfare Act and the Department of Health and Human Services. All rats were provided with food and water ad lib and were housed in an air-conditioned room under a reversed light-dark cycle (light on 10 p.m. through 10 a.m.). They were allowed 1 wk or more to adapt to the reverse light-dark cycle. During the adaptation period, the rats were handled in preparation for the treatment procedures. The rats were then subjected to gonadectomy and cannula implantation as described elsewhere (20). In brief, rats were first ovariectomized or castrated under Nembutal (40–45 mg/kg) anesthetization and then implanted with double guide cannulae (Plastics One, Roanoke, VA) aiming at the VMN [coordinates for the tips of dummy cannulae: AP, –2.4; lateral, ±0.7; and depth, –9.4; skull leveled, according to Paxinos and Watson (21)]. After the surgery, their health was monitored daily until the behavioral test 1 wk or more later.
The rats were tested weekly for lordosis. Each rat received a one- or two-pulse treatment 2 d before a test. Each pulse consisted of an insertion of inner cannulae containing a test agent for a chosen period specified in Results. The interval between the two pulses was 5 h. The rats, tamed by previous handling, were gently restrained with experimenter's hand without any sedation or anesthetization during the brief insertion and removal of inner and dummy cannulae. Test agents included crystalline 17β-estradiol (E), β-estradiol 6-(O-carboxymethyl)-oxime:BSA (E-BSA or E′), PKA-specific activator, Sp-Adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (Sp-cAMP or PKAa), PKC activators, phorbol 12,13-diacetate (PDAc), and phorbol 12-myristate 13-acetate (PMA), cholesterol and BSA. All agents were obtained from Sigma. E, cholesterol, and BSA were filled by tamping inner cannulae on the agents for 8–10 times. E-BSA was dissolved in phosphate buffer pH 8.0 and, in most experiments, was filtered after a procedure proven to remove free estradiol (11). PDAc was dissolved in distilled water and other PK activators were dissolved in DMSO initially. Filtered and unfiltered E-BSA (fE′ and E′, respectively), PKA, and PKC activators were subsequently dissolved in a thin layer of 3% agar-saline, with the resulting concentrations of 0.5 mg/ml, 333 μM, and 333 μM, respectively. Inner cannulae were filled by punching out the agent-containing agar-saline. To avoid drying out and to cut down E release from E-BSA, only the fresh agent-containing agar-saline was used. All fillings were carried out immediately before the insertion. To avoid possible cross contamination, separate sets of inner cannulae were used for each test agent. Filled inner cannulae were inspected under a dissecting microscope right before and after an insertion. No filling fallout was ever observed. Occasionally, there was blood stain on the cannula tips. Results from such insertions were excluded.
Forty-four hours after the midpoint between the two pulses, each rat was injected with progesterone (0.5 mg per rat, s.c.) and tested for lordosis 4–6 h later. The test was conducted during the dark phase of the light-dark cycle in a dimly lighted (red light) room, where three stud male rats were each placed in a testing arena at least 10 min before a test. A test subject was then placed in one of the testing arenas. The subject was moved from arena to arena, if necessary, to facilitate the testing, which was terminated when the subject was mounted 10 times or when the test reached 10 min, whichever came first. The frequency of lordosis occurrence in response to mounting was calculated as lordosis quotient [LQ = (number of lordosis/number of mounts) × 100]. Any rat that did not show lordosis was given an injection of E (10 μg/rat, s.c.) to guarantee sensitivity to estrogen.
A rat showing a high LQ after an insertion of estrogen can conceivably have an unintended deposit of estrogen in the hypothalamus. All such rats were retested the following week with progesterone but without any estrogen treatment. If they still showed lordosis, their results were excluded from analyses. This was observed only occasionally. Also, some rats, with cannulae located in the third ventricle or gone through the brain bottom, were also excluded because they clearly missed the VMN.
Three series of experiments were conducted. In the first series, 13 groups of female (n in group = 5–22, except two groups) and one group of male (n = 9) rats were used to study the relationship between membrane and genomic actions. Each of the exceptional female groups had two rats, which were treated with estrogen in the VMN for 8 consecutive hours or with two 2-h pulses of estrogen, respectively, and were extremely receptive. Two control groups, Ctrl 1 and Ctrl 2, were observed. In Crtl 1, E-BSA was replaced with cholesterol, BSA, or simply blank. The results of these controls were indistinguishable and are pooled (n = 16). In Crtl 2, E was replaced with cholesterol (n = 9). In the second series, the dose-response study, 4–11 female rats were used for each dose. The last series includes three experiments that studied PDAc (n = 12), PMA (n = 4), and PKAa (n = 6), respectively. In experiments with PDAc and PKAa, female rats were divided evenly into two subgroups and treated in counterbalanced order. For controls (n = 14) the PK activators were replaced with vehicle (saline or DMSO).
At the end of a test series, each rat was anesthetized with urethane (1.6 g/kg, i.p.), and 0.2 μl of a dye (Pontamine sky blue, 2% in 0.5 mM Na-acetate) was infused over 2 min into each side of the hypothalamus to mark the insertion sites. The brain was then removed and fixed with Zamboni for histological examination. Rats with insertion sites outside the ventromedial hypothalamus were excluded from analyses.
Results were analyzed with one-way ANOVAs or matched t tests (statistica, StatSoft, Tulsa, OK), with P < 0.05 regarded as significant.
Results
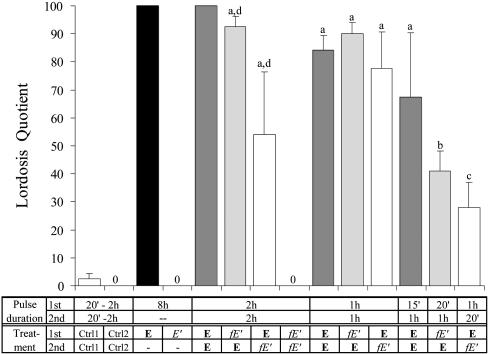
A Short Pulse of E-BSA Can Potentiate the Lordosis-Inducing Genomic Action of a Second Pulse of E. Application of E in VMN for 8 consecutive hours induced a very high level of lordosis behavior (LQ = 100) in both of the female rats tested, indicating that 8 h is more than sufficient for E to induce the lordosis behavior. But similar application of E-BSA for 8 h did not induce any lordosis in any of the females tested (Fig. 1). Because E-BSA is membrane-impermeable, it is obvious from this contrast that a membrane action alone, even for 8 h, is not sufficient to induce lordosis. To assess the possibility that a discontinuous treatment is more effective, six females were treated with two 2-h pulses of E-BSA, and none showed any lordosis.
Fig. 1.
Results of one- and two-pulse treatments of female rats with various combinations of E, E-BSA (E′, unfiltered; fE′, filtered), and control agents. Control group 1 (Ctrl 1) includes results from the following treatments: chol + E, O + E, BSA + E, and E + BSA. Control group 2 (Ctrl 2) includes chol + fE′ and fE′+ chol. As positive controls, in four rats each treated with E for 8 h or with E + E at 2 h each, very strong lordosis and full LQ were confirmed. For other groups, the number of rats ranges from 5 to 22. One-way ANOVA, compared with controls: a, P < 0.0005; b, P < 0.005; and c, P < 0.05; and compared with each other: d vs. d, P < 0.05. 0 (zero), no lordosis; Chol, cholesterol.
Treatment with one pulse of estrogen for 1 or 2 h combined with another pulse of control agents such as cholesterol (chol 1st + E 2nd, or chol + E, in Fig. 1), BSA (E + BSA and BSA + E), or empty cannulae (O + E) was ineffective to induce lordosis. Results from these control treatments are pooled together and are referred to as control 1 (Ctrl 1) in Fig. 1. But two pulses of E induced high receptivity whether the pulse duration was 2 h each, 1 h each, or even 15 min the first and 1 h the second pulse (Fig. 1, E + E′s). Thus, two short pulses of estrogen can act synergistically to induce lordosis.
In contrast to females, males are insensitive to estrogen. Nine males were given an 8-h estrogen insertion, and only four showed some weak lordoses (lordosis strength averaged at 0.78 in a scale of 0–3) with the average LQ of 14.5. Eight males were treated with two 2-h pulses of estrogen, and again, only four showed some weak lordoses (average strength and LQ are 0.75 and 16.5, respectively). These results are significantly different from respective female results (P < 0.0001 for both).
Similarly, one pulse of filtered E-BSA combined with one pulse of the control agent, cholesterol (fE′ + chol and chol + fE′, pooled as Ctrl 2 in Fig. 1), was not effective. However, a combination of filtered E-BSA in the first pulse and E in the second pulse (fE′ + E) was just as effective as two E pulses at every pulse duration paradigm (Fig. 1). In fact, the fE′ pulse could be as short as 20 min.
Surprisingly, reversing the order, with E in the first pulse and filtered E-BSA in the second pulse (E + fE′), a treatment order that was ineffective in neuroblastoma cells (7), was also effective in inducing lordosis. These results indicate that a pulse of membrane-bound E action as short as 20 min can potentiate the genomic action, whether it was applied before or after E itself.
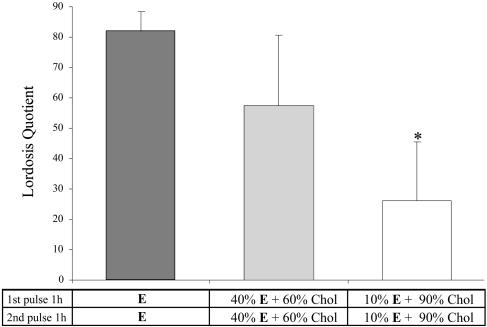
The Lordosis-Inducing Effect of E Was Dose-Dependent. Early in the study, a dose-response experiment was conducted to determine whether lower doses of E can be used. Crystalline E was mixed thoroughly with cholesterol to produce 40% or 10% E by weight. As shown in Fig. 2, the lordosis-inducing efficiency or the level of LQ induced was dose-dependent. At lower doses, variability was much greater than when pure E was used. Because lowering efficiency might obliterate the chance for synergy between membrane and genomic estrogen action, and because the high variance associated with lower doses would create difficulties for experimental comparisons, crystalline estrogen was used throughout the rest of the study.
Fig. 2.
Results of dose–response experiments. E at 100% induced high levels of receptivity in all rats tested. It tended to be more effective than 40% E and is significantly more so than 10% E. With the two lower doses, there are large individual variations. Chol, cholesterol. *, P < 0.02, compared with E + E, one-way ANOVA.
Potentiation of Genomic Actions by E-BSA Is Mimicked by Either PKA or PKC Activation. To evaluate the hypothesis generated from our previous studies in neuroblastoma (7) and MCF-7 cells (N. Devidze, D.W.P. and L.-M.K., unpublished data), that the membrane actions of estrogen activated both PKA and PKC to potentiate genomic actions, three experiments were conducted. In these experiments, rats were divided evenly into two subgroups and all treatment pulse durations were 1 h. The first experiment was designed to study the PKC activator, PDAc. In the first weekly test, one subgroup of rats received PDAc in the first and E in the second pulse (PDAc + E); the other subgroup received vehicle (V, saline) and E in the first and second pulses (V + E), respectively. For the second weekly test, the treatments for the two groups were reversed, so that each rat served as its own control. PDAc + E treatment induced lordosis in every rat, with LQ ranging from 30 to 100, averaging at 68.33 (Fig. 3). In contrast, V + E treatment did not induce any lordosis in any rat (Fig. 3). In the second experiment, a subgroup of animals tested with PDAc were tested with another PKC activator, PMA, for comparison. PDAc tended to be more effective than PMA (P < 0.03, one-tailed, matched t test) (Fig. 3). In the same experiment, two additional rats were used as control; they received V (DMSO) + E or E + V in alternative weeks. Neither control treatment was effective.
Fig. 3.
Potentiation of the lordosis-inducing action of E by PK activators. V + E group includes control treatments of (saline + E), (DMSO + E), and (E + DMSO). All treatment groups are highly significantly different from the control group (P < 0.001, one-way ANOVA). There is no significant difference among the four experimental groups. V, vehicle; PDAc and PMA, PKC activators, both 333 μM in agar-saline; Sp-cAMP, 333 μM in agar-saline.
The last experiment was conducted to determine whether the PKA activator, Sp-cAMP (or PKAa), can also mimic E-BSA, and if positive, whether it, like E-BSA, is effective when administered after an E pulse (i.e., in the second pulse rather than the first). One-half of the rats were given PKAa + E (PKAa in the first and E in the second pulse) in the first and E + PKAa in the next weekly test; another half received same treatment but in reversed order. Importantly, both orders of treatment were effective in inducing lordosis, with LQ induced by PKAa + E tending to be higher (Fig. 3).
Discussion
With the induction of lordosis in whole animals as the end point of action, one long (8 h) pulse of E induced full receptivity in female but very low LQ in males. One long or two short pulses of E-BSA were totally ineffective (Fig. 1). The fact that E, even when applied directly in the highly sensitive VMN (15), did not override the sex difference, indicates that it maintains physiologically relevant specificity. The ineffectiveness of the long pulse of membrane-acting E-BSA (see below) shows that membrane actions alone, even for 8 straight h, are insufficient to induce lordosis.
The above conclusion is also indicated by two-pulse experiments. All of the two-pulse treatments that have E in at least one of the pulses and an active agent in the other pulse, such as E + E, fE′ + E, E + fE′, PDAc + E, PMA + E, PKAa + E, and E + PKAa were effective in inducing lordosis. In contrast, when neither pulse was E, such as fE′ + fE′, no lordosis was induced. Likewise, when E was combined with an inactive agent, such as saline, DMSO, BSA, cholesterol, or a blank, no lordosis was observed either. Therefore, although genomic actions induced by E are required for the induction of lordosis (13, 14), those sensitive to only a short pulse of E of up to 2 h were not sufficient. Instead, synergistic actions from E-BSA or activators of PKA and PKC are necessary.
In the current study, E-BSA, which is membrane impermeable, was used to limit estrogenic actions to cell membrane. Even if E-BSA were contaminated with free E, the finding that 8 h or 2h + 2 h of E-BSA was ineffective, indicates that the conjugated E, even without filtering, contained no or too little free E to induce transcription. Nevertheless, the E-BSA was filtered with a proven procedure for removing free E (11) in all but the initial long-pulse experiment. E can dissociate from E-BSA over time, but the rate is very low (≈0.00063% per ml per h) (22). Calculating based on this rate and the amount we used in one inner cannula (0.17 μl of 2 ml of agar-saline that contains 1 mg of filtered E-BSA), the amount of E released at the end of a period (20 min) sufficient for the potentiation of genomic actions would be 0.6 × 10–17 g or 0.131 × 10–12 M. At this concentration, E was unable to stimulate transcription even in the very E-responsive MCF-7 cell line (N. Devidze, D.W.P. and L.-M.K., unpublished data). Thus, it is safe to state that the actions by E-BSA are membrane actions, and the synergism between the filtered E-BSA and E represents the potentiation of behaviorally relevant genomic action by membrane actions. In turns, that this occurs in the whole animal indicates that this type of hormonal potentiation is physiologically meaningful.
In the present study, against our expectation, membrane actions potentiated the genomic action regardless of whether they were applied before or after E itself. This was true for the PKAa as well as E-BSA. Theoretically, this was unexpected because in our previous study with neuroblastoma cells, the transcriptional potentiation occurred only when membrane actions preceded genomic actions (7). The exact reason for this contrast is not known, but it may be due to the nature or the quantity of ERs. Both ER-containing VMN neurons (23) and MCF-7 cells (24) contain high density of constituent ERs. The genomic action induced by such ERs may be sustained long enough to be potentiated subsequently by membrane actions that occur later. In contrast, neuroblastoma cells have very little or no ER and required transfection of ERα. The number or molecular properties of ERs after transfection may be such that genomic actions are too short to be potentiated by later membrane actions.
Consistent with our two previous studies by using cell lines in vitro, the potentiation of the lordosis-inducing genomic actions by membrane actions could be mimicked by the activation of PKA or PKC. These and other findings (1–5) suggest that the membrane actions of estrogen activate both PKA and PKC. Whether, in the present experiments, they were activated in series, as proposed by Kelly and coworkers (25, 26), or in parallel remains to be determined. Whichever it is, the activation of PKs appears to comprise a common set of core mechanisms in synergistic actions between the membrane and genomic effects of estrogen. This theoretical formulation does not exclude the involvement of other signaling pathways that also can be activated by the membrane actions of estrogen (6).
Acknowledgments
We thank Michael Englander and Magdalena Bogun for technical assistance. This work was supported by National Institutes of Health Grant HD 05751.
Abbreviations: E, estradiol; E-BSA, E conjugated to BSA; ER, estrogen receptor; PKA, protein kinase A; PKAa, PKA activator; VMN, ventromedial nucleus; LQ, lordosis quotient; PMA, phorbol 12-myristate 13-acetate; PDAc, phorbol 12,13-diacetate.
References
- 1.Cato, A. C., Nestl, A. & Mink, S. (2002) Sci. Signal Transduction Knowledge Environ. 138, RE9. [DOI] [PubMed] [Google Scholar]
- 2.Kelly, M. J. & Levin, E. R. (2001) Trends Endocrinol. Metab. 12, 152–156. [DOI] [PubMed] [Google Scholar]
- 3.Levin, E. R. (2001) J. Appl. Physiol. 91, 1860–1867. [DOI] [PubMed] [Google Scholar]
- 4.Simoncini, T. & Genazzani, A. R. (2003) Eur. J. Endocrinol. 148, 281–292. [DOI] [PubMed] [Google Scholar]
- 5.Watson, C. S. & Gametchu, B. (1999) Proc. Soc. Exp. Biol. Med. 220, 9–19. [DOI] [PubMed] [Google Scholar]
- 6.Lee, S. J. & McEwen, B. S. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 569–591. [DOI] [PubMed] [Google Scholar]
- 7.Vasudevan, N., Kow, L. M. & Pfaff, D. W. (2001) Proc. Natl. Acad. Sci. USA 98, 12267–12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo, Z., Krucken, J., Benten, W. P. & Wunderlich, F. (2002) J. Biol. Chem. 277, 7044–7050. [DOI] [PubMed] [Google Scholar]
- 9.Morales, A., Diaz, M., Ropero, A. B., Nadal, A. & Alonso, R. (2003) Eur. J. Neurosci. 18, 2505–2514. [DOI] [PubMed] [Google Scholar]
- 10.Razandi, M., Pedram, A., Greene, G. L. & Levin, E. R. (1999) Mol. Endocrinol. 13, 307–319. [DOI] [PubMed] [Google Scholar]
- 11.Stevis, P. E., Deecher, D. C., Suhadolnik, L., Mallis, L. M. & Frail, D. E. (1999) Endocrinology 140, 5455–5458. [DOI] [PubMed] [Google Scholar]
- 12.Abraham, I. M., Han, S. K., Todman, M. G., Korach, K. S. & Herbison, A. E. (2003) J. Neurosci. 23, 5771–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons, B., Rainbow, T. C., Pfaff, D. W. & McEwen, B. S. (1982) Endocrinology 110, 620–624. [DOI] [PubMed] [Google Scholar]
- 14.Pfaff, D. W. (1999) Drive (MIT Press, Cambridge, MA).
- 15.Davis, P. G., Krieger, M. S., Barfield, R. J., McEwen, B. S. & Pfaff, D. W. (1982) Endocrinology 111, 1581–1586. [DOI] [PubMed] [Google Scholar]
- 16.Harris, J. & Gorski, J. (1978) Endocrinology 103, 240–245. [DOI] [PubMed] [Google Scholar]
- 17.Clark, A. S. & Roy, E. J. (1983) Physiol. Behav. 4, 561–565. [DOI] [PubMed] [Google Scholar]
- 18.Parsons, B., McEwen, B. & Pfaff, D. (1982) Endocrinology 110, 613–619. [DOI] [PubMed] [Google Scholar]
- 19.Sodersten, P., Pettersson, A. & Eneroth, P. (1983) Endocrinology 112, 1883–1885. [DOI] [PubMed] [Google Scholar]
- 20.Kow, L.-M., Brown, H. E. & Pfaff, D. W. (1994) Brain Res. 660, 241–248. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos, G. & Watson, C. (1982) The Rat Brain in Stereotaxic Coordinates (Academic, London).
- 22.Binder, M. (1984) Histochem. J. 16, 1003–1023. [DOI] [PubMed] [Google Scholar]
- 23.Pfaff, D. & Keiner, M. (1973) J. Comp. Neurol. 151, 121–158. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz, K. B., Costlow, M. E. & McGuire, W. L. (1975) Steroids 26, 785–795. [DOI] [PubMed] [Google Scholar]
- 25.Kelly, M. J. & Wagner, E. J. (1999) Trends Endocrinol. Metab. 10, 369–374. [DOI] [PubMed] [Google Scholar]
- 26.Qiu, J., Bosch, M., Tobias, S., Grandy, D., Scanlan, T., Ronnekleiv, O. & Kelly, M. (2003) J. Neurosci. 23, 9529–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]