Abstract
Background
Limited information exists on the intermediate-term graft patency and 5-year clinical outcomes of patients receiving saphenous vein grafts with multiple (m-SVG) versus single distal targets (s-SVG) during coronary artery bypass graft (CABG) surgery in the current era.
Methods and Results
We studied the association of the use of m-SVG versus s-SVG conduits with 1-year SVG failure (defined as ≥75% angiographic stenosis) and 5-year clinical events (death; death or myocardial infarction [MI]; and death, MI, or revascularization) in 3014 patients undergoing their first CABG surgery enrolled in the Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT) IV. Of 3014 patients enrolled in PREVENT IV, 1045 (34.7%) had ≥1 m-SVGs during CABG. Vein graft failure at 1-year was higher for m-SVG compared with s-SVG (adjusted odds ratio 1.24, 95% confidence interval 1.03 to 1.48). At 5 years, the adjusted composite of death, MI (including perioperative MI), or revascularization (hazard ratio 1.15, 95% confidence interval 1.00 to 1.31) and death or MI (hazard ratio 1.21, 95% confidence interval 1.03 to 1.43) were significantly higher in patients receiving m-SVGs.
Conclusions
In patients undergoing first CABG surgery, the use of m-SVG was associated with a higher 1-year vein graft failure rate and trends toward worse clinical outcomes. Additional studies are needed to better understand the most appropriate conduit to improve long-term graft patency and clinical outcomes of patients undergoing CABG surgery. In the meantime, these data should encourage the use of s-SVG over m-SVG when feasible.
Keywords: coronary artery bypass grafting, bypass grafts, vascular patency, outcomes
Saphenous vein grafts (SVGs) with single proximal and multiple distal anastomoses (m-SVG) are often used for bypassing occluded coronary arteries in patients undergoing coronary artery bypass graft (CABG) surgery.1–8 The use of m-SVG has been shown to allow complete revascularization with limited SVG material necessary for multiple bypasses in the increasingly complex patients with diffuse coronary artery disease or in those referred for a repeat procedure, which constitutes a significant proportion of patients referred for surgical coronary revascularization.1–8 Additionally, as it requires only 1 proximal anastomosis, a m-SVG conduit permits shorter revascularization time compared with multiple single SVG (s-SVG, single proximal and distal anastomoses) conduits in unstable patients undergoing emergent or salvage CABG surgery.1–8
Although prior studies have evaluated the short- and long-term outcomes of patients receiving m-SVG compared with s-SVG during CABG,1–8 most of these studies were small, single-center investigations that did not have systematic angiographic follow-up, and almost all preceded the current era, in which modern medical therapy and surgical advancements have been shown to improve outcomes in patients undergoing CABG surgery. Accordingly, the objectives of the present study were (1) to evaluate the differences in the patency of m-SVG versus s-SVG conduits at 1 year and (2) to evaluate the differences in 5-year outcomes of patients receiving m-SVG compared with those receiving only s-SVG during CABG surgery.
Methods
Project of Ex-Vivo Vein Graft Engineering via Transfection (PREVENT) IV Trial and Patient Population
The details of the PREVENT IV trial and its findings have been published previously.9,10 In brief, PREVENT-IV was a phase-3, multicenter, randomized, double-blind, placebo-controlled trial to assess the efficacy of edifoligide, an oligonucleotide decoy that binds to and inhibits E2F transcription factors, and thereby was thought to prevent neointimal hyperplasia and SVG failure. A total of 3014 patients undergoing primary CABG surgery with at least 2 planned SVGs at 107 sites in the United States were randomly assigned between August 2002 and October 2003 to receive ex vivo autologous vein graft treatment with either edifoligide or placebo before implantation of these conduits. The first 2400 patients enrolled were scheduled to return for angiography 12 to 18 months after surgery. Major exclusion criteria included prior cardiac or planned concomitant valve surgery (because of the increased early mortality associated with these procedures), vasculitis or another nonatherosclerotic cause of coronary artery disease, hypercoagulable state, involvement in another investigational drug or device study within 30 days, or a comorbid illness that would make 5-year survival unlikely. Institutional review board approval was obtained at all sites, and all patients gave written informed consent before participating. For the purpose of this analysis, we included all patients enrolled in PREVENT-IV. Vein graft conduits that had a single proximal anastomosis but >1 distal connection were regarded as m-SVG. In PREVENT-IV, the use of m-SVG or s-SVG for coronary bypass was left to the discretion of the surgeon.
Outcome Measures
The main angiographic outcome measure for this analysis was per-graft incidence of SVG failure, defined as stenosis of ≥75% or occlusion of a vein graft assessed by quantitative follow-up coronary angiography 12 to 18 months after surgery. Failure of any part of an m-SVG was considered graft failure. Patients in the angiographic cohort who underwent angiography for clinical reasons before 12 months had passed and met the above end point did not have additional protocol angiography. Patients who died before angiography could be performed were not included in the angiographic end point. Secondary angiographic end points included per graft incidence of SVG occlusion. All angiograms in PREVENT-IV were interpreted at the PERFUSE Angiographic Core Laboratory (Boston, MA) using standard quantitative coronary angiographic techniques.
The main clinical end point for this analysis was the composite of death, MI, or repeat revascularization at 5 years. All patients were contacted via mail or telephone at 6 and 9 months and at 1, 2, 3, 4, and 5 years after CABG surgery. For those who reported a possible MI or revascularization procedure, additional medical records were obtained from their hospitals. All suspected MIs and revascularization procedures were adjudicated by a blinded independent clinical events committee using prespecified criteria. Perioperative MI was defined as a creatine kinase-MB (CK-MB) >10 times the upper limit of normal (ULN) or >5 times the ULN, with new Q waves longer than 30 ms in 2 contiguous leads, or, if postoperative CK-MB samples were not available, new Q waves longer than 30 ms in 2 contiguous leads. Perioperative MI was diagnosed if CK-MB was elevated within 24 hours of surgery when there was not an interval clinical event and when the elevation was not attributable to a preoperative MI. Postoperative MI was defined as either spontaneous (CK-MB >2 times the ULN or new Q waves >30 ms in 2 contiguous leads), after percutaneous coronary intervention (CK-MB >3 times the ULN or new Q waves >30 ms in 2 contiguous leads), or after CABG surgery (CK-MB >10 times the ULN or >5 times the ULN with new Q waves >30 ms in 2 contiguous leads). For patients for whom CK-MB samples and electrocardiograms were not available, MI could be defined by the presence of myocardial infarction, heart attack, or similar term in the medical record documenting that an MI had occurred after the initial CABG surgery.
Statistical Analysis
All analyses were performed with SAS software (SAS Institute Inc, Cary, NC). Baseline characteristics, surgery, and hospital care characteristics were summarized as frequencies and percentages for categorical variables and as medians and 25th and 75th percentiles for continuous variables. Differences in characteristics between patients with m-SVG and s-SVG were assessed using the Wilcoxon rank-sum test (for continuous variables) and the χ2 or Fisher exact test (for categorical variables). All tests of significance were 2-tailed.
For the per-graft end points of ≥75% stenosis and occlusion, general estimating equation techniques were used to adjust for correlation between grafts within a patient. Covariates adjusted for were those that were available before or at the time of CABG and included weight, duration of surgery, harvest technique, target vessel quality, and graft quality. A P value of <0.05 was considered statistically significant.
Cumulative event rates for the major adverse clinical outcomes were calculated using the Kaplan-Meier method. The statistical significance of differences in outcomes between the 2 groups was assessed with the log-rank test. In addition, covariate adjusted analyses of outcomes were assessed using the Cox proportional hazards model. Covariates adjusted for were those that were available before or at the time of CABG and included age, sex, race, history of congestive heart failure, creatinine clearance, recent MI (within 30 days of enrollment), weight, height, diastolic blood pressure, on-pump surgery, length of surgery, harvesting technique, and use of internal mammary artery (IMA) conduit. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated with the Cox model. Given that there was no effect of edifoligide on SVG failure rate or on clinical outcomes in PREVENT-IV, we did not adjust for treatment assignment in the evaluation of graft failure rates or clinical events.
We also performed a number of sensitivity analyses. First, we evaluated per-graft failure and occlusion rates in patients with good-quality distal targets. Second, we excluded patients with emergent or emergent salvage procedures during their index CABG and evaluated the composite of death, MI, or revascularization in patients with and without m-SVG. Furthermore, in order to provide insights into the relationship between m- and s-SVG failure and outcomes, we evaluated an adjusted composite end point of death, MI, or revascularization in patients receiving follow-up angiography, dividing them into the following groups: only s-SVG and ≥1 s-SVG failure; m-SVG with or without s-SVG with no vein graft failure; m-SVG and s-SVG with ≥1 s-SVG failure but no m-SVG failure; m-SVG with or without s-SVG with ≥1 m-SVG failure but no s-SVG failure; and m-SVG and s-SVG with ≥1 m-SVG and ≥1 s-SVG failure (referent group s-SVG with no vein graft failure). For this last analysis, events occurring before protocol angiography were excluded. Time to event/censoring was calculated on the basis of time from protocol angiography.
All authors had full access to and take full responsibility for the integrity of the data. All authors have read and agreed to the manuscript as written.
Results
Baseline Characteristics, Surgical Features, and In-Hospital Care
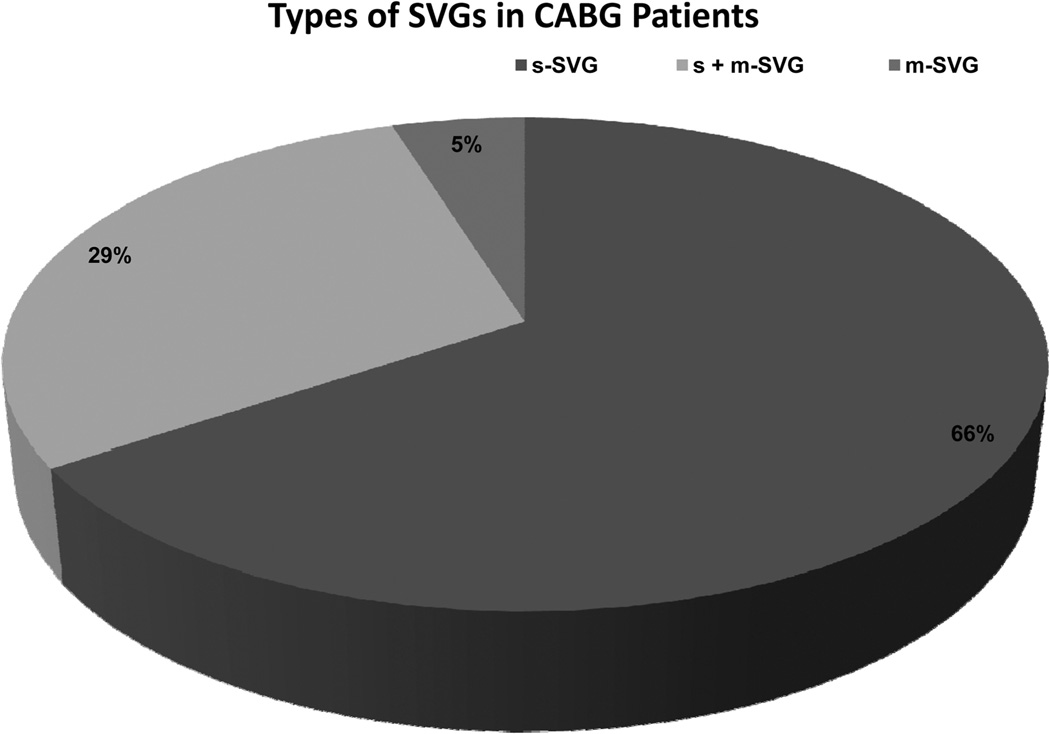
Of 3014 patients enrolled in PREVENT IV, 1045 (34.7%) had m-SVG during CABG (4.9% only m-SVG, 29.8% both m-SVG and s-SVG) (Figure 1). Table 1 shows the baseline characteristics of the 2 groups with and without m-SVG. The median age of patients in the 2 groups was similar with more men in the m-SVG group. These patients also had marginally higher weight and height (perhaps because of the higher proportion of men). Most comorbid conditions, including hypertension, diabetes mellitus, chronic lung disease, prior congestive heart failure, prior stroke, prior peripheral and cerebrovascular disease, liver disease, renal insufficiency, cardiogenic shock, and prior cancer, did not differ significantly in the 2 groups. Patients with m-SVG had a statistically significant (but clinically insignificant) lower diastolic blood pressure. Similarly, these patients had marginally lower left ventricular ejection fraction and a higher prevalence of 2- or 3-vessel or left main coronary artery disease, whereas there were no differences in other presenting features, such as heart rate, systolic blood pressure, New York Heart Association class, and creatinine clearance between the 2 groups.
Figure 1.
Types of vein graft conduits used in PREVENT-IV patients. CABG indicates coronary artery bypass surgery; m-SVG, saphenous vein graft with >1 distal target; and s-SVG, saphenous vein graft with only 1 distal target.
Table 1.
Baseline Characteristics
| Characteristics | Overall (N = 3014) |
No m-SVG (N = 1969) |
m-SVGs (N = 1045) |
P |
|---|---|---|---|---|
| Age, median, y (25th, 75th percentiles) | 64 (56, 71) | 63 (56, 70) | 64 (56, 71) | 0.406* |
| Female sex, % | 20.9 | 22.8 | 17.3 | <0.001 |
| Race, nonwhite, % | 9.1 | 9.1 | 9.1 | 0.275 |
| Weight, median, kg (25th, 75th percentiles) | 88 (77, 100) | 87 (76, 100) | 89 (77, 101) | 0.046* |
| Height, median, cm (25th, 75th percentiles) | 175 (168, 180) | 174 (168, 180) | 175 (168, 180) | 0.011* |
| Medical history, % | ||||
| Hypertension | 75.1 | 74.4 | 76.5 | 0.203 |
| Diabetes mellitus | 37.8 | 36.9 | 39.4 | 0.177 |
| Current smoking | 22.9 | 23.0 | 22.7 | 0.166 |
| Hyperlipidemia | 76.3 | 75.5 | 77.9 | 0.134 |
| Chronic lung disease | 15.8 | 15.6 | 16.1 | 0.728 |
| Preoperative atrial fibrillation/flutter | 7.0 | 6.6 | 7.8 | 0.204 |
| MI, any | 42.2 | 42.2 | 42.4 | 0.899 |
| MI within 3 mo | 38.8 | 38.3 | 39.5 | 0.528 |
| Prior PCI | 0 | 0 | 0 | |
| Congestive heart failure | 9.7 | 9.5 | 10.0 | 0.721 |
| Prior stroke | 5.5 | 5.8 | 4.9 | 0.296 |
| PVD | 12.2 | 12.0 | 12.6 | 0.635 |
| Cerebrovascular disease | 12.7 | 12.7 | 12.6 | 0.959 |
| History of liver disease | 1.8 | 1.8 | 1.8 | 0.936 |
| Renal insufficiency | 2.2 | 2.1 | 2.2 | 0.903 |
| Cardiogenic shock | 0.8 | 0.8 | 0.9 | 0.770 |
| Cancer | 8.2 | 8.5 | 7.7 | 0.429 |
| Presenting features | ||||
| Heart rate, median, bpm (25th, 75th percentiles) | 70 (62, 80) | 70 (62, 80) | 71 (62, 80) | 0.222* |
| SBP, median, mm Hg (25th, 75th percentiles) | 134 (120, 149) | 133 (120, 149) | 134 (120, 150) | 0.276* |
| DBP, median, mm Hg (25th, 75th percentiles) | 75 (67, 82) | 76 (66, 82) | 74 (68, 82) | 0.029* |
| Peoperative NYHA class, % | 0.374 | |||
| I | 40.2 | 40.7 | 37.3 | |
| II | 33.4 | 33.3 | 33.8 | |
| III | 18.0 | 17.9 | 18.8 | |
| IV | 8.4 | 8.1 | 10.2 | |
| LVEF, median, % (25th, 75th percentiles) | 50 (40, 60) | 50 (42, 60) | 50 (40, 60) | 0.018* |
| No. of diseased vessels ≤2 or left main (>75% stenosis), % |
79.4 | 78.3 | 81.6 | 0.031 |
| Baseline creatinine, median, mg/dL (25th, 75th percentiles) |
1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 0.049 |
| Creatinine clearance, median, mL/min (25th, 75th percentiles) |
89 (70, 112) | 88 (69, 112) | 89 (70, 112) | 0.724* |
Non-parametric test.
DBP indicates diastolic blood pressure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; m-SVG, saphenous vein graft with >1 distal target; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; and SBP, systolic blood pressure.
Patients with m-SVG were more likely to have on-pump surgery with a longer duration of cardiopulmonary bypass time, were more likely to have endoscopic SVG harvesting, and had lower use of IMA as a conduit (Table 2). The quality of target vessels (graded as good, fair, and poor) was similar in the 2 groups. Postoperative duration on a ventilator was longer in the m-SVG group, with no difference in the intensive care unit or hospital length of stay. The quality of the vein graft used for bypass was similar in the 2 cohorts. Use of evidence-based medications was high and similar in the 2 groups.
Table 2.
Surgery and Hospital Care
| Characteristics | Overall (N = 3014) |
No m-SVG (N = 1969) |
m-SVGs (N = 1045) |
P |
|---|---|---|---|---|
| Emergent/emergent salvage surgery | 2.9 | 3.1 | 0.802 | |
| IMA graft | 92.3 | 93.7 | 89.9 | <0.001 |
| Surgery duration, median, min (25th, 75th percentiles) | 231 (193, 272) | 226 (190, 267) | 237 (201, 280) | <0.001† |
| Cardiopulmonary bypass, % | 78.9 | 74.7 | 86.8 | <0.001 |
| Duration of cardiopulmonary bypass, median, min (25th, 75th percentiles) |
100 (79, 123) | 97 (77, 121) | 104 (82, 127) | <0.001 |
| Endoscopic harvesting technique | 58.4 | 55.7 | 63.5 | <0.001 |
| Postoperative duration, median (25th, 75th percentiles) | ||||
| Ventilator, h | 8 (5, 14) | 7 (5, 13) | 8 (5, 14) | 0.025 |
| Intensive care unit stay, h | 26 (22, 47) | 26 (22, 47) | 26 (22, 48) | 0.674 |
| Hospital stay, d | 6 (5, 8) | 6 (5, 8) | 6 (5, 8) | 0.524 |
| Worst target-artery quality | 0.296 | |||
| Good | 43.0 | 42.8 | 43.3 | |
| Fair | 35.9 | 35.3 | 37.1 | |
| Poor | 21.2 | 21.9 | 19.6 | |
| Worst graft quality | 0.474 | |||
| Good | 70.8 | 70.4 | 71.7 | |
| Fair | 24.1 | 24.2 | 23.9 | |
| Poor | 5.0 | 5.4 | 4.4 | |
| Medications continued at 30 d | ||||
| Aspirin | 90.5 | 91.2 | 89.3 | 0.094 |
| Thienopyridine | 21.9 | 22.8 | 20.2 | 0.109 |
| ACE inhibitor | 35.8 | 34.9 | 37.4 | 0.165 |
| Angiotensin II receptor blocker | 6.7 | 6.4 | 7.3 | 0.399 |
| β-blockers | 78.7 | 78.3 | 79.6 | 0.401 |
| HMG-CoA reductase inhibitor | 72.9 | 73.2 | 72.3 | 0.633 |
m-SVG indicates saphenous vein graft with >1 distal target; IMA, internal mammary artery; ACE, angiotensin-converting enzyme; and MHG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A.
Angiographic Results
In the overall PREVENT-IV population, the proportion of patients assigned to the angiographic cohort was similar among patients with m-SVG and s-SVG (n = 826 [79%] and 1574 [80%], respectively). Within the cohort that was scheduled to return for angiographic follow-up, the proportion that actually returned for angiography was similar in patients with and without m-SVG (80% versus 79%) (Table 3).
Table 3.
Angiographic Results
| m-SVGs | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|
| Per-Graft Angiographic End Points | Yes (n = 709) | No (n = 3602) | OR (95% CI) | P | OR (95% CI) | P |
| SVG stenosis ≥75%, % | 30.5 | 24.1 | 1.29 (1.08, 1.54) | 0.006 | 1.24 (1.03, 1.48) | 0.025 |
| SVG occlusion | 27.7 | 21.0 | 1.36 (1.13, 1.64) | 0.002 | 1.30 (1.08, 157) | 0.009 |
| Grafts with “good” target arteries—per graft angiographic end point, n |
431 | 2284 | ||||
| SVG stenosis ≥75% | 29.2 | 20.8 | 1.49 (1.20, 1.87) | 0.001 | 1.40 (1.13, 1.76) | 0.006 |
| SVG occlusion | 27.0 | 18.0 | 1.64 (1.30, 2.06) | <0.001 | 1.53 (1.21, 1.93) | 0.001 |
Values are percentages unless otherwise indicated.
Adjusted for weight, duration of surgery, harvest technique, target-vessel quality, and graft quality.
m-SVG indicates saphenous vein graft with >1 distal target; OR, odds ratio; CI, confidence interval; and SVG, saphenous vein graft.
Saphenous vein graft failure was lowest in patients with isolated s-SVG (41.6%), highest in those with isolated m-SVG (50.6%), and intermediate in those with m- and s-SVG (46.0%). One-year follow-up angiographic results revealed that the adjusted rates of SVG failure (defined as stenosis ≥75%) and/or occlusion was higher for m-SVG compared with s-SVG. A sensitivity analysis restricting our angiographic end points to only SVGs attached to a good target vessel also showed higher per-graft failure rates for m-SVG compared with s-SVG. There was no interaction between treatment assignment and s-SVG or m-SVG conduit use in multivariate analysis.
Clinical Events
Of the clinical events evaluated in the PREVENT-IV study through 30 days, only perioperative MI was 40% higher in patients with an m-SVG (8.6% versus 12.0%), with no difference in other events. Mortality at 30 days was low and similar (1.2%) in the 2 groups.
Five-year major adverse cardiac events are shown in Table 4. The use of m-SVG was associated with a significantly higher incidence of the combined end point of death, MI, or revascularization and death or MI even after adjusting for differences in prognostically important baseline variables and differences in IMA use. Furthermore, when we restricted the analysis to the angiographic cohort, the composite of death, MI, or revascularization remained significantly higher in the m-SVG group, related mostly to the higher incidence of perioperative MI (Table 5). Finally, when we evaluated the composite of death, MI, or revascularization in patients without emergent/emergent salvage CABG, our findings remained consistent with the overall results (adjusted HR 1.15, 95% CI 1.01 to 1.32).
Table 4.
Five-Year Major Adverse Clinical Events in Overall PREVENT-IV Population With or Without the m-SVGs
| m-SVGs | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|
| Clinical Events | Yes (n = 1045) | No (n = 1969) | HR (95% CI) | P | HR (95% CI) | P |
| Death, MI (including perioperative MI), or revascularization, n |
364 | 603 | 1.17 (1.03, 1.33) | 0.018 | 1.15 (1.00, 1.31) | 0.045 |
| 5-y event rate, % | 35.2 | 31.1 | ||||
| Death or MI (including perioperative MI), n | 262 | 398 | 1.27 (1.08, 1.48) | 0.003 | 1.21 (1.03, 1.43) | 0.020 |
| 5-y event rate, 5 | 25.4 | 20.5 | ||||
| Death, MI (excluding perioperative MI), or revascularization, n |
282 | 484 | 1.11 (0.95, 1.28) | 0.182 | 1.10 (0.94, 1.28) | 0.231 |
| 5-y event rate, % | 27.5 | 25.1 | ||||
| Death or MI (excluding perioperative MI), n | 161 | 252 | 1.21 (0.99, 1.47) | 0.063 | 1.15 (0.94, 1.42) | 0.178 |
| 5-y event rate, % | 15.8 | 13.1 | ||||
| Death or revascularization, n | 270 | 468 | 1.09 (0.94, 1.27) | 0.253 | 1.08 (0.92, 1.26) | 0.348 |
| 5-y event rate, % | 26.3 | 24.3 | ||||
| Death | 127 | 210 | 1.13 (0.91, 1.41) | 0.266 | 1.09 (0.86, 1.37) | 0.480 |
| 5-y event rate, % | 12.5 | 10.9 | ||||
Adjusted for age, sex, race, history of congestive heart failure, creatinine clearance, recent MI (within 30 d of enrollment), weight, height, diastolic blood pressure, on-pump surgery, length of surgery, harvesting technique, and use of internal mammary artery conduit.
m-SVG indicates saphenous vein graft with >1 distal target; HR, hazard ratio; CI, confidence interval; and MI, myocardial infarction.
Table 5.
Five-Year Major Adverse Clinical Events in Patients With and Without m-SVGs in the Angiographic Cohort: All Events (Including Those Before, During, and After Protocol Angiography)
| m-SVGs | Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|---|
| Clinical Events | Yes (n = 826) | No (n = 1574) | HR (95% CI) | P | HR (95% CI) | P | |
| Death, MIs or revascularizations, n | 300 | 481 | 1.24 (1.07, 1.43) | 0.004 | 1.20 (1.03, 1.39) | 0.018 | |
| 5-y event rate, % | 5-y event rate | 36.7 | 31.0 | ||||
| Deaths or MI, n | # of events | 215 | 318 | 1.32 (1.11, 1.57) | 0.002 | 1.27 (1.06, 1.52) | 0.011 |
| 5-y event rate, % | 5-y event rate | 26.3 | 20.5 | ||||
| Death or revascularization, n | # of events | 226 | 370 | 1.19 (1.01, 1.40) | .0043 | 1.16 (0.97, 1.38) | 0.098 |
| 5-y event rate, % | 5-y event rate | 27.8 | 23.9 | ||||
| All death, n | # of events | 108 | 165 | 1.25 (0.98, 1.60) | 0.070 | 1.21 (0.94, 1.56) | 0.140 |
| 5-y event rate, % | 5-y event rate | 13.4 | 10.7 | ||||
Adjusted for age, sex, race, history of congestive heart failure, creatinine clearance, recent MI (within 30 d of enrollment), weight, height, diastolic blood pressure, on-pump surgery, length of surgery, harvesting technique, and use of internal mammary artery conduit.
m-SVG indicates saphenous vein graft with >1 distal target; HR, hazard ratio; CI, confidence interval; and MI, myocardial infarction.
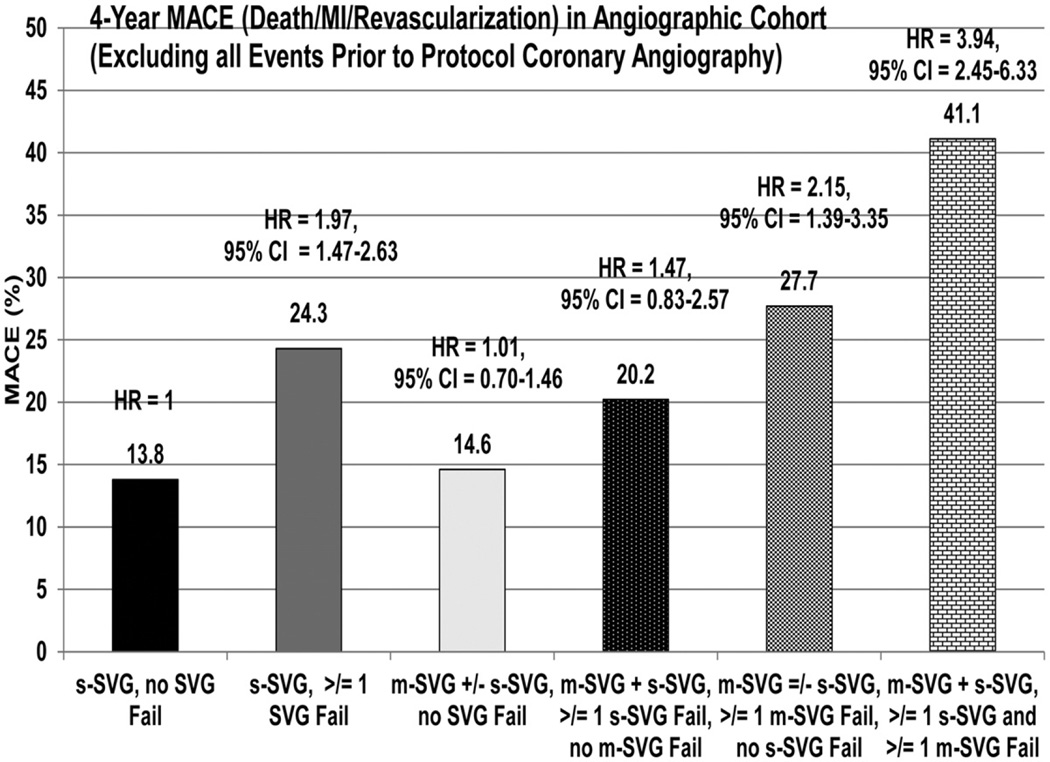
Table 6 shows adjusted major adverse event rates for patients in various SVG groups in the coronary angiographic cohort excluding events before the cardiac catheterization, and Figure 2 demonstrates the adjusted composite event rate of death, MI, or revascularization (excluding events before coronary angiography). The adjusted composite rates were similar for the groups with either s-SVG and those with m-SVG with or without s-SVG who had no graft failure. In contrast, this event was significantly higher in patients with m-SVG and s-SVG in whom ≥1 s-SVG failed, but without any failure of m-SVG, and in those with m-SVG with or without s-SVG in whom >1 m-SVG failed, but without any failure of s-SVG. The highest events were observed among patients who had both s-SVG and m-SVG and in whom both types of vein graft failed.
Table 6.
Association of 4-Year Major Adverse Clinical Events in Various SVG Groups With and Without Graft Failure in the Angiographic Cohort
| Outcomes | s-SVG Only and No Graft Failures (n = 700) |
s-SVG Only and ≥1 Graft Failure (n = 499) |
m-SVG With or Without s-SVG and No Graft Failures (n = 341) |
s-SVG and m-SVG With ≥1 s-SVG Failure and No m-SVG Failures (n = 89) |
m-SVG With or Without s-SVG with ≥1 m-SVG Failure and No s-SVG Failure (n = 123) |
s-SVG and m-SVG With 1 m-SVG and ≥1 m-SVG Failures (n = 77) |
|---|---|---|---|---|---|---|
| Events that include only those during or after protocol angiography (excludes events before this), n |
682 | 425 | 332 | 85 | 107 | 59 |
| Death, MI or revascularization |
13.8 | 24.3 | 14.6 | 20.2 | 27.7 | 41.1 |
| Death or MI | 7.0 | 8.4 | 9.5 | 4.9 | 11.8 | 6.9 |
| Death or revascularization |
13.4 | 23.6 | 13.7 | 18.9 | 26.8 | 41.1 |
| Death | 5.8 | 7.2 | 7.4 | 3.6 | 9.9 | 5.2 |
Values are presented as percentages, unless otherwise indicated.
SVG, saphenous vein graft; s-SVG = saphenous vein graft with only 1 distal target; m-SVG, saphenous vein graft with >1 distal target; and MI, myocardial infarction.
Figure 2.
Vein graft failure and outcomes in various patient subgroups. MACE indicates major adverse cardiac event; MI, myocardial infarction; HR, hazard ratio; m-SVG, saphenous vein graft with >1 distal target; CI, confidence interval; and s-SVG, saphenous vein graft with only 1 distal target.
Discussion
Study Findings
Our data suggest that roughly one third of patients undergoing their first CABG surgery receive an m-SVG conduit. Patients who received a composite SVG during their first CABG surgery were more likely to have these grafts fail and had a trend for higher death, MI, or repeat revascularization in the 5 years after surgery. Analyses of data from the angiographic cohort suggest that any SVG failure was associated with poor outcomes. Perhaps the higher clinical adverse event rates with the use of m-SVG were a reflection of higher vein graft failure rates in the m-SVG group compared with the s-SVG group.
Comparisons With Prior Studies
Prior studies evaluating the short- and long-term patency and outcomes of m-SVG compared with s-SVG1–8,11–15 have had disparate results, with some demonstrating excellent shortand long-term patency5,7,11–13 and others suggesting no difference8,14 or even worse6,15 long-term patency with m-SVG. Studies that evaluated clinical events suggested contradictory findings, similar to those seen for vein graft failure, with 1 study showing a survival advantage11 and another showing no difference8 in survival between patients with m-SVG and those with s-SVG. These differences in vein graft patency and outcomes were attributed to variable patient mix, different levels of surgical expertise with the technique, variation in surgical procedure and graft harvesting techniques, sequence of grafting, quality of native vessels, and differences in the use of IMA conduits. Patency of m-SVGs has been shown to be lower in diabetic patients, those with end-to-side versus side-to-side anastomosis, and those with poor-quality target vessel, particularly when the most distal end-to-side anastomosis was made to a vessel with poor rather than good target quality.11,13,16
Other differences between our study and previous studies may account for the lower patency rates for m-SVG in our study. All of the above studies were single-center investigations where surgeons had significantly greater expertise in and a preference for performing m-SVG.1–8,11–16 In PREVENT-IV, patients were enrolled at 107 sites across the United States where preferences, expertise, and practice patterns likely differed significantly.9,10 For instance, the pump time for patients receiving m-SVG in our study was considerably longer when compared with several of the single-center investigations.1–8,11–16 Unlike PREVENT-IV, earlier studies did not have systematic collection of perioperative cardiac biomarkers to ascertain perioperative MI or systematic follow-up angiography, which could have resulted in significant bias favoring 1 group over the other. We defined m-SVG failure as failure of any of the limbs of these grafts, a definition that was not consistently used in previous studies. Almost all of the previous studies failed to account for the differences in IMA use, a factor shown to be strongly linked with outcomes.17
Clinical Implications
Complete arterial revascularization during CABG surgery remains a highly desirable but very optimistic goal, even in the current era.18 Thus, ≥1 SVG conduits continue to be used in >95% of patients undergoing CABG surgery. Saphenous vein grafts remain more commonly used than arterial grafts for emergent or salvage procedures where high starting flow is desirable immediately or when arterial conduits have been exhausted by previous surgery or are of poor quality. As noted above, m-SVGs offer several advantages, including conservation of graft material, shorter time for revascularization, more complete revascularization, and hemodynamic advantages that include higher flow velocity in the proximal segment.19 More than 3 decades after its introduction,1,2 controversy plagues the use of m-SVG conduits with studies providing contradictory information about long-term patency and outcomes.1–8,11–16 Much has been written about theoretical advantages of various refinements in the use of m-SVG (eg, Y grafts versus sequential grafts, side-to-side anastomosis to a small branch or poor-quality native artery, and end-to-side anastomosis to larger major epicardial or good-quality vessel), but none of these refinements have been uniformly tested in large numbers of patients.4,11,16 Thus, the techniques relative to m-SVG use remain variable and at the discretion of surgeons. The differences in graft patency and clinical outcomes between prior studies and our data are likely a result of this variance in clinical practice.
The use of m-SVG may be unavoidable in circumstances where graft material is limited. However, in PREVENT-IV, the availability of graft material was less of an issue, yet one-third of the patients received m-SVG, suggesting that many surgeons still prefer their use. Rigorous evaluation and refinement of the current technique is needed so that long-term patency of m-SVG can be improved in contemporary cardiac surgical practice with the ultimate goal of improving patient outcomes. The higher failure rates of m-SVG (and poor outcomes of vein graft failure in general) demonstrated in our study suggests that the use of s-SVG should be preferred over m-SVG when feasible. Although preliminary data have supported the use of arterial conduits as composite grafts during CABG,13,14 no large study has definitely proven their advantages. These hypotheses require testing in adequately designed studies.
Limitations
This retrospective, post hoc, observational analysis of the PREVENT-IV data should be considered hypothesis generating. We did not formally correct for multiple comparisons. The observational nature of the analysis limits inferences about causation. As with most retrospective studies, some data were missing or incomplete, and the influence of this on the study results cannot be ascertained. The influence of unmeasured confounders or long-term adherence to guideline-based secondary prevention treatments on outcomes could not be evaluated. In multivariable analysis, we adjusted for harvesting technique to account for the differences in the rate of endoscopic vein harvesting versus open technique among multiple versus single distal target vein grafts. However, given recent data from PREVENT-IV suggesting the association of endoscopic vein graft harvesting technique with higher vein graft failure and worse outcomes than with open technique,20 the possibility that the difference in harvesting techniques may have influenced vein graft failure as well as outcomes cannot be entirely excluded. We did not have separate information on Y versus sequential graft use. We also did not have information on the location of stenosis or occlusion in m-SVGs. Lesions proximal to all distal anastomoses are intuitively more likely to have an impact on adverse clinical events than those in the distal segment of sequential or any single limb of Y m-SVGs. Inclusion of these 2 m-SVG groups together in the failed m-SVG cohort is likely to have only biased our findings toward null (one would anticipate better outcomes with failure of 1 distal attachment compared with failure of >1 distal attachments). Finally, our findings are most applicable to patients undergoing their first CABG surgery.
Conclusions
Compared with s-SVG conduits, the use of m-SVG conduits was associated with a higher rate of 1-year vein graft failure in patients undergoing first CABG. The 5-year composite of death, MI (including perioperative MI), or revascularization also tended to be higher in patients receiving m-SVG. The trend toward worse clinical outcomes in patients with m-SVG compared with those receiving s-SVG appeared to be related to the higher clinical event rate associated with any SVG failure (m- or s-SVG), with higher m-SVG failure rates translating into trends toward worse outcomes in patients with m-SVG. These findings should stimulate further studies to identify why m-SVGs have a higher failure rate and to better understand the most appropriate conduit to improve longterm graft patency and clinical outcomes of patients undergoing CABG surgery. In the meantime, the use of s-SVG over m-SVG should be encouraged when feasible.
CLINICAL PERSPECTIVE.
Saphenous vein grafts (SVGs) with single proximal and multiple distal anastomoses (m-SVG), often used in patients undergoing coronary artery bypass graft (CABG) surgery, allow complete revascularization with limited SVG material necessary for multiple bypasses and permit shorter revascularization time compared with multiple single SVG (s-SVG, single proximal and distal anastomoses) conduits in unstable patients undergoing emergent or salvage CABG surgery. However, limited information exists on the intermediate-term graft patency and 5-year clinical outcomes of patients receiving m-SVG versus s-SVG during CABG in the current era. We studied the association of the use of m-SVG versus s-SVG conduits with 1-year SVG failure (defined as ≥75% angiographic stenosis) and 5-year clinical events (death; death or myocardial infarction; and death, myocardial infarction, or revascularization) in 3014 patients undergoing their first CABG surgery enrolled in the Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT) IV trial. Of 3014 patients enrolled in PREVENT IV, 1045 (34.7%) had ≥1 m-SVGs during CABG. We found that in patients undergoing first CABG surgery, the use of m-SVG was associated with a higher 1-year vein graft failure rate and trends toward worse clinical outcomes. Our data call for additional studies to better understand the most appropriate conduit to improve long-term graft patency and clinical outcomes of patients undergoing CABG surgery. In the meantime, these data should encourage the use of s-SVG over m-SVG when feasible.
Acknowledgments
The authors wish to thank Elizabeth E.S. Cook of the Duke Clinical Research Institute for her editorial assistance.
Sources of Funding
PREVENT-IV was funded by Corgentech Inc, San Francisco, CA, and analysis was funded by the Duke Clinical Research Institute, Durham, NC. Corgentech Inc had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data, or preparation, review, or approval of the manuscript. Dr Alexander was supported in part by grant U01-HL088953 from the National Institutes of Health Cardiothoracic Surgical Trials Network.
Drs Gibson, Harrington, and Califf have received significant research grants from Corgentech and Bristol-Myers Squibb. Dr Peterson has received significant research grants from Merck/Schering Plough, Bristol-Myers Squibb, and the Society of Thoracic Surgery.
Footnotes
Disclosures
Please see https://dcri.org/about-us/conflict-of-interest for additional conflict of interest information for DCRI faculty. The other authors report no conflicts.
References
- 1.Flemma RJ, Johnson WD, Lepley D., Jr Triple aorto-coronary vein bypass for coronary insufficiency. Arch Surg. 1971;103:82–83. doi: 10.1001/archsurg.1971.01350070108026. [DOI] [PubMed] [Google Scholar]
- 2.Bartley TD, Bigelow JC, Page US. Aortocoronary bypass grafting with multiple sequential anastomoses to a single vein. Arch Surg. 1972;105:915–917. doi: 10.1001/archsurg.1972.04180120092017. [DOI] [PubMed] [Google Scholar]
- 3.Sewell WH, Sewell KV. Technique for the coronary snake graft operation. Ann Thorac Surg. 1976;22:58–65. doi: 10.1016/s0003-4975(10)63954-9. [DOI] [PubMed] [Google Scholar]
- 4.Grondin CM, Limet R. Sequential anastomoses in coronary grafting: technical aspects and early and late angiographic results. Ann Thorac Surg. 1977;23:1–8. doi: 10.1016/s0003-4975(10)64059-3. [DOI] [PubMed] [Google Scholar]
- 5.Meurala H, Valle M, Hekali P, Somer K, Frick MH, Harjola PT. Patency of sequential versus single vein grafts in coronary bypass surgery. Thorac Cardiovasc Surg. 1982;30:147–151. doi: 10.1055/s-2007-1022233. [DOI] [PubMed] [Google Scholar]
- 6.Kieser TM, FitzGibbon GM, Keon WJ. Sequential coronary bypass grafts: long-term follow-up. J Thorac Cardiovasc Surg. 1986;91:767–772. [PubMed] [Google Scholar]
- 7.Eschenbruch EM, Pabst F, Tollenaere P, Roskamm H, Schuziger M. The significance of coronary topography for operative technique and tactics in multiple myocardial revascularization with jump-grafts. Thorac Cardiovasc Surg. 1981;29:206–211. doi: 10.1055/s-2007-1023478. [DOI] [PubMed] [Google Scholar]
- 8.Meeter K, Veldkamp R, Tijssen JG, van Herwerden LL, Bos E. Clinical outcomes of single versus sequential grafts in coronary bypass operations at ten years’ follow-up. J Thorac Cardiovasc Surg. 1991;101:1076–1081. [PubMed] [Google Scholar]
- 9.Alexander JH, Ferguson TB, Jr, Joseph DM, Mack MJ, Wolf RK, Gibson CM, Gennevois D, Lorenz TJ, Harrington RA, Peterson ED, Lee KL, Califf RM, Kouchoukos NT. The PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) trial: study rationale, design, and baseline patient characteristics. Am Heart J. 2005;150:643–649. doi: 10.1016/j.ahj.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 10.PREVENT IV Investigators. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 11.Christenson JT, Simonet F, Schmuziger M. Sequential vein bypass grafting: tactics and long-term results. Cardiovasc Surg. 1998;6:389–397. doi: 10.1016/s0967-2109(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 12.Vural KM, Sener E, Tasdemir O. Long-term patency of sequential and individual saphenous vein coronary bypass grafts. Eur J Cardiothorac Surg. 2001;19:140–144. doi: 10.1016/s1010-7940(00)00629-1. [DOI] [PubMed] [Google Scholar]
- 13.Oz BS, Iyem H, Akay HT, Bolcal C, Yokusoglu M, Kuralay E, Demirkilic U, Tatar H. Mid-term angiographic comparison of sequential and individual anatomosis techniques for diagonal artery. J Card Surg. 2006;21:471–474. doi: 10.1111/j.1540-8191.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 14.Cho KR, Kim JS, Choi JS, Kim KB. Serial angiographic follow-up of grafts one year and five years after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006;29:511–516. doi: 10.1016/j.ejcts.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Goldman S, Zadina K, Krasnicka B, Moritz T, Sethi G, Copeland J, Ovitt T, Henderson W. Predictors of graft patency 3 years after coronary artery bypass graft surgery. J Am Coll Cardiol. 1997;29:1563–1568. doi: 10.1016/s0735-1097(97)82539-9. [DOI] [PubMed] [Google Scholar]
- 16.Christenson JT, Schmuziger M. Sequential venous bypass grafts: results 10 years later. Ann Thorac Surg. 1997;63:371–376. doi: 10.1016/s0003-4975(96)01059-4. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RH, Honeycutt E, Shaw LK, Milano CA, Smith PK, Harrington RA, Sketch MH., Jr Clinical and angiographic correlates of short- and long-term mortality in patients undergoing bypass grafting. Am J Cardiol. 2007;100:1538–1542. doi: 10.1016/j.amjcard.2007.06.053. [DOI] [PubMed] [Google Scholar]
- 18.Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, Hart JC, Herrmann HC, Hillis LD, Hutter AM, Jr, Lytle BW, Marlow RA, Nugent WC, Orszulak TA. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2004;110:e340–e437. [PubMed] [Google Scholar]
- 19.O’Neill MJ, Jr, Wolf PD, O’Neill TK, Montesano RM, Waldhausen JA. A rationale for the use of sequential coronary artery bypass grafts. J Thorac Cardiovasc Surg. 1981;81:686–690. [PubMed] [Google Scholar]
- 20.Lopes RD, Hafley GE, Allen KB, Ferguson TB, Peterson ED, Harrington RA, Mehta RH, Gibson CM, Mack MJ, Koucoukos NT, Califf RM, Alexander JH. Association between endoscopic versus open vein graft harvesting and long-term angiographic and clinical outcomes in patients undergoing coronary artery bypass graft surgery. N Engl J Med. 2009;361:235–244. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]