Abstract
Illumination of dark-adapted barley plants with low light transiently induced a large nonphotochemical quenching of chlorophyll fluorescence. This reaction was identified as a form of high-energy-state quenching. Its appearance was not accompanied by zeaxanthin synthesis but was associated with a reversible inactivation of a fraction of photosystem II (PSII) centers. Both the fluorescence quenching and PSII inactivation relaxed in parallel with the activation of the Calvin cycle. We interpret the induction of this phenomenon as due to the generation of a quenched state in the PSII core complex. This reaction is probably caused by the transient overacidification of the thylakoid lumen, whereas its dissipation results from the relaxation of both the pH gradient across the thylakoid membrane and redox pressure upon activation of carbon fixation. At saturating light intensities, inactivation of PSII was still observed at the onset of illumination, although its recovery did not result in dissipation of high-energy quenching, which presents typical characteristics of an antenna-associated quenching at steady state. Reaction-center quenching seems therefore to be a common transient feature during illumination, being replaced by other phenomena (photochemical or antenna quenching and photoinhibition), depending on the balance between light and carbon fixation fluxes.
The absorption of light in excess of the capacity of the photosynthetic electron transport chain can be harmful to plants (reviewed in refs. 1 and 2). The capacity of that chain, however, is variable, depending on both environmental conditions (e.g., low temperature inhibits electron transport) and the degree of activation of the Calvin cycle, the major sink for reductants and ATP. Calvin cycle activity notably varies after the transition from darkness to light, when accumulation of metabolites and activation of enzymes is required. Consequently, a particular light intensity may be barely enough to promote photosynthesis under full potential of CO2 assimilation and yet excessive when that flow is restricted.
Under conditions of excess light, processes are initiated that are thought to protect the chloroplasts from damage. Prominent among these is the phenomenon described as nonphotochemical quenching of chlorophyll fluorescence (NPQ) (1). This term, however, describes a collection of processes, playing a variety of different roles in the regulation of photochemistry, including state transitions [phosphorylation-related migration of chlorophyll-binding proteins (light-harvesting complex II, LHCII) between photosystem (PS) II and PSI (3)], photoinhibition (slowly reversible damage to PSII reaction centers) (4), and pH-dependent (or high-energy state) quenching (qE) resulting from an increase in the thermal dissipation capacity of the light-collecting apparatus (1, 5, 6).
Quantitatively the most important component of NPQ, the mechanism of high-energy-state quenching, has been the subject of intense research for many years. By definition, qE depends on the presence of a pH gradient across the thylakoid membrane (ΔpH) and, specifically, on the pH in the thylakoid lumen (7, 8). It is characterized by its sensitivity to uncouplers of ΔpH in isolated systems (9, 10) and to N,N′-dicyclohexylcarbodiimide, a protein modification reagent that covalently binds to protonatable residues (11). The existence of such quenching in higher plant leaves is defined kinetically as the fast phase in the relaxation of total NPQ after a period of illumination (12). Early studies of qE focused on the site of quenching. Two classes of model were proposed, depending on whether the quencher was assumed to be associated with the reaction center (13) or to occur within the chlorophyll antenna bed of PSII (14). Although in vitro evidence for both models exists, the lack of clear data supporting the occurrence of reaction-center quenching in vivo (15), as well as the isolation of mutants of both higher plants and green algae highly perturbed in NPQ development because of deficiencies in the xanthophyll cycle (6), has brought about a general consensus that the major form of quenching occurs in the PSII antenna.
In this paper, we present evidence that reaction-center NPQ is transiently induced during illumination of dark-adapted barley plants, even when low light intensities are used. This NPQ may be extensive during the first seconds of illumination. Under steady-state conditions, the phenomenon disappears at low light, whereas it is replaced by antenna quenching at higher intensities or even leads to the generation of a stable inhibited state of PSII at supersaturating irradiance (≈10 times the saturating value). The choice between these different possibilities is modulated by the balance between the light flux and the dissipation ability of the carbon fixation apparatus.
Materials and Methods
Growth Conditions. Young seedlings of barley (Hordeum vulgaris) and Arabidopsis thaliana plants were grown on soil at an irradiance of 100 μE m–2·s–1 [μE, microeinsteins (1 E = 1 mol of photons)] white light under a 12-h dark/12-h light regime, at a temperature of 25°C until development of the second leaf. They were dark-adapted for at least 4 h before measurements.
Fluorescence, Oxygen Evolution, and CO2 Assimilation Measurements. Fluorescence emission was measured on attached leaves by using a PAM fluorometer (Walz, Effeltrich, Germany). Light was provided by a red source (SDL diode laser, emission peak at 690 nm). Maximal fluorescence was induced by a saturating pulse of white light (1 s, 2,200 μEm–2·s–1). Alternatively, a halogen lamp (Schott KL1500, Mainz, Germany), filtered by using a Wratten filter (no. 55) was used to provide light at 520 nm. The fluorometer used in Fig. 1 operates in the reflection mode, detecting fluorescence emitted by the same side of the leaf that is illuminated. It selects emission from the top cell layers within the leaf, i.e., the most illuminated ones. Conversely, most of our other experiments were performed in transmission mode, where all leaf layers were sampled. Therefore, we used green light when probing the effect of low light illumination. Because this light is less efficiently absorbed than red light, it should be homogeneously absorbed throughout the leaf. When tested for its ability to induce reversible NPQ, this source proved to be as efficient as the red sources (Fig. 1 A). We checked that the reversibility of the quenching during illumination inversely correlated with light intensity in both cases (data not shown). However, we were not able to saturate or oversaturate electron flow with green light and therefore used the red source in these conditions.
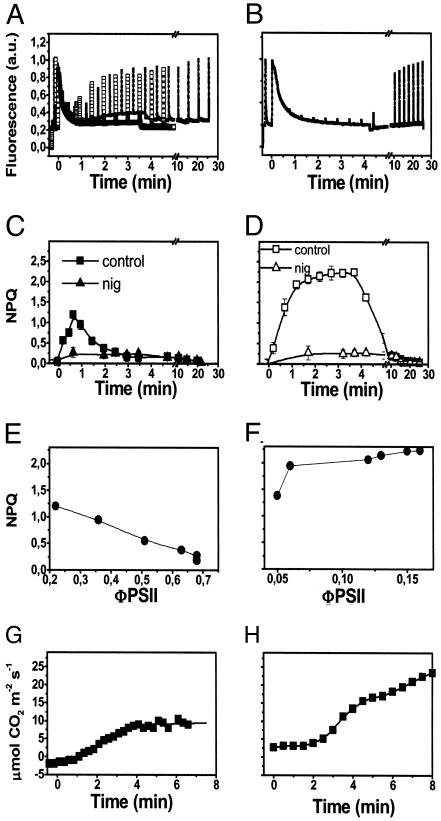
Fig. 1.
Light-induced NPQ generation in barley leaves, under low and high photon flux. (A and B) Chlorophyll fluorescence emission in wild-type leaves illuminated with ≈100 μEm–2·s–1 green light (solid symbols) or red light (open symbols) (A) and ≈1,000 μE m–2·s–1 red light (B) for 4 min, and then dark-adapted for a further 20 min. (C and D) Calculations of NPQ. Each point represents the mean of three separate experiments with standard error bars. NPQ was calculated as (Fm-Fm′)/Fm′, where Fm represents the maximum fluorescence emission recorded in dark-adapted leaves, and Fm′ represents the maximum fluorescence value recorder at discrete time intervals during illumination. Squares, control; triangles, nigericin-treated sample. Nigericin (100 μM) was added to scratched leaves (see Materials and Methods) 5 min before illumination, and then NPQ was evaluated as above. Fv/Fm values were 0.75 ± 0.05 and 0.7 ± 0.06 in the case of scratched leaves treated with water (control) and with nigericin, respectively. (E and F) Relationship between NPQ relaxation during illumination (estimated from traces as in A and B) and the increase of the noncyclic electron flow (ΦPSII = ΔF/Fm) (19). (G and H) Time course of CO2 assimilation during illumination under the same conditions as in A and B, respectively. a.u., arbitrary units.
Oxygen evolution was measured in leaf disks by using a naked platinum electrode, as described in ref. 16. Oxygen evolution followed excitation with a laser pulse at 695 nm. CO2 fixation was measured with an infrared gas analyzer (CIRAS-1, PP Systems, Hertfordshire, U.K.).
Spectroscopic Measurements. Spectroscopic measurements were performed on attached leaves at room temperature by using a laboratory-built spectrophotometer (17). Actinic light was provided by the same sources used in the case of fluorescence. Light-induced absorption changes were measured as absorption of flashed monochromatic light at discrete times. PSII charge separation was measured as the extent of the electrochromic signal at 515–545 nm, 100 μs after excitation with single turnover laser pulses at 695 nm. This signal is linearly proportional to reaction-center photochemistry (18). PSII contribution was deduced as the difference between the signal measured in the absence and in the presence of the PSII inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). Hydroxylamine was added to destroy the manganese cluster responsible for oxygen evolution and to slow down recombination between the donor and acceptor side of PSII (20), which would preclude correct estimation of the PSI/PSII ratio. Both compounds were introduced by gently scratching the leaves (21). This method proved most efficient for homogeneous and reproducible penetration of chemicals, as evidenced by measurements of fluorescence increase upon addition of DCMU with a fluorescence video imaging system (data not shown).
Pigment Analysis. The pigments were extracted with 80% acetone and then separated and quantified by HPLC (22) and by fitting of the acetone extract with the spectra of individual pigments (23).
Results
Reversible Fluorescence Quenching Is Induced by Subsaturating Light in Barley Leaves. Illumination of attached barley leaves under a constant CO2 atmosphere of 360 ppm at a low photon flux density (100 μmol m–2·s–1 of red light at 690 nm) resulted in the development of a large NPQ, appearing during the first seconds of illumination (Fig. 1 A). Approximately 80% of this quenching reversed rapidly during the first 3–4 min of illumination (Fig. 1 A). No further changes were observed at steady state (data not shown), with the remaining part of the quenching reverting only upon returning the plants to darkness. Illumination with saturating light (1,000 μmol m–2·s–1 of red light at 690 nm) resulted, conversely, in the generation of a larger quenching that did not relax during illumination (Fig. 1B).
The red actinic light used here is highly absorbed by chloroplasts. Therefore, it produces a large gradient of light within the leaf at low intensity. This might result in heterogeneous illumination of chloroplasts and NPQ response. Indeed, chloroplasts that are closer to the light source receive a rather high amount of light and are therefore more prone to undergo NPQ than the ones that are far from the light source. For this reason, we chose green light sources for the next series of experiments in which low light illumination was used but kept red light sources when we needed higher intensities to reach saturating light conditions (see Materials and Methods for details). Fig. 1 A shows that the two light sources provided the same NPQ response when used at low intensity in the reflection mode.
The dark relaxation of both the transient quenching in low light and the steady-state quenching in saturating light was very fast, with ≈80% of fluorescence being recovered within 1 min. Because of its fast relaxation, most of the quenching observed in both light conditions could be ascribed to a form of qE (12). The remaining fraction of NPQ showed a slower dark relaxation time (≈20 min) and was probably due to state transitions (12). That qE was indeed the most prominent form of NPQ in both conditions is also confirmed by the sensitivity of the fluorescence quenching to ΔpH dissipation (9, 10); addition of the ionophore nigericin abolished most of the quenching in both cases (Fig. 1 C and D, triangles).
The relaxation of the transient nonphotochemical quenching of fluorescence during illumination with low light correlated with the onset of photosynthetic activity, as evidenced by the increase in the quantum yield of noncyclic electron flow (ΦPSII, Fig. 1E) (19). This increase of the photosynthetic activity occurred in the same time scale as the activation of the Calvin cycle (Fig. 1 G and H). Conversely, a rise in photosynthetic activity also was observed at saturating light, but no relaxation of nonphotochemical quenching was induced in these conditions. As a consequence, no parallel could be drawn between the recovery of fluorescence emission and the activation of the Calvin cycle in high-light-treated plants.
Transient Fluorescence Quenching Is Not Related to Carotenoid Deepoxidation. The extent of qE is generally correlated with changes in the carotenoid composition of the LHCII antenna. Specifically, the light-induced ΔpH activates a lumen-located deepoxidase that becomes membrane-bound and converts violaxanthin into zeaxanthin at low pH levels (24, 25).
This conversion is strictly related to the increased thermal dissipation capacity of the PSII antenna proteins during the development of qE (26). We measured the correlation between the amount of zeaxanthin synthesized in plants and the appearance of both the reversible and irreversible qE. Table 1 shows that under the conditions of illumination used in Fig. 1, no deepoxidation of xanthophyll was detectable at low light intensity. Conversely, a substantial conversion was observed upon illumination with high light, in agreement with previous reports (26). As a consequence, no correlation could be established between the generation of the large and reversible qE at low light and carotenoid deepoxidation.
Table 1. Carotenoid deepoxidation after illumination of barley leaves.
| Neo. | Viola. | Anthera. | Zea. | Lut. | β-Car. | |
|---|---|---|---|---|---|---|
| Dark-adapted | 9.68 ± 0.36 | 15.17 ± 1.48 | ND | ND | 28.07 ± 1.34 | 14.78 ± 0.66 |
| Green light, 30 s | 9.63 ± 0.09 | 15.97 ± 1.45 | ND | ND | 28.48 ± 0.76 | 14.19 ± 0.63 |
| Red light, 4 min | 9.25 ± 0.16 | 12.33 ± 1.01 | 1.37 ± 0.02 | 4.20 ± 0.93 | 29.37 ± 0.77 | 13.5 ± 1.25 |
Plants were illuminated as in Fig. 1, and then leaves were frozen in liquid nitrogen. Pigment analysis was performed on whole-leaf extract by HPLC pigment analysis, as described in Materials and Methods. Values are moles of pigment per 100 moles of chlorophyll a + b. Light intensities were as in Fig. 1. ND, not detectable. Neo., neoxanthin; Viola., violaxanthin; Anthera., antheraxanthin; Zea., zeaxanthin; Lut., lutein; β-car., β carotene.
Reaction-Center Quenching Is Reversibly Generated During Illumination with both Limiting and Saturating Light Intensities. The NPQ observed in low light is different from that observed under steady-state conditions at higher light; it is reversible during illumination at low light intensity and not correlated with the synthesis of zeaxanthin. To assess whether it can be ascribed to a form of reaction-center quenching similar to that described in vitro in ref. 13, we measured the photochemical activity of PSII before and during development of the reversible quenching by using the generation of a field-indicating electrochromic shift around 515 nm as a measure of PSII activity (see Materials and Methods for details).
Quenching was induced by illumination as in Fig. 1, and PSII activity was measured 10 s after a variable period of preillumination. This protocol was chosen to allow an almost complete reoxidation of the quinone acceptor of PSII, QA, whereas most of the fluorescence quenching would be preserved (data not shown). A single turnover laser pulse, the intensity of which was either saturating or subsaturating (≈15% of the maximum), was used to detect PSII activity. Quenching in the antenna should only be associated with loss of activity upon illumination with the subsaturating laser flash because of the reduced light-harvesting capacity. In contrast, an identical degree of inhibition is expected at both saturating and subsaturating intensities in the case of reaction-center quenching, which affects the intrinsic photochemical capacity of the reaction centers independently of the light intensity used.
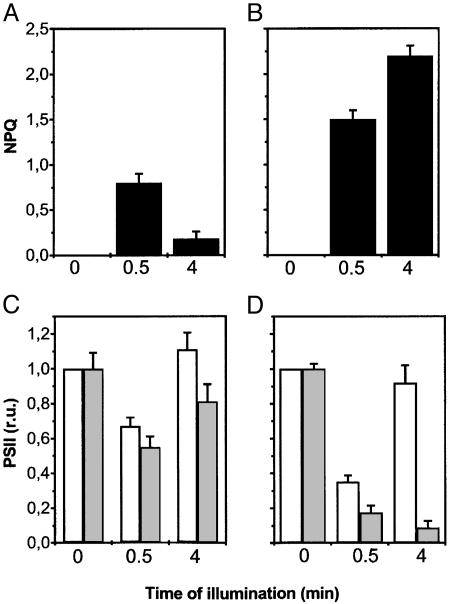
Fig. 2 shows that preillumination with the low-intensity green light for 30 s resulted in a decrease of PSII activity, which could be detected at both saturating (white columns) and subsaturating (gray columns) flash intensities. The effect was somewhat more marked at the lower flash intensity (Fig. 2C). The decreased PSII activity observed at saturating flash intensity disappeared when the preillumination was prolonged to 4 min (i.e., the time required to induce the recovery of most of the fluorescence quenching) (Fig. 2 A), whereas the effect observed at the subsaturating flash intensity was only partially removed by this treatment (Fig. 2C). When the saturating light was used to preilluminate leaves as in Fig. 1B, a substantial loss of PSII activity (larger than in low-light conditions) (Fig. 2D) was also observed after 30 s of preillumination, in parallel with the generation of a large NPQ (Fig. 2B). Whereas PSII activity detected with the saturating laser flash recovered substantially at steady state, as already observed in the case of low-light preillumination, neither fluorescence quenching (Fig. 2B) nor PSII activity detected by using the subsaturating laser flash (Fig. 2D) showed any relaxation. This result suggests that a stable antenna quenching replaced the transient NPQ under saturating steady-state illumination. At supersaturating light intensities (10,000 μmol m–2·s–1, i.e., 10 times the saturation value), a sustained decrease in PSII activity also was observed. This phenomenon, however, was not reversible during illumination and required >30 min of dark incubation before reversal could be detected (data not shown). We attribute this decrease in PSII activity to the generation of a PSII photoinhibited state.
Fig. 2.
Nonphotochemical quenching after 30-s and 4-min illumination of wild-type barley leaves (A and B) and changes in the activity of PSII complexes in barley leaves during a dark–light transition (C and D). PSII activity was estimated as the extent of the electrochromic signal as described in Materials and Methods. Dark-adapted leaves were measured as a reference for 100% activity of PSII. Then, activity was measured 10 s after a given time of illumination (30 s or 4 min), and the relative variation of activity was calculated. White and gray columns represent PSII activity detected with a saturating or a subsaturating single turnover laser flash, respectively. Data refer to three different experiments, each repeated three times. Experimental conditions were as in Fig. 1. At time = 0, the ratio between the 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU)/hydroxylamine-sensitive (PSII) and -insensitive (PSI) component was equal to 1 (data not shown), indicating normal accumulation of both PSs in our plants. No changes in the fraction of the signal insensitive to DCMU and hydroxylamine addition were observed, suggesting no changes in PSI photochemical activity. (A and C) Preillumination as in Fig. 1A. (B and D) Preillumination as in Fig. 1B. r.u., relative units.
Reaction-Center Quenching Is Associated with Inactivation of a Fraction of PSII Complexes. Two possibilities might explain the relationship between fluorescence quenching and inhibition of PSII activity that is transiently observed in illuminated leaves. Illumination might partially inhibit all of the reaction centers present in the membranes at the same time, or it might inactivate a limited fraction of the centers, generating thermal traps within the thylakoid membranes. In both cases, photochemical activity would be decreased and fluorescence yield reduced.
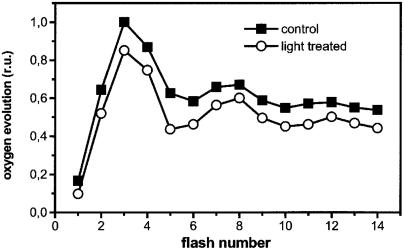
To distinguish between these two possibilities, we measured flash-induced oxygen evolution from intact leaves that were preilluminated with low light, as in Fig. 1 A. Fig. 3 shows that oxygen evolution was reduced by ≈20% after preillumination, as compared with leaves that were illuminated with the same sequence of laser flashes (one every 10 s) in the absence of preillumination. We detected no major changes in the period of four oscillations of oxygen evolution (16). Thus, preillumination did not modify the recombination properties of all centers, which would result in an increased damping of the period 4 oscillation. We conclude that ≈20% of the reaction centers were reversibly converted into thermal traps during a dark–light transition.
Fig. 3.
Consequences of the reversible fluorescence quenching on oxygen evolution by barley leaves. Leaf disks were gently scratched and then placed on a naked oxygen electrode. Oxygen evolution was induced by saturating laser flashes fired at the frequency of 1 Hz. Squares, dark-adapted leaves; circles, preilluminated leaves. Preillumination was for 30 s as in Fig. 1A. Traces represent the average of five different recordings, standard deviation being smaller than data point size.
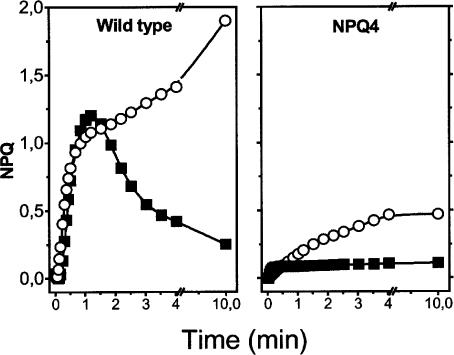
Transient Quenching Is Affected in the npq4 Mutant of A. thaliana. A major contribution to our understanding of the mechanism of qE generation in plants came from the genetic approach that provided several mutants of A. thaliana severely impaired in NPQ generation. The most extensive effect was observed with the npq4 mutant devoid of the Lhc-like protein PsbS (27). When tested for its capacity to generate reversible fluorescence quenching at low light, the npq4 strain appeared to be completely inhibited compared with the wild type of A. thaliana, which behaved as barley (Fig. 4). In this mutant, the extent of quenching was also largely reduced in high light, in agreement with previous reports (27).
Fig. 4.
NPQ measurements in A. thaliana wild-type and npq4 mutant. Squares, low light as in Fig. 1 A; circles, high light as in Fig. 1B; other conditions as in Fig. 1.
Discussion
Properties of the Transient NPQ Generated upon a Dark–Light Transition in Vascular Plants. Here, we present evidence indicating that a substantial nonphotochemical quenching of fluorescence is induced in dark-adapted plants under illumination at subsaturating light intensities. The nonphotochemical quenching disappears during illumination, being rapidly replaced by a photochemical quenching.
This transient NPQ is largely dominated by a process occurring within the PSII reaction center. Its generation is not related to violaxanthin deepoxidation (Table 1), but rather it correlates with a decrease of PSII photochemistry (Fig. 2) and oxygen evolution (Fig. 3). Moreover, it is observed in leaves of the chlorina F2 mutant of barley, which is devoid of most of the peripheral antennae complexes (data not shown) Therefore, it is very likely that the transient qE reported here occurs within the PSII core complex.
The same phenomenon observed under low light develops in saturating conditions at the onset of illumination. Under this light regime, however, the nonphotochemical quenching of fluorescence does not relax during illumination but is converted into an antenna quenching process, which affects the light harvesting of PSII rather than photochemistry, and is accompanied by zeaxanthin generation at steady state.
Although our physiological characterization of transient quenching demonstrates that it differs from a classical antenna quenching, its disappearance in the npq4 mutant raises the possibility of a common origin for these two forms of quenching. PsbS is indeed considered the key effector of antenna quenching in chloroplasts (27).
Two hypotheses can be proposed to explain the influence of PsbS on both our reaction-center and antenna quenching forms. One is to assume that reaction-center and antenna quenching really represent two distinct phenomena, but the generation of the former is an absolute prerequisite for the development of the latter. Accordingly, PsbS might be actively involved in the generation of reaction-center quenching, and its removal would be responsible for the absence of both forms of quenching. PsbS is an Lhc-type protein (27), the role of which in the generation of qE is not fully understood. It is able to bind N,N′-dicyclohexylcarbodiimide (28), a qE inhibitor (11), and likely able to associate with carotenoids (29). PsbS can associate with either the antenna or the PSII core complex, thanks to a pH-dependent shift between a monomeric and a homodimeric form (30). PsbS might therefore be involved in promoting or stabilizing a conformation of the PSII core suitable to promote fluorescence quenching, thus promoting generation of antenna qE. This conformational change might be related to the modification induced by a luminal pH acidification, which results in the release of Ca2+ from the oxygen-evolving complex, and in the shift in the redox midpoint potentials of the primary quinone acceptor QA (31). They are accompanied by a reduction in both PSII-mediated electron flow and in fluorescence emission, as required for the generation of the reaction-center quenching studied here.
Alternatively, it can be proposed that, although different from a physiological point of view, both the reaction-center and the antenna quenching arise from the generation of energy quenchers within the PSII antenna, related for instance to changes in the aggregation state of LHCII (32, 33). The efficiency of the quencher generated under reaction-center conditions should be much stronger than in antenna quenching conditions to compete successfully with photochemistry for excitation energy at saturating light. Although this possibility cannot be ruled out a priori, it seems rather unlikely. Indeed, the presence of such an efficient quencher would have dramatic consequences on photochemistry under limiting light. This is, however, not the case, as clearly illustrated by the data of Fig. 2, where PSII activity measured with subsaturating laser flashes is still largely present under conditions that generate reaction-center quenching.
Modulating the Distribution Between Reaction-Center and Antenna Quenching Processes. The involvement of ΔpH in triggering transient NPQ is indicated by both its fast dark relaxation and sensitivity to the ionophore nigericin. Overacidification of the thylakoid lumen is expected to occur during the first seconds of illumination because carbon-fixation through the Calvin cycle, consuming ATP, is largely inactive. At the same time, two processes are likely to contribute to the generation of ΔpH under such conditions: electron transport to oxygen, producing superoxide, and cyclic electron transport around PSI. The former is known to make a minor contribution to the overall electron flux under steady-state conditions but might be substantial during the first seconds of illumination when normal regulatory processes are not fully induced (34). The latter has recently been shown to occur at a high rate in dark-adapted leaves after a dark-to-light transition (17).
Conversely, activation of the Calvin cycle is expected to decrease ΔpH because of the increased ATP turnover rate. This decrease in ΔpH would be mainly responsible for switching off the reaction-center quenching at low light, as evidenced by the correlation between activation of CO2 assimilation, the increase of ΦPSII, and relaxation of the quenching (Fig. 5B). However, a more careful kinetic analysis of the increase of ΦPSII and the relaxation of NPQ suggests that the former actually precedes the latter (P.J., unpublished results) implying that the relaxation of the ΔpH is not simply controlled by an increased usage of ATP for the Calvin cycle (35).
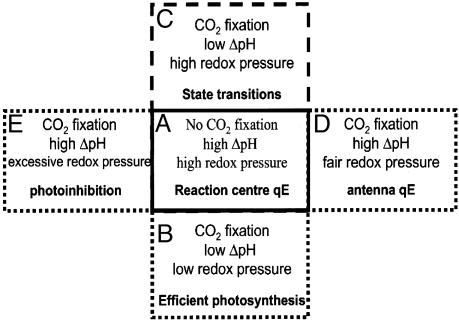
Fig. 5.
Schematic description of NPQ generation in vascular plants. See text for further explanations.
Low light that overexcites PSII will lead to reduction of the plastoquinone pool, inducing a fluorescence emission decrease by means of phosphorylation of LHCs and their migration to PSI, a process known as “state transitions” (3). Sustained illumination with higher irradiances, sufficient to saturate carbon fixation, would lead to the maintenance of a stable and high ΔpH, resulting in the deepoxidation of violaxanthin to form zeaxanthin and the subsequent generation of antenna-based nonphotochemical quenching (Fig. 5D). Upon illumination of leaves with very high irradiances, inhibition of PSII reaction centers was no longer reversible in the dark (data not shown), indicating that, under such conditions, photoinhibition of PSII occurs. This finding suggests that the “reaction-center” quenching state might represent an intermediate step in photoinhibition (Fig. 5E), as also suggested by the similarity between the characteristics of the reversibly inhibited state reported here and those of the reversible state of photoinhibition characterized in ref. 36.
Abbreviations: qE, high-energy quenching of fluorescence; PS, photosystem; NPQ, nonphotochemical quenching of fluorescence; LHC, light-harvesting complex; ΔpH, pH gradient across the thylakoid membrane.
References
- 1.Horton, P., Ruban, A. V. & Walters, R. G. (1996) Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684. [DOI] [PubMed] [Google Scholar]
- 2.Heber, U., Bukhov, N. G., Shuvalov, V. A., Kobayashi, Y. & Lange, O. L. (2001) J. Exp. Bot. 52, 1999–2006. [DOI] [PubMed] [Google Scholar]
- 3.Allen, J. F. (1992) Biochim. Biophys. Acta 1098, 275–335. [DOI] [PubMed] [Google Scholar]
- 4.Osmond, B., Badger, M., Maxwell, K., Bjorkman, O. & Leegood, R. (1997) Trends Plant Sci. 2, 119–121. [Google Scholar]
- 5.Demmig-Adams, B. & Adams, W. W. (1996) Trends Plant Sci. 1, 21–26. [Google Scholar]
- 6.Niyogi, K. K. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 333–359. [DOI] [PubMed] [Google Scholar]
- 7.Wraight, C. A. & Crofts, A. R. (1970) Eur. J. Biochem. 17, 319–327. [DOI] [PubMed] [Google Scholar]
- 8.Briantais, J. M., Vernotte, C. & Krause, G. H. (1979) Biochim. Biophys. Acta 548, 128–138. [DOI] [PubMed] [Google Scholar]
- 9.Krause, G. H., Laasch, H. & Weis, E. (1988) Plant Physiol. Biochem. 26, 445–452. [Google Scholar]
- 10.Oxborough, K. & Horton, P. (1988) Biochim. Biophys. Acta 934, 135–143. [Google Scholar]
- 11.Walters, R. G., Ruban, A. V. & Horton, P. (1994) Eur. J. Biochem. 226, 1063–1069. [DOI] [PubMed] [Google Scholar]
- 12.Walters, R. G. & Horton, P. (1991) Photosynth. Res. 27, 121–133. [DOI] [PubMed] [Google Scholar]
- 13.Weis, E. & Berry, J. (1987) Biochim. Biophys. Acta 894, 198–208. [Google Scholar]
- 14.Rees, D., Noctor, G. D. & Horton, P. (1990) Photosynth. Res. 25, 199–211. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, G. N. & Krieger, A. (1994) Photosynth. Res. 41, 371–379. [DOI] [PubMed] [Google Scholar]
- 16.Joliot, P., Barbieri, G. & Chabaud, R. (1969) Photochem. Photobiol. 10, 309–331. [Google Scholar]
- 17.Joliot, P. & Joliot, A. (2002) Proc. Natl. Acad. Sci. USA 99, 10209–10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junge, W. & Witt, H. T. (1968) Z. Naturforsch. 24b, 1038–1041. [Google Scholar]
- 19.Genty, B., Briantais, J.-M. & Baker, N. R. (1989) Biochim. Biophys. Acta 990, 87–92. [Google Scholar]
- 20.Bennoun, P. (1970) Biochim. Biophys. Acta 216, 357–363. [DOI] [PubMed] [Google Scholar]
- 21.Barkan, A. (1988) Methods Enzymol. 297, 38–56. [Google Scholar]
- 22.Gilmore, A. M. & Yamamoto, H. Y. (1991) Plant Physiol. 96, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croce, R., Canino, G., Ros, F. & Bassi, R. (2002) Biochemistry 41, 7334–7343. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto, H. Y. (1979) Pure Appl. Chem. 51, 639–648. [Google Scholar]
- 25.Pfundel, E. E., Renganathan, M., Gilmore, A. M., Yamamoto, H. Y. & Dilley, R. A. (1994) Plant Physiol. 106, 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demmig-Adams, B. & Adams, W. W. I. (1992) Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 599–626. [Google Scholar]
- 27.Li, X. P., Bjorkman, O., Shih, C., Grossman, A. R., Rosenquist, M., Jansson, S. & Niyogi, K. K. (2000) Nature 403, 391–395. [DOI] [PubMed] [Google Scholar]
- 28.Dominici, P., Caffarri, S., Armenante, F., Ceoldo, S., Crimi, M. & Bassi, R. (2002) J. Biol. Chem. 277, 22750–22758. [DOI] [PubMed] [Google Scholar]
- 29.Aspinall-O'Dea, M., Wentworth, M., Pascal, A., Robert, B., Ruban, A. & Horton, P. (2002) Proc. Natl. Acad. Sci. USA 99, 16331–16335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergantino, E., Segalla, A., Brunetta, A., Teardo, E., Rigoni, F., Giacometti, G. M. & Szabo, I. (2003) Proc. Natl. Acad. Sci. USA 100, 15265–15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger, A. & Rutherford, A. W. (1997) Biochim. Biophys. Acta 1319, 91–98. [Google Scholar]
- 32.Zer, H., Vink, M., Shochat, S., Herrmann, R. G., Andersson, B. & Ohad, I. (2003) Biochemistry 42, 728–738. [DOI] [PubMed] [Google Scholar]
- 33.Horton, P., Ruban, A. V. & Wentworth, M. (2000) Philos. Trans. R. Soc. London B 355, 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke, J. E. & Johnson, G. N. (2001) Planta 212, 808–816. [DOI] [PubMed] [Google Scholar]
- 35.Kanazawa, A. & Kramer, D. M. (2002) Proc. Natl. Acad. Sci. USA 99, 12789–12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohad, I., Koike, H., Shochat, S. & Inoue, Y. (1988) Biochim. Biophys. Acta 933, 288–298. [Google Scholar]