Abstract
HIV remains a highly important public health and clinical issue despite many recent advances in attempting to develop a cure, which has remained elusive for most people infected with HIV. HIV disease can be controlled with pharmacologic therapies; however, these treatments are expensive, may have severe side effects, and are not curative. Consequently, an improved means to control or eliminate HIV replication is needed. Cytotoxic T lymphocytes (CTLs) play a critical role in controlling viral replication and are an important part in the ability of the immune response to eradicate most viral infections. There are considerable efforts to enhance CTL responses in HIV-infected individuals in hopes of providing the immune response with armaments to more effectively control viral replication. In this review, we discuss some of these efforts and focus on the development of a gene therapy-based approach to engineer hematopoietic stem cells with an HIV-1-specific chimeric antigen receptor, which seeks to provide an inexhaustible source of HIV-1-specific immune cells that are MHC unrestricted and superior to natural antiviral T cell responses. These efforts provide the basis for further development of T cell functional enhancement to target and treat chronic HIV infection in hopes of eradicating the virus from the body.
Keywords: : CD8+ T cells, chimeric antigen receptors, engineered immunity, HIV immunity, immunotherapy
While the current treatment of HIV infection with antiretroviral drugs has been largely successful in lowering viral loads, increasing CD4 T cell counts, and decreasing morbidities and mortalities, even the most successful of therapies fails to eradicate the virus from the body. In addition, antiretroviral therapy (ART) is expensive and associated with significant toxicities. Due to the durable persistence of long-term viral reservoirs, if therapy is terminated, virus replication and disease progression resume, requiring patients to remain on these medications permanently.
As is widely known, to date, there has only been a single reported case of cured chronic HIV infection in an adult (known as Timothy Brown), through bone marrow transplant from a donor lacking the normal gene for CCR5, which is a cell receptor required, in addition to CD4, for most strains of HIV to infect cells. However, the mortality rate of this procedure is about 15–20%, and matched bone marrow with this genetic profile is almost nonexistent for most ethnic groups, rendering this approach impractical for broader clinical applicability. The mechanism(s) of the success of this therapy is also not clear as it is likely that some combination of the myeloablation procedure, graft-versus-host response, and CCR5-deficient (HIV refractory) cellular reconstitution is responsible for the clearance of HIV infection. These unknowns also make repeating this cure approach very difficult.
Several recent studies have attempted to remove CCR5 from hematopoietic stem cells (HSCs) and/or deliver anti-HIV genes to protect cells from HIV infection in humans, but these studies face limitations due to unknowns regarding levels of transduced cell engraftment required to generate an HIV-resistant immune system. Further, attempts to treat individuals with myeloablation and allogeneic stem cells have resulted in short-term suppressed viral replication, followed by reemergence of the virus in the absence of ART.1 In addition, while most transmitted strains of HIV utilize the CCR5 molecule as a coreceptor for infection, it is also well known that HIV can mutate and evolve in the host to utilize other coreceptors other than CCR5, including the CXCR4 molecule, thus limiting the protective effects of the lack of CCR5 expression on the cell. Thus, recapitulating the parameters that allowed Timothy Brown to clear HIV has proven difficult and the lack of successful ART to clear the virus reiterates a need for a therapeutic strategy to cure HIV infection.
The HIV-specific T cell response is a critical component in naturally controlling HIV viral replication following infection; however, due to a variety of reasons, including the insufficient generation of enough numbers and breadth of T cells and the maintenance of the functional T cell response during the infection, the T cell response is incapable of clearing the infection. CD8+ cytotoxic T lymphocytes (CTLs) partially control HIV in almost all infected persons, but eventually fail due to viral mutation, downregulation of viral antigen presentation, lack of CD4+ T cell help, and CTL clonal exhaustion and dysfunction. Since the first reports of HIV-1-specific CTLs in 1987,2 it has become generally accepted that CTL antiviral activity is critical to immune containment of infection, although usually incomplete. Key evidence of this has been observed in the SIV-macaque model in studies that cannot be performed in humans where CD8 depletion in vivo results in loss of viral immune containment.3–6 In human HIV infection, there are observations of human leukocyte antigen (HLA)-associated footprints or evidence of preferential types of responses and specifically driven viral evolution in particular HIV epitopes.7,8
CTLs play an important role in controlling acute HIV infection and in lowering viral loads and the pressure that they place on viral evolution [outside of the Envelope (Env) gene] is evident as it is mostly driven by these cells.9–13 The importance of CTLs in controlling viremia is additionally evident, in that the HLA class I locus is the strongest host genetic determinant of disease progression,14 and CTLs have been observed to exert potent antiviral activity through killing of HIV-1-infected cells in vitro.15,16 Thus, although ultimately unsuccessful in clearing the infection and in preventing disease progression, CTLs are a key immune mechanism for the clearance of HIV-1-infected cells in vivo. However, the ability of CTLs to control HIV in rare persons and their efficacy against other viruses indicate that overcoming these barriers should allow successful CTL-mediated control of HIV.
An increasingly popular approach to treat infections or malignancies has involved the redirection or reprogramming of the immune response through genetic manipulation of immune cells that allow the targeting of these cells to specific antigens of interest.17 There are currently a number of clinical trials in place that involve the redirection of T cells to target a variety of different types of malignancies;18 however, there remains a strong desire to tap into the potential of redirecting T cells to target HIV infection.
A popular strategy to enhance host immunity is to genetically modify peripheral blood cells with an antigen-specific molecularly cloned T cell receptor (TCR) or a chimeric TCR-based molecule containing a ligand receptor binding domain that can redirect cells to target specific antigens. In the case of HIV infection, antigen-specific TCRs from HIV-reactive T cells obtained from infected individuals have been identified, cloned, and used to modify peripheral cells from the same patient.19–22
While attempts to modify peripheral T cells with HIV-specific TCRs have been largely experimental and ineffective therapeutically thus far, genetically modified CD8 cells have in fact exhibited enhanced and polyfunctional immune responses against viral antigens in vivo and these cells have been demonstrated to have an increased ability to control HIV infection.21,22 A potential benefit of this approach is due to the specificity of the TCR, which would likely limit issues with tolerance or self-reactivity. However, an inherent limitation to this approach is the absolute requirement for a specific HLA molecule to present antigen to the T cell, limiting each TCR to persons with the right HLA type. Further, HIV has evolved means to counteract HLA presentation to CTLs and thereby limit their efficacy. Perhaps a more important caveat for engineered TCR-based gene therapy is the ability of HIV to escape HIV-specific T cell responses by mutation. Thus, there are significant advantages and disadvantages with this approach.
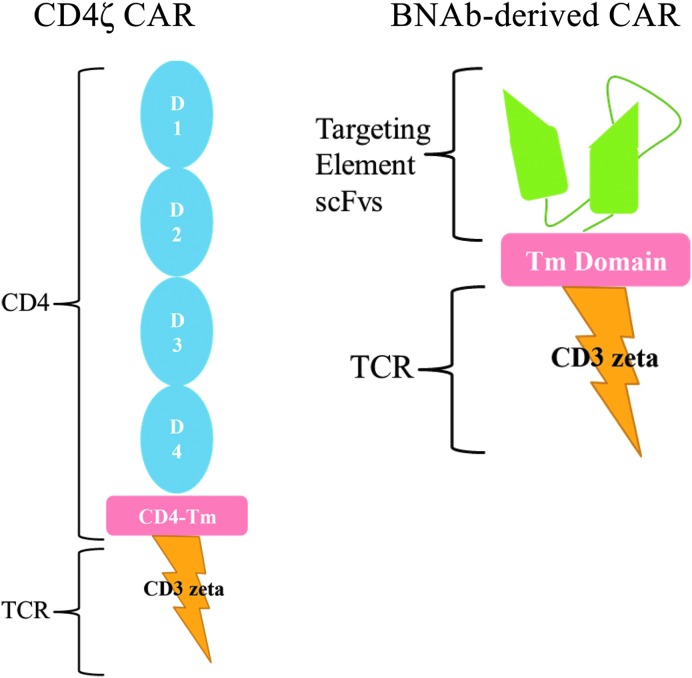
Another approach for immune engineering is to utilize a chimeric antigen receptor (CAR) specific for HIV in place of TCRs. HIV-specific CARs have an antigen binding domain specific for HIV and an internal TCR signaling domain (Fig. 1). When they bind the target antigen, which occurs directly without the need for presentation by HLA molecules, they trigger cellular activation similar to that triggered by TCR ligation. Thus, this approach could be used in HIV-infected persons of any HLA type. Antitumor CARs have been safely utilized in peripheral T cells and have produced antitumor responses in the treatment of malignancies.17 For HIV infection, only one CAR has been previously tested in clinical trials. This reagent involved the use of a CAR containing the extracellular and transmembrane domains of CD4 fused to the CD3-ζ signaling domain. Activation of modified T cells occurs following CD4 binding viral gp120. This protein was expressed in modified human peripheral T cells using a gamma-retroviral vector in peripheral T cells and evaluated in three clinical trials23–25 more than 20 years ago. Treatment was well tolerated and safe, but these studies were all confounded by the concurrent administration of combination ART, although it appeared in one trial that tissue viral replication was reduced. Further, a significant problem with this approach was that the reinfused gene-modified T cells were premorbid and likely dysfunctional due to massive ex vivo expansion and persisted only at low levels. Both approaches using a molecularly cloned HIV-specific TCR and an HIV-specific CAR are currently under investigation, and each has its potential benefits and drawbacks in the development of an effective protocol to enhance antiviral T cell responses.
FIG. 1.
HIV-specific chimeric antigen receptors (CARs). The left figure depicts the CD4ζ CAR, which comprised the entire natural extracellular domain [domains (D) 1–4] and transmembrane domain of the CD4 molecule fused to the CD3ζ signaling domain. The right figure depicts a broadly neutralizing antibody (BMAb)-derived CAR, which contains an HIV-recognizing extracellular single-chain variable fragment (scFv). The scFv is a fusion protein of the variable regions of the heavy (VH) and light chains (VL) of an HIV-specific neutralizing antibody that is usually linked together with a short linker peptide and is the targeting element of the CAR. This is fused to some sort of a transmembrane domain (for instance, of the CD8 molecule) as well as the CD3ζ signaling domain. Additional signaling domains from costimulatory molecules can also be included in CAR constructs to increase the potency of the signal (not shown).
While the manipulation and modification of peripheral blood T cells have certain benefits (including the ease of obtaining high numbers of cells and their ability to be manipulated ex vivo), there are several disadvantages (including the manipulation process causing cellular dysfunction and potentially low levels of engraftment of the genetic modification and persistence of genetically modified cells). The use of HSCs would provide for potential improvements over peripheral T cell modification, including the long-term maintenance of functional gene-modified effector T cells of both CD4+ and CD8+ lineages. A stem cell-based gene therapy approach using a molecularly cloned HIV-specific TCR or CAR would allow proper thymic selection of modified cells and exclusion of endogenous TCR surface expression, eliminating the risk of generating self-reactive TCR through mispairing.26 A stem cell-based gene therapy approach would allow for long-lived continuously renewable immunity capable of continuously generating cells that develop into HIV-targeting T cells that could potentially overcome the barriers that inhibit the eradication of HIV from the body.
We have previously demonstrated that we can engineer HIV-specific T cell responses utilizing a stem cell-based gene therapy approach with a molecularly cloned T cell receptor (TCR) that targets HIV.27–29 We determined that the modification of a human HSC with a lentiviral vector containing an anti-HIV TCR can direct the differentiation of mature, polyfunctional HIV-specific T cells in human thymic tissue in vivo in the SCID-hu mouse, a humanized mouse model that recapitulates human T cell development and thymic selection.27 These cells, carrying the transgenic anti-HIV TCR, survived hematopoietic differentiation and thymopoiesis and developed into cells capable of killing HIV-infected cells ex vivo. This initial proof of concept demonstrated for the first time that a human TCR could be used in this manner to direct human T cell differentiation. Interestingly, this study demonstrated the importance of HLA-matched tissue as tissues that were not matched to the TCR specificity of HLA-A*0201 (the HLA usage of that particular TCR) did not allow T cell differentiation past the immature stage of T cell development, presumably through the absence of positive selection.
Follow-up studies to this demonstrated that an HIV-specific TCR is capable of reducing HIV replication in vivo.28 In this approach, we utilized the surrogate humanized bone marrow, fetal liver, and thymus (BLT) mouse model to demonstrate that human CD34+ HSCs can be genetically modified with a lentiviral vector containing a molecularly cloned TCR specific to HIV and subsequently develop into mature fully functional CTLs that reconstitute the peripheral immune system.28 We further demonstrated that these HIV-specific CTLs could effectively lower viral loads following HIV infection. These cells underwent normal developmental processes, including their maturation into T cells in the human thymus, and responded to HIV infection in vivo in a highly active and normal manner. These studies demonstrated that the modification of an HSC with HIV-specific TCR and the subsequent development of HIV-specific T cells was a feasible approach and was biologically possible in producing immune cells that were capable of lowering viral loads and killing HIV-infected cells. However, this technology is again somewhat limited as it requires TCRs that match the individual's HLA molecules due to the process known as MHC restriction.
As mentioned above, CARs may be superior to TCRs as they bypass the need for MHC restriction. The inherent limitations to using a molecularly cloned TCR are the absolute requirement for a specific HLA molecule to present antigen to the T cell, limiting each TCR to persons with the right HLA type as well as the fact that HIV has evolved means to counteract HLA presentation to CTLs and thereby limit their efficacy. To overcome these issues associated with HLA restriction of an HIV-specific TCR, we explored the notion of utilizing an HIV-specific CAR in a stem cell-based approach.
The initial studies in this investigation used the aforementioned CD4ζ CAR as a prototype anti-HIV CAR.30–32 The use of this CD4ζCAR has important advantages, in that it has been found to be a safe reagent in multiple long-term clinical trials with over 500 patient years of clinical safety data33,34 and this is strong evidence that it does not induce cytokine storms that have been an unwanted element with other CAR-based approaches in treating malignancies.35,36 It is also unlikely to generate escape variants of HIV envelope as the loss of CD4 binding of an escape variant will likely have a dramatic effect on viral fitness.
We and others have done extensive testing of the antiviral function of CD8+ T cells transduced with CD4ζ CAR, finding that transduced cells are capable of killing HIV-infected cells and suppressing viral replication30–32,34,37 and (unlike the HIV-specific TCR) that this activity is independent of HLA-I molecules.38 CARs can also function in CD4+ T cells to act as HIV-1-specific helper cells. IL-2 and IFN-γ are induced in CD4ζ CAR-transduced CD4+ cells when cocultured with infected cells. Interestingly, possibly one reason that antiviral efficacy was minimal in previous trials with the CD4ζ CAR was that it was erroneously thought that CD4ζ CAR-expressing cells were not infected by HIV.34 We and others tested whether HIV can infect CD8+ cells expressing the CD4ζ CAR molecule and determined that CD8+ cells, which now express CD4 through transduction of CD4ζ CAR, are susceptible to HIV infection.30,39 In this regard, genetic engineering with CD4ζ CAR alone could be rendered useless if CTL can now be infected by HIV. CD4 can also be induced on CD8+ cells following TCR stimulation, allowing infection and killing of the CTL.40,41 Further, destruction of developing or supporting immune cells such as engineered CD4+ helper cells would limit effectiveness of CTLs. Thus, we have combined CD4ζ CAR with anti-HIV reagents to confer protection from HIV infection.
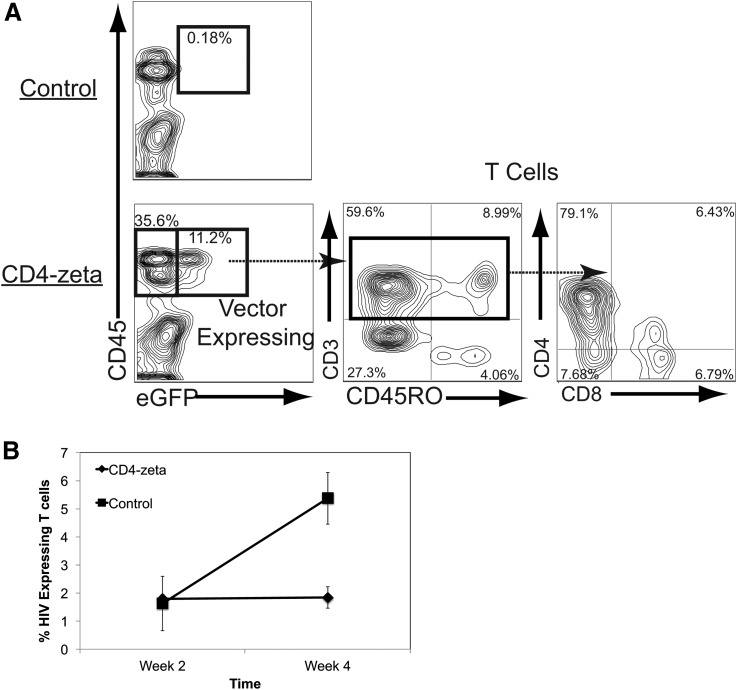
We introduced two shRNAs, one that downregulates CCR5 and one that downregulates HIV expression by targeting the LTR region into the CD4ζ-expressing vector. Using this combinational vector, we successfully diminished the susceptibility to HIV infection in vitro and in vivo through the CD4ζ CAR molecule.30 To test a proof of concept that HSC transduced with a CAR would proceed successfully through thymopoiesis, we transplanted HSCs modified with a lentiviral vector containing the CD4ζ CAR into the humanized BLT mouse. We observed the efficient development in the peripheral blood of mature T cells expressing the lentiviral vector marker gene (green fluorescent protein) and the CD4ζ gene (Fig. 2A). We saw reduced viral loads in animals that received the CD4ζ CAR when compared with control animals (Fig. 2B). We also saw significant expansion and effector/memory cell differentiation of CD4ζ CAR cells following HIV infection, indicating full functional responses of these cells.30 Interestingly, those cells that expressed the highest levels of the transgenic CD4ζ CAR appeared to have shut down their endogenous TCR expression, indicating that the transgenic CD4ζ CAR became the sole TCR on these cells, likely by activating the allelic exclusion machinery in these cells.
FIG. 2.
(A) T cell development of human hematopoietic stem cells (HSCs) modified with CD4ζ CAR. CD34+ human fetal liver-derived HSCs were modified with a lentiviral vector expressing the CD4ζ CAR and green fluorescent protein (GFP) and were transplanted into humanized NOD-SCID-common gamma chain −/− (NSG)-bone marrow, fetal liver, and thymus (BLT) mice. Twelve seeks following implantation of these cells, before HIV infection, CAR-expressing cells were identified on human CD45+ cells by GFP expression (left panels, control untransduced animals are shown on the top panel) and were gated to identify human CD3+CD45RO+ effector/memory markers expressing T cells (middle panel) and CD4+ and CD8+ T cells (right panel). Note that in the BLT mouse, T cell differentiation is skewed toward the CD4+ T cell lineage. (B) Untransduced (control) mice or mice transduced with the CD4ζ CAR were then infected with HIV-1NLHSA-HA, which is a variant of HIVNL4-3 that contains the murine heat-stable antigen containing the hemagglutinin (HA) epitope from influenza cloned into the vpr gene to allow cell surface detection of viral expression.28,43 The data represent the % of HSA-HA-expressing cells 2 and 4 weeks following infection (n = 4). This proof of principle establishes the ability of CD4ζ CAR expression to allow hematopoietic development into function T cells from early progenitor cells without deleterious effects and functionally inhibit viral replication in vivo. CAR, chimeric antigen receptor.
Following HIV infection in these animals, we found that levels of cells expressing HIV and viral loads were significantly diminished in animals expressing the CAR versus control animals. We are currently investigating and have made significant progress in the use of this CAR in the nonhuman primate model, where modification of HSCs and subsequent T cell development appears to be well tolerated and safe in early studies (not shown). In summary, we have established an efficient system to closely examine the development of genetically engineered HIV-specific T cells from HSCs in vivo and demonstrated the feasibility of the use of molecularly cloned HIV-specific TCR and CARs in this approach.
Recently, we and others have developed CARs that utilize antigen-binding components derived from the sequences of HIV-specific broadly neutralizing antibodies (BNAbs) specific for a variety of HIV epitopes.32,42 Some of these CARs also contain additional signaling sequences, which provide costimulatory capability upon ligand binding, to achieve a more robust activation event upon ligation with the target epitope. Upon assessment in a suite of assays, each of these CARs is functional; however, efficiency varies with each ligand-binding moiety, each assay used, and the virus strain being employed. Importantly, Liu et al.42 recently reported that a CAR utilizing the ligand binding domain of VRC01, a broadly neutralizing anti-HIV antibody, reduced viral rebound following removal of antiretrovirals in an in vitro latency model. Thus, these molecules are effective at depleting HIV-expressing cells and reducing virus replication in vitro, but they have not yet been tested in in vivo models through a stem cell-based approach. These latter studies are in progress with several of these BNAb-derived molecules in our laboratory. Importantly, having several reagents that effectively target a variety of HIV epitopes, when used in combination, may decrease the ability of the virus to escape from this type of approach.
In summary, a variety of CARs specific against HIV are under development and pre-clinical testing. These reagents may be useful in peripheral cell or stem cell-based approaches and have considerable potential for use in shock and kill as well as long-term immune surveillance strategies.
Acknowledgments
This work was funded by grants from amfAR ARCHE awards (grant nos. 108688-54-RGRL, 108929-54-RGRL); the NIH, grant no. AI078806 (SGK); the UCLA Center for AIDS Research (CFAR), grant no. P30AI28697; the California Center for Regenerative Medicine, grant no. TR4-06845; and the UC Multi-campus Research Program and Initiatives, California Center for Antiviral Drug discovery (CCADD).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: Report of 2 cases. Ann Intern Med 2014;161:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker BD, Chakrabarti S, Moss B, et al. HIV-specific cytotoxic T-lymphocytes in seropositive individuals. Nature 1987;328:345–348 [DOI] [PubMed] [Google Scholar]
- 3.Egelhofer M, Brandenburg G, Martinius H, et al. Inhibition of human immunodeficiency virus type 1 entry in cells expressing gp41-derived peptides. J Virol 2004;78:568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matano T, Shibata R, Siemon C, Connors M, Lane HC, Martin MA. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol 1998;72:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin X, Bauer DE, Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med 1999;189:991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 1999;283:857–860 [DOI] [PubMed] [Google Scholar]
- 7.Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci U S A 1994;91:9770–9774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science 2002;296:1439–1443 [DOI] [PubMed] [Google Scholar]
- 9.Allen TM, Altfeld M, Geer SC, et al. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J Virol 2005;79:13239–13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen TM, Yu XG, Kalife ET, et al. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J Virol 2005;79:12952–12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feeney ME, Tang Y, Pfafferott K, et al. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. J Immunol 2005;174:7524–7530 [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, McNevin J, Cao J, et al. Selection on the human immunodeficiency virus type 1 proteome following primary infection. J Virol 2006;80:9519–9529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, McNevin J, Zhao H, et al. Evolution of human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitopes: Fitness-balanced escape. J Virol 2007;81:12179–12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International HIVCS, Pereyra F, Jia X, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010;330:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang OO, Kalams SA, Rosenzweig M, et al. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T-lymphocytes. J Virol 1996;70:5799–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang OO, Kalams SA, Trocha A, et al. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: Evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol 1997;71:3120–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fesnak AD, June CH, Levine BL. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat Rev Cancer 2016;16:566–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holzinger A, Barden M, Abken H. The growing world of CAR T cell trials: A systematic review. Cancer Immunol Immunother 2016:1–18. doi: 10.1007/S00262-016-1895-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph A, Zheng JH, Chen K, et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J Virol 2010;84:6645–6653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper LJ, Kalos M, Lewinsohn DA, Riddell SR, Greenberg PD. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J Virol 2000;74:8207–8212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varela-Rohena A, Carpenito C, Perez EE, et al. Genetic engineering of T cells for adoptive immunotherapy. Immunol Res 2008;42:166–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varela-Rohena A, Molloy PE, Dunn SM, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med 2008;14:1390–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deeks SG, Wagner B, Anton PA, et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther 2002;5:788–797 [DOI] [PubMed] [Google Scholar]
- 24.Mitsuyasu RT, Anton PA, Deeks SG, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood 2000;96:785–793 [PubMed] [Google Scholar]
- 25.Walker RE, Bechtel CM, Natarajan V, et al. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood 2000;96:467–474 [PubMed] [Google Scholar]
- 26.Vatakis DN, Arumugam B, Kim SG, Bristol G, Yang O, Zack JA. Introduction of exogenous T-cell receptors into human hematopoietic progenitors results in exclusion of endogenous T-cell receptor expression. Mol Ther 2013;21:1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitchen SG, Bennett M, Galic Z, et al. Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice. PLoS One 2009;4:e8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitchen SG, Levin BR, Bristol G, et al. In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells. PLoS Pathog 2012;8:e1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitchen SG, Shimizu S, An DS. Stem cell-based anti-HIV gene therapy. Virology 2011;411:260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhen A, Kamata M, Rezek V, et al. HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol Ther 2015;23:1358–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhen A, Rezek V, Youn C, et al. Stem-cell based engineered immunity against HIV infection in the humanized mouse model. J Vis Exp 2016. doi: 10.3791/54048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali A, Kitchen SG, Chen IS, Ng HL, Zack JA, Yang OO. HIV-1-Specific chimeric antigen receptors based on broadly neutralizing antibodies. J Virol 2016;90:6999–7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012;4:132ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts MR, Qin L, Zhang D, et al. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood 1994;84:2878–2889 [PubMed] [Google Scholar]
- 35.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 2014;20:119–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang OO, Tran AC, Kalams SA, Johnson RP, Roberts MR, Walker BD. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proc Natl Acad Sci U S A 1997;94:11478–11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Severino ME, Sarkis PT, Walker BD, Yang OO. Chimeric immune receptor T cells bypass class I requirements and recognize multiple cell types relevant in HIV-1 infection. Virology 2003;306:371–375 [DOI] [PubMed] [Google Scholar]
- 39.Kamata M, Kim PY, Ng HL, et al. Ectopic expression of anti-HIV-1 shRNAs protects CD8(+) T cells modified with CD4zeta CAR from HIV-1 infection and alleviates impairment of cell proliferation. Biochem Biophys Res Commun 2015;463:216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitchen SG, Jones NR, LaForge S, et al. CD4 on CD8+ T cells directly enhances effector function and is a target for HIV infection. Proc Natl Acad Sci U S A 2004;101:8727–8732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitchen SG, Korin Y, Roth MD, Landay A, Zack JA. Costimulation of CD8+ lymphocytes induces CD4 expression and allows HIV-1 infection. J Virol 1998;72:9054–9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B, Zou F, Lu L, et al. CAR-T cells guided by the single-chain Fv of a broadly neutralizing antibody specifically and effectively eradicate the reactivated virus-latently-infected CD4+ T-lymphocytes isolated from HIV-1-infected individuals receiving suppressive cART. J Virol 2016;90:9712–9724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jamieson BD, Zack JA. In vivo pathogenesis of a human immunodeficiency virus type 1 reporter virus. J Virol 1998;72:6520–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]