Abstract
An effective approach to HIV cure will almost certainly require a combination of strategies, including some means of reducing the latent HIV reservoir. Because the integrated HIV provirus represents the major source of viral persistence and reactivation, one attractive approach is the direct targeting of provirus for disruption or excision using targeted endonucleases, such as CRISPR/Cas9, zinc finger nucleases, TAL effector nucleases, or meganucleases (homing endonucleases). This article highlights some of the challenges for successful endonuclease therapy for HIV, including optimization of enzyme activity and specificity, the possible emergence of viral resistance, and most importantly, efficient in vivo delivery of the enzymes to a sufficient portion of the latent reservoir.
Keywords: : CRISPR/Cas9, endonuclease, gene editing, gene therapy, HIV cure, provirus
Introduction
As of 2016, there remains only a single well-documented case of HIV cure. The cure of Timothy Ray Brown, or the “Berlin Patient,” continues to energize research in the field, at the same time powerfully demonstrating that cure is a plausible goal. Although so far research in this area has not led to additional cures, our knowledge base has expanded greatly, and we now have a greater understanding of the conceptual framework by which cure might be achieved. Specifically, it now appears that cure (in this case defined as a long-term period of undetectable viremia in the absence of antiviral therapy) might be achieved by a combination of two complementary strategies—reduction of the reservoir of latent HIV, together with augmentation of immune or other mechanisms to control any rare reactivation events arising from the remaining cells harboring HIV. This article will briefly address current concepts regarding the HIV reservoir and methods to estimate its size, the importance of reservoir size in HIV cure efforts, and finally, means to reduce the HIV reservoir, with particular emphasis on the use of designer-targeted endonucleases to directly disable latent virus within reservoir cells.
Estimating the Size of the HIV Reservoir
HIV establishes latency in long-lived memory T cells. Since these cells can reactivate virus and contribute to HIV rebound after antiretroviral therapy is stopped, the size of the latent reservoir is important in HIV cure efforts. Quantitation of the reservoir is complicated by the fact that various measures of the reservoir give markedly different estimates as to its size. Simple polymerase chain reaction-based measures of individuals with long-term HIV suppression by antiviral therapy suggest that on the order of 100 per 1 × 106 purified resting T cells contain integrated HIV DNA.1 However, it is important to appreciate that simple DNA measurements overestimate the relevant reservoir—most of these cells contain defective HIV and cannot give rise to infectious HIV; thus, these cells do not represent a threat for clinical recurrence. To address this limitation, many groups have developed variations on the viral outgrowth assay, or VOA. In the VOA, cells potentially containing HIV sequences are stimulated in an attempt to force HIV production; by combining this with limiting dilution analysis, the goal is to obtain a measure of replication-competent virus. By VOA, the HIV reservoir has been estimated to consist of ∼1 per 1 × 106 purified resting T cells.1,2 Unfortunately, the VOA suffers from what is essentially the converse problem as DNA-based assays. Specifically, the VOA underestimates the relevant HIV reservoir, because only a fraction of HIV with an apparently intact sequence can be reactivated during a given assay.3 Thus, the size of the relevant HIV reservoir, in this case defined as the reservoir that represents a risk of clinical HIV recurrence after antiviral therapy is halted, in actuality is somewhere between the values provided by DNA assay and the VOA. At present, this reservoir is estimated to be as much as 60-fold higher than the VOA value,3 but direct measurement continues to prove elusive.
Reservoir Size Is a Critical Aspect of HIV Cure
The prevailing initial thought was that HIV cure would require elimination of all functionally intact virus in the body, whereas more recent modeling efforts suggest that lifelong freedom from HIV reactivation after cessation of antiviral therapy might be achieved with less than complete eradication. This is based on the assumption that HIV reactivation from rare reservoir cells is a stochastic process, in which a given cell reactivating HIV is statistically unlikely to successfully “seed” a self-sustaining and, ultimately, systemic reactivation of virus. Work from Hill et al. suggests that durable remission might be possible with as little as a four-log reduction of the reservoir.4 Others have suggested an even more optimistic situation in which long-term remission might be possible with only a two-log reduction, if host immunity is sufficient.5 In any event, this work reinforces the importance of accurate estimation of the size of the HIV reservoir in HIV cure efforts.
Approaches to Reduce the HIV Reservoir
Given the importance of reservoir size in efforts to cure HIV, a number of reservoir reduction approaches have been suggested, including various cell and gene therapy strategies discussed elsewhere in this issue. Hematopoietic cell transplantation can clearly induce a large reduction in the HIV reservoir,6 and it has been responsible for extended HIV remissions and the single well-documented case of durable cure.7 Other cell and gene therapy approaches have been suggested to reduce the HIV reservoir, possibly as the effector component of “shock and kill” strategies,8,9 which seek to reactivate latent virus to allow recognition and destruction of latently infected cells. Perhaps the most direct use of gene therapy, however, would be to directly target the integrated HIV for disruption or excision. Recent advances in genome engineering suggest that this might be possible, if a few critical roadblocks can be surmounted (Fig. 1).
FIG. 1.
Challenges in proviral disruption for HIV cure. (A) Efficient delivery of anti-HIV endonuclease to latently infected cells; (B) maximizing enzyme activity; (C) avoidance of viral resistance. Modified from Ref. 30.
Designer Endonucleases: A Pathway to Eliminating HIV?
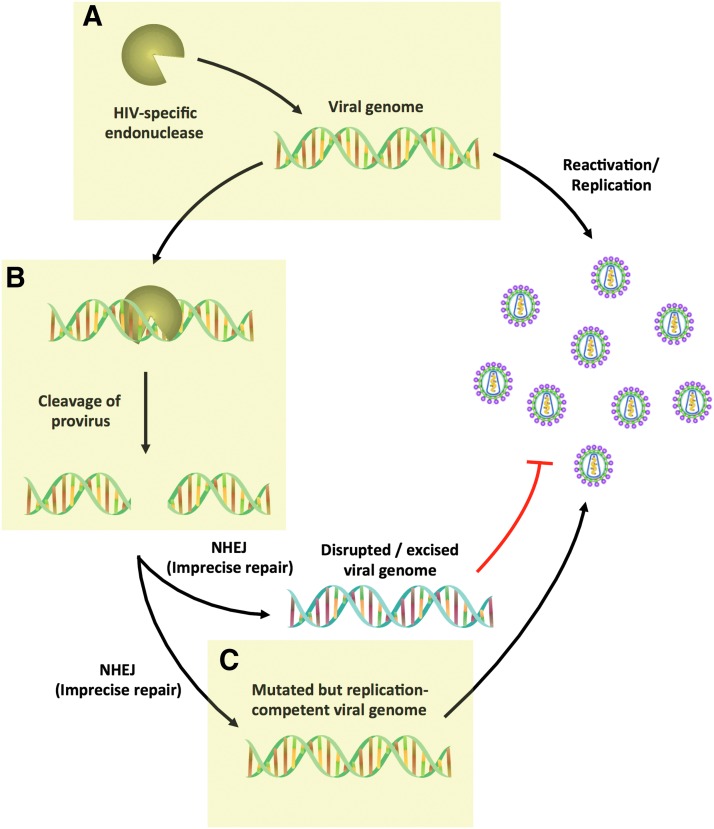
Boiled down to its essence, the problem with HIV is the presence of integrated HIV sequences in long-lived immune cells. Since it is these integrated sequences that give rise to new HIV during recurrence after antiviral therapy is stopped, disrupting or removing these sequences, in principle, should lead to cure. This concept moved from the realm of conjecture to possibility with the development of so-called “targeted endonucleases,” a set of protein families including zinc finger nucleases (ZFNs), meganucleases (also called homing endonucleases), TAL effector nucleases (TALENs), and CRISPR/Cas9. Each of these protein families shares the ability to recognize specified DNA sequences with high specificity, and they then induce the formation of DNA double-strand breaks (DSBs). Non-homologous end joining repair of the broken DNA by cellular mechanisms leads to insertions or deletions of the DNA at the targeted site. In principle, then, inducing insertions or deletions within an essential HIV gene sequence should prevent production of new virus. The plausibility of such an approach has now been shown by several groups who have demonstrated the process in vitro (reviewed in Ref. 9). Taken together, this work has raised optimism that such an approach might prove successful. At the same time, however, it has also identified several critical issues that must be successfully addressed before such an approach can be a realistic means to cure HIV.
Delivery: The Main Challenge for Endonuclease Therapy
Perhaps the greatest challenge in designed endonuclease therapy for HIV is delivering the enzyme to the relevant cells. Based on the estimates of the reservoir reduction needed for HIV cure, at least a substantial majority of cells containing integrated HIV would need to have endonuclease delivered to them. Complicating the issue is the fact that unlike other cell and gene therapy approaches, delivery must occur in vivo, since the relevant cells are scattered throughout the body in lymph nodes, gut, lung, and elsewhere. One potential delivery approach is the use of adeno-associated virus (AAV) vectors, which have been shown to be able to deliver designer endonucleases to latent herpes simplex virus in trigeminal ganglia of mice, leading to disruption of latent viral sequences.10 AAV can transduce T cells in vitro, and a recent report using a transgenic mouse containing integrated HIV sequences showed that AAV-delivered endonucleases could result in disruption of viral sequences in a variety of tissues.11 However, the lack of specific targeting of AAV, combined with the large number of cells in the body, raises questions as to the applicability of AAV toward HIV cure in humans.
Several other approaches have been suggested for delivering targeted endonucleases. Viral vectors other than AAV are a leading possibility, especially lentiviruses. Lentiviruses have a potential advantage in that they can be more specifically targeted to T cells or other immune cells, for example, by pseudotyping with measles hemagglutinin.12 However, the efficiency with which delivery occurs in vivo appears to be limited, and it is unclear whether targeting of an adequate proportion of reservoir cells will be possible in vivo, especially given the effort required to generate large quantities of viral vectors. Other approaches that may be more amenable to large-scale production, such as synthetic nanoparticles or cellular exosomes, may be attractive, particularly if they can be modified with targeting ligands promoting preferential uptake into the relevant cell types comprising the HIV reservoir.
Maximizing the Efficacy of Targeted Endonuclease Activity
Ideally, once the endonuclease has entered the appropriate cell, disruption of the target sequence could be confidently assumed to go to completion. In practice, however, this is clearly not the case. For HIV, introduction of a single targeted endonuclease has been reported to result in disruption of ∼10–50% of provirus.13–15 Incomplete disruption may occur because repair of the induced DSBs is generally precise, and as such, the original target gene sequence may be retained even after multiple rounds of cleavage and repair over the duration of endonuclease expression. If so, increasing endonuclease expression levels, persistence, or specific activity may result in higher mutagenesis, as does the inclusion of DNA end–processing enzymes such as Trex2.16,17 However, such efforts may yield only incremental improvements, and targeting a single site for non-homologous end joining-induced mutation may be incapable of achieving the required >99% disruption needed for HIV cure.
An alternative approach to achieving high levels of HIV disruption is the use of paired cleavage events within the HIV genome. This originally arose from the use of enzymes targeting HIV long terminal repeat (LTR) sequences present at both ends of the integrated HIV genome. It was observed that this frequently led to excision of the intervening portions of the HIV genome, a result clearly desirable for HIV cure. Importantly, the levels of excision seen have generally exceeded the levels of mutagenesis in single-nuclease approaches.18–21 This is presumably because closely spaced pairs of DNA DSBs lead to release of the intervening HIV sequence, making it much less likely that cellular DNA repair mechanisms can restore the intact integrated viral sequence. Although originally observed in the LTR-targeting approaches described earlier, high efficiency of excision can also be achieved by using Cas9 with two or more guide RNAs targeting non-LTR sequences, suggesting that this approach may be widely applicable.
Resistance
Another potential problem with designed endonuclease therapy is the possibility of viral resistance. Although a given target sequence is unlikely to be perfectly conserved in all the HIV strains circulating worldwide, this approach should benefit from targeting highly conserved regions of the virus, tailoring the choice of endonuclease based on viral genotype at the target site, and perhaps by targeting multiple regions of HIV simultaneously.22–24 Such approaches should also address the issue of viral quasispecies present within an infected individual.
An additional concern is the possibility that viral resistance might emerge as a result of the endonuclease therapy itself. The induced insertions and deletions introduced into the viral target site may not themselves result in a loss of viral fitness if they maintain the frame of the encoded protein, as illustrated by insertion of a tyrosine after a 3 bp insertion into the viral reverse transcriptase after ZFN therapy.25 Similar resistance mutants have been shown to emerge and replicate in cultures undergoing treatment with HIV-targeted Cas9.26–28 Fortunately, it appears that the emergence of resistance can be successfully managed in a manner that is analogous to traditional antiviral therapy, by simultaneously targeting multiple sites within the viral genome. Although ten or more sites may be required if targeting is limited to the LTR,22 attacking other more conserved sites elsewhere in the genome may allow fewer targets to suffice.
Conclusions
It is nearly certain that any effective approach to HIV cure will rely on a combination of strategies, and the most promising of such combinations includes a component of reservoir reduction. Direct targeting of integrated provirus using targeted endonucleases is an intuitively attractive concept for reservoir reduction, but several challenges have been identified, including optimization of enzyme activity and specificity, possible emergence of viral resistance, and delivery of the enzymes to a sufficient portion of the cells harboring latent virus.
Of these, it seems the main challenge to bringing endonuclease therapy to reality for HIV will be ensuring effective delivery. Current approaches are likely to be insufficient, and workers in the field should broadly consider a wide variety of possible approaches, including improved AAV, lentivirus, or other viral vectors; nanoparticle-based delivery, especially systems offering selective delivery to CD4-expressing cells; and cell-based delivery systems.
Progress in developing in vivo delivery systems that can efficiently target transgenes to immune cells would also benefit other aspects of the HIV cure effort. For example, one of the main limitations of cell-based gene therapy such as CCR5 disruption is the need for complex ex vivo manipulation of immune cells or stem cells.29 The ability to perform such cell manipulation in a completely in vivo manner would allow approaches such as CCR5 disruption to be much more easily scaled into resource-limited settings. Similarly, the ability to preferentially target latency-reversing agents, antiproliferatives, or other agents to reservoir cells may increase their efficacy and safety in HIV cure efforts.
Acknowledgments
This work was funded in part by NIH grants U19-AI-96111, UM1-AI-126623, and P30-AI027757. The author thanks Martine Aubert and Harshana De Silva Feelixge for helpful comments on this article.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 2013;9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crooks AM, Bateson R, Cope AB, et al. Precise quantitation of the latent HIV-1 reservoir: Implications for eradication strategies. J Infect Dis 2015;212:1361–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013;155:540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill AL, Rosenbloom DI, Fu F, Nowak MA, Siliciano RF. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci U S A 2014;111:13475–13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway JM, Perelson AS. Post-treatment control of HIV infection. Proc Natl Acad Sci U S A 2015;112:5467–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henrich TJ, Hu ZX, Li JZ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis 2013;207:1694–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009;360:692–698 [DOI] [PubMed] [Google Scholar]
- 8.June CH, Levine BL. T cell engineering as therapy for cancer and HIV: Our synthetic future. Philos Trans R Soc Lond B Biol Sci 2015;370:20140374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spragg C, De Silva Feelixge H, Jerome KR. Cell and gene therapy strategies to eradicate HIV reservoirs. Curr Opin HIV AIDS 2016;11:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aubert M, Madden EA, Loprieno M, et al. In vivo disruption of latent HSV by designer endonuclease therapy. JCI Insight 2016;1:e88468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaminski R, Bella R, Yin C, et al. Excision of HIV-1 DNA by gene editing: A proof-of-concept in vivo study. Gene Ther 2016;23:690–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Q, Uhlig KM, Muth A, et al. Exclusive transduction of human CD4+ T cells upon systemic delivery of CD4-targeted lentiviral vectors. J Immunol 2015;195:2493–2501 [DOI] [PubMed] [Google Scholar]
- 13.Aubert M, Ryu BY, Banks L, Rawlings DJ, Scharenberg AM, Jerome KR. Successful targeting and disruption of an integrated reporter lentivirus using the engineered homing endonuclease Y2 I-AniI. PLoS One 2011;6:e16825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedlak RH, Liang S, Niyonzima N, et al. Digital detection of endonuclease mediated gene disruption in the HIV provirus. Sci Rep 2016;6:20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu W, Lei R, Le Duff Y, et al. The CRISPR/Cas9 system inactivates latent HIV-1 proviral DNA. Retrovirology 2015;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aubert M, Boyle NM, Stone D, et al. In vitro inactivation of latent HSV by targeted mutagenesis using an HSV-specific homing endonuclease. Mol Ther Nucleic Acids 2014;3:e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Certo MT, Gwiazda KS, Kuhar R, et al. Coupling endonucleases with DNA end-processing enzymes to drive gene disruption. Nat Methods 2012;9:973–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu X, Wang P, Ding D, et al. Zinc-finger-nucleases mediate specific and efficient excision of HIV-1 proviral DNA from infected and latently infected human T cells. Nucleic Acids Res 2013;41:7771–7782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebina H, Kanemura Y, Misawa N, et al. A high excision potential of TALENs for integrated DNA of HIV-based lentiviral vector. PLoS One 2015;10:e0120047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W, Kaminski R, Yang F, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci U S A 2014;111:11461–11466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao HK, Gu Y, Diaz A, et al. Use of the CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells. Nat Commun 2015;6:6413. [DOI] [PubMed] [Google Scholar]
- 22.Dampier W, Nonnemacher MR, Sullivan NT, Jacobson JM, Wigdahl B. HIV excision utilizing CRISPR/Cas9 technology: Attacking the proviral quasispecies in reservoirs to achieve a cure. MOJ Immunol 2014;1:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffer JT, Aubert M, Weber ND, Mintzer E, Stone D, Jerome KR. Targeted DNA mutagenesis for the cure of chronic viral infections. J Virol 2012;86:8920–8936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiffer JT, Swan DA, Stone D, Jerome KR. Predictors of hepatitis B cure using gene therapy to deliver DNA cleavage enzymes: A mathematical modeling approach. PLoS Comput Biol 2013;9:e1003131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Silva Feelixge HS, Stone D, Pietz HL, et al. Emergence of treatment-resistant infectious HIV after genome-directed antiviral endonuclease therapy. Antiviral Res 2016;126:90–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, Zhao N, Berkhout B, Das AT. CRISPR-Cas9 can inhibit HIV-1 replication but NHEJ repair facilitates virus escape. Mol Ther 2016;24:522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoder KE, Bundschuh R. Host double strand break repair generates HIV-1 strains resistant to CRISPR/Cas9. Sci Rep 2016;6:29530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Pan Q, Gendron P, et al. CRISPR/Cas9-derived mutations both inhibit HIV-1 replication and accelerate viral escape. Cell Rep 2016;15:481–489 [DOI] [PubMed] [Google Scholar]
- 29.Kiem HP, Jerome KR, Deeks SG, McCune JM. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell Stem Cell 2012;10:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone D, Niyonzima N, Jerome KR. Genome editing and the next generation of antiviral therapy. Hum Genet 2016;135:1071–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]