Abstract
Human travel can shape infectious disease dynamics by introducing pathogens into susceptible populations or by changing the frequency of contacts between infected and susceptible individuals. Quantifying infectious disease–relevant travel patterns on fine spatial and temporal scales has historically been limited by data availability. The recent emergence of mobile phone calling data and associated locational information means that we can now trace fine scale movement across large numbers of individuals. However, these data necessarily reflect a biased sample of individuals across communities and are generally aggregated for both ethical and pragmatic reasons that may further obscure the nuance of individual and spatial heterogeneities. Additionally, as a general rule, the mobile phone data are not linked to demographic or social identifiers, or to information about the disease status of individual subscribers (although these may be made available in smaller-scale specific cases). Combining data on human movement from mobile phone data–derived population fluxes with data on disease incidence requires approaches that can tackle varying spatial and temporal resolutions of each data source and generate inference about dynamics on scales relevant to both pathogen biology and human ecology. Here, we review the opportunities and challenges of these novel data streams, illustrating our examples with analyses of 2 different pathogens in Kenya, and conclude by outlining core directions for future research.
Keywords: spatial epidemiology, Big Data, mobile phones, human mobility
It has long been recognized that the spatial spread of many human infectious diseases is affected by how, where, and when people are moving; for example, human movement has been shown to play a key role in the dynamics of influenza [1, 2], cholera [3], malaria [4, 5], measles [6], dengue [7–9], polio [10], and Ebola [11, 12]. Human travel can affect pathogen dynamics in two main ways: movement may introduce pathogens into susceptible populations, or movement may increase the contact between susceptible and infected individuals; both may result in an outbreak or an increase in the prevalence of the disease. Despite the long-recognized relationship between mobility and infectious disease dynamics [4, 6, 13–15], models that directly incorporate travel data into disease models remain rare, partly because of a lack of appropriate data.
Historically, infectious disease models have often assumed that populations within a spatially defined area (eg, a city) are well mixed, meaning that every individual has an equal probability of interacting with every other individual. When human movement is explicitly considered, the focus tends to be on movement between such populations (eg, using assumptions from metapopulation dynamics to link movement between cities or countries [6, 7, 16–18]), and between-population movements have been quantified using relatively simple parametric forms (ie, gravity models and radiation models [7, 19]). Such simplistic assumptions about both scales of movement (eg, within cities and between cities) may not always reflect human behavior, and recent studies have revealed that they often fail to describe population mixing on local scales [8, 20–24]. This suggests that spatial, temporal, and immunological heterogeneities known to influence population disease dynamics will require more nuanced framing to capture spatial and temporal fluctuations in incidence. In turn, this calls for more-detailed data about human movement and mixing patterns [25].
Without such mobility data, one option has been to use disease data to indirectly infer travel patterns, for example by building transmission models around a phenomenological core designed to reflect movement through so-called social space. A classic example is the use of seasonal fluctuations in measles transmission to capture children's movement in and out of school within cities [26]. Analogously, nonparametric forms that make no assumptions about the role of city size or distance between cities can be used to characterize the overall connectivity of cities [27], indicative of overall fluxes of human movement. Despite the success of these approaches, their usefulness hinges on the resolution of existing disease incidence data and pathogen-specific ecology (eg, high transmissibility), which raises questions about how generalizable this approach is across pathogens with varying transmission routes. In many cases, more-detailed data on human movement and mixing will be necessary to understand human movements underlying disease ecology.
Until recently, data sets reflecting human mobility dynamics on spatial and temporal scales that could be related to disease incidence were challenging to obtain, particularly in low-income settings, where the burden of infectious diseases is high [4, 9, 28]. However, recent years have seen a substantial change in our understanding of human mobility across contexts. Much of this progress has been predicated on harnessing a novel and tremendously powerful source of information: call data records (CDRs) from mobile phones [5, 12, 29–31]. The unique power of this lens onto human mobility is partly a function of the astonishing ubiquity of mobile phone ownership. In 2014, there were an estimated 3.6 billion unique mobile phone subscribers, with a penetration rate of about 50% of the global population [32]. Even in the most resource-poor settings, such as Sub-Saharan Africa, an estimated 39% of the population owns a mobile phone, and mobile phone adoption is steadily increasing [32]. Of course, considerable inequities remain, and mobile phone ownership rates remain incredibly heterogeneous, particularly in many low-resource settings. For example, in Sub-Saharan Africa, Botswana has more mobile phones than individuals (167 phones per 100 individuals), whereas Eritrea has the lowest mobile phone ownership rate on the continent, with 7 phones per 100 individuals [33]. Nonetheless, these data provide an unprecedented opportunity to quantify human travel through the sheer scale of individuals reflected in this data stream and the broad diversity of relevant contexts for which this information is available.
Here, we focus specifically on the power of this novel source of information for inference into infectious disease dynamics and where it both builds on and extends previous work. We start by describing the data afforded by mobile phone CDRs, discuss the challenges and opportunities of linking this to infectious disease data, and conclude by outlining future directions for research in this area.
THE PROMISE OF MOBILE PHONE DATA
Every time an individual makes a call or sends a text via a short messaging service (SMS), it will be routed through the closest mobile phone tower in their network. Most operators maintain CDRs, which list the tower and the code for the subscriber identification module (SIM) card involved in each call or SMS-based text. If these data are available in conjunction with a map of relevant towers, then the locations of each call can be identified, and from this, movement of the individual between different calls can be derived. With appropriate safeguards in place to ensure the anonymity of callers, and with agreement of the regulators, operators can make these data available relatively easily for end users to extract individual patterns of movement. Currently, access for research and public health purposes has primarily been through negotiated agreements between operators and research groups. The notable exception has been the effort by Orange Telecom as part of the Data for Development Challenge, although these data included restrictions on the data available and their usefulness, but, overall, access to these data continues to limit research, and obtaining access is a nontrivial and time-consuming task.
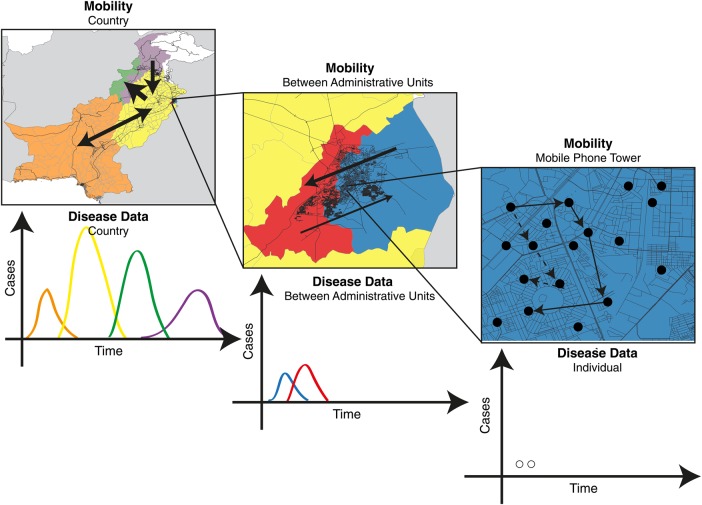
If the hurdle of obtaining access to CDRs can be overcome, depending on the rate of calling of the focal individual, the resulting data can be highly detailed, yielding insight into the individual's movement trajectories across days and weeks, and from spatial scales ranging from city blocks to a country (Figure 1). To date, mobility data derived from mobile phones have been used to understand the spatial transmission of malaria [5, 34], dengue [29], cholera [35], rubella [36], Ebola [12], and human immunodeficiency virus (HIV) infection [37].
Figure 1.
A schematic describing the spatial scales of mobility measured using mobile phone calling data (map, above) and indicating what associated infectious disease data might look like (time course, below). Using mobile phone CDRs, an individual subscriber's location can be geolocated on the tower level (far right); however, this may be difficult to use in conjunction with the locations and timings of individual cases, given highly sporadic incidence. These mobility and infectious disease data can be aggregated to larger spatial areas, such as those between administrative units (middle panel), where patterns of incidence may resolve into clearer outbreaks, as, for example, when lags between outbreaks might map onto the flow of many individuals between larger spatial units.
Mobile phone data are not perfect, and there are biases in terms of who owns phones, as well as subtleties in factors such as how and when calls are made and the possibility that individuals own multiple SIM cards [38]. On very local scales, the spatial and temporal accuracy of CDRs is determined by both tower density and the calling behavior of individuals and is likely to have limited usefulness in disentangling the travel patterns of individuals that are relevant to local disease transmission. These features of the data motivate a shift in focus from very local to broader movement patterns, which are likely to be less biased by calling frequency, ownership biases, and geographic coverage. This also makes data sharing more attractive to operators, because on larger spatial scales CDRs can be aggregated to such an extent that privacy is highly secure and less of a concern. Mechanistically, however, large-scale population shifts are less easy to incorporate into models of infectious disease, since it is less clear when and where the movement patterns quantified on these scales are relevant to transmission. More research is needed to establish the most promising uses of these data, but they undoubtedly provide one of the most powerful, scalable, and real-time data sets on human mobility available. Further, the first analyses demonstrating the power of these data for understanding infectious disease dynamics are beginning to be produced, yielding a proof of concept and hinting at a road map for future studies.
CONNECTING MOBILE PHONE DATA TO INFECTIOUS DISEASES
Many infectious diseases require contact between individuals for transmission to occur (eg, directly transmitted infections, like measles, rubella, or influenza, or sexually transmitted diseases, like HIV infection). Others may require a vector for transmission to humans (eg, infections like dengue or malaria), yet even these generally rely on human movement for wide-scale spread [14]. In general, these broad dependencies tend to be relatively well characterized. As a result, for a focal pathogen, a first impulse, given access to the enormously detailed movement data that mobile phone CDRs make available, is to simulate patterns of spread through space and time by using individual human movement traces combined with the pathogen's known biological aspects (eg, overall life cycle, magnitude of transmission, and epidemiological inoculation rate). However, for many pathogens, the extent to which mobile phone CDRs capture movement relevant to disease transmission remains unclear. Combined with the paucity of existing disease data, this makes simulation studies nearly impossible to validate. Further, even if the premise were sound, such approaches inevitably run into issues of parameter sensitivity and propagation of error often encountered in simulation of highly detailed reductionist models [39]. Inclusion of this level of detail results in the loss of the principal advantages of aggregation: the ability to treat statistical ensembles of diverse elements as groups for which mean properties convey the essence of individual behavior. Aggregation is thus likely to be an important component of successful simulation of an infectious disease across spatial and temporal scales, and identifying the appropriate scale of aggregation becomes an interesting (and as yet unresolved) problem in its own right (see below).
More frequently, researchers working at the interface between human mobility and infectious disease are motivated by existing incidence data for a particular disease. The challenge then becomes to connect patterns of movement to infectious disease data of varying spatial resolutions and temporal scales. A one-to-one connection between a particular infectious disease case and a particular movement pattern is never likely to be tractable, because subscriber anonymity must remain a major preoccupation in this field and because such detailed infectious disease data are unlikely to be available in almost any setting of interest. Even if we can pinpoint specific infectious cases with powerful accuracy, we will likely miss many others through underreporting or existence of asymptomatic individuals, which will prevent direct inference about transmission for individual cases. It is also likely that the exact spatial location where cases occurred is likely to have been aggregated to a broader setting as part of the surveillance system (eg, recording at the local hospital where cases were treated), so that even for the cases that are reported, the spatial scale may be less resolved than the mobility data. As a result, analysis of fluxes of infectious disease cases often inevitably occurs at an aggregated spatial (and often temporal) scale.
While this loss of spatial detail seems to waste the potential of mobile phone data, core biological characteristics of many pathogens mean that aggregation may actually be essential to interpreting drivers of incidence. In particular, many pathogens of interest are sufficiently transmissible that subtle heterogeneities in contact will only minimally modify transmission trajectories, and broad fluxes of movement will be much more relevant to dynamics [40]. For example, decades of work aiming to identify the effects of family size or density on measles transmission have produced very little in terms of meaningful patterns, suggesting that broad fluxes may be the most relevant scale for understanding this pathogen. For many other pathogens, although heterogeneity in mobility may shape transmission, it is one factor among many driving heterogeneities. Patient immune status, for example, is likely to be an important yet heterogeneous driver, particularly for partially immunizing infections, but there are often very few data available with which to characterize this. As a consequence, once again, aggregation can strengthen inference by averaging over unknown but important additional heterogeneities.
There is no a priori correct level of aggregation for analysis of human mobility and infectious disease dynamics. The right framing for any particular study will depend on the target question (introduction events [12]? aggregation and transmission [36]?) and the biological features of the pathogen and is likely to be dictated to a large degree by the scale of infectious disease data available. We illustrate this below, using contrasting examples for 2 different pathogens and mobility data from CDRs in Kenya.
RELATING MOBILITY PATTERNS DERIVED FROM MOBILE PHONE DATA TO INFECTIOUS DISEASE DYNAMICS
We studied both malaria [5] and rubella [36] dynamics within Kenya to understand the role of travel on transmission (for rubella) and parasite introductions. Analyses were constructed around individual mobility data derived from mobile phone data. Incidence data for both infections was available at broad spatial scales (districts for rubella, and settlements for malaria). Rubella data were also extremely underreported [41], such that district-level data were extremely erratic (resulting in statistical uncertainties as detailed above), and consequently we further aggregated this information to the province scale. The availability of detailed data on fluctuations of rubella across the year informed the focal question for this pathogen: the degree to which seasonal fluctuations in travel reflected seasonal fluctuations in infectious disease transmission at the scale of provinces. Despite the very high scale of spatial aggregation, we found a significant correlation, suggesting that the large-scale temporal fluctuations in movement captured by CDRs captures disease-relevant mobility for this pathogen.
For malaria, disease data were derived from parasite prevalence maps and used in conjunction with travel between settlements to understand the flow of malaria parasites. In contrast to the rubella analysis, information on seasonal fluctuations was unavailable for the disease data, and these results were temporally aggregated to represent yearly values. The focus was on identifying locations most at risk of reintroduction of malaria parasites, and the inference here builds off known aspects of the biological characteristics and prevalence estimates of malaria parasites. Combined with validation of the role of mobility data on introductions at smaller spatial scales (validation required very little or no local transmission and therefore was spatially constrained), we could identify locations at risk for parasite introduction and areas where those parasites originate. Overall, this delineation of malaria source and sink populations provides powerful guidance relative to public health investment.
In both cases, the explicit information on human travel provided by CDRs improved our understanding of the spatial transmission of these diseases. The key to effectively harnessing the available information lay in framing a question that allowed the spatial and temporal scales of available infectious disease data to be productively confronted by the mobility data. This type of success has been replicated with CDRs in malaria [34], dengue [29], cholera [35], Ebola [12], and HIV infection [37] and, by extending data availability, promises to be extended yet further. Overall, so far, inference has proceeded by indirectly linking mobility to infectious disease data or through simple models that directly introduce mobility data into infectious disease models. When, where, and whether it makes sense to make the jump to fully integrated simulation models that account for additional sources of spatial and temporal heterogeneity and that can be formally statistically evaluated and tested against infected disease incidence remains an open question.
CHALLENGES AND OPPORTUNITIES
These two examples illustrate that epidemiologically relevant data can be extracted from mobile phone data that outperform previous assumptions about the dynamics of human travel. This evidence opens the way to extending these analyses to increase their applicability in a public health setting, such as by explicitly simulating the outcome of health interventions. However, there are a number of challenges that must be met to ensure the success of this program (Table 1).
Table 1.
Key Challenges and Opportunities to Relate Mobility Derived From Mobile Phone Data to Infectious Disease Data
| Variable | Mobility Derived From Mobile Phone Data | Infectious Disease Data |
|---|---|---|
| Key challenges | ||
| Data availability | Difficult to obtain access to these data | Spatially aggregated case reports, often from national surveillance or smaller-scale studies, for which access may be more or less straightforward |
| Biases | Ownership biases, likely not capturing the entire population; cannot measure spatial movements finer than tower-level spatial resolution; commonly measures movements when individuals have made/received a call or a text via short messaging service | Low reporting rates, asymptomatic individuals, and variable healthcare seeking may reduce or bias cases that enter the surveillance systems; this is then often spatial and temporally aggregated |
| Key opportunities | ||
| Generalizability | Across countries and populations | Inferred mobility might be relevant across multiple pathogens |
| Spatial and temporal scales | Individual movements, fine-scale movements | Inferred mobility might be applicable at uniquely small scales (disease dynamics in parts of a city) and time horizons and might allow a more pointed definition of disease risk |
| New data sources | Global positioning system data from smartphones, understanding the relationship between social connections | New sources of disease data, including genetic and serological data |
| Public health | Improving data access for public health officials for public health interventions | A better understanding of spatial epidemiology for public health planning |
Data Access
Access to mobile phone data remains difficult and is limited by telecom regulations in many countries. However, as the public health usefulness of these data continues to be demonstrated, mobile phone operators are becoming more receptive to providing access to these types of data for development projects, most notably the Data for Development Challenge, primarily sponsored by Orange Telecom. In this instance, anonymized subsets, often restricted in time, the number of subscribers, or spatial coverage, of mobile phone calling data were made available for Senegal and Cote d′Ivoire (information available at: http://www.d4d.orange.com). These data were available to approved researchers who had requested access and signed a contract about their uses. Although the data are logistically and technically difficult to obtain, they can often be used across a range of pathogens that only required a single time point at which the mobile phone data had been obtained from the operator. A clear opportunity in the future is to make these data and results available in near real time to public health officials.
Who Is Captured in These Data?
Mobile phone owners represent a biased sample of the population, and there is always the concern that we are missing the people most at risk through focus on this data stream. In particular, young children are unlikely to be represented [42]. We have previously shown that although ownership is biased, mobility estimates are not significantly skewed by this bias [43]. Additional work needs to be done to understand how representative CDR-derived mobility patterns are of general travel patterns. In particular, future work should continue to explore whether there are particular regions of a country (eg, rural areas) or socioeconomic levels that are underrepresented in these data and identify ways to adjust these biases.
What Movement Patterns Are Captured?
Locational data are actively collected when the subscriber makes or receives a call or text via SMS and are consequently biased by calling behavior. In previous work, we have shown that fewer locations are captured when individuals make fewer communications; the mobility patterns calculated from these data are not biased toward more or less mobile estimates. As smartphone adoption increases, the possibility of capturing global positioning system data from mobile phones emerges, although this imposes additional logistical, technical, and privacy issues.
Defining Spatial and Temporal Disease Heterogeneity Across Pathogens
Research based around CDRs can inform but must also be informed by existing knowledge of spatial and temporal disease heterogeneity in incidence and susceptibility, both of which shape and are shaped by transmission dynamics. For example, understanding the role of human travel in introducing a novel pathogen into completely susceptible populations is relatively tractable if the incidence if infection in the source population is well known. The core question becomes quantifying travel between populations, and the data to inform this might be timing of outbreaks across a span of differently remote populations [18]. Inference may be more complex for endemic, seasonal diseases such as measles or rubella, where the signature of pathogen arrival is less clear (the arrival of a single infectious individual has negligible impact on the epidemic trajectory where an outbreak is already occurring, as opposed to a situation in which it sparks the outbreak). Previous work has characterized school-related movements as driving transmission at local scales for infections like measles and rubella. This result indicates that processes governing local transmission (eg, school terms) will be different from those governing pathogen movement at larger scales (eg, travel between cities). This insight could be used to inform the appropriate spatial scale of aggregation for an analysis using CDRs (assuming sufficiently spatially resolved infectious disease data), breaking apart the local (eg, city-scale) transmission, and focusing on between-city transmission, using the CDR data [25]. One emerging opportunity in this area may be to relate travel and epidemiological dynamics to phylogeographic analyses, particularly in the context of expanding availability of genetic data.
General Insights
Mobility inferred from mobile phone data, particularly across many different countries and continents, can be used to understand general population-level travel patterns and variability across geographic locations. These types of data provide an unprecedented opportunity to generate an understanding of how predictable travel patterns are and the magnitude of population-level variability in travel. How robust and stable are travel patterns? Without mobility data, researchers often use a simple spatial interaction model to characterize movements. Developing new models that can be fit to data from many different countries is a burgeoning area of research, as is the question of identifying the relationship between travel on fine versus coarse temporal and spatial scales.
CONCLUSION
Human travel underlies infectious disease transmission. Mobile phone data provide an unprecedented opportunity to quantify human travel and directly relate these population dynamics to understand infectious diseases. As additional data sets become available and our understanding of spatial heterogeneity in disease incidence and transmission improves, mobile phone data may play a key role in quantifying population-level behavior that can be used by researchers and public health officials to change our understanding and ability to predict future disease outbreaks. By expanding the field to look at human mobility across spatial and temporal scales for multiple countries and pathogens, mobile phone data may provide an additional lens to help understand infectious disease epidemiology and identify how to account for human population dynamics in the spread of infectious diseases. The power of mobile phone CDRs must be allied with carefully posed questions that reflect the varying scale and availability of infectious disease data across a range of pathogens.
Notes
Acknowledgments. Many of the key themes emerged following discussion at a workshop funded by the Princeton Institute for International and Regional Studies.
Financial support. This work was supported by the James S. McDonnell Foundation (to A. W.), the Wellcome Trust (Sustaining Health Grant 106866/Z/15/Z to A. W., C. O. B., and C. J. E. M.), the Bill and Melinda Gates Foundation (to C. J. E. M.), and the Models of Infectious Disease Agent Study program (cooperative agreement 1U54GM088558 to C. O. B. and A. W.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gog JR, Ballesteros S, Viboud C et al. Spatial Transmission of 2009 Pandemic Influenza in the US. PLoS Comput Biol 2014; 10:e1003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson NM, Cummings DA, Cauchemez S et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 2005; 437:209–14. [DOI] [PubMed] [Google Scholar]
- 3.Mari L, Bertuzzo E, Righetto L et al. Modelling cholera epidemics: the role of waterways, human mobility and sanitation. J R Soc Interface 2012; 9:376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prothero RM. Disease and mobility: a neglected factor in epidemiology. Int J Epidemiol 1977; 6:259–67. [DOI] [PubMed] [Google Scholar]
- 5.Wesolowski A, Eagle N, Tatem AJ et al. Quantifying the impact of human mobility on malaria. Science 2012; 338:267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grenfell BT, Bjornstad ON, Kappey J. Travelling waves and spatial hierarchies in measles epidemics. Nature 2001; 414:716–23. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DA, Irizarry RA, Huang NE et al. Travelling waves in the occurrence of dengue haemorrhagic fever in Thailand. Nature 2004; 427:344–7. [DOI] [PubMed] [Google Scholar]
- 8.Stoddard ST, Forshey BM, Morrison AC et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A 2013; 110:994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoddard ST, Morrison AC, Vazquez-Prokopec GM et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis 2009; 3:e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilder-Smith A, Leong WY, Lopez LF et al. Potential for international spread of wild poliovirus via travelers. BMC Med 2015; 13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poletto C, Gomes MF, Pastore y, Piontti A et al. Assessing the impact of travel restrictions on international spread of the 2014 West African Ebola epidemic. Euro Surveill 2014; 19:pii:20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesolowski A, Buckee CO, Bengtsson L, Wetter E, Lu X, Tatem AJ. Commentary: containing the ebola outbreak - the potential and challenge of mobile network data. PLoS Curr 2014; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson ME. Travel and the emergence of infectious diseases. Emerg Infect Dis 1995; 1:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatem AJ, Rogers DJ, Hay SI. Global transport networks and infectious disease spread. Adv Parasitol 2006; 62:293–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley S. Large-scale spatial-transmission models of infectious disease. Science 2007; 316:1298–301. [DOI] [PubMed] [Google Scholar]
- 16.Keeling MJ, Danon L, Vernon MC, House TA. Individual identity and movement networks for disease metapopulations. Proc Natl Acad Sci U S A 2010; 107:8866–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colizza V, Barrat A, Barthelemy M, Valleron AJ, Vespignani A. Modeling the worldwide spread of pandemic influenza: baseline case and containment interventions. PLoS Med 2007; 4:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tizzoni M, Bajardi P, Poletto C et al. Real-time numerical forecast of global epidemic spreading: case study of 2009 A/H1N1pdm. BMC Med 2012; 10:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia Y, Bjornstad ON, Grenfell BT. Measles metapopulation dynamics: a gravity model for epidemiological coupling and dynamics. Am Nat 2004; 164:267–81. [DOI] [PubMed] [Google Scholar]
- 20.Ajelli M, Poletti P, Melegaro A, Merler S. The role of different social contexts in shaping influenza transmission during the 2009 pandemic. Sci Rep 2014; 4:7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tizzoni M, Sun K, Benusiglio D, Karsai M, Perra N. The scaling of human contacts and epidemic processes in metapopulation networks. Sci Rep 2015; 5:15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eubank S, Guclu H, Kumar VS et al. Modelling disease outbreaks in realistic urban social networks. Nature 2004; 429:180–4. [DOI] [PubMed] [Google Scholar]
- 23.Ajelli M, Goncalves B, Balcan D et al. Comparing large-scale computational approaches to epidemic modeling: agent-based versus structured metapopulation models. BMC Infect Dis 2010; 10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apolloni A, Poletto C, Colizza V. Age-specific contacts and travel patterns in the spatial spread of 2009 H1N1 influenza pandemic. BMC Infect Dis 2013; 13:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tizzoni M, Bajardi P, Decuyper A et al. On the use of human mobility proxies for modeling epidemics. PLoS Comput Biol 2014; 10:e1003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjornstad ON, Finkenstadt BF, Grenfell BT. Dyanmics of measles epidemics: estimating scale of transmission rates using a time series SIR model. Ecol Monogr 2002; 72:169–84. [Google Scholar]
- 27.Bjornstad ON, Grenfell BT. Hazards, spatial transmission and timing of outbreaks in epidemic metapopulations. Environ Ecol Stat 2008; 15:265–77. [Google Scholar]
- 28.Wesolowski A, O'Meara WP, Eagle N, Tatem AJ, Buckee CO. Evaluating spatial interaction models for regional mobility in sub-Saharan Africa. PLoS Comput Biol 2015; 11:e1004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wesolowski A, Qureshi T, Boni MF et al. Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc Natl Acad Sci U S A 2015; 112:11887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu X, Bengtsson L, Holme P. Predictability of population displacement after the 2010 Haiti earthquake. Proc Natl Acad Sci U S A 2012; 109:11576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez MC, Hidalgo CA, Barabasi AL. Understanding individual human mobility patterns. Nature 2008; 453:779–82. [DOI] [PubMed] [Google Scholar]
- 32.GSMA. The mobile economy 2015. London, United Kingdom: GSMA, 2015. [Google Scholar]
- 33.World Bank. Mobile cellular subscriptions. In: International Telecommunication Union, World Telecommunication/ICT development report and database. Washington, DC: World Bank, 2015. [Google Scholar]
- 34.Tatem AJ, Huang Z, Narib C et al. Integrating rapid risk mapping and mobile phone call record data for strategic malaria elimination planning. Malar J 2014; 13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bengtsson L, Gaudart J, Lu X et al. Using mobile phone data to predict the spatial spread of cholera. Sci Rep 2015; 5:8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wesolowski A, Metcalf CJ, Eagle N et al. Quantifying seasonal population fluxes driving rubella transmission dynamics using mobile phone data. Proc Natl Acad Sci U S A 2015; 112:11114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isdory A, Mureithi EW, Sumpter DJ. The impact of human mobility on HIV transmission in Kenya. PLoS One 2015; 10:e0142805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wesolowski A, Eagle N, Noor AM, Snow RW, Buckee CO. Heterogeneous mobile phone ownership and usage patterns in Kenya. PLoS One 2012; 7:e35319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasa Y, Viggo A, Levin S. Aggregation in model ecosystems. I. Perfect aggregation. Ecol Modell 1987; 37:287–302. [Google Scholar]
- 40.Ferrari MJ, Grais RF, Bharti N et al. The dynamics of measles in sub-Saharan Africa. Nature 2008; 451:679–84. [DOI] [PubMed] [Google Scholar]
- 41.Metcalf CJ, Munayco CV, Chowell G, Grenfell BT, Bjornstad ON. Rubella metapopulation dynamics and importance of spatial coupling to the risk of congenital rubella syndrome in Peru. J R Soc Interface 2011; 8:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall JM, Toure M, Ouedraogo AL et al. Key traveller groups of relevance to spatial malaria transmission: a survey of movement patterns in four sub-Saharan African countries. Malar J 2016; 15:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wesolowski A, Eagle N, Noor AM, Snow RW, Buckee CO. The impact of biases in mobile phone ownership on estimates of human mobility. J R Soc Interface 2013; 10:20120986. [DOI] [PMC free article] [PubMed] [Google Scholar]