Abstract
It is encouraging to observe that a search for publications on “asymmetric dimethylarginine (ADMA)” in PubMed, as updated on June 2016, yielded >2500 items, 24 years after a splendid paper published by Vallance et al in which the authors proposed that ADMA accumulation could be a cardiovascular risk factor in chronic kidney diseases. ADMA is the endogenous inhibitor of nitric oxide synthase and is related to endothelial dysfunction, which plays an important role in vascular damage elicited by various cardiometabolic risk factors. Although current knowledge suggests that ADMA has critical central roles in renal diseases, there are still unexplained details. The present article aims to provide a review on ADMA and its relation as a biomarker in nephrologic diseases. We aimed to systematize articles in which ADMA levels were assessed in order to clarify its role in many diseases and establish its reference values in different populations.
Keywords: asymmetric dimethylarginine, biomarker, endothelial dysfunction, nitric oxide, oxidative stress, renal failure
Introduction
In 1970, long before the discovery of nitric oxide (NO), Kakimoto and Akazawa were the first pioneers to isolate and describe N-N, N-G- and N-G, N′-G dimethylarginine from human urine. Today, these substances are known as asymmetric dimethylarginine (ADMA; NG,NG-dimethyl-l-arginine) and symmetric dimethylarginine (SDMA; a structural isomer of ADMA).1 Vallance et al first demonstrated that methylated arginines, capable of inhibiting NO synthesis, circulate in plasma and also showed that patients with end-stage renal disease (ESRD) on hemodialysis had higher ADMA levels than controls. The study was a milestone and game changer. After these findings, the possibility of these compounds acting as endogenous regulators of l-arginine to NO pathway in health and diseases raised considerably.2,3
ADMA is a naturally occurring post-translationally modified form of arginine that is generated in all cells during physiological protein turnover. This molecule has a low molecular weight (202 Da), and it is a relatively stable substance. It can also diffuse between cells (easy entry–exit), is excreted in urine, and can be found in tissues and cells.2,4 Intracellular entry of arginine can be supplied through the Y+ transporter – cationic amino acid transporter.4
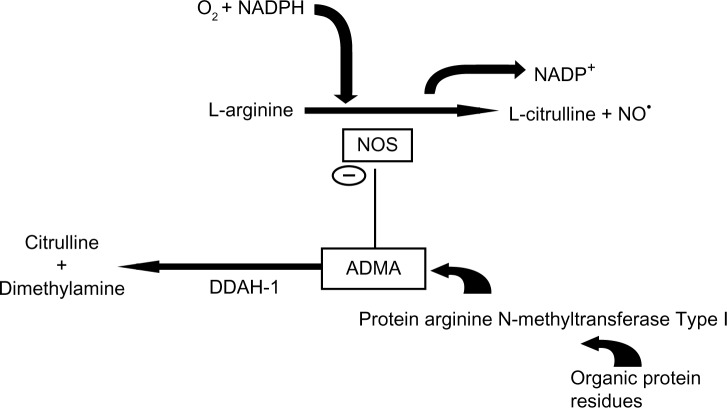
ADMA, but not SDMA, has a crucial role as an endogenous inhibitor of nitric oxide synthase (NOS; EC 1.14.13.39) by competing with l-arginine as a substrate (Fig. 1). NO has key roles in the pathophysiology of endothelial dysfunction, in the progression of atherosclerosis, and in cardiovascular diseases.5 NO, also known as endothelium-derived relaxing factor or EDRF, is a simple gaseous molecule with countless remarkable physiological functions. NO is also an important messenger in many vertebrate signal-transduction processes. It is produced endogenously from arginine in a complex reaction that is catalyzed by NOS. NADPH and O2 are required for the synthesis of NO (Fig. 1).6 l-arginine/NO biosynthetic pathway is involved in many physiological and pathophysiological processes. They include neurotransmission, vascular tone (by stimulating NO-sensitive guanylyl cyclase), regulation of gene transcription, mRNA translation, and production of post-translational modifications of proteins.7
Figure 1.
Interaction of ADMA between the L-arginine NO pathway and its metabolism by DDAH. DDAH-1 is the predominant isoform in the proximal tubules of the kidney and in the liver, which are major sites of ADMA metabolism.68,71
Arginine is a basic amino acid whose ionized group is a guanidinium. The side chains of this amino acid are fully protonated at pH 7. On the other hand, ADMA is created by protein methylation, a type of post-translational modification. This reaction is catalyzed by an enzyme set called protein arginine N-methyltransferase (PRMT; Fig. 1). These enzymes can transfer a methyl group from S-adenosyl methionine to the guanidino nitrogen of arginine.8,9 Although the origin of free ADMA in the circulation is not definitely resolved, ADMA-containing proteins are considered the exclusive source of free ADMA. Free ADMA is generally regarded as the major endogenous regulator of NOS activity.10 Human beings generate ∼300 µmol of ADMA per day.11 ADMA is eliminated from the body either by renal excretion or by degradation to dimethylamine and citrulline by the enzyme dimethylarginine dimethylaminohydrolase (DDAH).12 Renal excretion accounts for only 20% of ADMA elimination. The primary route of elimination (80%) is the metabolism of ADMA through the enzyme DDAH.1 Most ADMA, but not SDMA, is degraded to citrulline and dimethylamine by DDAH, which is widely distributed throughout the body, particularly in the liver and kidney (Fig. 1).13,14 Reduction of NO production in renal patients due to elevated ADMA levels can be attributed to reduced DDAH activity along with endothelial dysfunction and oxidative stress.14
In clinical studies, a strong correlation between increased ADMA levels and impaired endothelial-dependent vasodilatation and cardiovascular morbidity and mortality has been documented in different populations, including in patients with renal disease.8 Renal diseases, which are closely linked to endothelial dysfunction and are associated with cardiovascular morbidity and mortality, were the first among diseases in which elevated plasma levels of ADMA were reported.2 Nowadays, in addition to renal diseases, elevated or impaired ADMA levels have been detected in a large number of diseases such as atherosclerosis, asthma, complications of diabetes, psoriasis, Parkinson’s disease, migraines, preeclampsia, carbon monoxide poisoning, Duchenne muscular dystrophy, rheumatoid arthritis, obstructive sleep apnea, renal transplantation, and systemic sclerosis (Table 1). Endothelial dysfunction and free-radical damage are the intersection events throughout the course of these diseases and role of ADMA. Table 1 summarizes the role of ADMA in the aforementioned diseases.
Table 1.
Latest information about wide range of diseases and their association with ADMA (in alphabetical order).
| DISEASE STATE | CORRELATION OF ADMA WITH DISEASE STATUS OR RELEVANCE OF ADMA WITH THE DISEASE | TYPE OF SAMPLE | ANALYSIS METHOD | REFERENCES |
|---|---|---|---|---|
| Asthma | Potentiation of lung inflammation in a mouse model of allergic asthma by ADMA15 | Lung lavage fluid and cell examination | DDAH expression by quantitative PCR and immunohistochemistry | Elizabet Klein et al. 2010 |
| Cardiovascular diseases | Positive correlation of serum ADMA and negative correlation serum NO with the presence and severity of coronary artery disease, as ADMA being a strong prognostic value16 | Serum | Reverse phase HPLC | Shivkar RR and Abhang SA 2014 |
| Carbon monoxide poisoning | Risk marker of cardio toxicity and also correlation with oxidative stress markers and cardiac damage biomarker heart-type fatty acid binding protein 3 in carbon monoxide poisoning17 | Blood sample | Competitive ELISA | Abass MA et al. 2016 |
| Complications in diabetes mellitus | New and meaningful biomarker of cardiovascular complications in DM patients, markedly elevated ADMA with cardiovascular complications18 | Plasma | Subject and method section has not been given separately | Konya H et al. 2015 |
| Duchenne muscular dystrophy | Positive correlations between ADMA and stage of the disease19 Lower concentrations of plasma ADMA on steroid medication patients compared to non-treated patients19 | Plasma and urine | GC–MS or GC-MS/MS | Hörster I et al. 2015 |
| Migraine | Higher ADMA concentrations in migraine patients with silent white matter lesion when compared to lesion-free patients and controls20 | Serum | HPLC | Erdélyi-Bótor S et al. 2016 |
| Obstructive sleep apnea | Increased ADMA levels in obstructive sleep apnea patients reflecting the deterioration of vascular endothelial function.21 | Serum | Commercially available ELISA kit | Sunnetcioglu A et al. 2016 |
| Parkinson’s disease | High ADMA levels in patients with Parkinson’s disease22 | Serum | ELISA | Kirbas S et al. 2016 |
| Preeclampsia | Significantly higher levels of ADMA in patients with impaired placental perfusion when compared to women with normal doppler waveforms23 Significantly higher ADMA concentrations in both placenta and maternal serum in severe preeclampsia patients24 | Plasma23 serum and placental homogenate24 | HPLC23ELISA24 | Savvidou MD et al. 2003 Zheng JJ et al. 2016 |
| Psoriasis | ADMA as an indicator of the disease severity25 | Serum | Ultra high-performance liquid chromatography/electro spray ionization-tandem mass spectrometry | Bilgiç Ö et al. 2015 |
| Rheumatoid arthritis | Significant association between homocysteine and ADMA in rheumatoid arthritis patients26 | Serum | Commercially available ELISA kit | Dimitroulas T et al. 2016 |
| Systemic sclerosis | Elevated ADMA is a particular feature of diffuse systemic sclerosis when compared to limited systemic sclerosis27 | Serum | Commercially competitive ELISA kit | Dooley A et al. 2006 |
Biochemical Measurement Methods of ADMA
The methods for ADMA measurement may often produce widely differing results, and few methods simultaneously offer satisfactory accuracy and precision with aspect of method validation (specificity, sensitivity, precision, accuracy, recovery, and linearity). Establishment of accurate and inclusionary reference values in different biological materials is substantial for both clinicians and researchers. Since it is related to cardiovascular risk management, high analytical precision has extreme importance to discriminate between normal and even slightly elevated concentrations of ADMA in all types of biological samples. Currently available methods for determination of ADMA include gas chromatography coupled with mass spectrometry (GC–MS),28 high-performance liquid chromatography (HPLC) with fluorescence detection after derivatization,29 HPLC with mass spectrometric detection (LC-MS and LC-MS/MS) underivatized30,66,67 or after derivatization,31 and capillary electrophoresis (CE) coupled with ultraviolet32,33 or CE coupled with mass-spectrometric detection.34,35 Zinellu et al.32,33 later proposed a modified electrophoresis method in which both sample cleanup and derivatization procedures have been eliminated, thus reducing pre-analytical time and sample loss and also removing the typical problems due to derivatives’ instability. Mass spectrometric detection, either in combination with GC or LC, has been increasingly used but these methods are not ideal for routine clinical purposes because the procedures are time-consuming and the instrumentation is not always available in routine clinical laboratories.
All of these aforementioned methods are laborious and have a long turnaround period. To overcome these issues, Schulze et al developed and validated an easy to perform enzyme-linked immunosorbent assay (ELISA) method for the determination of ADMA levels in serum, plasma, or other biological fluids. Their ADMA ELISA kit is based on the principles of a competitive immunoassay.36 Other than this, there are numerous ready-to-use ELISA kits that are commercially available for detection of ADMA. However, it should be kept in mind that an important study confirmed that ELISA measurements can overestimate ADMA levels in patients who have glomerular filtration rate (GFR) <30 mL/minute when compared to gold-standard liquid chromatography–electrospray tandem mass spectrometry.37 All new and emerging methods should be compared with gold-standard liquid chromatography–tandem mass spectrometry.
Discussion: ADMA as a Biomarker in Renal Diseases
The usage of biomarkers in research and in clinical routine practice as diagnostic tools has become commonplace. Their presence as primary end points in clinical trials is still a topic of interest.38 Biomarkers are functional variants or objective quantitative indices of a biological process, which predict or reflect the evolution or predisposition to a disease or response to therapy.39 Simply, a biomarker should provide an objective measurable value, and this value should give information about the consequences of the disease or health status of the subject.
Kidneys have dual function in ADMA metabolism; they excrete ADMA and possess high levels of DDAH.40 As ADMA is considered as a “uremic toxin,” there is a growing datum demand from clinicians and researchers regarding levels of ADMA for nephrologic diseases.41 It was shown that circulating ADMA concentrations decrease slowly and moderately during dialysis. After dialysis, circulating ADMA concentrations increase again, a phenomenon called rebound, and ADMA can reach higher levels compared to the baseline.10 Increased ADMA levels in both early renal disease and ESRD are reported in many studies.4 In a prospective observational follow-up study involving patients with type 1 diabetic nephropathy, increased ADMA was associated with a decline in GFR.42 Patients with chronic kidney disease (CKD) are especially at increased risk of cardiovascular morbidity and mortality. Plasma ADMA levels may predict the progression of renal injury and guide the clinician during the follow-up of patients with early-stage CKD. This phenomenon could explain the high cardiovascular risk that typically accompanies renal diseases. Increased ADMA has been associated with more rapid renal disease progression and mortality.40,43 Possible mechanisms by which ADMA is involved in the pathogenesis of renal diseases4 are:
Reduction in glomerular blood flow and ultrafiltration due to decrease in renal NO by ADMA,14,44,45
Vasoconstriction and increase in blood pressure, by impairing the endothelial-dependent relaxation and increasing the adhesion ability of endothelial cell,11
Disturbance in intraglomerular hemodynamic state and impairment in both tubular glomerular retrograde regulation and the renal adaptation to sympathetic activity (sympathetic over activity),46,70
Increased renal oxidative stress and elevated renal levels of superoxide anion together with increased ADMA levels in serum,47,69
Induction of glomerular fibrosis and vascular fibrosis, apparently by increased collagen type I and II and fibronectin deposition,47
Impaired amino acid metabolism due to abnormalities in synthesis or excretion,14
Increased systolic blood pressure due to reduced plasma nitrite/nitrate levels, NO stable end-products, and a lower vasorelaxant reaction of the aortic tissues to accumulative acetylcholine concentrations, and18
Renal parenchymal damage, resulting in reduced renal DDAH expression and activity.4
ADMA and its role in renal incidences can be examined in detail in Table 2.
Table 2.
ADMA and its role in renal incidences (in alphabetical order).
| NEPHROLOGIC DISEASES OR MAJOR TRADITIONAL RISKS FOR NEPHROLOGIC DISEASES | BRIEF EXPLANATION FOR THE ROLE OF ADMA | REFERENCES |
|---|---|---|
| CKD | – Higher ADMA levels in all stages of CKD48 – Elevated levels of ADMA in children and adolescents with high blood pressure and with chronic renal failure49,50 – Prediction the progression of CKD51,52 |
Reddy YS et al. 2015 Goonasekera CD et al. 1997 Brooks ER et al. 2009 Wang, S et al. 2007 Kielstein JT and Fliser D 2007 |
| Hypertension | – Observation of increased ADMA concentration in humans with essential hypertension compared to normotensive healthy subjects53 | Perticone F et al. 2010 |
| Proteinuria | – ADMA levels related with proteinuria due to impaired NO production and endothelial dysfunction in non-diabetic renal patients with normal GFR54 – Impaired NO production is a characteristic feature of endothelial dysfunction and ADMA appears related to proteinuria55 |
Caglar K et al. 2006 Sharma M et al. 2009 |
| Renal transplantation | – Increased ADMA as a significant risk factor for increased plasma creatinine concentration and graft failure in renal transplantation56 – Renal transplantation not only improved renal functions but also made a great improvement in ADMA levels, NO activity, and oxidative stress and inflammation indicators57 – Plasma ADMA association with increased risk of all causes of mortality in renal transplant recipients58 |
Zhang W et al. 2009 Abedini S et al. 2010 Frenay AR et al. 2015 |
| Polycystic kidney disease | – Serum ADMA increase early in the course of CKD in patients with autosomal-dominant polycystic kidney disease even before a reduction in GFR59 | Kielstein JT et al. 2002 |
Reference interval of serum ADMA levels was determined as 0.22–0.88 µmol/L (2.5th and 97.5th percentile values) in a recent study involving 120 healthy subjects aged between 34 and 61 years (mean age: 47.4 years).60 Analogous to this information, Schulze et al.36 found a mean ADMA level of 0.65 µmol/L in healthy subjects’ plasma by the help of ELISA. Ekim et al used HPLC to determine normal serum ADMA levels in a control group comprising 34 healthy volunteers (20 females and 14 males) with an average age of 46 years. They reported mean serum ADMA concentration as 0.37 µmol/L in their study.61 Tsikas et al measured plasma ADMA (0.39 ± 0.06 µmol/L) and urine ADMA (3.4 ± 1.1 µmol/mmol creatinine). Their study included a healthy volunteer group consisting of five males, aged 27–64 years (mean: 33 years), and seven females, aged 24–43 years (mean: 31 years). They used a gas chromatography–tandem mass spectrometry method for the accurate quantification of ADMA in human plasma using de novo synthesized [2H3]-methyl ester ADMA as the internal standard.28 Zhang et al performed a study regarding ADMA and its role in renal transplantation. They recruited a control group consisting of 36 people (20 males and 16 females, mean age: 46 ± 13 years) and measured mean ADMA as 0.49 ± 0.1 µmol/L by LC-fluorescence method.56 In a study completed by Weaving et al in 2008, methylated arginines in human plasma and urine were measured by tandem mass spectrometry without need for chromatography or sample derivatization. Nine healthy volunteers were included in the study (mean age: 20.9 ± 2.5 years). Concentration of mean plasma ADMA was 0.39 ± 0.13 µmol/L and 24-hour urine ADMA was 47.8 ± 8.7 µmol/24 hours.62 The most important source of difference in these numbers can be attributed to the method of choice applied by researchers. Age group and gender discrepancy of the studies can also add extra difference.
If concentrations of ADMA in plasma and urine of children and adolescents are to be considered, there is lack of large-scale studies. Cerbone et al investigated serum ADMA levels in 39 subclinical hypothyroidism patients and their counterpart healthy controls. ADMA concentration was reported as 0.60 ± 0.16 µmol/L for controls.63 Another recent and valuable study involving 30 otherwise healthy children of both sexes, aged 6–18 years, who underwent minor surgery (hernia, phimosis, bone fractures, tympanostomy tube insertion, etc.) was performed to analyze plasma and urine ADMA levels. The plasma level was found as 0.61 µmol/L (0.42–1.10 µmol/L), and urine level was found as 89.8 µmol/L (51.8–162.3 µmol/L).64 Brooks et al examined the relationship between these ADMA levels and estimated GFR in children and adolescents (n = 28) with stage 2–3 CKD and in matched intra-familial controls (n = 10, mean age: 11.3 ± 4.7 years). The plasma level of ADMA was measured as 0.8 ± 0.2 µmol/L in controls.50 Other than plasma levels of ADMA, another biochemical aspect of the NO pathway can be the analysis of ratios. Arg/ADMA, Arg/SDMA, and ADMA/SDMA ratios are drawing increasing attention lately. El-Sadek et al conducted a research recently in which the results showed significantly higher Arg/ADMA and Arg/SDMA and significantly lower ADMA/SDMA ratios in chronic kidney pediatric patients compared to controls.65
Challenges and Future Directions
Recent research and current information on ADMA have increased considerably both in basic and clinical settings during the previous three decades. ADMA is a good candidate to be accepted as a mediator, as a regulator, and also as a novel biomarker in many aspects. Confusion regarding the role of ADMA being a predictive biomarker and/or a prognostic biomarker can only be solved with larger and preferably randomized controlled studies including pediatric population. These studies should also focus on the mechanism of action extensively. Our increasing knowledge of the routes of synthesis and metabolism of ADMA will provide new horizons for novel mechanisms of acute or chronic renal diseases and will allow us to identify potential therapeutic opportunities through this pathway. Further studies are also needed to establish robust reference intervals of serum and urine ADMA for different ages. ADMA may exert additional largely unrevealed physiological or pathologic functions that are waiting to be enlightened.
Acknowledgments
We acknowledge the authors of many excellent and valuable studies that we were unable to cite due to limitations. We also thank Dr. David T. Thomas for his valuable contribution during English editing process.
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 699 words, excluding any confidential comments to the academic editor.
FUNDING: Author discloses no external funding sources.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the author was invited to submit this paper.
Author Contributions
Conceived and designed the experiments: MES. Analyzed the data: MES. Wrote the first draft of the manuscript: MES. Made critical revisions: MES. The author reviewed and approved of the final manuscript.
REFERENCES
- 1.Kielstein JT, Fliser D, Veldink H. Asymmetric dimethylarginine and symmetric dimethylarginine: axis of evil or useful alliance? Semin Dial. 2009;22(4):346–50. doi: 10.1111/j.1525-139X.2009.00578.x. [DOI] [PubMed] [Google Scholar]
- 2.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 3.Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20(suppl 12):S60–2. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 4.Raptis V, Kapoulas S, Grekas D. Role of asymmetrical dimethylarginine in the progression of renal disease. Nephrology (Carlton) 2013;18:11–21. doi: 10.1111/j.1440-1797.2012.01659.x. [DOI] [PubMed] [Google Scholar]
- 5.Tousoulis D, Georgakis MK, Oikonomou E, et al. Asymmetric dimethylarginine: clinical significance and novel therapeutic approaches. Curr Med Chem. 2015;22(24):2871–901. doi: 10.2174/0929867322666150625095046. [DOI] [PubMed] [Google Scholar]
- 6.Costa ED, Rezende BA, Cortes SF, Lemos VS. Neuronal nitric oxide synthase in vascular physiology and diseases. Front Physiol. 2016;7:206. doi: 10.3389/fphys.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fliser D, Kielstein JT, Haller H, Bode-Böger SM. Asymmetric dimethylarginine: a cardiovascular risk factor in renal disease? Kidney Int Suppl. 2003;84:S37–40. doi: 10.1046/j.1523-1755.63.s84.11.x. [DOI] [PubMed] [Google Scholar]
- 9.Jahan S, Davie JR. Protein arginine methyltransferases (PRMTs): role in chromatin organization. Adv Biol Regul. 2015;57:173–84. doi: 10.1016/j.jbior.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Sitar ME, Kayacelebi AA, Beckmann B, Kielstein JT, Tsikas D. Asymmetric dimethylarginine (ADMA) in human blood: effects of extended haemodialysis in the critically ill patient with acute kidney injury, protein binding to human serum albumin and proteolysis by thermolysin. Amino Acids. 2015;47(9):1983–93. doi: 10.1007/s00726-015-1991-4. [DOI] [PubMed] [Google Scholar]
- 11.Achan V, Broadhead M, Malaki M, et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–9. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 12.MacAllister RJ, Parry H, Kimoto M, et al. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br J Pharmacol. 1996;119:1533–40. doi: 10.1111/j.1476-5381.1996.tb16069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nijveldt RJ, Teerlink T, van Guldener C, et al. Handling of asymmetrical dimethylarginine and symmetrical dimethylarginine by the rat kidney under basal conditions and during endotoxaemia. Nephrol Dial Transplant. 2003;18:2542–50. doi: 10.1093/ndt/gfg452. [DOI] [PubMed] [Google Scholar]
- 14.Aldámiz-Echevarría L, Andrade F. Asymmetric dimethylarginine, endothelial dysfunction and renal disease. Int J Mol Sci. 2012;13(9):11288–311. doi: 10.3390/ijms130911288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein E, Weigel J, Buford MC, Holian A, Wells SM. Asymmetric dimethylarginine potentiates lung inflammation in a mouse model of allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2010;299(6):816–25. doi: 10.1152/ajplung.00188.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shivkar RR, Abhang SA. Ratio of serum asymmetric dimethyl arginine (ADMA)/nitric oxide in coronary artery disease patients. J Clin Diagn Res. 2014;8(8):04–6. doi: 10.7860/JCDR/2014/7849.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abass MA, Arafa MH, El-Shal AS, Atteia HH. Asymmetric dimethylarginine and heart-type fatty acid-binding protein 3 are risk markers of cardiotoxicity in carbon monoxide poisoning cases in Zagazig university hospitals. Hum Exp Toxicol. 2016 doi: 10.1177/0960327116646621. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Konya H, Miuchi M, Satani K, et al. Asymmetric dimethylarginine, a biomarker of cardiovascular complications in diabetes mellitus. World J Exp Med. 2015;5(2):110–9. doi: 10.5493/wjem.v5.i2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hörster I, Weigt-Usinger K, Carmann C, et al. The L-arginine/NO pathway and homoarginine are altered in Duchenne muscular dystrophy and improved by glucocorticoids. Amino Acids. 2015;47(9):1853–63. doi: 10.1007/s00726-015-2018-x. [DOI] [PubMed] [Google Scholar]
- 20.Erdélyi-Bótor S, Komáromy H, Kamson DO, et al. Serum L-arginine and dimethylarginine levels in migraine patients with brain white matter lesions. Cephalalgia. 2016 doi: 10.1177/0333102416651454. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Sunnetcioglu A, Asker S, Alp HH, Gunbatar H. Increased asymmetric dimethylarginine and ischemia-modified albumin levels in obstructive sleep apnea. Respir Care. 2016;61(8):1038–43. doi: 10.4187/respcare.04472. [DOI] [PubMed] [Google Scholar]
- 22.Kirbas S, Kirbas A, Tufekci A, et al. Serum levels of homocysteine, asymmetric dimethylarginine and nitric oxide in patients with Parkinson’s disease. Acta Clin Belg. 2016;71(2):71–5. doi: 10.1080/17843286.2016.1138592. [DOI] [PubMed] [Google Scholar]
- 23.Savvidou MD, Hingorani AD, Tsikas D, Frolich JC, Vallance P, Nicolaides KH. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003;361:1511–7. doi: 10.1016/S0140-6736(03)13177-7. [DOI] [PubMed] [Google Scholar]
- 24.Zheng JJ, Wang HO, Huang M, Zheng FY. Assessment of ADMA, estradiol, and progesterone in severe preeclampsia. Clin Exp Hypertens. 2016;38(4):347–51. doi: 10.3109/10641963.2015.1089880. [DOI] [PubMed] [Google Scholar]
- 25.Bilgiç Ö, Altınyazar HC, Baran H, Ünlü A. Serum homocysteine, asymmetric dimethyl arginine (ADMA) and other arginine-NO pathway metabolite levels in patients with psoriasis. Arch Dermatol Res. 2015;307(5):439–44. doi: 10.1007/s00403-015-1553-3. [DOI] [PubMed] [Google Scholar]
- 26.Dimitroulas T, Sandoo A, Hodson J, Smith J, Douglas KM, Kitas GD. Associations between asymmetric dimethylarginine, homocysteine, and the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism (rs1801133) in rheumatoid arthritis. Scand J Rheumatol. 2016;45(4):267–73. doi: 10.3109/03009742.2015.1086433. [DOI] [PubMed] [Google Scholar]
- 27.Dooley A, Gao B, Bradley N, et al. Abnormal nitric oxide metabolism in systemic sclerosis: increased levels of nitrated proteins and asymmetric dimethylarginine. Rheumatology (Oxford) 2006;45:676–84. doi: 10.1093/rheumatology/kei276. [DOI] [PubMed] [Google Scholar]
- 28.Tsikas D, Schubert B, Gutzki FM, Sandmann J, Frölich JC. Quantitative determination of circulating and urinary asymmetric dimethylarginine (ADMA) in humans by gas chromatography tandem mass spectrometry as methyl ester tri(N-pentafluoropropionyl) derivative. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;798(1):87–99. doi: 10.1016/j.jchromb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Blackwell S, O’Reilly DS, Talwar DK. HPLC analysis of asymmetric dimethylarginine (ADMA) and related arginine metabolites in human plasma using a novel non-endogenous internal standard. Clin Chim Acta. 2009;401(1–2):14–9. doi: 10.1016/j.cca.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 30.D’Apolito O, Paglia G, Tricarico F, et al. Development and validation of a fast quantitative method for plasma dimethylarginines analysis using liquid chromatography-tandem mass spectrometry. Clin Biochem. 2008;41:1391–5. doi: 10.1016/j.clinbiochem.2008.08.075. [DOI] [PubMed] [Google Scholar]
- 31.Davids M, Swieringa E, Palm F, et al. Simultaneous determination of asymmetric and symmetric dimethylarginine, L monomethylarginine, l-arginine, and L-homoarginine in biological samples using stable isotope dilution liquid chromatography tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2012;900:38–47. doi: 10.1016/j.jchromb.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Zinellu A, Sotgia S, Zinellu E, et al. High-throughput CZE-UV determination of arginine and dimethylated arginines in human plasma. Electrophoresis. 2007;28(12):1942–8. doi: 10.1002/elps.200600534. [DOI] [PubMed] [Google Scholar]
- 33.Zinellu A, Sotgia S, Usai MF, Pintus G, Deiana L, Carru C. Improved method for plasma ADMA,SDMA, and arginine quantification by field-amplified sample injection capillary electrophoresis UV detection. Anal Bioanal Chem. 2011;399:1815–21. doi: 10.1007/s00216-010-4580-0. [DOI] [PubMed] [Google Scholar]
- 34.Desiderio C, Rossetti DV, Messana I, Giardina B, Castagnola M. Analysis of arginine and methylated metabolites in human plasma by field amplified sample injection capillary electrophoresis tandem mass spectrometry. Electrophoresis. 2010;31:1894–902. doi: 10.1002/elps.200900690. [DOI] [PubMed] [Google Scholar]
- 35.Boelaert J, Schepers E, Glorieux G, Eloot S, Vanholder R, Lynen F. Determination of asymmetric and symmetric dimethylarginine in serum from patients with chronic kidney disease: UPLC-MS/MS versus ELISA. Toxins (Basel) 2016;8(5):E149. doi: 10.3390/toxins8050149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulze F, Wesemann R, Schwedhelm E, et al. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin Chem Lab Med. 2004;42(12):1377–83. doi: 10.1515/CCLM.2004.257. [DOI] [PubMed] [Google Scholar]
- 37.Pecchini P, Malberti F, Mieth M, et al. Measuring asymmetric dimethylarginine (ADMA) in CKD: a comparison between enzyme-linked immunosorbent assay and liquid chromatography-electrospray tandem mass spectrometry. J Nephrol. 2012;25(6):1016–22. doi: 10.5301/jn.5000085. [DOI] [PubMed] [Google Scholar]
- 38.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–6. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.FitzGerald GA. Measure for measure: biomarker standards and transparency. Sci Transl Med. 2016;8(343):343fs10. doi: 10.1126/scitranslmed.aaf8590. [DOI] [PubMed] [Google Scholar]
- 40.Alpoim PN, Sousa LP, Mota AP, Rios DR, Dusse LM. Asymmetric Dimethylarginine (ADMA) in cardiovascular and renal disease. Clin Chim Acta. 2015;2(440):36–9. doi: 10.1016/j.cca.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Vanholder R, De SR, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–43. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 42.Lajer M, Tarnow L, Jorsal A, Teerlink T, Parving HH, Rossing P. Plasma concentration of asymmetric dimethylarginine (ADMA) predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2008;31:747–52. doi: 10.2337/dc07-1762. [DOI] [PubMed] [Google Scholar]
- 43.Briet M, Burns KD. Chronic kidney disease and vascular remodelling: molecular mechanisms and clinical implications. Clin Sci (Lond) 2012;123:399–416. doi: 10.1042/CS20120074. [DOI] [PubMed] [Google Scholar]
- 44.Kielstein JT, Simmel S, Bode-Böger SM, et al. Suppressor dose asymmetric dimethylarginine modulates renal functions in humans through nitric oxide synthase inhibition. Kidney Blood Press Res. 2004;27(3):143–7. doi: 10.1159/000078838. [DOI] [PubMed] [Google Scholar]
- 45.Cabral PD, Garvin JL. Luminal flow regulates NO and O2(−) along the nephron. Am J Physiol Renal Physiol. 2011;300(5):F1047–53. doi: 10.1152/ajprenal.00724.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 2006;187(4):433–46. doi: 10.1111/j.1748-1716.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- 47.Mihout F, Shweke N, Bigé N, et al. Asymmetric dimethylarginine (ADMA) induces chronic kidney disease through a mechanism involving collagen and TGF-b1 synthesis. J Pathol. 2011;223:37–45. doi: 10.1002/path.2769. [DOI] [PubMed] [Google Scholar]
- 48.Reddy YS, Kiranmayi VS, Bitla AR, Krishna GS, Rao PV, Sivakumar V. Nitric oxide status in patients with chronic kidney disease. Indian J Nephrol. 2015;25(5):287–91. doi: 10.4103/0971-4065.147376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goonasekera CD, Rees DD, Woolard P, Frend A, Shah V, Dillon MJ. Nitric oxide synthase inhibitors and hypertension in children and adolescents. J Hypertens. 1997;15:901–9. doi: 10.1097/00004872-199715080-00015. [DOI] [PubMed] [Google Scholar]
- 50.Brooks ER, Langman CB, Wang S, et al. Methylated arginine derivatives in children and adolescents with chronic kidney disease. Pediatr Nephrol. 2009;24:129–34. doi: 10.1007/s00467-008-0972-1. [DOI] [PubMed] [Google Scholar]
- 51.Wang S, Vicente FB, Miller A, Brooks ER, Price HE, Smith FA. Measurement of arginine derivatives in pediatric patients with chronic kidney disease using high-performance liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med. 2007;45:1305–12. doi: 10.1515/CCLM.2007.277. [DOI] [PubMed] [Google Scholar]
- 52.Kielstein JT, Fliser D. The past, presence and future of ADMA in nephrology. Nephrol Ther. 2007;3(2):47–54. doi: 10.1016/j.nephro.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Perticone F, Sciacqua A, Maio R, et al. Endothelial dysfunction, ADMA and insulin resistance in essential hypertension. Int J Cardiol. 2010;142(3):236–41. doi: 10.1016/j.ijcard.2008.12.131. [DOI] [PubMed] [Google Scholar]
- 54.Caglar K, Yilmaz MI, Sonmez A, et al. ADMA, proteinuria, and insulin resistance in non-diabetic stage I chronic kidney disease. Kidney Int. 2006;70:781–7. doi: 10.1038/sj.ki.5001632. [DOI] [PubMed] [Google Scholar]
- 55.Sharma M, Zhou Z, Miura H, et al. ADMA injures the glomerular filtration barrier: role of nitric oxide and superoxide. Am J Physiol Renal Physiol. 2009;296:F1386–95. doi: 10.1152/ajprenal.90369.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang W, Zhou C, Xie J, Chen B, Chang L. Serum asymmetric dimethylarginine and endothelial function after renal transplantation. J Cent South Univ (Med Sci) 2009;34:289–94. [PubMed] [Google Scholar]
- 57.Abedini S, Meinitzer A, Holme I, et al. Asymmetrical dimethylarginine is associated with renal and cardiovascular outcomes and all-cause mortality in renal transplant recipients. Kidney Int. 2010;77:44. doi: 10.1038/ki.2009.382. [DOI] [PubMed] [Google Scholar]
- 58.Frenay AR, van den Berg E, de Borst MH, et al. Plasma ADMA associates with all-cause mortality in renal transplant recipients. Amino Acids. 2015;47(9):1941–9. doi: 10.1007/s00726-015-2023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kielstein JT, Boger RH, Bode-Boger SM, et al. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol. 2002;13:170–6. doi: 10.1681/ASN.V131170. [DOI] [PubMed] [Google Scholar]
- 60.Kusnierova P, Vsiansky F, Pleva L, Plevova P, Safarcik K, Svagera Z. Reference intervals of plasma matrix metalloproteinases 2, 3, and 9 and serum asymmetric dimethylarginine levels. Scand J Clin Lab Invest. 2015;75(6):508–13. doi: 10.3109/00365513.2015.1057760. [DOI] [PubMed] [Google Scholar]
- 61.Ekim M, Sekeroglu MR, Balahoroglu R, Ozkol H, Ekim H. Roles of the oxidative stress and ADMA in the development of deep venous thrombosis. Biochem Res Int. 2014;2014:703128. doi: 10.1155/2014/703128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaving G, Rocks BF, Bailey MP, Titheradge MA. Arginine and methylated arginines in human plasma and urine measured by tandem mass spectrometry without the need for chromatography or sample derivatisation. J Chromatogr B. 2008;874:27–32. doi: 10.1016/j.jchromb.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Cerbone M, Capalbo D, Wasniewska M, et al. Effects of L-thyroxine treatment on early markers of atherosclerotic disease in children with subclinical hypothyroidism. Eur J Endocrinol. 2016;175(1):11–9. doi: 10.1530/EJE-15-0833. [DOI] [PubMed] [Google Scholar]
- 64.Andrade F, Llarena M, Lage S, Aldámiz-Echevarría L. Quantification of arginine and its methylated derivatives in healthy children by liquid chromatography-tandem mass spectrometry. J Chromatogr Sci. 2015;53(5):787–92. doi: 10.1093/chromsci/bmu126. [DOI] [PubMed] [Google Scholar]
- 65.El-Sadek AE, Behery EG, Azab AA, et al. Arginine dimethylation products in pediatric patients with chronic kidney disease. Ann Med Surg (Lond) 2016;9:22–7. doi: 10.1016/j.amsu.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vicente FB, Vespa G, Miller A, Haymond S. Quantification of arginine and its methylated derivatives in plasma by high-performance liquid chromato graphy tandem mass spectrometry (LC-MS/MS) Methods Mol Biol. 2016;1378:21–30. doi: 10.1007/978-1-4939-3182-8_3. [DOI] [PubMed] [Google Scholar]
- 67.Pettersson A, Uggla L, Backman V. Determination of dimethylated arginines in human plasma by high performance liquid chromatography. J Chromatogr B. 1997;692:257–62. doi: 10.1016/s0378-4347(96)00525-7. [DOI] [PubMed] [Google Scholar]
- 68.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine: dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24:1023–30. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 69.Sydow K, Schwedhelm E, Arakawa N, et al. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res. 2003;57(1):244–52. doi: 10.1016/s0008-6363(02)00617-x. [DOI] [PubMed] [Google Scholar]
- 70.Wiswedel I, Peter D, Gardemann A, Carluccio F, Hampl H, Siems W. Serum concentrations of F2-isoprostanes and 4-hydroxynonenal in hemodialysis patients in relation to inflammation and renal anemia. Biomark Insights. 2008;27(3):419–28. doi: 10.4137/bmi.s363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293(6):3227–45. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 72.Gathiaka S, Boykin B, Cáceres T, Hevel JM, Acevedo O. Understanding protein arginine methyltransferase 1 (PRMT1) product specificity from molecular dynamics. Bioorg Med Chem. 2016;24(20):4949–60. doi: 10.1016/j.bmc.2016.08.009. [DOI] [PubMed] [Google Scholar]