Abstract
Elucidating the mechanism of resistance to biotic and abiotic stress is of great importance in cotton. In this study, a gene containing the NAC domain, designated GbNAC1, was identified from Gossypium barbadense L. Homologous sequence alignment indicated that GbNAC1 belongs to the TERN subgroup. GbNAC1 protein localized to the cell nucleus. GbNAC1 was expressed in roots, stems, and leaves, and was especially highly expressed in vascular bundles. Functional analysis showed that cotton resistance to Verticillium wilt was reduced when the GbNAC1 gene was silenced using the virus-induced gene silencing (VIGS) method. GbNAC1-overexpressing Arabidopsis showed enhanced resistance to Verticillium dahliae compared to wild-type. Thus, GbNAC1 is involved in the positive regulation of resistance to Verticillium wilt. In addition, analysis of GbNAC1-overexpressing Arabidopsis under different stress treatments indicated that it is involved in plant growth, development, and response to various abiotic stresses (ABA, mannitol, and NaCl). This suggests that GbNAC1 plays an important role in resistance to biotic and abiotic stresses in cotton. This study provides a foundation for further study of the function of NAC genes in cotton and other plants.
Keywords: NAC transcription factor, Verticillium wilt, VIGS, biotic and abiotic stress, cotton
Cotton is an important economic crop, but it is susceptible to Verticillium wilt, a soil-borne vascular disease that can result in devastating losses of yield and quality; the leaves turn yellow, the plant defoliates, and even dies when infected by V. dahliae (Sink and Grey 1999). Currently, the molecular mechanisms of resistance to Verticillium wilt remain unclear.
NAC protein is involved in various biotic (pathogen attack) and abiotic stress responses (salinity, temperature, and drought), as well as in developmental processes including cell division (Kim et al. 2006), embryo development (Duval et al. 2002), leaf senescence (Breeze et al. 2011), vascular vessels (Yamaguchi et al. 2010), seed development (Sperotto et al. 2009), lateral root development (Xie et al. 2000), fiber development (Ko et al. 2007), and shoot apical meristem formation (Kim et al. 2007). The cotton stress-responsive NAC transcription factor also plays important roles in the stress response to both abiotic and biotic stresses by coordinating phytohormone signaling networks (He et al. 2016).
The NAC protein family is a large group of plant transcription factors and its name is derived from the three initially discovered genes: NAM (no apical meristem), ATAF1/2, and CUC2 (cup-shaped cotyledon) (Duval et al. 2002; Grant et al. 2010; Ooka et al. 2003; Su et al. 2013). There are more than 117 NAC genes in Arabidopsis (Nuruzzaman et al. 2010), 151 in Oryza sativa (Nuruzzaman et al. 2010), 154 in G. raimondii (Shang et al. 2013), and 60 full-length putative NAC genes in G. hirsutum L (Syed et al. 2014). NAC proteins contain a highly conserved NAC domain, consisting of about 150 amino acids in the N-terminus, and a divergent transcriptional regulatory domain in the C-terminus (Aida et al. 1997; Ooka et al. 2003). Based on an analysis of the structure of the NAC domain in rice and Arabidopsis, NAC proteins were divided into two large groups (I and II). There are 14 subgroups (TERN, ONAC022, and SENU5, etc.) in group I and four subgroups (ANAC001, ONAC003, ONAC001, and ANAC063) in group II (Ooka et al. 2003). The NAC domain contains five subdomains (A–E), and it plays an important role in DNA binding, formation of homodimers or heterodimers, and nuclear localization (Olsen et al. 2005).
A recent study found that the overexpression of a rice NAC gene (SNAC1) could improve drought and salt tolerance in transgenic cotton (Liu et al. 2014b). AtLOV1, an Arabidopsis NAC transcriptional factor, regulates the cold response and flowering time (Yoo et al. 2007). AhNAC2-overexpressing tobacco plants had improved salt tolerance (Liu et al. 2011). NTL8, an Arabidopsis NAC protein, regulates GA3-mediated salt signaling in seed germination (Kim et al. 2008). ANAC019 and ANAC055 are transcription activators regulating the JA-induced expression of defense genes (Bu et al. 2008). The SiNAC gene is involved in stress and developmental regulation in plants (Puranik et al. 2011). In Arabidopsis, some NAC related proteins (NST1, NST2, and NST3) were shown to regulate secondary cell wall biosynthesis (Mitsuda et al. 2005, 2007; Mitsuda and Ohme-Takagi 2008). GhXND1, a NAC transcription factor in G. hirsutum, may be related to the regulation of plant xylem development (Li et al. 2014).
Studies suggest that the NAC proteins play a pivotal role in the plant innate immune system, in terms of both systemic acquired resistance and basic defense. Grapevine NAC1 is involved in the regulation of the disease resistance response (Le Hénanff et al. 2013). Rice NAC genes (OsNAC6, ONAC122, RIM1, and ONAC131) were reported to have a regulatory function in disease resistance against Magnaporthe oryzae and rice dwarf virus (Nakashima et al. 2007; Motoyasu et al. 2009; Yoshii et al. 2010; Sun et al. 2013a). Recent research demonstrated that VpNAC1 may function as a positive regulator in resistance to Erysiphe cichoracearum and Phytophthora parasitica var. nicotianae Tucker (Zhu et al. 2012). HvNAC6 in barley can improve resistance against virulent Blumeria graminis f. sp. hordei and enhance penetration resistance (Chen et al. 2013; Jensen et al. 2007).
In this study, we identified the GbNAC1 gene from previous transcriptome sequencing of G. barbadense. The function of GbNAC1 was analyzed using VIGS and overexpression approaches. Results revealed that GbNAC1 plays important roles in resistance to Verticillium wilt and abiotic stress in cotton.
Materials and Methods
Materials and growth conditions
Cotton varieties Xinhai 15 and Xinhai 16 (G. barbadense L.) were cultivated in a constant temperature incubator under long day conditions (16 hr light/8 hr dark) with about 80% relative humidity at 28/25° (day/night). Arabidopsis thaliana (ecotype Columbia-0) was grown in a 16 hr light/8 hr dark photoperiod with about 125 μE m−2s−1 light at 23°. Nicotiana benthamiana was grown in an incubator maintained at 25° with a 14 hr light/10 hr dark photoperiod for use after 3 wk. V. dahliae strain V991 (highly aggressive) was cultivated on potato dextrose agar (PDA) at 25° for 1 wk, and was then inoculated into Czapek medium until the concentration of conidia was 107/ml for use.
Preparation of cDNA and gDNA
Total RNA was extracted from whole Xinhai 15 (G. barbadense L.) plants using the Plant RNA EASYspin Plus Kit (Aidlab, Beijing, China), according to the manufacturer’s instructions. First strand cDNA was synthesized using the Superscript II RNase H-Reverse Transcriptase Kit (Takara Biotechnology, Dalian, China). Genomic DNA was isolated from young cotton leaves using the Plant Genomic DNA Kit (Tian Gen, Beijing, China).
Cloning of GbNAC1 and phylogenetic trees
Using a unigene fragment (unigene25035_kv-1) and in silico cloning, the cDNA sequence of GbNAC1 was obtained. Primers (GbNAC1F: 5′-ATGGTTGCAGAGCTTGCTGG-3′; GbNAC1R: 5′-TTATATTTCATTAATTTGTC-3′) were designed to amplify the cDNA template from Xinhai 15 and this sequence was verified. The open reading frame (ORF) was predicted by ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/). Multi-sequence alignments were obtained using DNAMAN software. MEGA software was used to construct phylogenetic trees with the neighbor-joining (NJ) method.
Putative NAC-TF genes were identified from protein data of G. raimondii UIbr, which was downloaded from the Cotton Genome Project (http://cgp.genomics.org.cn/) by HMMER. The NJ phylogenetic tree generated for the ORF region of NAC-TF genes and motifs was annotated using MEME (http://meme-suite.org/tools/meme) (Bailey and Elkan 1994).
Quantitative RT-PCR analysis
Cotton RNA was extracted from roots, stems, euphyllas, cotyledons, and stem apices from Xinhai 15 and Xinhai 16 at the two-true-leaf growth stage. Each Xinhai 15 seedling was inoculated with V. dahliae spore suspension (2 × 107 spores/ml) through injured roots, while control seedlings were treated with distilled water in the same way. Euphyllas were collected from three repeats after treatment for 0, 1, 4, 8, 12, 24, 36, 48, and 72 hr. RNA was isolated from leaves and qRT-PCR was performed.
qRT-PCR was performed with three replicates using an ABI 7300 Real Time PCR system and SYBR Premix Ex Taq (Takara). The cotton ubiquitin gene (GbUBQ7F: 5′-GAAGGCATTCCACCTGACCAAC-3′; GbUBQ7R: 5′- CTTGACCTTCTTCTTCTTGTGCTTG-3′) was used as a standard control. Gene-specific primers (qGbNAC1F: 5′-GACCTTGAGCCTTGGGACC-3′; qGbNAC1R: 5′-CTTCCCTCTCTTGTCTTGGTGTA-3′) were used for amplification.
Subcellular localization
The GbNAC1 protein was amplified using the nlsGbNAC1F (5′-AAAAAGCAGGCT ATGGTTGCAGAGCTTGCTGG-3′, attB1 adaptor sequence underlined) and nlsGbNAC1R (5′-AGAAAGCTGGGTC TATTTCATTAATTTGTC-3′, attB2 adaptor sequence underlined) primers. The PCR product was cloned into the pK7FWG2.0 vector using the BP and LR reactions. The constructs were cloned into Agrobacterium tumefaciens strain GV3101, while empty pK7FWG2.0 was used as a control. The agrobacterial culture suspensions for both control and constructs were injected into the underside of N. benthamiana leaves cultured for 3 wk using a 20 ml needleless syringe. After dark incubation of the injected tobacco for 48 hr, the injected leaves were observed with a confocal laser scanning microscope (Zeiss LSM710; Carl Zeiss, Oberkochen, Germany). Then, 4, 6-diamino-2-phenyl indole (DAPI) was used to stain cell nuclei.
Promoter analysis
The promoter sequence was amplified from gDNA using the pGbNAC1F (5′-AAAAAGCAGGCTGACTTGTAAACTGGTGCCTAT-3′, attB1 adaptor sequence underlined) and pGbNAC1R (5′-AGAAAGCTGGGTGTAGCTGATCTATACGTGTTGT-3′, attB1 adaptor sequence underlined) primers. The PCR product was cloned into the pKGWFS7.0 vector using the BP and LR reactions. The constructed plasmid was cloned into Ag. tumefaciens strain GV3101. Transformation of Arabidopsis plants was performed using the floral dip method. For selection, seeds were planted in aseptic conditions on MS agar containing 25 mg/L kanamycin. The selected positive seedlings were histochemically stained for GUS activity based on the method of Jefferson (1987).
Construction of the VIGS vector and transient expression
A 266 bp gene-specific fragment from GbNAC1 was PCR amplified using vGbNAC1F (5′-CCGGAATTCTCATACTTGTAGACCAAAGGAAC-3′, EcoRI restriction site underlined) and vGbNAC1R (5′-CGGGGTACCAGTGATAGCATAGAAAAGCAATA-3′, KpnI restriction site underlined) primers. The PCR product was cloned into the pTRV2 vector to produce TRV:GbNAC1. Then the recombinant plasmids pTRV1, pTRV2, and TRV:GbNAC1 were transformed into Ag. tumefaciens strain GV3101.
The VIGS transient expression methods followed Gao et al. (2011). VIGS-infiltrated seedlings were allowed to grow for 2 wk until the two-true-leaf stage and then leaves were collected and stored at −80°. Seedlings were simultaneously inoculated with a V. dahliae spore suspension (2 × 107 spores/ml) through injured roots and disease progression was analyzed.
Construction of the plant overexpression vector and generation of transgenic plants
The ORF of GbNAC1 was amplified from cDNA with oeGbNAC1F (5′-GCTCTAGAATGGTTGCAGAGCTTGCTGG-3′, XbaI restriction site underlined) and oeGbNAC1R (5′-GGACTAGTTTATATTTCATTAATTTGTCCCCA-3′, SpeI restriction site underlined) primers. The PCR fragment was cloned into the super-pCAMBIA1300 vector. The super-pCAMBIA1300:GbNAC1 construct was transformed into Ag. tumefaciens GV3101. Transformation of Arabidopsis plants was performed using the floral dip method. For selection, seeds were planted in aseptic conditions on MS agar containing 25 mg/L hygromycin for three generations. T3 lines displaying 100% hygromycin resistance were considered homozygous, and were used to observe the phenotype of transgenic plants and for further experiments.
Overexpression of GbNAC1 in Arabidopsis and responses to abiotic and biotic stresses
To further explore the function of GbNAC1, homozygous seeds from T3 lines of overexpressing transgenic plants were used for abiotic stress treatment. To observe germination and plant growth, seeds from wild-type and transgenic plants were sown in triplicate on blank MS medium and MS medium with 120 mM NaCl, 240 mM mannitol, or 1 μM ABA. Seeds were incubated for 2–3 d at 4° in darkness to break dormancy, and then were transferred to the culture room (21°). Germination was recorded daily for 5 d, when the radicle emerged. Wild-type and transgenic plants were photographed after 10 d.
Overexpressing transgenic plants were also used to study the function of GbNAC1 in response to V. dahliae. Wild-type and transgenic Arabidopsis plants were cultivated in a growth chamber (according to the above-mentioned growth conditions). After 25 d, plants were gently removed from the vermiculite and the roots were washed with sterile water. Then, the roots were dipped in a fresh V. dahliae spore suspension of 107 spores/ml. The seedlings were replanted in pathogen-free vermiculite after inoculation and cultivated under normal conditions. Seedling growth was monitored until disease symptoms appeared.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Characterization of the GbNAC1 gene
A 1159 bp unigene containing a NAM domain was obtained through screening differentially expressed genes involved in the cotton defense response to V. dahliae (Sun et al. 2013b). The assembled sequence obtained after in silico cloning was the same as the original unigene sequence. Through T-vector cloning and sequencing, a verified ORF of 543 bp (GenBank ID: KP317496) encoding a protein of 180 amino acids was obtained using ORF Finder.
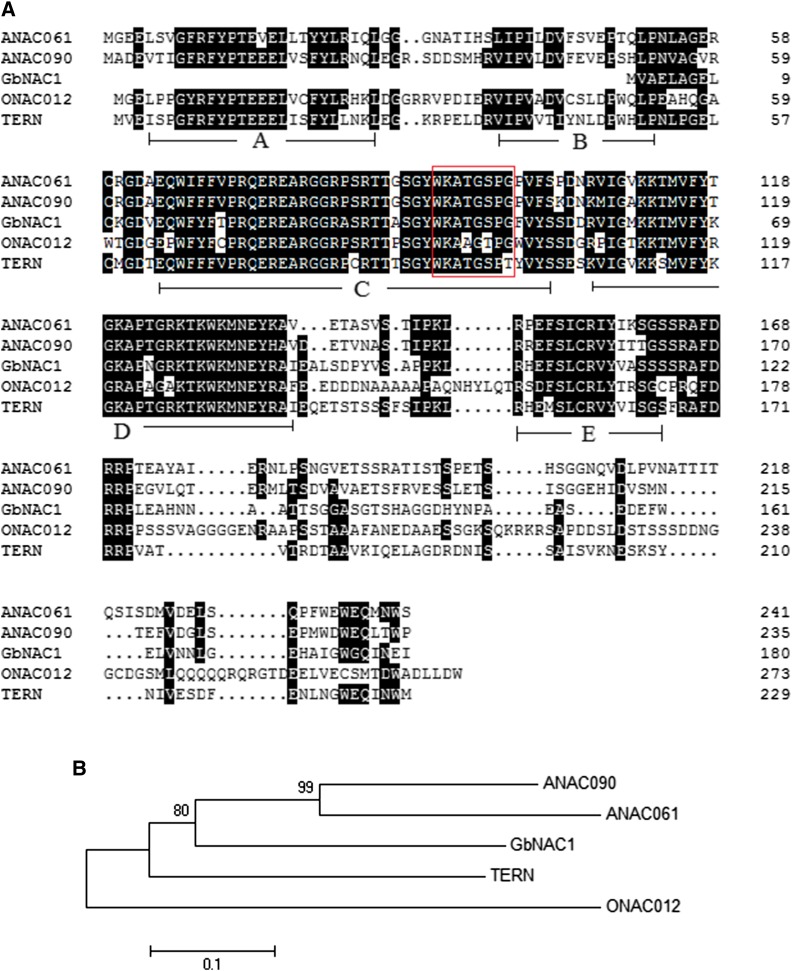
GbNAC1 belongs to the TERN subgroup of the NAC-TF family, according to the multiple alignment (Figure 1A) and phylogenetic tree (Figure 1B). The typical NAC domain, located at the N-terminus, contains five subdomains (A–E), but GbNAC1 lacks subdomains A and B. NAC proteins also have a conserved sequence, WKATGSPG.
Figure 1.
Characterization of GbNAC1. (A) Amino acid sequence alignment of GbNAC1 with other TERN group NAC proteins by DNAMAN software. Identical amino acids are highlighted in black and the conservative domain is boxed in red. The proteins involved are ANAC061 (NP_001118771.1) and ANAC090 (NP_197630.1) from Arabidopsis, TERN (XP_009759500.1) from N. tabacum, and ONAC012 (NP_001055672.1) from O. sativa. The location of five conserved substructural domains (A–E) is shown below the sequences. (B) Phylogenetic relationship of above NAC proteins. Bootstrap values were 500 replicates with neighbor-joining method using MEGA 5.0.
Phylogenetic tree and conserved motif analysis of GbNAC1 and G. raimondii NAC-TF genes
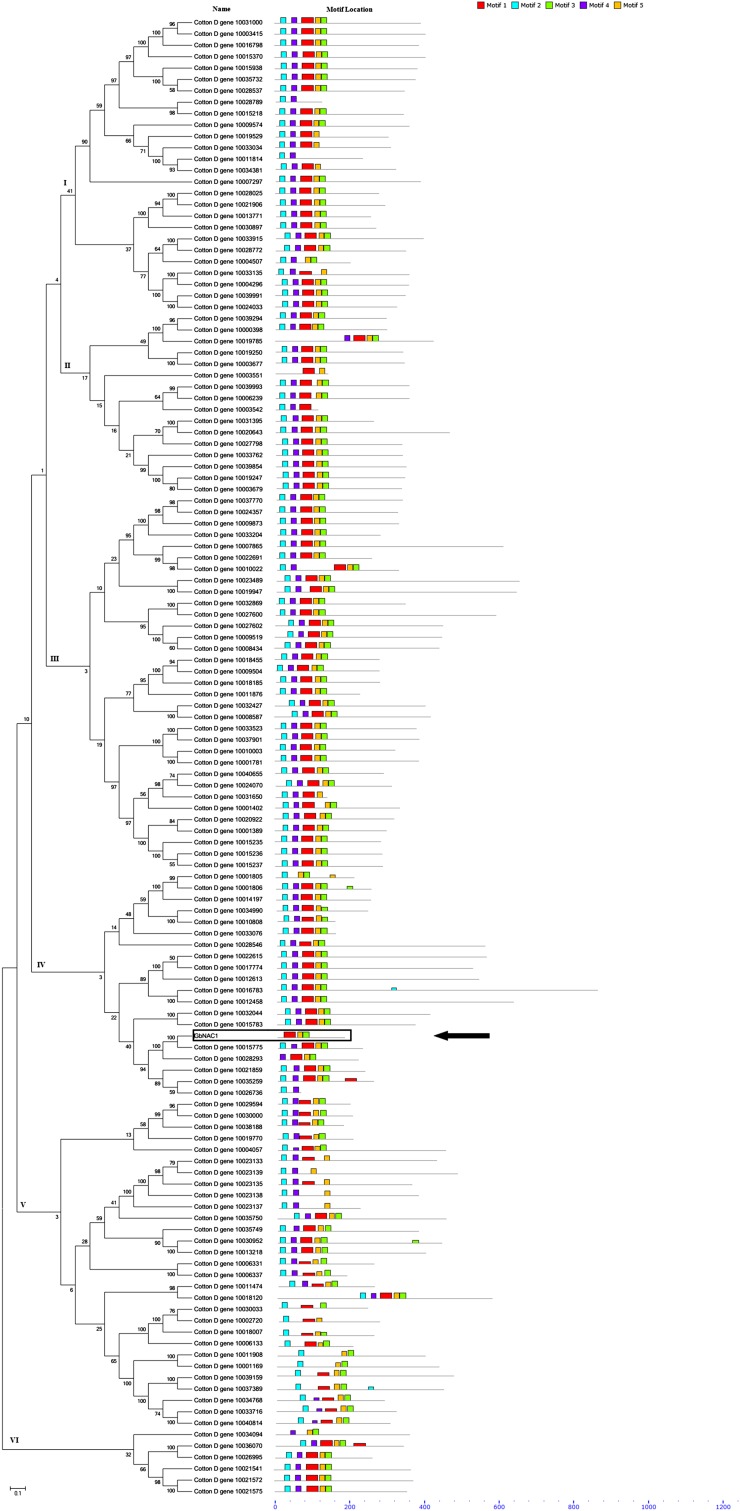
To determine the location of GbNAC1 in cotton, a phylogenetic tree of 129 NAC-TF genes from G. raimondii using HMMER 3.0 software (E-value < e−10 and deleting those proteins without a NAC domain) was constructed. The result indicates that GbNAC1 is close to the Cotton D gene 10015775 (Figure 2). The MEME program was used to predict putative conserved motifs, and five motifs were found at the N-terminus corresponding to the five NAC subdomains. As expected, GbNAC1 lacks the first and the second motif, namely the A and B subdomains. The phylogenetic tree shows six distinct subfamilies (I, II, III, IV, V, and VI) and each subfamily was consistent with similar conserved motifs.
Figure 2.
Phylogenetic relationships and conserved motifs of GbNAC1 and the other NAC genes in G. raimondii. The phylogenetic tree was constructed using MEGA 5.0 by NJ method with 500 bootstrap replicates on a multiple alignment of 129 amino acid sequences of NAC genes from G. raimondii and GbNAC1. Schematic diagram of the conserved motifs were showed by MEME. Each motif is represented by a colored box and the black lines represent the nonconserved sequences. GbNAC1 is marked by a black box and a black arrow.
Subcellular localization of GbNAC1
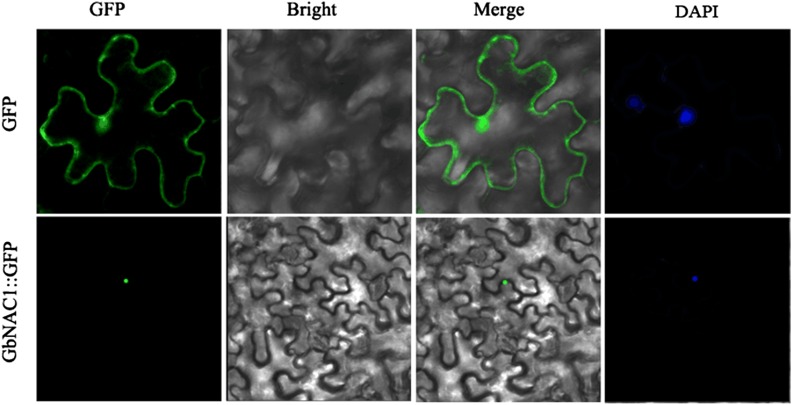
To determine the intracellular localization of GbNAC1, the GbNAC1::GFP fusion gene was constructed and transferred into N. benthamiana leaves. As shown in Figure 3, the fusion protein targeted to the nucleus of transgenic tobacco epidermal cells and the DAPI staining verified this result, indicating that the GbNAC1 protein is localized in the cell nucleus.
Figure 3.
Subcellular localization of GbNAC1 in a tobacco epidermal cell visualized by Zeiss LSM710.
Expression pattern of GbNAC1 in cotton tissues and induction by V. dahliae
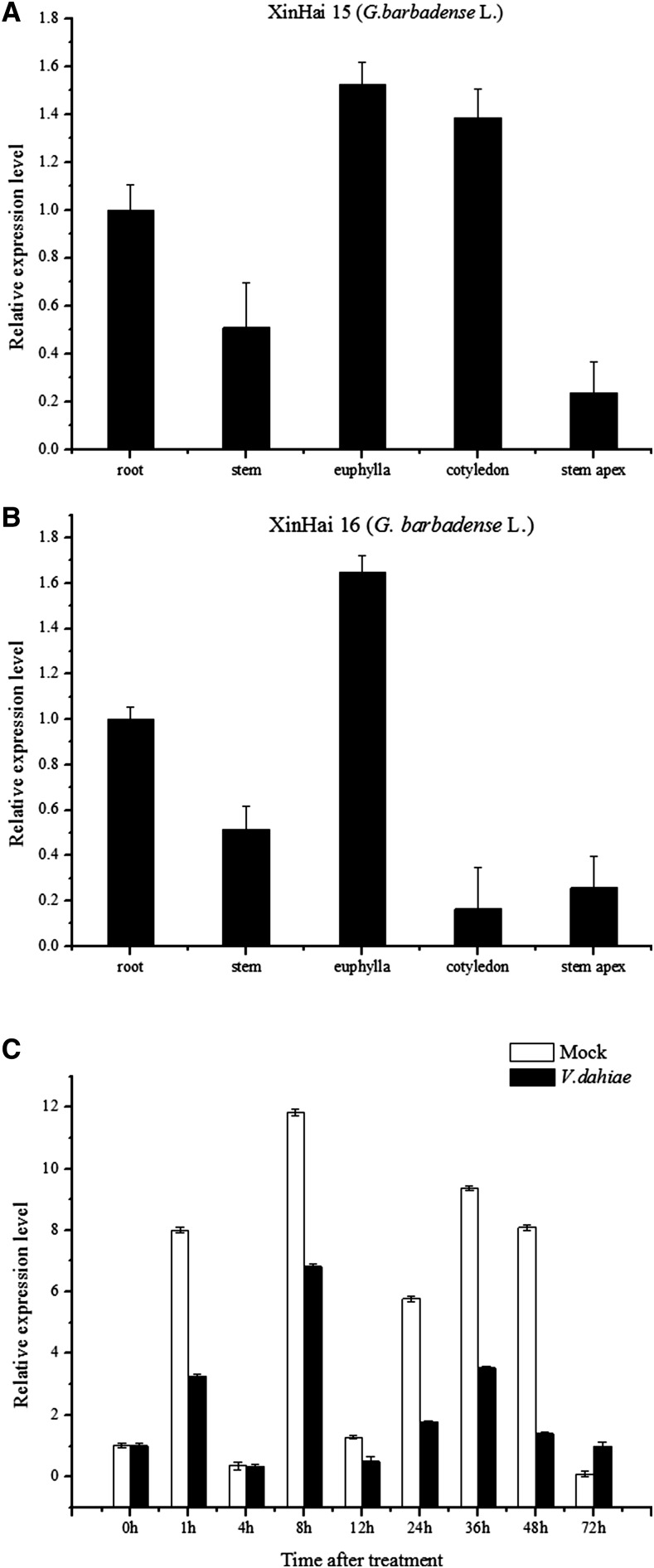
To investigate the tissue-specific expression pattern of GbNAC1, qRT-PCR was performed in two cultivars, Xinhai 15 and Xinhai 16. The results indicated that GbNAC1 was differentially expressed in different organs (Figure 4A, B). Expression was high in euphyllas of both cultivars. However, the expression level of GbNAC1 was high in cotyledons of Xinhai 15, but low in Xinhai 16. It was identically expressed in roots, stems, and stem apices in both cultivars.
Figure 4.
qRT-PCR analysis of expression profile of GbNAC1. (A) Tissue-specific expression pattern of GbNAC1 in Xinhai 15. (B) Tissue-specific expression pattern of GbNAC1 in Xinhai 16. (C) Expression of GbNAC1 in response to V. dahliae. The leaves were collected at 0, 1, 4, 8, 12, 24, 36, 48, and 72 hr after inoculation, respectively. Cotton UBQ7 was used as an internal control. qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
After inoculation of Xinhai 16 with V. dahliae, the expression of GbNAC1 in leaves of control and treated plants differed over time. The expression level decreased in infected plants compared to control from 1–48 hr, but increased at 72 hr (Figure 4C).
Promoter analysis of GbNAC1 in transgenic Arabidopsis
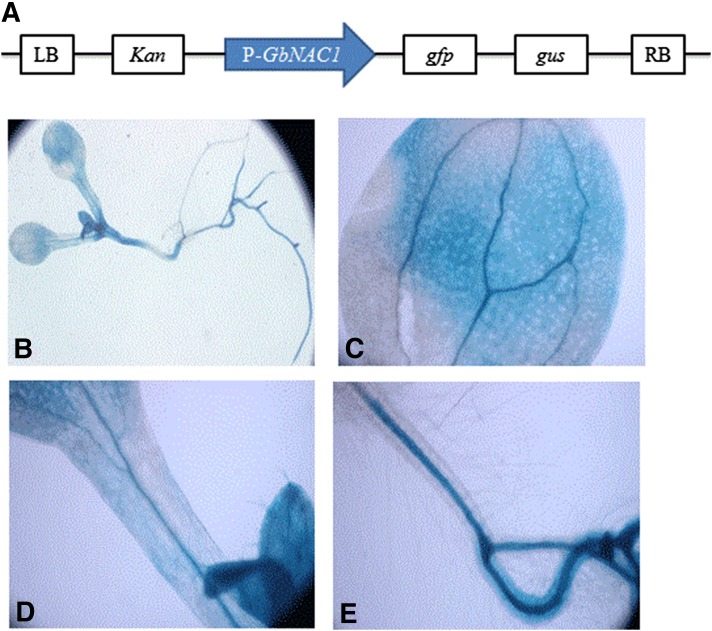
To elucidate the spatial expression of GbNAC1, GUS activity was analyzed under the control of the GbNAC1 promoter. Histochemical staining in transgenic Arabidopsis seedlings showed that GbNAC1 expression was present in leaves, stems, and roots (Figure 5A), and was particularly high in vascular bundles (Figure 5, B–D).
Figure 5.
Histochemical GUS staining of different tissues in pGbNAC1:: GUS Arabidopsis. (A) Schematic diagram of pGbNAC1:: GUS. Drawing is not to scale. (B) Staining of whole transgenic plant. Staining of leaf (C), stem (D), and root (E) tissues.
Silencing of GbNAC1 reduced resistance to V. dahliae
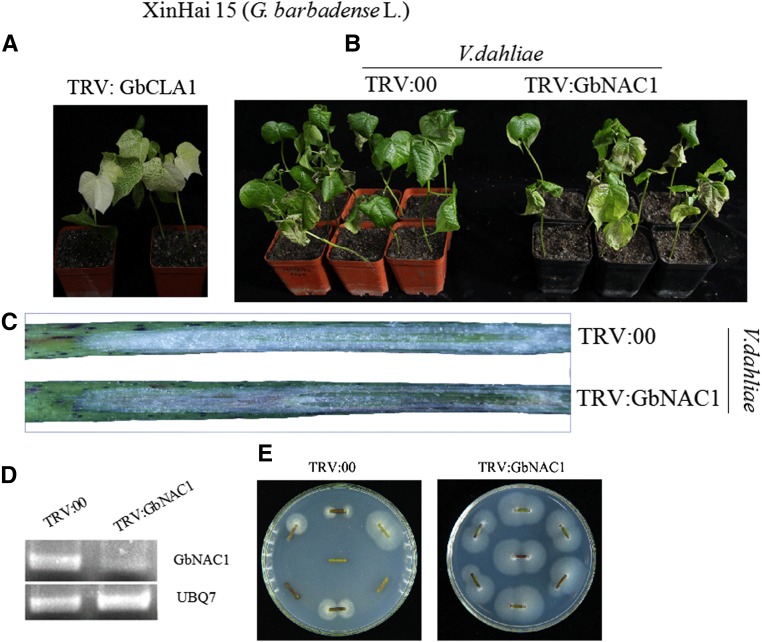
To explore the function of GbNAC1 in cotton plant defense against V. dahliae, VIGS was used to silence the gene expression of GbNAC1. The GbCla1 gene was used as a visual marker for VIGS to monitor efficiency and reliability. The true leaves displayed an albino phenotype after cotyledons were hand-infiltrated by Agrobacterium carrying TRV:GhCla1 (Figure 6A). Compared to the expression of GbNAC1 in TRV:00 controls, expression in TRV:GbNAC1 plants was suppressed after 2 wk (Figure 6D). After inoculation with V. dahliae strain V991, typical disease symptoms were seen in TRV:00 plants and TRV:GbNAC1 plants after 10 d; however, disease symptoms, including leaf chlorosis and necrosis, were more severe in TRV:GbNAC1 plants than in TRV:00 plants (Figure 6B and Supplemental Material, Figure S1). In addition, if a plant is infected by V. dahliae, xylem vessels turn brown. The diagonal plane of the stem was more apparent in TRV:GbNAC1 plants than in TRV:00 plants (Figure 6C), and the fungal renewal cultivation showed that the disease conditions of TRV:GbNAC1 plants were more serious compared with controls (Figure 6E). The increase in the disease index indicated that downregulation of GbNAC1 via VIGS reduced V. dahliae resistance in cotton (Figure 6F).
Figure 6 V.
dahliae-resistant analysis of GbNAC1-silencing (TRV: GbNAC1) and control (TRV: 00) in Xinhai15 (G. barbadense L.). (A) The albino phenotype of true leaves after 10 d with the VIGS method. (B) The disease phenotype of TRV: 00 plants and TRV: GbNAC1 plants by inoculation with V. dahliae after 10 d. (C) Diagonal plane of stem by inoculation with V. dahliae after 10 d. (D) RT-PCR analysis of the expression of GbNAC1 in TRV: GbNAC1 and TRV: 00. Cotton gene UBQ7 was used as an internal control. (E) The fungal renewal cultivation of V. dahliae inoculation stem sections on PDA medium. (F) Disease index after inoculation with V. dahliae. PDA, potato dextrose agar; RT-PCR, reverse transcription-polymerase chain reaction; VIGS, virus-induced gene silencing.
Overexpression of GbNAC1 enhanced resistance against V. dahliae infection in Arabidopsis
To further determine the functions of GbNAC1, the phenotype of the T3 generation of overexpressing transgenic Arabidopsis plants was observed compared to wild-type plants. According to the semiquantity, the GbNAC1 was overexpressed (Figure 7B). Overexpressing plants confirmed the function of GbNAC1 in resistance against V. dahliae. During V. dahliae infection, wild-type plants and transgenic plants displayed different phenotypes. Wild-type plants were susceptible to V. dahliae; however, GbNAC1 transgenic plants exhibited enhanced resistance against infection, with less chlorosis and necrosis after 10 d (Figure 7A).
Figure 7.
Disease-resistant analysis of overexpressing transgenic Arabidopsis. (A) The resistance of GbNAC1 to V. dahliae in overexpressed Arabidopsis. (B) Semiquantity expression of GbNAC1 in wild-type (WT) and overexpression transgenic plants. Arabidopsis gene Actin was used as an internal control.
Phenotype of transgenic Arabidopsis and response to abiotic stress
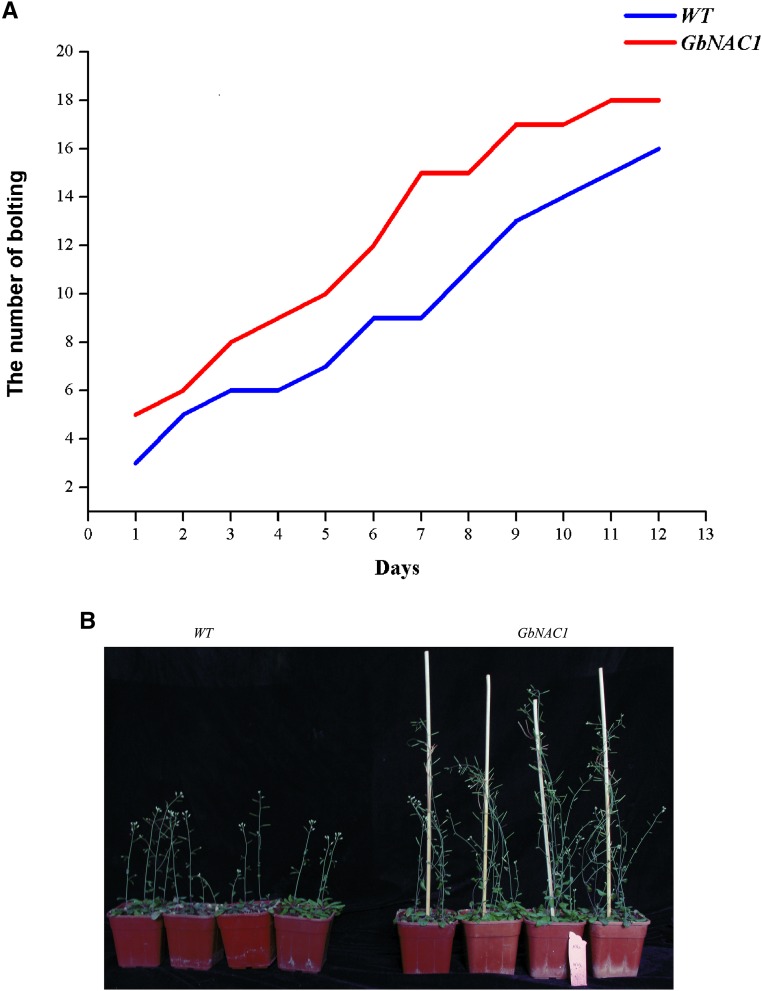
Compared with wild-type plants, transgenic plants grew quickly in the seedling stage (Figure 8A) and overexpressing plants reach the bolting stage earlier. In addition, transgenic Arabidopsis plants were taller when mature (Figure 8B). The results indicate that GbNAC1 is involved in plant growth and development.
Figure 8.
Phenotype analysis of overexpressing transgenic Arabidopsis. (A) Statistical analysis of the amount of bolting in wild-type (WT) and transgenic plants. (B) Phenotype of wild-type and overexpression transgenic mature plants.
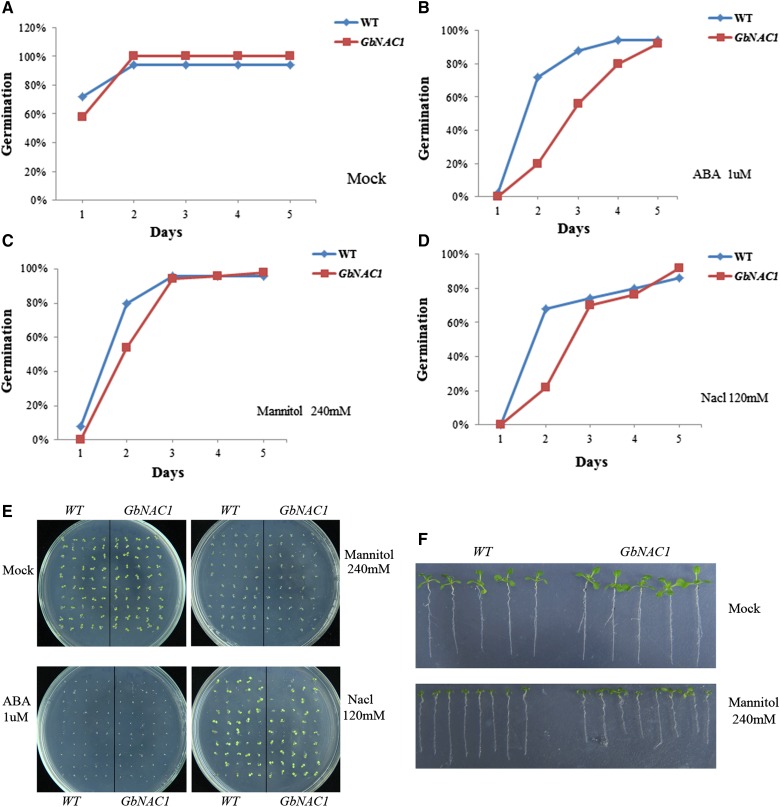
Different stress treatments revealed differences in germination between overexpressing Arabidopsis plants and wild-type. Under normal conditions, the germination rate of transgenic plants was higher than wild-type within the first 5 d (Figure 9A). Under 1 μM ABA, the germination of transgenic plants was delayed and the rate was lower than that of wild-type (Figure 9B). There was no obvious difference in germination between transgenic plants and wild-type in 240 mM mannitol (Figure 9C). In 120 mM NaCl, the germination rate of transgenic plants was lower, and then higher, than that of wild-type (Figure 9D). The germination conditions of transgenic and wild-type plants under different abiotic stresses are displayed in Figure 9E. Wild-type and transgenic seedlings showed different growth characteristics after germination in the mannitol treatment. Transgenic seedling roots were longer than wild-type under normal growth conditions (Figure 9F). Both wild-type and transgenic seedlings were inhibited by osmotic stress, but transgenic plants were more sensitive than wild-type plants (Figure 9F). The results suggested that GbNAC1 is also involved in the downregulation of abiotic stress responses.
Figure 9.
Analysis of transgenic Arabidopsis under stress conditions. Seed germination of wild-type and transgenic plants on (A) blank MS or MS-containing (B) ABA (1 μM), (C) mannitol (240 mM), and (D) NaCl (120 mM), respectively. (E) Germination condition of wild-type and transgenic on plants MS containing blank, ABA, Mannitol, and NaCl after 5 d. (F) Phenotype of wild-type and transgenic plant seedlings on MS (blank and Mannitol, respectively). ABA, abscisic acid; MS, mannitol salt; WT, wild-type.
Discussion
NAC is an important terrestrial plant-specific transcription factor family that is involved in a wide range of regulatory roles in the biotic and abiotic stress response, growth, and development. In recent years, a number of NAC transcription factors have been reported to be involved in biotic stress responses.
Based on the previous transcriptome sequencing of sea-island cotton in response to V. dahliae (Sun et al. 2013b), we identified the differential expression of a unigene fragment (designated as GbNAC1). GbNAC1 encodes a NAC protein with 180 amino acids containing three NAC substructure domains (C–E) (Figure 1A and Figure 2). GbNAC1 lacks the A and B substructure domains, which might, to some extent, influence the function of GbNAC1, though the potential influence of this lack of domains is unknown. According to a previous study, substructure domains C and D contain nuclear localization signals (Olsen et al. 2005); thus, GbNAC1 was localized in the nucleus of transgenic tobacco epidermal cells (Figure 3). Furthermore, based on the homologous sequence alignment of TERN NAC-TFs, these NAC proteins have a conserved WKATGSPG sequence, similar to WRKYGQK from WRKY proteins (Figure 1A), which suggests that this conserved domain may function in DNA binding and that the NAC-TF and WRKY TF families may have a common evolutionary ancestor (Yabuki et al. 2005; Babu et al. 2006).
GbNAC1 was expressed in roots, stems, and leaves in both Xinhai 15 and Xinhai 16 (two varieties of G. barbadense L.), and was especially highly expressed in leaves (Figure 4). The GUS results showed that the expression of GbNAC1 was focused in the vascular bundle (Figure 5). As reported previously, V. dahliae invades from the root system, entering and spreading upward through vessels, damaging those vessels and hindering the transportation of water, thereby inducing the disease symptoms (Sal’kova and Guseva 1965). During V. dahliae infection, GbNAC1 expression initially decreased, then increased at 72 hr, indicating that GbNAC1 expression in vascular bundles may be involved in the response to V. dahliae.
Overexpression of NTL6, an active NAC protein, increased resistance to Pseudomonas syringae in transgenic Arabidopsis (Seo et al. 2010). SISRN1, a tomato NAC gene, was involved in the response to Botrytis cinerea (Liu et al. 2014a). NTP1 and NTP2, two NAC proteins from potato and N. benthamiana, are involved in the response to P. infestans (McLellan et al. 2013). In this study, the function of GbNAC1 in resistance to V. dahliae was examined using VIGS and overexpression in Arabidopsis. The VIGS result showed that resistance to V. dahliae was reduced when GbNAC1 was silenced in the resistant variety (Xinhai15). Furthermore, GbNAC1-overexpressing Arabidopsis plants showed enhanced resistance to Verticillium wilt compared to wild-type plants. This demonstrates that GbNAC1 plays a positive regulatory role in resistance to V. dahliae. This study serves as a foundation for the further functional study of NAC genes in cotton. Overexpression of OsNAC9 could enhance drought resistance under the control of the RCc3 promoter in rice (Redillas et al. 2012). Not all NAC proteins were positive regulators of abiotic stress; for example, ATAF1 played a negative role in drought stress (Lu et al. 2007).
In this study, GbNAC1-overexpressing Arabidopsis plants showed more vigorous growth both as seedlings and mature plants, especially in terms of bolting, than wild-type plants. However, in the presence of abiotic stressors, such as NaCl, ABA, and mannitol, wild-type plants fared better than overexpressing plants in terms of seed germination and seedling growth. These results suggest that GbNAC1 is involved in plant growth and development and also responds to biotic and abiotic stress, though the function of NAC genes in cotton requires further study.
Supplementary Material
Acknowledgments
This work was supported by the National Key Research and Development Program of China (NO: 2016YFD0101900), the National Science Foundation of China (grants 31571724, 31071461, and 31470354), the National Key Basic Special Funds (2012CB1143001), and a 2014 Henan outstanding talent project grant (154200510006).
Footnotes
Supplemental material is available online at http://www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.034512/-/DC1/.
Communicating editor: J. D. Faris
Literature Cited
- Aida M., Ishida T., Fukaki H., Fujisawa H., Tasaka M., 1997. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9: 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu M. M., Iyer L. M., Balaji S., Aravind L., 2006. The natural history of the WRKY-GCM1 zinc fingers and the relationship between transcription factors and transposons. Nucleic Acids Res. 34: 6505–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T. L., Elkan C., 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2: 28–36. [PubMed] [Google Scholar]
- Breeze E., Harrison E., McHattie S., Hughes L., Hickman R., et al. , 2011. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23: 873–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q., Jian H., Li C. B., Zhai Q., Zhang J., et al. , 2008. Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid signaled defense responses. Cell Res. 18: 756–767. [DOI] [PubMed] [Google Scholar]
- Chen Y. J., Perera V., Christiansen M. W., Holme I. B., Gregersen P. L., et al. , 2013. The barley HvNAC6 transcription factor affects ABA accumulation and promotes basal resistance against powdery mildew. Plant Mol. Biol. 83: 577–590. [DOI] [PubMed] [Google Scholar]
- Duval M., Hsieh T. F., Kim S. Y., Thomas T. L., 2002. Molecular characterization of AtNAM: a member of the Arabidopsis NAC domain superfamily. Plant Mol. Biol. 50: 237–248. [DOI] [PubMed] [Google Scholar]
- Gao X. Q., Britt R. C., Jr, Shan L. B., He P. 2011. Agrobacterium-mediated virus-induced gene silencing assay in cotton. J. Vis. Exp. 54: e2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant E. H., Fujino T., Beers E. P., Brunner A. M., 2010. Characterization of NAC domain transcription factors implicated in control of vascular cell differentiation in Arabidopsis and Populus. Planta 232: 337–352. [DOI] [PubMed] [Google Scholar]
- He X., Zhu L., Xu L., Guo W., Zhang X., 2016. GhATAF1, a NAC transcription factor, confers abiotic and biotic stress responses by regulating phytohormonal signaling networks. Plant Cell Rep. 35(10): 2167–2179. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., 1987. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Report. 5: 387–405. [Google Scholar]
- Jensen M. K., Rung J. H., Gregersen P. L., Gjetting T., Fuglsang A. T., et al. , 2007. The HvNAC6 transcription factor: a positive regulator of penetration resistance in barley and Arabidopsis. Plant Mol. Biol. 65: 137–150. [DOI] [PubMed] [Google Scholar]
- Kim S. G., Kim S. Y., Park C. M., 2007. A membrane-associated NAC transcription factor regulates salt-responsive flowering via FLOWERING LOCUS T in Arabidopsis. Planta 226: 647–654. [DOI] [PubMed] [Google Scholar]
- Kim S. G., Lee A. K., Yoon H. K., Park C. M., 2008. A membrane-bound NAC transcription factor NTL8 regulates gibberellic acid-mediated salt signaling in Arabidopsis seed germination. Plant J. 55: 77–88. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Kim S. G., Park J. E., Park H. Y., Lim M. H., et al. , 2006. A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. Plant Cell 18: 3132–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J. H., Yang S. H., Park A. H., Lerouxel O., Han K. H., 2007. ANAC012, a member of the plant-specific NAC transcription factor family, negatively regulates xylary fiber development in Arabidopsis thaliana. Plant J. 50: 1035–1048. [DOI] [PubMed] [Google Scholar]
- Le Hénanff G., Profizi C., Courteaux B., Rabenoelina F., Gérard C., et al. , 2013. Grapevine NAC1 transcription factor as a convergent node in developmental processes, abiotic stresses, and necrotrophic /biotrophic pathogen tolerance. J. Exp. Bot. 64: 4877–4893. [DOI] [PubMed] [Google Scholar]
- Li W., Huang G. Q., Zhou W., Xia X. C., Li D. D., et al. , 2014. A cotton (Gossypium hirsutum) gene encoding a NAC transcription factor is involved in negative regulation of plant xylem development. Plant Physiol. Biochem. 83: 134–141. [DOI] [PubMed] [Google Scholar]
- Liu B., Ouyang Z. G., Zhang Y. F., Li X. H., Hong Y. B., et al. , 2014a Tomato NAC transcription factor SlSRN1 positively regulates defense response against biotic stress but negatively regulates abiotic stress response. PLoS One 9: e102067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. Z., Li X. L., Jin S. X., Liu X. Y., Zhu L. F., et al. , 2014b Overexpression of rice NAC gene SNAC1 improves drought and salt tolerance by enhancing root development and reducing transpiration rate in transgenic cotton. PLoS One 9: e86895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. L., Xu K. D., Zhao L. J., Pan Y. Z., Jiang B. B., et al. , 2011. Overexpression of a novel chrysanthemum NAC transcription factor gene enhances salt tolerance in tobacco. Biotechnol. Lett. 33: 2073–2082. [DOI] [PubMed] [Google Scholar]
- Lu P. L., Chen N. Z., An R., Su Z., Qi B. S., et al. , 2007. A novel drought- inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the express ion of stress-responsive genes in Arabidopsis. Plant Mol. Biol. 63: 289–305. [DOI] [PubMed] [Google Scholar]
- McLellan H., Boevink P. C., Armstrong M. R., Pritchard L., Gomez S., et al. , 2013. An RxLR effector from Phytophthora infestans prevents re-localisation of two plant NAC transcription factors from the endoplasmic reticulum to the nucleus. PLoS Pathog. 9: e1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda N., Ohme-Takagi M., 2008. NAC transcription factors NST1 and NST3 regulate pod shattering in a partially redundant manner by promoting secondary wall formation after the establishment of tissue identity. Plant J. 56: 768–778. [DOI] [PubMed] [Google Scholar]
- Mitsuda N., Seki M., Shinozaki K., Ohme-Takagi M., 2005. The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17: 2993–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda N., Iwase A., Yamamoto H., Yoshida M., Seki M., et al. , 2007. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 19: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyasu Y., Takumi S., Muneo Y., Takahiko H., Akio M., et al. , 2009. Disruption of a novel gene for a NAC-domain protein in rice confers resistance to rice dwarf virus. Plant J. 57: 615–625. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Tran L. P., Nguyen D. V., Fujita M., Maruyama K., et al. , 2007. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 51: 617–630. [DOI] [PubMed] [Google Scholar]
- Nuruzzaman M., Manimekalai R., Sharoni A. M., Satoh K., Kondoh H., et al. , 2010. Genome-wide analysis of NAC transcription factor family in rice. Gene 465: 30–44. [DOI] [PubMed] [Google Scholar]
- Olsen A. N., Ernst H. A., Leggio L. L., Skriver K., 2005. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. 10: 1360–1385. [DOI] [PubMed] [Google Scholar]
- Ooka H., Satoh K., Doi K., Nagata T., Otomo Y., et al. , 2003. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 10: 239–247. [DOI] [PubMed] [Google Scholar]
- Puranik S., Bahadur R. P., Srivastava P. S., Prasad M., 2011. Molecular cloning and characterization of a membrane associated NAC family gene, SiNAC from foxtail millet [Setaria italica (L.) P. Beauv]. Mol. Biotechnol. 49: 138–150. [DOI] [PubMed] [Google Scholar]
- Redillas M. C., Jeong J. S., Kim Y. S., Jung H., Bang S. W., et al. , 2012. The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 10: 792–805. [DOI] [PubMed] [Google Scholar]
- Sal’kova E. G., Guseva N. N., 1965. The role of pectolytic enzymes of the Verticillium dahliae fungus in the development of cotton wilt. Dokl. Akad. Nauk SSSR 163: 515–522 (in Russian). [PubMed] [Google Scholar]
- Seo P. J., Kim M. J., Park J. Y., Kim S. Y., Jeon J., et al. , 2010. Cold activation of a plasma membrane-tethered NAC transcription factor induces a pathogen resistance response in Arabidopsis. Plant J. 61: 661–671. [DOI] [PubMed] [Google Scholar]
- Shang H. H., Li W., Zou C. S., Yuan Y. L., 2013. Analyses of the NAC transcription factor gene family in Gossypium raimondii UIbr.: chromosomal location, structure, phylogeny, and expression patterns. J. Integr. Plant Biol. 55: 663–676. [DOI] [PubMed] [Google Scholar]
- Sink K. C., Grey W. E., 1999. A root-injection method to assess Verticillium wilt resistance of peppermint (Mentha × piperita L.) and its use in identifying resistant somaclones of cv. Black Mitcham. Euphytica 106: 223–230. [Google Scholar]
- Sperotto R. A., Ricachenevsky F. K., Duarte G. L., Boff T., Lopes K. L., et al. , 2009. Identification of up-regulated genes in flag leaves during rice grain filling and characterization of OsNAC5, a new ABA-dependent transcription factor. Planta 230: 985–1002. [DOI] [PubMed] [Google Scholar]
- Su H., Zhang S., Yuan X., Chen C., Wang X. F., et al. , 2013. Genome-wide analysis and identification of stress-responsive genes of the NAM-ATAF1, 2–CUC2 transcription factor family in apple. Plant Physiol. Biochem. 71: 11–21. [DOI] [PubMed] [Google Scholar]
- Sun L., Zhang H., Li D., Huang L., Hong Y., et al. , 2013a Functions of rice NAC transcriptional factors, ONAC122 and ONAC131, in defense responses against Magnaporthe grisea. Plant Mol. Biol. 81: 41–56. [DOI] [PubMed] [Google Scholar]
- Sun Q., Jiang H. Z., Zhu X. Y., Wang W. N., He X. H., et al. , 2013b Analysis of sea-island cotton and upland cotton in response to Verticillium dahliae infection by RNA sequencing. BMC Genomics 14: 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed T. S., Pang C. Y., Hussain A., Fan S. L., Song M. Z., et al. , 2014. Molecular cloning and functional analysis of NAC family genes associated with leaf senescence and stresses in Gossypium hirsutum L. Plant Cell Tissue Organ Cult. 117: 167–186. [Google Scholar]
- Xie Q., Frugis G., Colgan D., Chua N. H., 2000. Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Genes Dev. 14: 3024–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuki T., Aoki M., Seki E., Matsuda T., Tomo Y., et al. , 2005. Solution structure of an Arabidopsis WRKY DNA binding domain. Plant Cell 17: 944–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Goue N., Igarashi H., Ohtani M., Nakano Y., et al. , 2010. VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NACDOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol. 153: 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. Y., Kim Y., Kim S. Y., Lee J. S., Ahn J. H., 2007. Control of flowering time and cold response by a NAC-domain protein in Arabidopsis. PLoS One 2: e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii M., Yamazaki M., Rakwal R., Kishi-Kaboshi M., Miyao A., et al. , 2010. The NAC transcription factor RIM1 of rice is a new regulator of jasmonate signaling. Plant J. 61: 804–815. [DOI] [PubMed] [Google Scholar]
- Zhu Z. G., Shi J. L., He M. Y., Cao J. L., Wang Y. J., et al. , 2012. Isolation and functional characterization of a transcription factor VpNAC1 from Chinese wild Vitis pseudoreticulata. Biotechnol. Lett. 34: 1335–1342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.