Abstract
Antibody–cytokine fusion proteins, often referred to as immunocytokines, represent a novel class of biopharmaceutical agents that combine the disease-homing activity of certain antibodies with the immunomodulatory properties of cytokine payloads. Originally, immunocytokines were mainly developed for cancer therapy applications. More recently, however, the use of anti-inflammatory cytokines for the treatment of chronic inflammatory conditions and to treat autoimmune diseases has been considered. This review analyzes basic principles in the design of immunocytokines and describes the most advanced products in preclinical and clinical development.
Introduction
Chronic inflammation is generally associated with a persistent dysregulation of immune cells, causing considerable damage to tissues and organs. Chronic inflammatory disorders cover a wide range of diseases, the incidence of which is continuously increasing. Some of these disorders have been associated with increased risk of other health threatening maladies, for example, cardiovascular events or certain cancer types [1,2]. Many inflammatory conditions (e.g. rheumatoid arthritis) are not curable and there is an urgent need for more-efficacious therapeutic agents.
Immunocytokines: a strategy to improve potency and selectivity of cytokine-based products
Cytokines are a group of small immunomodulatory proteins that regulate the activity of immune cells in health and disease. These proteins can be released not only by leukocytes but also by other cell types, including fibroblasts, endothelial cells and other stromal cells. In most cases, cytokines act locally in an autocrine or paracrine fashion, binding with high affinity to cognate receptors and regulating immune cell activity. In pathological conditions, such as cancer or septic shock, cytokines can also act on distant organs, influencing a variety of biological processes such as vascular permeability, mobilization of metabolites, control of body temperature and leukocyte development, to name just a few [3].
Cytokines are crucially important in a variety of pathological conditions and the antibody-based blockade of proinflammatory cytokines [e.g. tumor necrosis factor (TNF), interleukin (IL1)b, IL12, IL17, IL23] or their cognate receptors (e.g. IL6R) has led to the development of successful biopharmaceutical products (Table 1). For example, TNF blockers represent the best-selling class of all pharmaceutical products, as a consequence of the substantial benefit offered to patients with chronic inflammatory conditions such as rheumatoid arthritis, psoriatic arthritis, psoriasis, ankylosing spondylitis, Crohn’s disease and ulcerative colitis [4].
Table 1. Approved cytokine-based pharmaceutical products.
| Cytokine | Name | Trade name | Indication | Refs |
|---|---|---|---|---|
| Cytokine-blocking monoclonal antibodies | ||||
| TNF | Adalimumab | Humira® | Rheumatoid arthritis | [95–110] |
| Infliximab | Remicade® | Psoriatic arthritis, colitis, Crohn’s disease, psoriasis, ankylosing spondylitis | ||
| Certolizumab | Cimzia® | |||
| Golimumab | Simponi® | |||
| IL1b | Canakinumab | Ilaris® | Arthritis, cryopyrin-associated periodic syndromes | [111,112] |
| IL2R | Daclizumab | Zenapax® | Renal transplant rejection | [113] |
| IL12/IL23 | Ustekinumab | Stelara® | Psoriasis | [114] |
| IL17A | Secukinumab | Cosentyx® | Psoriasis | [115] |
| IL6R | Tocilizumab | Actemra® | Rheumatoid arthritis | [116] |
| IL6 | Siltuximab | Sylvant® | Castleman’s disease | [117] |
| Therapeutic cytokine products | ||||
| IL2 | Aldesleukin | Proleukin® | Renal cell carcinoma | [118,119] |
| Metastatic melanoma | ||||
| IL11 | Oprelvekin | Neumega® | Thrombocytopenia | [120] |
| IFNα | IFNα-2b | IntronA® | Hepatitis | [121–127] |
| Viraferon® | Melanoma | |||
| PEG-IFNα-2b | PegIntron® | Kaposi sarcoma | ||
| PEG-IFNα-2a | Pegasys® | Hematologic cancer | ||
| IFNβ | IFNβ-1b | Betaseron® | Multiple sclerosis | [128–130] |
| IFNβ-1a | Avonex® | |||
| Rebif® | ||||
| PEG-IFNβ-1a | Plegridy® | |||
| IFNγ | IFNγ-1b | Actimmune® | Chronic granulomatous disease | [131] |
| GM-CSF | Filgrastim | Neupogen® | Neutropenia | [132,133] |
| Sargramostim | Leukine® | |||
| TNFα | Tasonermin | Beromun® | Soft tissue sarcoma | [134] |
A summary of approved cytokine-based pharmaceuticals comprising cytokine blocking agents and recombinant cytokine therapeutics.
In addition to serving as targets for the development of blocking agents, the potent agonistic activity of certain cytokines has prompted their industrial and clinical development as recombinant biopharmaceuticals (Table 1). IL2 has been approved for the treatment of advanced melanoma and renal cell carcinoma and received orphan drug designation for the treatment of primary immunodeficiency disease. Interferon (IFN)α has received marketing authorization for oncological conditions such as renal cell carcinoma, melanoma and Kaposi’s sarcoma and also for the treatment of hepatitis, cirrhosis, viral infections and genital warts. IFNγ is being used for chronic granulomatous disease and osteopetrosis, whereas IFNβ represents a leading therapeutic agent for the treatment of multiple sclerosis. The colony-stimulating factors GM-CSF and G-CSF are important therapeutic agents for neutrophil recovery after bone marrow and stem cell transplantation. Recombinant TNFα is being used for the isolated limb perfusion treatment of patients with inoperable sarcomas, and IL11 received marketing authorization for prevention of chemotherapy-induced thrombocytopenia.
Therapeutic strategies centered on antibody-based blocking agents can display a limited pharmaceutical benefit (or excessive toxicities) when several cytokines contribute to a given pathological condition [5]. By contrast, the use of recombinant cytokines as therapeutic agents can suffer from certain limitations. For example, receptor expression by many types of cells and tissues could lead to substantial toxicities, especially for potent proinflammatory cytokines. Alternatively, the inability to reach desired concentrations at the site of disease might limit pharmaceutical activity [6].
In an attempt to improve their potency and selectivity, cytokines can be fused to antibodies (or antibody fragments), serving as pharmacodelivery vehicles. The resulting fusion proteins (referred to as immunocytokines) are finding an increasing number of applications in the treatment of cancer and other diseases. We have previously reviewed the use of proinflammatory immunocytokines for cancer treatment [7,8]. In this review, we analyze the potential and challenge of immunocytokines for the treatment of nononcological conditions, with a main focus on chronic inflammation and autoimmunity.
Cytokines as payloads for nononcological applications
Immunocytokines represent a class of therapeutic agents with the potential to modulate immunity at the site of disease and a number of payloads can be considered for product development. Indeed, the considerable amount of preclinical and clinical data, available for the therapeutic use of unmodified cytokine products, could provide inspiration for the development of targeted immunocytokines. For example, IL10 and IL4 have extensively been studied in the clinical setting, after having shown promising results in preclinical animal models of various diseases. Recombinant human IL10 (tenovil) has been extensively studied for use in rheumatoid arthritis, Crohn’s disease, organ transplantation, hepatitis and psoriasis [9]. Although a clear superiority compared with placebo control groups was observed for some indications (e.g. rheumatoid arthritis), disease remissions were rarely observed and for this reason the product was not advanced to Phase III clinical studies. However, limiting toxicities were not observed and the treatment was generally well tolerated. The fast clearance of the product and the inability to localize at the site of disease selectively could have contributed to a suboptimal therapeutic performance [9]. Dose-limiting toxicities were observed for recombinant IL4, and the product failed to show sufficient activity in patients with cancer [10–13]. By contrast, treatment of psoriasis patients with IL4 (as a single agent or in combination with IL10 and IL11) led to a substantial reduction in disease symptoms, at doses that were well tolerated (0.2–0.5 μg/kg) [14,15]. In a mouse model of collagen-induced arthritis, suppression of clinical symptoms and delay of disease progression was achieved by administration of recombinant murine IL4 [16]. Arthritis-suppressing effects were further confirmed in subsequent animal studies [17,18]. Clinical data on rheumatoid arthritis, however, have not been described.
In practice, the choice of cytokine payloads for the development of therapeutic agents in nononcological conditions remains largely unexplored, as illustrated in the following sections. Cytokines are particularly intriguing proteins for the design of agonistic pharmaceutical products, because they often display different biological activities, as a function of their concentration and the concentration of other components (e.g. other cytokines, pathogen-associated molecular patterns) at the site of disease. Transforming growth factor (TGF)β, for example, suppresses cell proliferation and stimulates excessive extracellular matrix (ECM) growth at high doses. By contrast, at low doses, the same protein induces opposite effects, with excessive cell proliferation, underproduction of ECM components and impaired wound healing [19].
Furthermore, TGFβ inhibits IL1β and IL8 expression in macrophages but induces it in endothelial cells [20]. Similarly, TNF promotes angiogenesis at low doses but causes intraluminal blood coagulation and blocks the formation of new vessels at high doses [21]. A more global picture of opposing effects of a single cytokine has been demonstrated in an experiment using the collagen-induced animal model of arthritis. Injections of low doses of IL12 led to worsening of the disease whereas administration of high doses of the same cytokine significantly improved the arthritic score [22].
Cytokines can have pro- and anti-inflammatory properties. Levels of IL1, IL6 and TNFα, for example, are substantially elevated in inflammatory conditions and these cytokines are considered to be proinflammatory proteins. By contrast, IL10 is generally considered an anti-inflammatory cytokine, with the potential to suppress immunity in physiological (e.g. pregnancy) and pathological (e.g. cancer) conditions [23,24]. However, immunological processes in inflammation are not a simple interplay between two opposing classes of actors. The immunological environment, the target cell and the concentration of the cytokine itself influence the overall response [25–27].
Besides using naturally occurring cytokine payloads, mutants generated using protein-engineering techniques could provide some benefit in certain applications. As reported for the cytokines IFN, IL2, IL4, IL13 and IL15, mutations in key residues within the cytokine can alter the binding affinity to the cognate receptor, modulate cytokine activity and/or promote a selective interaction with different subsets of leukocytes [28].
Immunocytokine formats and target antigens
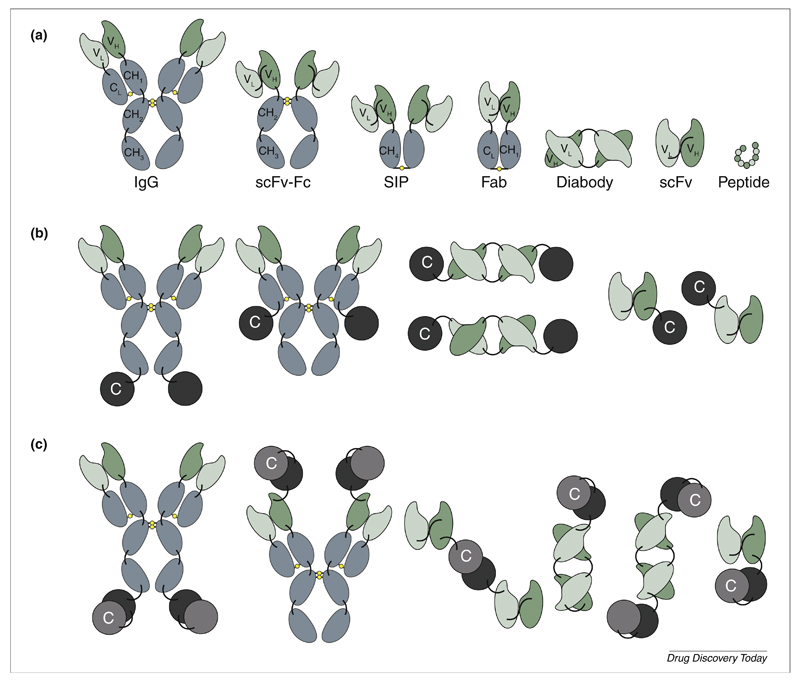
Antibodies can be used as full immunoglobulins or as antibody fragments for the selective pharmacodelivery of cytokine payloads, as shown in Fig. 1a. The IgG format promotes a long circulatory half-life in blood, as a result of size and Fc recycling. The molecular weight of 150 kDa exceeds the renal clearance threshold of 70 kDa and prevents the protein from being eliminated via the kidney. Additionally, the interaction with the neonatal Fc receptor leads to a continuous recycling process [29].
Figure 1.
(a) Schematic illustration of different recombinant binding moiety formats suitable for immunocytokine development. (b) Frequently used immunocytokine formats, including IgG heavy chain fusion, IgG light chain fusion, C- and N-terminal diabody fusion, C- and N-terminal scFv fusion (left to right). C: cytokine. (c) Various immunocytokine formats for heterodimeric cytokine payloads, which have been used for the development of IL12-based immunocytokines [8,42].
The bivalent nature of IgGs is considered to be advantageous, because it can lead to a longer residence time at the site of disease, increasing the functional affinity to the cognate antigen. High blood levels of cytokine payloads, promoted by their fusion to IgG molecules, might not always be desirable for pharmaceutical applications, because they could lead to side-effects and decrease in vivo selectivity. However, fairly short circulatory half-lives (1.6–8.2 hours) for IgG–cytokine fusion proteins have been reported in clinical studies, owing to reasons that are not yet fully understood [30,31]. One possible explanation for this could be cytokine-specific receptor-mediated clearance, which has been described for an IgG-based fusion to IL2 [32]. For some applications, antibody fragments might be preferable to achieve a long residence time of the product at the site of disease, while being rapidly cleared from the circulation. A variety of antibody formats could be considered, with different biochemical properties (e.g. avidity, size, Fc functionality) influencing in vivo behavior (e.g. retention time at the site of disease, tissue penetration, blood clearance). In most cases, bivalent antibody fragments are preferred for pharmacodelivery applications, because they promote a longer residence time on the target antigen. Rapid blood clearance inevitably reduces the accumulation at the site of disease, but typically leads to better in vivo selectivity (i.e. higher target:non-target organ ratios) at early time points, as evidenced by quantitative biodistribution studies with radiolabeled protein preparations [33–35]. Single-chain Fv fragments (scFv) are particularly attractive antibody fragments for immunocytokine development, because they can lead to monomeric or dimeric (diabody) structures, depending on the linker used to connect the VH and VL domain in the protein [36,37]. Monomeric scFv fragment units are often used when the cytokine payload is multimeric (e.g. members of the TNF superfamily) [38]. A summary of biodistribution properties for immunocytokines based on intact antibodies and antibody fragments has previously been reported [8].
The arrangement of antibody and cytokine moieties within the recombinant protein also influences immunocytokine performance (Fig. 1b). In most cases, cytokines and antibodies can be modified at their N- and C-terminal extremities without loss of function [39]. For some cytokines, however, a free N or C terminus can be important for function. In addition, heterodimeric cytokines (e.g. members of the IL12 superfamily) can offer additional design possibilities, depending on the assembly of the two cytokine subunits (Fig. 1c) [40–43]. When considering IgG-based immunocytokines, C-terminal fusions to the heavy or to the light chains have been proposed [44]. Indeed, depending on the length of the linker, fusions to the light chain can favor different specificities toward certain cytokine receptor types. This has been demonstrated using an IL2-based immunocytokine targeting ganglioside GD2. One variant of the light-chain-fused immunocytokine exhibited 1000-fold increased selectivity to the high affinity αβγ IL2 receptor compared with the heavy-chain-fused immunocytokine [44].
Antibody-based pharmacodelivery applications typically require specific antigens that are abundantly expressed at the site of disease but are virtually undetectable in normal adult tissues. In principle, accessible antigens on the cell surface or components of the modified ECM could be considered. The identification of targets for antibody-based pharmacodelivery approaches facilitates product development. Accessible antigens are conveniently identified and quantified using perfusion-based in vivo biotinylation techniques. In this approach (which confirmed the value of fibronectin and tenascin-C variants as accessible targets) [45,46] endothelial proteins and ECM components can be enriched using streptavidin-based capture reagents and analyzed by mass spectrometry [47]. Alternatively, transcriptomic studies of endothelial cells using microarray chips to determine mRNA expression levels can be performed to discover new putative target antigens. Not all transcriptomic technologies can be used to study alternative splicing processes. For example, methods that detect only a small portion of the mRNA molecule (e.g. most commercial microarrays and serial analysis of gene expression) fail to detect the insertion or the omission of an exon into the corresponding transcript. Furthermore, mRNA levels are not always predictive of protein abundance.
Macrophages and neutrophils seem to be promising sources of target structures because they are abundant at the site of inflammation. Apart from that, antigens on their surface can be shared with circulating leukocytes leading to loss of targeting and systemic toxicities. However, CD64, an internalizing antigen, abundantly present on macrophages, monocytes and their progenitors, has been shown to be a promising target. Treatment with CD64-targeting antibody–toxin fusions in animal models of skin inflammation, rheumatoid arthritis, ischemia-induced kidney injury and cancer have been shown to be effective in ameliorating disease [48]. The F4/80 antigen, a marker of murine macrophage populations, has been shown to be another suitable structure to target macrophages in mice. Radiolabeled antibody preparations accumulated in tumors, but to large extent also in the kidney, spleen and liver [49]. The human homolog, EMR1, however, is not expressed on macrophages but on mature blood and tissue eosinophil granulocytes, which makes this approach hardly translatable to man [50]. For eosinophilic disorders, however, EMR1 has been shown to be a promising target because antibodies directed against EMR1 enhance killing of eosinophils by natural killer cells in non-human primates [51]. Besides infiltrating immune cells, components of the modified ECM generated during the inflammatory process are particularly attractive antigens for pharmacodelivery applications. In rheumatoid arthritis and osteoarthritis these structures can arise from reactive oxygen species mediated post-translational modification of collagen type II (ROS-CII). The antibody 1-11E generated against ROS-CII was shown to localize selectively at inflamed joints in a mouse model of arthritis [52]. A recent study in the collagen-induced model of arthritis revealed that radiolabeled antibodies specific to fibroblast-activating protein (FAP) accumulated at the sites of disease, with uptake values that correlated with severity of inflammation [53]. Previously, FAP had been shown to be a suitable target for imaging of carcinoma [54].
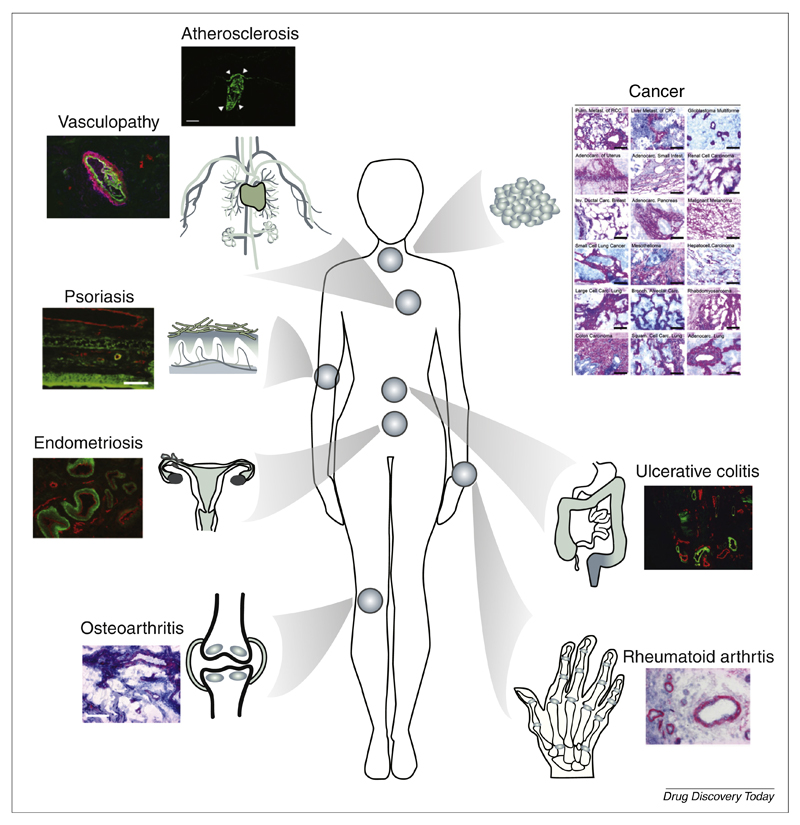
Alternatively spliced isoforms of fibronectin [containing extra-domain (ED)A or EDB] and tenascin-C [containing extra-domain A1 (TnC A1)], recognized by the human antibodies F8, L19 and F16, respectively, represent possibly the most characterized ECM components for pharmacodelivery applications [55,56]. These antigens are often found around new blood vessels. Indeed, angiogenesis is a characteristic feature of cancer and chronic inflammatory conditions, but otherwise a rare event in health. Splice isoforms of fibronectin and tenascin-C are usually undetectable in normal adult human tissues, with the exception of the placenta and uterus. Abundant expression, however, has been demonstrated not only in various cancer types but also in rheumatoid arthritis, osteoarthritis, ulcerative colitis, chronic skin inflammation, vasculopathy and endometriosis specimens (Fig. 2) [57–65].
Figure 2.
Pharmacodelivery applications for monoclonal antibodies, specific to the extra-domain A (EDA) of fibronectin, an antigen that is virtually undetectable in normal adult tissues [87]. Tissue stainings adapted, with permission, from [46,57,59,61,62,67].
Preclinical studies with immunocytokines for nononcological indications
Preclinical studies using IL10 fused to the L19 antibody (directed against EDB, L19–IL10) or to the F8 antibody [directed against EDA, F8–IL10 (dekavil)] in the diabody format revealed potent inhibition of the progression of established disease in a model of collagen-induced arthritis. Administration of L19–IL10 or F8–IL10 significantly lowered the arthritic score and led to reduced paw swelling compared with saline and the untargeted cytokine [62,65,66]. Moreover, combination of F8–IL10 with methotrexate or a murine analog of etanercept (TNFR2–Fc fusion) increased therapeutic activity in this setting [66,67]. Additionally, F8–IL10 significantly reduced lesion size in a mouse model of endometriosis [61].
IL10-based immunocytokine products are also described in a patent application of Roche-Glycart. For the treatment or prophylaxis of inflammatory bowel disease or rheumatoid arthritis, the cytokine was fused to an antibody specific to FAP, used in IgG format [68]. In another approach, the 1-11E antibody (directed against ROS-CII) in scFv format was fused to viral IL10, which is considered to be a less immunostimulatory variant of its human counterpart. The resulting fusion protein was able to inhibit disease progression in a mouse model of antigen-induced arthritis. Similar results were obtained by combining the antibody to the soluble portion of mTNFRII receptor fused to an Fc moiety [52,69].
IL4 represents a second attractive cytokine payload for certain inflammatory conditions and other nononcological diseases. An IL4 fusion to the F8 antibody in the diabody format [F8–IL4 (tetravil)] exhibited high therapeutic efficacy in mouse models of endometriosis, psoriasis and rheumatoid arthritis. Importantly, complete cures in a mouse model of rheumatoid arthritis were achieved when F8–IL4 was administered in combination with dexamethasone [67]. Moreover, F8–IL4 decreased ear swelling in imiquimod (IMQ)-induced and contact-hypersensitivity-induced mouse models of chronic skin inflammation. The therapeutic effect in the mouse was comparable to the one achieved using treatment with a murine version of etanercept [59]. Recent data further revealed that F8–IL4 could significantly reduce endometriotic lesion progression in an immunocompetent mouse model of the disease, whereas no effect could be observed for equimolar doses of untargeted IL4 [70].
As an alternative to antibody-based pharmacodelivery, a phage-display-derived cyclic peptide binding to inflamed synovial endothelium (SyETP) was fused to IL4, yielding a cytokine derivative that selectively accumulated in synovial tissue xenografts but not in skin xenografts in a model of human/SCID chimeric rheumatoid arthritis. Increasing the binding avidity of the fusion protein by adding three cyclic peptide units led to an increased uptake into the synovial tissue. In vivo activity of the fusion protein was also confirmed by analysis of phosphorylated signal transducer and activator of transcription (STAT)6 levels in the xenografts [71].
Besides being an important product in cancer treatment, IL2 can have a beneficial effect for certain anti-inflammatory strategies by stimulating regulatory T cells [72]. In a mouse model of atherosclerosis in apoE-deficient mice, administration of a fusion of IL2 to the L19 antibody induced rapid shrinkage of atherosclerotic plaques [73].
Naturally occurring homodimers of the p40 subunit of IL12 (IL12p40) have been reported to exert anti-inflammatory activity by antagonizing IL12 and IL23 [74–76]. An immunocytokine comprising IL12p40 and the F8 antibody has recently been shown to promote more-rapid clinical recovery and morphological improvement in the dextran sulfate sodium (DSS)-induced mouse model of colitis compared with mice treated with saline or cyclosporine A [57]. A summary of reported immunocytokines explored for nononcological conditions is shown in Table 2.
Table 2. Immunocytokines for nononcological conditions.
| Name | Target | Targeting moiety | Targeting assessment | Developmental stage (disease area) | Refs |
|---|---|---|---|---|---|
| IL10 | |||||
| L19–IL10 | EDB(+)FN | L19 (diabody) | Autoradiography and IVIS fluoroscopy Quantitative biodistribution study |
Preclinical (RA, psoriasis) | [63,65] |
| F8–IL10 (dekavil) | EDA(+)FN | F8 (diabody) | Autoradiography Quantitative biodistribution study |
Clinical Phase II (RA) Preclinical (endometriosis cardiac rejection) |
[61,62,66,87,135] |
| N/A | FAP | 4B9/4G8 (IgG/Fab) | Quantitative biodistribution study | N/A (RA, IBD) | [68] |
| vIL10 | |||||
| 1-11E/vIL10 | ROS-CII | 1-11E (scFv) | IVIS fluoroscopy | Preclinical (RA) | [69] |
| IL4 | |||||
| F8–IL4 (tetravil) | EDA(+)FN | F8 (diabody) | Autoradiography Quantitative biodistribution study |
Preparation for clinic (RA) Preclinical (psoriasis endometriosis cancer) |
[59,67,70,77] |
| IL4–TP/IL4–SP | Synovial endothel-ium | SyETP (peptide) | Nano-SPECT-CT | Preclinical (RA) | [71] |
| IL2 | |||||
| L19–IL2 | EDB(+)FN | L19 | Quantitative biodistribution | Preclinical (atherosclerosis) | [73,136] |
| IL12p40 | |||||
| F8–IL12p40 | EDA(+)FN | F8 (diabody) | Autoradiography | Preclinical (IBD) | [57] |
Summary of preclinically or clinically investigated immunocytokines for the treatment of nononcological conditions. Abbreviations: FN, fibronectin; RA, rheumatoid arthritis; IBD, inflammatory bowel disease.
Disease-homing properties of immunocytokines
The ability to localize selectively at the site of disease represents an important aspect for the development of immunocytokine products. Disease-homing properties of antibody products are best characterized in quantitative biodistribution studies, using radioiodinated protein preparations. In most cases, tumor-bearing animals are used for these studies, because it is easy to weigh neoplastic lesions and count radioactivity in those specimens. However, disease-homing properties in nononcological conditions can be adequately studied by noninvasive radioactive or fluorescent imaging techniques or by autoradiography [66,67]. Microscopic analysis of protein localization in tissue sections (e.g. by microautoradiography or by fluorescence microscopy) can provide complementary information on structures that can be reached in vivo by the product.
Using the F8 and L19 antibodies (specific to alternatively spliced isoforms of fibronectin that are conserved in mouse and man), biodistribution studies with radiolabeled immunocytokines in tumor-bearing mice have revealed three basic patterns of possible pharmacokinetic behavior. In the most favorable situation, the cytokine payload does not impair the disease-homing properties of the parental antibody at various concentration ranges, which are compatible with pharmacological activity. In the mouse this situation has been observed for various cytokine payloads, including IL2, IL4, IL6, IL10, IFNα and TNF, to name just a few [38,39,62,77,78]. For more-complex cytokines (e.g. IL12) biodistribution properties heavily depend on the format chosen for the fusion with antibody moieties [42].
For a number of payloads (e.g. GM-CSF, IFNγ) the ability of the corresponding immunocytokine product to localize at the site of disease was found to be dose dependent. The experience with two IFNγ fusion proteins was particularly informative. Derivatives of the L19 and F8 antibody failed to localize selectively to tumors in biodistribution studies when used at a dose of 5–15 μg per mouse. However, tumor-homing properties could be recovered in knockout mice devoid of IFNγ receptor, or after pre-administration of suitably high unlabeled doses of the fusion protein [79,80]. These observations suggest that, for some payloads, cytokine receptors can trap the therapeutic agent and that selective disease targeting might only become efficient once receptors in normal organs have been saturated. Indeed, simple in vitro tests based on the incubation of radiolabeled products with whole blood, followed by centrifugation and radioactive counting of supernatant and pellet, could provide valuable information about the in vivo performance of the corresponding protein.
In some cases, cytokine payloads (or other protein payloads) could completely abrogate the disease-homing properties of the parental antibody. Such an unfavorable situation has been observed with highly charged polypeptides [81–83] with very large fusion proteins [84] and with heavily glycosylated products [85]. Recent biodistribution studies with IL9-based immunocytokines revealed that protein production conditions in mammalian cells could have a profound influence on glycostructures (including sialylation) and on extravasation properties [86].
In many cases, cytokine payloads can be fused at the N and C terminus of recombinant antibody fragments, without substantial differences in biological activity and biodistribution properties [39]. However, because different pharmacokinetic profiles are often observed with different immunocytokine formats, it remains important to perform biodistribution studies before selecting a product candidate for industrial development programs.
Concluding remarks: immunocytokines in clinical development programs and emerging trends
To date, one immunocytokine for the treatment of nononcological diseases has entered the clinical phase. Dekavil (F8–IL10) is currently being studied in a Phase II clinical trial for the treatment of patients with rheumatoid arthritis. A Phase Ib study of dekavil in combination with methotrexate revealed an excellent tolerability and preliminary signs of activity [87,88]. In this study, 60% of treated patients showed a reduction of the arthritic score of 20% (ACR20), 32% of treated patients achieved ACR50 and 16% of treated patients even reached ACR70 [88]. A second immunocytokine [F8–IL4 (tetravil)] is currently completing safety toxicological testing in non-human primates before clinical testing in patients with endometriosis or with rheumatoid arthritis.
In principle, it would be conceivable to deliver cytokine payloads for a variety of pathological conditions, with the aim to boost or inhibit inflammation and leukocyte activity. In addition, cytokines can facilitate tissue-remodeling processes. Although this field of investigations is still in its infancy, the approach remains particularly attractive, not only in view of the potent activity that cytokines display in vivo but also because of the modular nature of product development activities. In most cases, antibodies for pharmacodelivery applications are chosen on the basis of their immunohistochemical properties and biodistribution patterns. Once a validated disease-homing antibody becomes available this agent can be systematically fused to many different payloads and the corresponding biopharmaceuticals can be studied in vivo. Comparative studies typically shed light on the beneficial or detrimental role that individual cytokine moieties might have on the pathological condition of interest. Indeed, emerging experimental evidence indicates that the combination of immunocytokine products could display a potent synergistic or antagonistic activity at the site of disease [77,84,89–91]. Immune responses are typically regulated by multiple signals and we anticipate that the combined use of multiple immunocytokine products will represent an important research focus in the near future.
The activity of disease-targeting immunocytokines heavily relies on a product’s ability to localize at the pathological site. Although tissue distribution properties are easy to study in animal models, the execution of imaging studies in patients is complicated by various types of hurdles. Nuclear medicine studies require radiolabeled preparations of the study drug, thus adding complexity to product development in terms of regulatory compliance, radiosafety and patient recruitment. Guidelines for easier clinical execution of microdosing studies have been released and Phase 0 clinical trials with radiolabeled antibody preparations have recently been reported [92]. Nonradioactive detection methodologies (e.g. near-infrared fluorescence imaging) might be attractive for the study superficial lesions (e.g. psoriatic lesions or inflamed joints in arthritis) [93,94] but also in this case the biopharmaceutical agent needs to be chemically modified for the execution of imaging studies.
In summary, antibody–cytokine fusion proteins represent an emerging class of biopharmaceutical agents for the treatment not only of cancer but also of other conditions, including chronic inflammatory processes and autoimmunity. In some cases (e.g. IL2, IL10) a human cytokine payload can be used in preclinical studies and in patients, but most of the times surrogate products (based on rodent cytokines) will be needed for studies in mice and rats, whereas the corresponding fully human fusion protein will be required for clinical applications. The ability to characterize disease-homing properties of the product efficiently and to assess optimal payloads (or payload combinations) will be crucial for pharmaceutical success.
Acknowledgments
Financial contribution from ETH Zürich, Swiss National Science Foundation, KTI MedTech Project and an ERC Advanced Grant (ZAUBERKUGEL) is gratefully acknowledged.
Footnotes
Conflicts of interest
Dario Neri is a co-founder and shareholder of Philogen, a biotech company that is developing derivatives of F8, L19 and F16 antibodies for clinical applications.
References
- 1.Roifman I, et al. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. 2011;27:174–182. doi: 10.1016/j.cjca.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 2.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–226. 229. discussion 230– 212. [PubMed] [Google Scholar]
- 3.Murphy K, et al., editors. Janeway’s Immunobiology. Garland Science; 2012. [Google Scholar]
- 4.Walsh G. Biopharmaceutical benchmarks 2014. Nat Biotechnol. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- 5.Genovese MC, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412–1419. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt SR, editor. Fusion Protein Technologies for Biopharmaceuticals: Applications and Challenges. Wiley; 2013. [PubMed] [Google Scholar]
- 7.Hess C, et al. Emerging classes of armed antibody therapeutics against cancer. MedChemComm. 2014;5:408–431. [Google Scholar]
- 8.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17:583–590. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Asadullah K, et al. Interleukin-10 therapy – review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz DM, et al. Subcutaneous interleukin-4 (IL-4) for relapsed and resistant non-Hodgkin lymphoma: a phase II trial in the North Central Cancer Treatment Group, NCCTG 91-78-51. Leuk Lymphoma. 2007;48:1290–1298. doi: 10.1080/10428190701355028. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead RP, et al. Phase II trial of recombinant human interleukin-4 in patients with advanced renal cell carcinoma: a southwest oncology group study. J Immunother. 2002;25:352–358. doi: 10.1097/00002371-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead RP, et al. Phase II trial of recombinant human interleukin-4 in patients with disseminated malignant melanoma: a Southwest Oncology Group study. J Immunother. 1998;21:440–446. doi: 10.1097/00002371-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Wiernik PH, et al. Phase II study of interleukin-4 in indolent B-cell non-Hodgkin lymphoma and B-cell chronic lymphocytic leukemia: a study of the Eastern Cooperative Oncology Group (E5Y92) J Immunother. 2010;33:1006–1009. doi: 10.1097/CJI.0b013e3181f5dfc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghoreschi K, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9:40–46. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 15.Roberti ML, et al. Immunomodulating treatment with low dose interleukin-4, interleukin-10 and interleukin-11 in psoriasis vulgaris. J Biol Regul Homeost Agents. 2014;28:133–139. [PubMed] [Google Scholar]
- 16.Horsfall AC, et al. Suppression of collagen-induced arthritis by continuous administration of IL-4. J Immunol. 1997;159:5687–5696. [PubMed] [Google Scholar]
- 17.Ho SH, et al. Protection against collagen-induced arthritis by electrotransfer of an expression plasmid for the interleukin-4. Biochem Biophys Res Commun. 2004;321:759–766. doi: 10.1016/j.bbrc.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Joosten LA, et al. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. 1999;1:81–91. doi: 10.1186/ar14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Border WA, Noble NA. Targeting TGF-beta for treatment of disease. Nat Med. 1995;1:1000–1001. doi: 10.1038/nm1095-1000. [DOI] [PubMed] [Google Scholar]
- 20.Kumar NM, et al. Induction of interleukin-1 and interleukin-8 mRNAs and proteins by TGF beta 1 in rat lung alveolar epithelial cells. J Cell Physiol. 1996;169:186–199. doi: 10.1002/(SICI)1097-4652(199610)169:1<186::AID-JCP19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Fajardo LF, et al. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140:539–544. [PMC free article] [PubMed] [Google Scholar]
- 22.Kasama T, et al. Biphasic regulation of the development of murine type II collagen-induced arthritis by interleukin-12: possible involvement of endogenous interleukin-10 and tumor necrosis factor alpha. Arthritis Rheum. 1999;42:100–109. doi: 10.1002/1529-0131(199901)42:1<100::AID-ANR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Mocellin S, et al. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 24.Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biedermann T, Rocken M. Pro- and anti-inflammatory effects of IL-4: from studies in mice to therapy of autoimmune diseases in humans. Ernst Schering Res Found Workshop. 2005;50:235–242. doi: 10.1007/3-540-26811-1_13. [DOI] [PubMed] [Google Scholar]
- 26.Scheller J, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 27.Cavaillon JM. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol. 2001;47:695–702. [PubMed] [Google Scholar]
- 28.Spangler JB, et al. Insights into cytokine-receptor interactions from cytokine engineering. Annu Rev Immunol. 2015;33:139–167. doi: 10.1146/annurev-immunol-032713-120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward ES, et al. Targeting FcRn for the modulation of antibody dynamics. Mol Immunol. 2015;67:131–141. doi: 10.1016/j.molimm.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King DM, et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22:4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribas A, et al. Phase I/II open-label study of the biologic effects of the interleukin-2 immunocytokine EMD 273063 (hu14.18-IL2) in patients with metastatic malignant melanoma. J Transl Med. 2009;7:68. doi: 10.1186/1479-5876-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzeng A, et al. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc Natl Acad Sci U S A. 2015;112:3320–3325. doi: 10.1073/pnas.1416159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borsi L, et al. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer. 2002;102:75–85. doi: 10.1002/ijc.10662. [DOI] [PubMed] [Google Scholar]
- 34.Adams GP, et al. Avidity-mediated enhancement of in vivo tumor targeting by single-chain Fv dimers. Clin Cancer Res. 2006;12:1599–1605. doi: 10.1158/1078-0432.CCR-05-2217. [DOI] [PubMed] [Google Scholar]
- 35.Wu AM, et al. Tumor localization of anti-CEA single-chain Fvs: improved targeting by non-covalent dimers. Immunotechnology. 1996;2:21–36. doi: 10.1016/1380-2933(95)00027-5. [DOI] [PubMed] [Google Scholar]
- 36.Huston JS, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holliger P, et al. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borsi L, et al. Selective targeted delivery of TNFalpha to tumor blood vessels. Blood. 2003;102:4384–4392. doi: 10.1182/blood-2003-04-1039. [DOI] [PubMed] [Google Scholar]
- 39.Hess C, Neri D. Tumor-targeting properties of novel immunocytokines based on murine IL1beta and IL6. Protein Eng Des Sel. 2014;27:207–213. doi: 10.1093/protein/gzu013. [DOI] [PubMed] [Google Scholar]
- 40.Gafner V, et al. An engineered antibody-interleukin-12 fusion protein with enhanced tumor vascular targeting properties. Int J Cancer. 2006;119:2205–2212. doi: 10.1002/ijc.22101. [DOI] [PubMed] [Google Scholar]
- 41.Halin C, et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20:264–269. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- 42.Pasche N, et al. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin Cancer Res. 2012;18:4092–4103. doi: 10.1158/1078-0432.CCR-12-0282. [DOI] [PubMed] [Google Scholar]
- 43.Sommavilla R, et al. Expression, engineering and characterization of the tumor-targeting heterodimeric immunocytokine F8-IL12. Protein Eng Des Sel. 2010;23:653–661. doi: 10.1093/protein/gzq038. [DOI] [PubMed] [Google Scholar]
- 44.Gillies SD. A new platform for constructing antibody–cytokine fusion proteins (immunocytokines) with improved biological properties and adaptable cytokine activity. Protein Eng Des Sel. 2013;26:561–569. doi: 10.1093/protein/gzt045. [DOI] [PubMed] [Google Scholar]
- 45.Borgia B, et al. A proteomic approach for the identification of vascular markers of liver metastasis. Cancer Res. 2010;70:309–318. doi: 10.1158/0008-5472.CAN-09-2939. [DOI] [PubMed] [Google Scholar]
- 46.Rybak JN, et al. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 2007;67:10948–10957. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 47.Strassberger V, et al. Chemical proteomic and bioinformatic strategies for the identification and quantification of vascular antigens in cancer. J Proteomics. 2010;73:1954–1973. doi: 10.1016/j.jprot.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Hristodorov D, et al. Macrophage-targeted therapy: CD64-based immunotoxins for treatment of chronic inflammatory diseases. Toxins. 2012;4:676–694. doi: 10.3390/toxins4090676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terry SY, et al. In-anti-F4/80-A3-1 antibody: a novel tracer to image macrophages. Eur J Nucl Med Mol Imaging. 2015;42:1430–1438. doi: 10.1007/s00259-015-3084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamann J, et al. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol. 2007;37:2797–2802. doi: 10.1002/eji.200737553. [DOI] [PubMed] [Google Scholar]
- 51.Legrand F, et al. The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J Allergy Clin Immunol. 2014;133:1439–1447. doi: 10.1016/j.jaci.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hughes C, et al. Human single-chain variable fragment that specifically targets arthritic cartilage. Arthritis Rheum. 2010;62:1007–1016. doi: 10.1002/art.27346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laverman P, et al. Immuno-PET and immuno-SPECT of rheumatoid arthritis with radiolabeled anti-fibroblast activation protein antibody correlates with severity of arthritis. J Nucl Med. 2015;56:778–783. doi: 10.2967/jnumed.114.152959. [DOI] [PubMed] [Google Scholar]
- 54.Fischer E, et al. Radioimmunotherapy of fibroblast activation protein positive tumors by rapidly internalizing antibodies. Clin Cancer Res. 2012;18:6208–6218. doi: 10.1158/1078-0432.CCR-12-0644. [DOI] [PubMed] [Google Scholar]
- 55.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 56.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5:436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 57.Bootz F, et al. Alternatively spliced EDA domain of fibronectin is a target for pharmacodelivery applications in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1908–1917. doi: 10.1097/MIB.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 58.Gutbrodt KL, et al. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med. 2013;5:201ra118. doi: 10.1126/scitranslmed.3006221. [DOI] [PubMed] [Google Scholar]
- 59.Hemmerle T, et al. Antibody-mediated delivery of interleukin 4 to the neovasculature reduces chronic skin inflammation. J Dermatol Sci. 2014;76:96–103. doi: 10.1016/j.jdermsci.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Pedretti M, et al. Comparative immunohistochemical staining of atherosclerotic plaques using F16, F8 and L19: three clinical-grade fully human antibodies. Atherosclerosis. 2010;208:382–389. doi: 10.1016/j.atherosclerosis.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 61.Schwager K, et al. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum Reprod. 2011;26:2344–2352. doi: 10.1093/humrep/der195. [DOI] [PubMed] [Google Scholar]
- 62.Schwager K, et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther. 2009;11:R142. doi: 10.1186/ar2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trachsel E, et al. A human mAb specific to oncofetal fibronectin selectively targets chronic skin inflammation in vivo. J Invest Dermatol. 2007;127:881–886. doi: 10.1038/sj.jid.5700653. [DOI] [PubMed] [Google Scholar]
- 64.Villa A, et al. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neovasculature in vivo. Int J Cancer. 2008;122:2405–2413. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- 65.Trachsel E, et al. Antibody-mediated delivery of IL-10 inhibits the progression of established collagen-induced arthritis. Arthritis Res Ther. 2007;9:R9. doi: 10.1186/ar2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doll F, et al. Murine analogues of etanercept and of F8-IL10 inhibit the progression of collagen-induced arthritis in the mouse. Arthritis Res Ther. 2013;15:R138. doi: 10.1186/ar4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hemmerle T. Antibody-based delivery of IL4 to the neovasculature cures mice with arthritis. Proc Natl Acad Sci U S A. 2014;111:12008–12012. doi: 10.1073/pnas.1402783111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duerner LJ, et al., inventors. Roche Glycart Ag. WO2014023673 A1. Interleukin-10 Fusion Proteins and Uses Thereof. 2014
- 69.Hughes C, et al. Targeting of viral interleukin-10 with an antibody fragment specific to damaged arthritic cartilage improves its therapeutic potency. Arthritis Res Ther. 2014;16:R151. doi: 10.1186/ar4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quattrone F, et al. The targeted delivery of interleukin 4 inhibits development of endometriotic lesions in a mouse model. Reprod Sci. 2015;22:1143–1152. doi: 10.1177/1933719115578930. [DOI] [PubMed] [Google Scholar]
- 71.Wythe SE, et al. Targeted delivery of cytokine therapy to rheumatoid tissue by a synovial targeting peptide. Ann Rheum Dis. 2013;72:129–135. doi: 10.1136/annrheumdis-2012-201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandenburg S, et al. IL-2 induces in vivo suppression by CD4(+)CD25(+)Foxp3(+) regulatory T cells. Eur J Immunol. 2008;38:1643–1653. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 73.Dietrich T, et al. Local delivery of IL-2 reduces atherosclerosis via expansion of regulatory T cells. Atherosclerosis. 2012;220:329–336. doi: 10.1016/j.atherosclerosis.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 74.Gillessen S, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 75.Gately MK, et al. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann N Y Acad Sci. 1996;795:1–12. doi: 10.1111/j.1749-6632.1996.tb52650.x. [DOI] [PubMed] [Google Scholar]
- 76.Shimozato O, et al. The secreted form of the p40 subunit of interleukin (IL)-12 inhibits IL-23 functions and abrogates IL-23-mediated antitumour effects. Immunology. 2006;117:22–28. doi: 10.1111/j.1365-2567.2005.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer. 2014;134:467–477. doi: 10.1002/ijc.28359. [DOI] [PubMed] [Google Scholar]
- 78.Frey K, et al. Antibody-based targeting of interferon-alpha to the tumor neovasculature: a critical evaluation. Integr Biol. 2011;3:468–478. doi: 10.1039/c0ib00099j. [DOI] [PubMed] [Google Scholar]
- 79.Hemmerle T, Neri D. The dose-dependent tumor targeting of antibody-IFNgamma fusion proteins reveals an unexpected receptor-trapping mechanism in vivo. Cancer Immunol Res. 2014;2:559–567. doi: 10.1158/2326-6066.CIR-13-0182. [DOI] [PubMed] [Google Scholar]
- 80.Ebbinghaus C, et al. Engineered vascular-targeting antibody-interferon-gamma fusion protein for cancer therapy. Int J Cancer. 2005;116:304–313. doi: 10.1002/ijc.20952. [DOI] [PubMed] [Google Scholar]
- 81.Halin C, et al. Tumor-targeting properties of antibody-vascular endothelial growth factor fusion proteins. Int J Cancer. 2002;102:109–116. doi: 10.1002/ijc.10674. [DOI] [PubMed] [Google Scholar]
- 82.Melkko S, et al. An antibody-calmodulin fusion protein reveals a functional dependence between macromolecular isoelectric point and tumor targeting performance. Int J Radiat Oncol Biol Phys. 2002;54:1485–1490. doi: 10.1016/s0360-3016(02)03927-5. [DOI] [PubMed] [Google Scholar]
- 83.Niesner U, et al. Quantitation of the tumor-targeting properties of antibody fragments conjugated to cell-permeating HIV-1 TAT peptides. Bioconjug Chem. 2002;13:729–736. doi: 10.1021/bc025517+. [DOI] [PubMed] [Google Scholar]
- 84.Halin C, et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003;63:3202–3210. [PubMed] [Google Scholar]
- 85.Hemmerle T, et al. A critical evaluation of the tumor-targeting properties of bispecific antibodies based on quantitative biodistribution data. Protein Eng Des Sel. 2012;25:851–854. doi: 10.1093/protein/gzs061. [DOI] [PubMed] [Google Scholar]
- 86.Venetz D, et al. Glycosylation profiles determine extravasation and disease-targeting properties of armed antibodies. Proc Natl Acad Sci U S A. 2015;112:2000–2005. doi: 10.1073/pnas.1416694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galeazzi M, et al. A Phase IB clinical trial in rheumatoid arthritis of DEKAVIL (F8-IL10), a novel anti-inflammatory immunocytokine. Ann Rheum Dis. 2014;73(Suppl. 2):675–676. [Google Scholar]
- 88.Galeazzi M, et al. DEKAVIL (F8-IL10), a novel therapeutic approach for rheumatoid arthritis: ongoing phase Ib clinical trial results. Ann Rheum Dis. 2015;74(Suppl. 2):726. [Google Scholar]
- 89.Balza E, et al. Therapy-induced antitumor vaccination in neuroblastomas by the combined targeting of IL-2 and TNFalpha. Int J Cancer. 2010;127:101–110. doi: 10.1002/ijc.25018. [DOI] [PubMed] [Google Scholar]
- 90.Gillies SD, et al. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother. 2002;51:449–460. doi: 10.1007/s00262-002-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hess C, Neri D. The antibody-mediated targeted delivery of interleukin-13 to syngeneic murine tumors mediates a potent anticancer activity. Cancer Immunol Immunother. 2015;64:635–644. doi: 10.1007/s00262-015-1666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heuveling DA, et al. Phase 0 microdosing PET study using the human mini antibody F16SIP in head and neck cancer patients. J Nucl Med. 2013;54:397–401. doi: 10.2967/jnumed.112.111310. [DOI] [PubMed] [Google Scholar]
- 93.Hilderbrand SA, Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14:71–79. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 94.Werner SG, et al. Inflammation assessment in patients with arthritis using a novel in vivo fluorescence optical imaging technology. Ann Rheum Dis. 2012;71:504–510. doi: 10.1136/annrheumdis-2010-148288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bissonnette R, et al. Efficacy and safety of adalimumab in patients with plaque psoriasis who have shown an unsatisfactory response to etanercept. J Am Acad Dermatol. 2010;63:228–234. doi: 10.1016/j.jaad.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 96.Breedveld FC, et al. The PREMIER study – a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 97.Papadakis KA. Adalimumab for the treatment of Crohn’s disease. Expert Rev Clin Immunol. 2006;2:11–15. doi: 10.1586/1744666X.2.1.11. [DOI] [PubMed] [Google Scholar]
- 98.van der Heijde D, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–2146. doi: 10.1002/art.21913. [DOI] [PubMed] [Google Scholar]
- 99.Danese S. Adalimumab for ulcerative colitis: a little is better than none? Inflamm Bowel Dis. 2012;18:793–794. doi: 10.1002/ibd.21773. [DOI] [PubMed] [Google Scholar]
- 100.Burgos-Vargas R. Juvenile onset spondyloarthropathies: therapeutic aspects. Ann Rheum Dis. 2002;61:iii33–iii39. doi: 10.1136/ard.61.suppl_3.iii33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chan JJ, Gebauer K. Treatment of severe recalcitrant plaque psoriasis with single-dose intravenous tumour necrosis factor-alpha antibody (infliximab) Australas J Dermatol. 2003;44:116–120. doi: 10.1046/j.1440-0960.2003.00656.x. [DOI] [PubMed] [Google Scholar]
- 102.Maini RN, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 103.Rossetti S, et al. The use of the anti-tumour necrosis factor monoclonal antibody-infliximab-to treat ulcerative colitis: implications and trends beyond the available data. Dig Liver Dis. 2004;36:426–431. doi: 10.1016/j.dld.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 104.van Dullemen HM, et al. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 105.Chimenti MS, et al. Profile of certolizumab and its potential in the treatment of psoriatic arthritis. Drug Des Dev Ther. 2013;7:339–348. doi: 10.2147/DDDT.S31658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Keystone E, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, Phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–3329. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 107.Landewe R, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rutgeerts P, et al. Certolizumab pegol, a monthly subcutaneously administered Fc-free anti-TNFalpha, improves health-related quality of life in patients with moderate to severe Crohn’s disease. Int J Colorectal Dis. 2008;23:289–296. doi: 10.1007/s00384-007-0395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oldfield V, Plosker GL. Golimumab: in the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. BioDrugs. 2009;23:125–135. doi: 10.2165/00063030-200923020-00005. [DOI] [PubMed] [Google Scholar]
- 110.Sandborn WJ, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 111.Kone-Paut I, et al. Sustained remission of symptoms and improved health-related quality of life in patients with cryopyrin-associated periodic syndrome treated with canakinumab: results of a double-blind placebo-controlled randomized withdrawal study. Arthritis Res Ther. 2011;13:R202. doi: 10.1186/ar3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schlesinger N, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis. 2012;71:1839–1848. doi: 10.1136/annrheumdis-2011-200908. [DOI] [PubMed] [Google Scholar]
- 113.Vincenti F, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med. 1998;338:161–165. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 114.Leonardi CL, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 115.Gisondi PC, et al. Efficacy and safety of secukinumab in chronic plaque psoriasis and psoriatic arthritis therapy. Dermatol Ther. 2014;4:1–9. doi: 10.1007/s13555-014-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Okuda Y. Review of tocilizumab in the treatment of rheumatoid arthritis. Biologics. 2008;2:75–82. doi: 10.2147/btt.s1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Rhee F, et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15:966–974. doi: 10.1016/S1470-2045(14)70319-5. [DOI] [PubMed] [Google Scholar]
- 118.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 119.West WH, et al. Multiple cycles of constant infusion recombinant interleukin-2 in adoptive cellular therapy of metastatic renal carcinoma. Mol Biother. 1989;1:268–274. [PubMed] [Google Scholar]
- 120.Isaacs C, et al. Randomized placebo-controlled study of recombinant human interleukin-11 to prevent chemotherapy-induced thrombocytopenia in patients with breast cancer receiving dose-intensive cyclophosphamide and doxorubicin. J Clin Oncol. 1997;15:3368–3377. doi: 10.1200/JCO.1997.15.11.3368. [DOI] [PubMed] [Google Scholar]
- 121.Baumgarten R, et al. Treatment of chronic hepatitis B with interferon alpha-2b. Gastroenterol J. 1990;50:124–128. [PubMed] [Google Scholar]
- 122.von Wussow P, et al. Intralesional interferon-alpha therapy in advanced malignant melanoma. Cancer. 1988;61:1071–1074. doi: 10.1002/1097-0142(19880315)61:6<1071::aid-cncr2820610603>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 123.Golomb HM, et al. Sequential evaluation of alpha-2b-interferon treatment in 128 patients with hairy cell leukemia. Semin Oncol. 1987;14:13–17. [PubMed] [Google Scholar]
- 124.Gill PS. Phase I/Ii trials of alpha-interferon alone or in combination with zidovudine as maintenance therapy following induction chemotherapy in the treatment of acquired immunodeficiency syndrome-related Kaposis-sarcoma. Semin Oncol. 1991;18:53–57. [PubMed] [Google Scholar]
- 125.Bottomley A, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma: a phase III randomized controlled trial of health-related quality of life and symptoms by the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol. 2009;27:2916–2923. doi: 10.1200/JCO.2008.20.2069. [DOI] [PubMed] [Google Scholar]
- 126.Reddy KR, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis. C Hepatology. 2001;33:433–438. doi: 10.1053/jhep.2001.21747. [DOI] [PubMed] [Google Scholar]
- 127.Eggermont AM, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–3818. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 128.Paty DW, et al. Interferon beta-1b is effective in relapsing-remitting multiple-sclerosis. 2. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- 129.Jacobs LD, et al. A phase III trial of intramuscular recombinant interferon beta as treatment for exacerbating-remitting multiple sclerosis: design and conduct of study and baseline characteristics of patients. Multiple Sclerosis Collaborative Research Group (MSCRG) Mult Scler. 2015;1:118–135. doi: 10.1177/135245859500100210. [DOI] [PubMed] [Google Scholar]
- 130.Khan UT, et al. PEGylated IFNbeta-1a in the treatment of multiple sclerosis. Expert Opin Biol Ther. 2015;15:1077–1084. doi: 10.1517/14712598.2015.1053206. [DOI] [PubMed] [Google Scholar]
- 131.Gallin JI. Interferon-gamma in the management of chronic granulomatous disease. Rev Infect Dis. 1991;13:973–978. doi: 10.1093/clinids/13.5.973. [DOI] [PubMed] [Google Scholar]
- 132.Sheridan WP, et al. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339:640–644. doi: 10.1016/0140-6736(92)90795-5. [DOI] [PubMed] [Google Scholar]
- 133.Gradishar WJ, et al. Clinical and cytogenetic responses to granulocyte-macrophage colony-stimulating factor in therapy-related myelodysplasia. Blood. 1992;80:2463–2470. [PubMed] [Google Scholar]
- 134.Eggermont AM, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg. 1996;224:756–764. doi: 10.1097/00000658-199612000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Franz M, et al. Targeted delivery of interleukin-10 to chronic cardiac allograft rejection using a human antibody specific to the extra domain A of fibronectin. Int J Cardiol. 2015;195:311–322. doi: 10.1016/j.ijcard.2015.05.144. [DOI] [PubMed] [Google Scholar]
- 136.Carnemolla B, et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99:1659–1665. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]