Abstract
Objective
While initially seemingly paradoxical due to the lack of nucleus, platelets possess a number of transcription factors that regulate their function through DNA-independent mechanisms. These include the Farnesoid X Receptor (FXR), a member of the superfamily of ligand-activated transcription factors that has been identified as a bile acid receptor. In this study, we show that FXR is present in human platelets and FXR ligands, GW4064 and 6-ECDCA, modulate platelet activation nongenomically.
Approach and Results
FXR ligands inhibited the activation of platelets in response to stimulation of collagen or thrombin receptors, resulting in diminished intracellular calcium mobilization and secretion, fibrinogen binding and aggregation. Exposure to FXR ligands also reduced integrin αIIbβ3 outside-in signaling and thereby reduced the ability of platelets to spread and to stimulate clot retraction. FXR function in platelets was found to be associated with the modulation of cGMP levels in platelets and associated downstream inhibitory signaling. Platelets from FXR-deficient mice were refractory to the actions of FXR agonists on platelet function and cyclic nucleotide signaling, firmly linking the non-genomic actions of these ligands to the FXR receptor.
Conclusion
This study provides support for the ability of FXR ligands to modulate platelet activation. The athero-protective effects of GW4064, with its novel antiplatelet effects, indicate FXR as a potential target for prevention of athero-thrombotic disease.
Keywords: Platelets, Farnesoid X Receptor, nongenomic, transcription factor, signal transduction
Introduction
The farnesoid X receptor/bile acid receptor (FXR; NR1H4) is a member of the nuclear receptor superfamily of ligand-activated transcription factors that binds and acts as heterodimer with retinoid X receptors that have also been found to be expressed in human platelets, and is highly expressed in liver, kidney, adrenal glands, intestine and vascular tissues. 1–2 FXR regulates the expression of genes involved in cholesterol and glucose homeostasis, liver regeneration and gastrointestinal defense. 3–4 FXR has also been shown to have anti-inflammatory and athero-protective effects after ligand stimulation. 5 Endogenous ligands of FXR are bile acids with ligands including chenodeoxycholic acid (CDCA) and deoxycholic acid (DCA). 6 Synthetic FXR ligands have also been identified, such as GW4064 and 6α-ethyl-chenodeoxycholic acid (6-ECDCA). 7–8 FXR regulates the transcription of target genes through the induction of the atypical nuclear receptor small heterodimer partner (SHP), which mediates some of the inhibitory effects of FXR ligands on bile acid and lipid metabolism.9–10
Platelets are anucleate blood cells with a central role in hemostasis but are also involved in inflammation, immunity, tumor progression and thrombosis. 11 Although lacking genomic DNA, platelets contain a diverse transcriptome, which allows signal-dependent protein translation and micro RNA processing.12–14 We and others have previously identified the presence of transcription factors in mammalian platelets, including peroxisome proliferator-activated receptors (PPARs) 15–16, retinoid X receptor (RXR) 17, glucocorticoid receptor (GR) 18, Liver X Receptor (LXR) 19, and the nuclear factor-κB (NF-κB). 20 Previous reports have demonstrated that FXR activation appears to protect against atherosclerotic plaque formation, 5, 21 but these effects are more pronounced than expected, based on its lipid-lowering actions alone. 22 We therefore hypothesized that the athero-protective effects of FXR ligands may be mediated in part through potential modulation of platelet function. In this report, we demonstrate that FXR is present in platelets and that FXR ligands inhibit a range of platelet functions and thrombus formation, suggesting a potential new target for prevention of atherosclerosis and thrombosis based on acute DNA-independent actions of this receptor in platelets.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Presence of FXR in Platelets
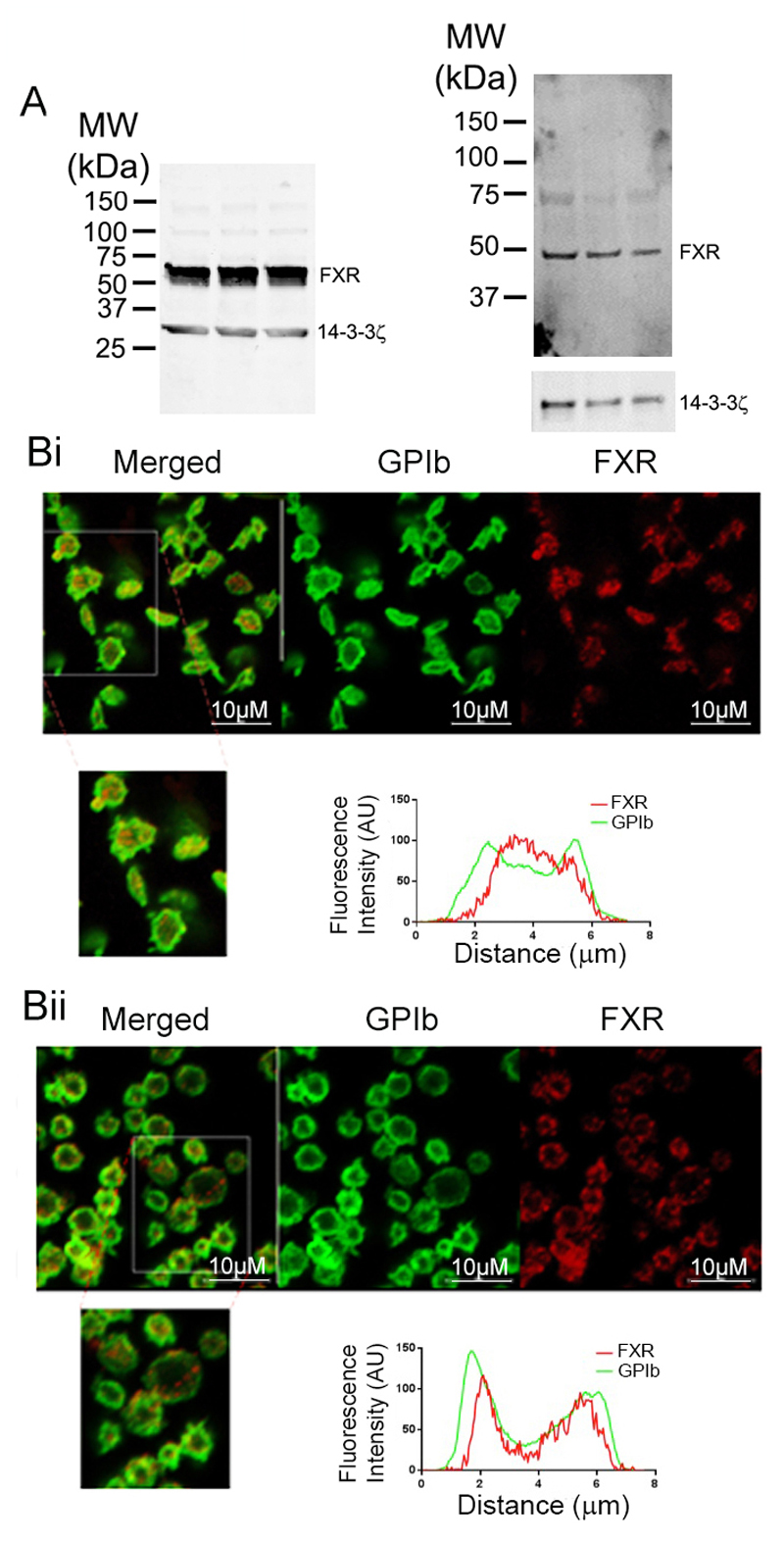
FXR protein expression was investigated in human and mouse platelets. Immunoblot analysis of cell lysates confirmed the presence of FXR in human and mouse platelets (Fig 1 A). The localization of FXR in human platelets was analyzed by immunofluorescence microscopy. In resting platelets FXR was found to be dispersed throughout the platelet cytoplasm in a punctate arrangement (Fig 1 Bi), while in response to U46619, a thromboxane A2 receptor (TP) agonist, the localization of FXR appeared to partially translocate towards the plasma membrane (Fig 1 Bii).
Figure 1.
FXR is expressed in human and mouse platelets. (A) Whole human and mouse platelet lysates, samples from 3 separate donors or mice, were immunoblotted (IB) to detect FXR. The localization of FXR in human platelets was analysed by immunofluorescence confocal microscopy. (B) Unstimulated (i) and stimulated (using U46619 (20µM) in the presence of Integrilin (4µM)) platelets (ii) in PRP were fixed in 4% (v/v) paraformaldehyde-PBS. Platelets were permeablised (in the presence of 0.2% triton X-100, donkey serum and 1% PBS-BSA) and stained for FXR (in red) and GPIb (in green). Visualisation of cells was performed using a Nikon A1-R confocal microscope and 100x oil immersion lens. In resting and activated platelets, FXR was found to be dispersed throughout the platelet volume in a punctate arrangement. Line plot analysis was performed across resting and stimulated platelets that also indicated partial re-localisation of FXR towards the plasma membrane following activation. The data for line plots are representative of >7 cells (representative data shown).
FXR ligands inhibit platelet aggregation and secretion
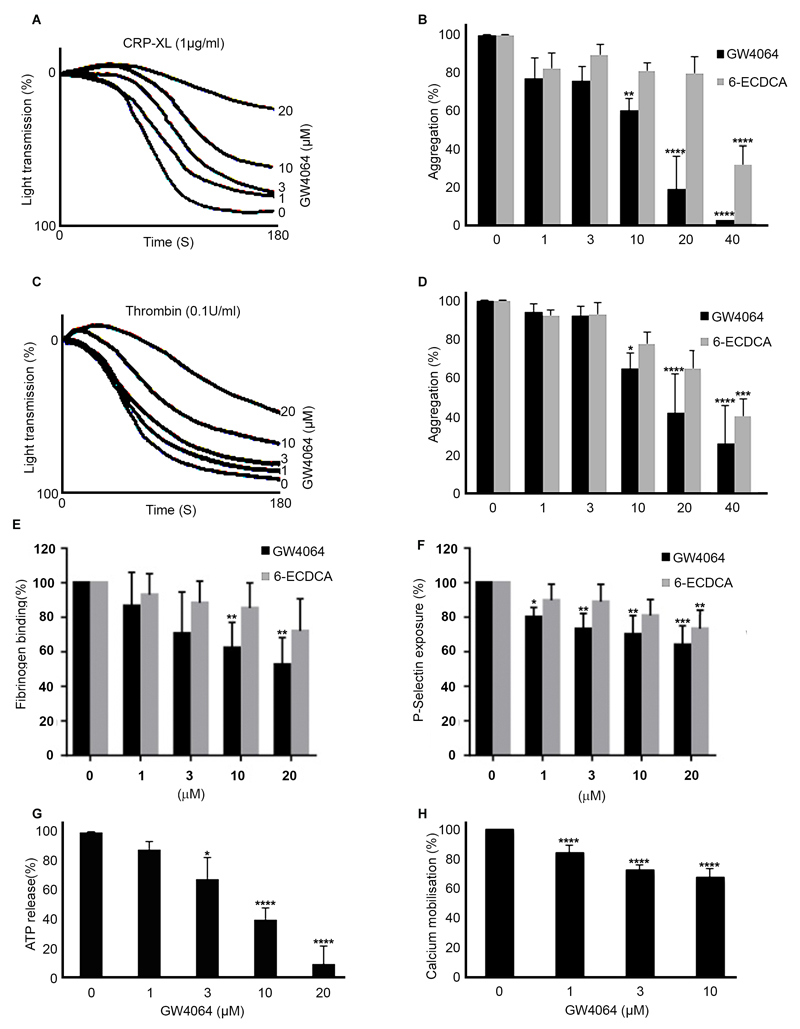
The effect of FXR-selective ligands GW4064 and 6-ECDCA on the aggregation of human washed platelets in response to activators of platelet function was explored. Platelet aggregation in response to CRP-XL (1 μg mL-1), a glycoprotein VI (GPVI)-collagen receptor-selective ligand, was found to be inhibited in a concentration-dependent manner by GW4064 (Fig 2 A-B). Inhibition of 22%, 40%, and 80% was observed with GW4064 (1, 10 and 20 μM), which was more potent than 6-ECDCA in inhibiting platelet aggregation. An increase in light transmission was observed on treatment with 10 and 20µM GW4064 that may be associated with platelet swelling. Consistent with inhibition of GPVI-mediated responses platelet aggregation in response to Collagen (0.5 µg mL-1) was also found to be inhibited in a concentration dependent-manner by GW4064. In contrast, high concentrations of the natural FXR ligand CDCA, was required to produce an inhibitory effect on collagen-stimulated platelets (supplemental Fig 1). Differences in the potencies of GW4064, 6-ECDCA and CDCA in inhibiting platelet aggregation were in line with the differences in potency reported of these agonists in other cell systems. 8, 23 To determine whether the target for FXR ligands is shared with other agonists, thrombin, that activates platelets via G-protein-coupled receptors was tested, and used at a concentration that was optimized to ensure a similar level of aggregation to that stimulated by CRP-XL. Lower levels of inhibition were noted with thrombin-induced platelet aggregation (0.1 U mL-1) following treatment with GW4064 and 6-ECDCA, although overall the inhibition profiles were similar. Inhibition of 5%, 35% and 55% was observed with GW4064 (1, 10 and 20 μM), (Fig 2 C, D). Aggregation monitored over an extended period of 5 min duration confirmed this effect to be inhibition rather than delay in aggregation (data not shown).
Figure 2.
FXR ligands inhibit platelet activation. Washed human platelets were treated for 5 min with increasing concentrations of FXR ligands, GW4064, 6-ECDCA or vehicle containing (DMSO (0.1% (v/v)), prior to stimulation for 180s with collagen-related peptide CRP-XL (1 μg mL-1) or Thrombin (0.1 U/mL) and aggregation measured at 37 °C under constant stirring conditions (A-D). The effect of GW4064 and 6-ECDCA on fibrinogen binding and P-selectin exposure prior to stimulation with CRP-XL (1 μg mL-1) were measured in human whole blood by Flow cytometry (E-F). Changes in ATP concentration released by washed platelets stimulatied for 180s with CRP-XL (1 μg mL-1) were used as a measure of dense-granule secretion and monitored simultaneously with aggregation in an optical lumi-aggregometer using a luciferase detection system (G). Calcium mobilization was assessed in Fura-2AM-loaded PRP pre-incubated with increasing concentration of GW4064 in the presence of EGTA to prevent influx of extracellular calcium and then stimulated with CRP-XL (1 μg mL-1) (H). Numerical data represent the percentage compared with control, mean ± SD (n=4).*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P≤0.001, (ANOVA with Bonferroni post test).
Platelet aggregation is dependent on conformational changes of integrin αIIbβ3 through inside-out signaling that results in an increase in its affinity for fibrinogen. 24 Thus, flow cytometry was used to measure fibrinogen binding to platelets, as a marker for activation of the integrin αIIbβ3. CRP-XL-stimulated fibrinogen binding was reduced in the presence of GW4064 (Fig 2 E), consistent with reduced aggregation and indicated the ability of the GW4064 to modulate inside-outside signaling to integrin αIIbβ3 in platelets. The effects of 6-ECDCA were insufficiently potent to elicit a statistically significant reduction in fibrinogen binding. Thrombus generation is supported and enhanced by the release of a number of substances from platelet alpha and dense granules, which are critical for the recruitment of additional platelets and for stabilization of the aggregate. 24–25 To analyze the effects of FXR agonist treatment on platelet granule secretion, α- and dense-granule secretion were assayed in the absence and presence of GW4064 or vehicle (containing DMSO (0.1% (v/v)). α-granule secretion was assessed by measuring the levels of P-selectin exposed on the surface of platelets after stimulation with CRP-XL (1 μg mL-1) by flow cytometry in human whole blood. GW4064 caused a concentration dependent inhibition of P-selectin exposure (Fig 2 F) reaching approximately 35% inhibition at a concentration of 20µM. Consistent with less potent effects on aggregation, 6-ECDCA exhibited only modest effects on P-selectin exposure, reaching statistical significance at a concentration of 20µM.
To investigate the role of FXR in dense granule secretion, ATP release was measured simultaneously with aggregation on washed platelet preparations using a luciferin-luciferase luminescence assay. GW4064 was found to reduce ATP secretion after CRP-XL stimulation (Fig 2 G). Cytosolic mobilization of calcium plays a fundamental role in various aspects of platelet function, including reorganization of the actin cytoskeleton necessary for shape change, degranulation and integrin αIIbβ3 affinity modulation. 26 We therefore examined the ability of FXR ligands to modulate intracellular mobilization of calcium. Fura-2AM-loaded PRP was pre-incubated with GW4064 (1-10 μM) or control (containing DMSO (0.1% (v/v)) for 5 min and then stimulated with CRP-XL (1 μg mL-1). Treatment with GW4064 was associated with a modest (in comparison with ATP secretion and other assays of function using washed platelets) inhibition of CRP-XL-stimulated peak calcium concentration (Fig 2 H).
The actions of GW4064 on platelets are mediated through FXR
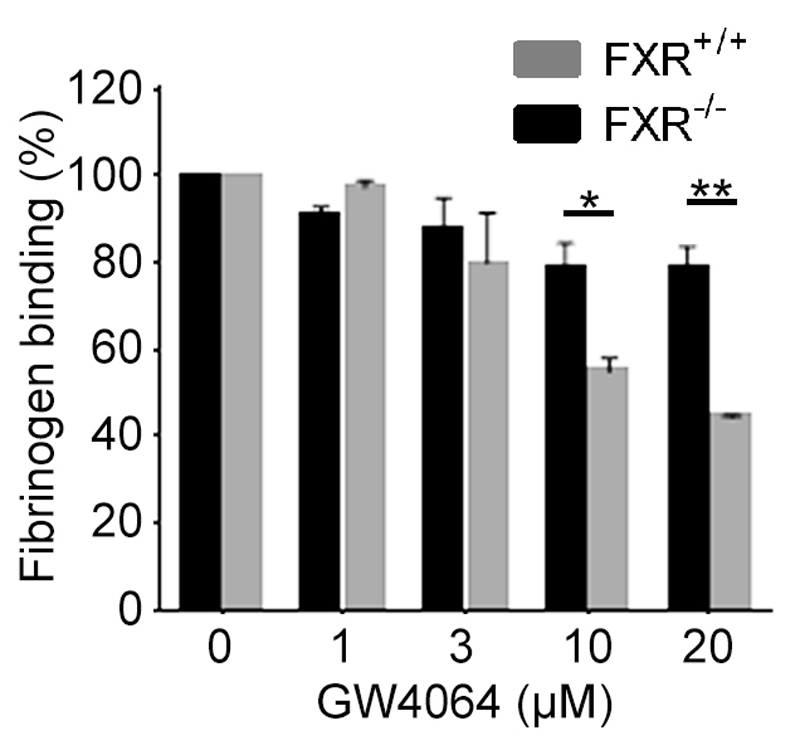
To confirm whether FXR is required for the inhibitory effect of FXR ligands on platelet function, the ability of GW4064 to inhibit platelet function in mice deficient in FXR was explored. We confirmed that the levels of integrin αIIbβ3, integrin α2β1, GPVI and GPIbα on the surface of FXR-/- platelets were similar to those from FXR+/+ mice (Supplemental data Fig 2). GW4064 (1-20 µM) treatment inhibited fibrinogen binding to FXR+/+ platelets upon stimulation with CRP-XL reaching approximately 50% inhibition at 20µM. This dramatic inhibition was not observed in FXR-/- platelets, although higher concentrations of GW4064 did cause a modest reduction in fibrinogen binding (Fig 3). These data confirm that the principal mode of action of FXR agonists on platelet function are mediated through binding to FXR
Figure 3.
The actions of GW4064 on platelets are mediated through FXR. Blood from FXR+/+ and FXR-/- mice was treated with GW4064 or vehicle (containing DMSO 0.1% (v/v)) for 20 min prior stimulation with CRP-XL (1 μg mL-1) and fibrinogen binding measured by flow cytometry. Data (median fluorescence intensity, expressed as a percentage of fibrinogen binding in the absence of GW4064) represent FXR-/- mice platelets compared with FXR+/+ mice platelets (control), mean ± SD (n=4), *P ≤ 0.05, **P ≤0.01 (t-test).
FXR ligand, GW4064 affects integrin αIIbβ3 mediated outside-in signalling
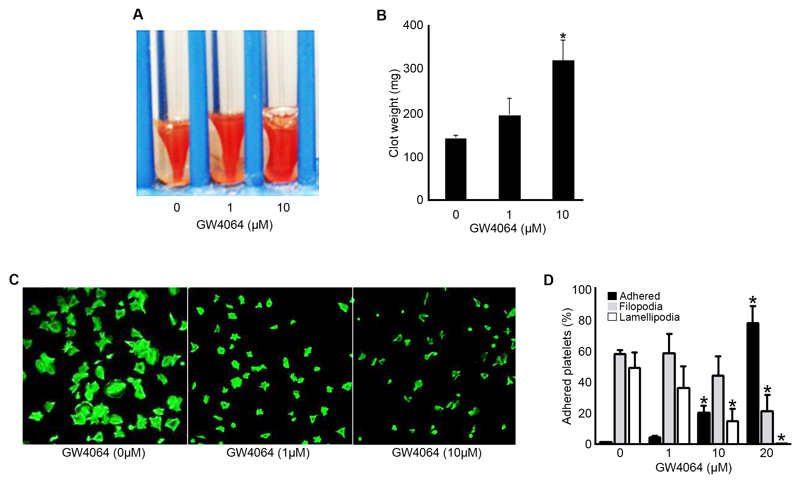
Following binding to fibrinogen, integrin αIIbβ3 clustering transduces signals (outside-in signalling) into platelets to allow spreading and in the latter phase of its formation, clot retraction. 27 The modulatory effects of GW4064 on outside-in integrin signalling through αIIbβ3 were assessed by the measurement of clot retraction and platelet spreading under static conditions. Clot formation was initiated by adding thrombin to platelet-rich plasma in the absence or presence of GW4064 (1, 10 μM), and the extent of clot retraction was monitored after 2 hours by measuring clot weight. Clot retraction was reduced in the presence of GW4064 (10 μM) at 2 hours (indicated by increased clot weight) compared with vehicle-treated samples (Fig 4 A-B). Consistent with this, GW4064-treated (10 μM) platelets were unable to adhere and spread on fibrinogen to the same extent as control platelets at 45 min. Most GW4064-treated platelets failed to progress beyond filopodia formation with only a few cells progressing to lamellapodia formation and full spreading (Fig 4 C-D). These data suggest that outside-in signalling through αIIbβ3, which controls the coordinated process of clot retraction, is also modulated by GW4064.
Figure 4.
GW4064 inhibits integrin αIIbβ3-mediated outside-in signalling. Effect of GW4064 on clot retraction was analyzed in vitro (A-B). Representative images of clot retraction at 2h in the presence and absence of GW4064 (1, 10 μM), (A). GW4064 affects spreading in a concentration-dependent manner. Washed human platelets were allowed to spread for 45 min in the presence and absence of GW4064 (1 – 20 μM) on 100 μg mL-1 fibrinogen-coated cover glasses and stained with Alexa fluor 488 labelled phalloidin prior to analysis by confocal microscopy (C). The images were analysed and the number of platelets found at different stages of platelet spreading were calculated (D). Numerical data represent the percentage compared with control, mean ± S.E.M. (n=4). *P ≤ 0.05, ANOVA with Bonferroni post test.
GW4064 inhibits Thrombus Formation and hemostasis
The integrin αIIbβ3 is critical for arterial thrombosis and hemostasis. Following platelet activation, the αIIbβ3 complex undergoes a conformational change that allows the adhesive protein fibrinogen to bind, forming a bridge between platelets that mediates platelet-platelet interactions and thrombus formation. 28–29
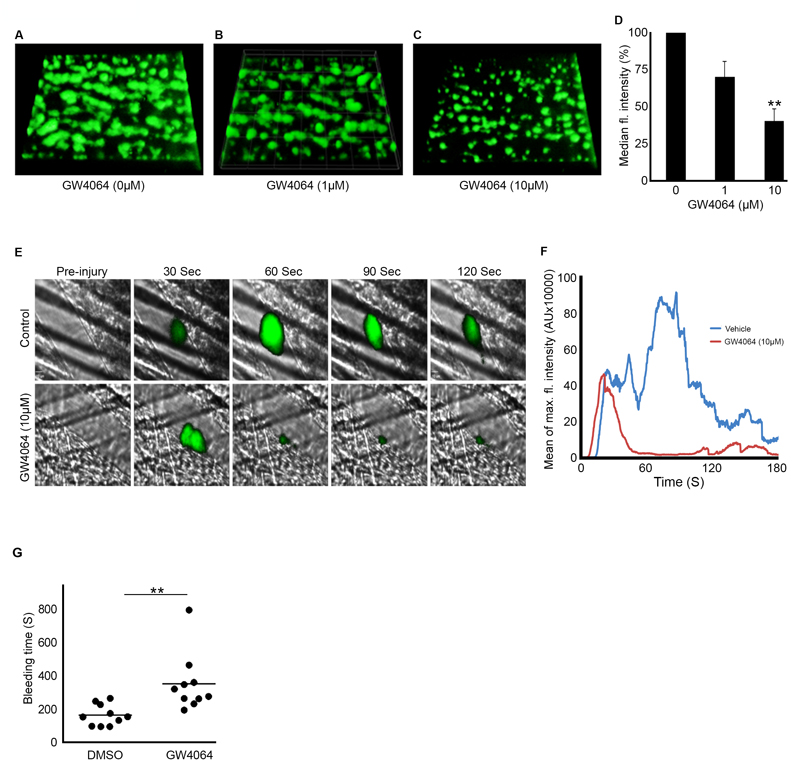
Given the ability of FXR ligands to regulate platelet function, we sought to determine the potential implications of GW4064 on thrombus formation. Analysis of thrombus formation in vitro was performed by fluorescence microscopy using DIOC6-labelled whole blood perfused under arterial flow conditions through Vena8 biochips coated with collagen, following pre-incubation with GW4064 (1,10 μM) or vehicle (containing DMSO (0.1% (v/v)) for 5 min. Blood was perfused for 10 min, after which thrombus development was assessed by measurement of fluorescence intensity. In comparison with control samples (Fig 5 A), 10 μM GW4064 inhibited the thrombus fluorescence intensity by 65% (Fig 5 C-D). These data suggest that GW4064 is able to modulate thrombus formation under arterial flow conditions in whole blood. To determine whether the effects of GW4064 on thrombus formation in vitro were due to inhibition of initial entrapment of platelets on collagen, or due to the inhibition of platelet aggregation and therefore thrombus growth, perfusion of blood was also performed in the presence and absence of the αIIbβ3 antagonist integrilin (4µM). In the presence of integrilin, GW4064 (10 μM) did not affect platelet adhesion to collagen suggesting that the FXR agonist does not modulate GPIb-dependent adhesion under flow (supplemental Fig 3). These data indicate that the inhibition of thrombus formation by GW4064 is likely due to its ability reduce platelet activation following adhesion.
Figure 5.
GW4064 inhibits thrombus formation and hemostasis. Human whole blood labelled with the (DiOC6)-lipophilic dye 3,3-dihexyloxacarbocyanine iodide was treated with vehicle (containing DMSO (0.1% (v/v)) (A) or GW4064 (1, 10 μM) (B-C) for 5 min and perfused through collagen-coated (400 μg mL-1) Vena8Biochips at a shear rate of 20 dyn/cm2. Thrombi were recorded at 10 min period through a series of images in the Z-plane through their full depth using a Nikon eclipse (TE2000-U) microscope, and thrombus fluorescence intensity was calculated using the Slidebook, version 5. The fluorescence intensity of thrombi obtained in the absence of GW4064 was taken as 100% (D). Cumulative data represent mean±SD (n=6), P * ≤ 0.05, **P ≤0.01 (t-test). In vivo thrombosis was assayed using a laser injury model by intravital microscopy. GW4064-estimated concentration (10 μM) or vehicle (containing DMSO (0.1% (v/v)) was administered intravenously to mice, and platelets were fluorescently labeled by injection of Alexa 488-conjugated anti-GPIb antibody. After laser-induced injury of the cremaster muscle arterioles, accumulation of platelets was assessed. Representative images of thrombi obtained from mice treated with or without GW4064 at different time intervals are shown (E). Mean fluorescence intensity was measured from control and GW4064-treated mice (n≥16 thrombi from 4 GW4064-treated and 4 control mice) (F). The effect of GW4064 on hemostasis of mice was analyzed by measuring the bleeding time after tail tip excision. The bleeding time obtained with vehicle-treated group was compared with GW4064-treated mice (G). Data represent mean±SD (n=10 for each vehicle and mice-treated group), Statistical analysis was performed using the nonparametric Mann-Whitney test (p=0.004).
To determine the potential impact of FXR on the acute regulation of platelet function in vivo, the effect of GW4064 on laser-induced thrombosis in mouse cremaster muscle arterioles was assessed. The effect of GW4064 infused intravenously prior to thrombus formation was explored and compared with thrombosis in vehicle-treated mice. Data analysis was performed for multiple thrombi formed in control and mice treated with GW4064. Following laser injury, thrombus formation was monitored for 180 seconds. The initiation of thrombus formation was accelerated slightly, although the continued growth or stability of the thrombus were found to be reduced substantially in GW4064-treated mice compared with controls (Fig 5 E-F). Inhibition of thrombus formation in the absence of endothelial cells (in vitro, Figure 5 A-D) suggests that the inhibitory effects of GW4064 on thrombi formed in vivo are likely to be principally due to diminished platelet function, although indirect effects on platelet function mediated by other cells cannot be excluded. Taken together, these data establish a role for FXR ligands in the regulation of thrombosis.
To assess the importance of FXR ligands for hemostasis, tail-bleeding assays were performed. The bleeding time after dissection of 1mm of tail tip was prolonged substantially in GW4064-treated mice (between 195 and 797 seconds) compared with controls (between 96 and 299 seconds) (Fig 5 G). These data are consistent with FXR agonists inhibiting platelet function and thereby suppressing hemostasis.
GW4064 modulates platelet cyclic nucleotide signaling
Due the inhibitory effects of FXR ligands on CRP-XL-induced aggregation, the phosphorylation levels of proteins involved early in the GPVI signaling pathway were assessed by immunoblot analysis. Platelet lysates were prepared after stimulation with CRP-XL (1 μg mL-1) in the presence of GW4064 (1-10 μM) or vehicle (containing DMSO (0.1% (v/v)). The levels of total platelet protein tyrosine phosphorylation were unaffected after GW4064 treatment as were the tyrosine phosphorylation levels of the tyrosine kinase Syk and adapter protein LAT (key early components in the GPVI signalling pathways, Supplementary Fig 4). Since early signaling stimulated by GPVI was unaffected and FXR ligands were found to inhibit the function platelet responses to GPVI agonists and thrombin, we explored whether FXR mediates its actions on platelets through the modulation of cyclic nucleotide signaling, mechanisms that provide powerful inhibition of platelet functional responses to a wide range of platelet activators.
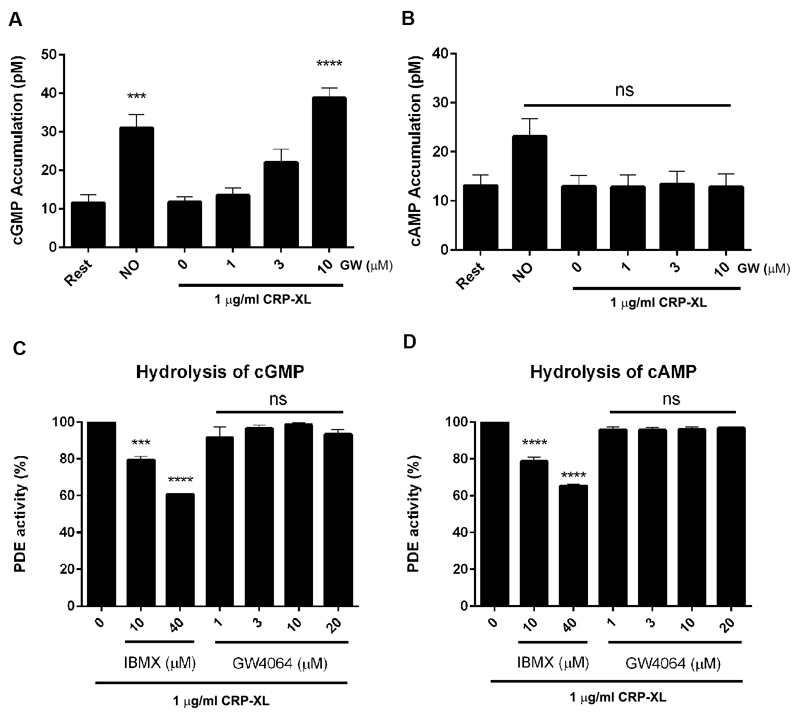
Nitric Oxide (NO) has both antithrombotic and vasodilatory effects. Under normal physiological conditions, the intact endothelium releases NO and prostacyclin to inhibit platelet adhesion and platelet aggregation by elevating the second messenger cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) respectively. 31–32 The levels of cGMP and cAMP in GW4064-treated platelets were therefore measured following stimulation with CRP-XL. We first confirmed that we were able to measure cyclic nucleotide signaling in our experimental system. Consistent with expectations, the levels of cGMP (Fig 6A) were elevated on addition of the NO-donor PAPA nonoate (10 μM). In agreement with a recent report, treatment with the NO donor also resulted in an increase in cAMP levels (Fig 6B). 35 The addition of GW4064 increased cGMP but not cAMP levels over the range of concentrations used in CRP-stimulated platelets (Fig 6A-B). Cyclic nucleotide levels are up regulated by synthesis through adenylyl cyclase and guanylyl cyclase and down regulated by degradation through phosphodiesterases (PDEs). 33 To establish if GW4064 increased cGMP levels were dependent on the modulation of PDEs, PDE activity was measured using cGMP and cAMP as substrates. The phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (IBMX), as expected, was able to inhibit PDE activity by 25% and 40% at concentrations of 10 and 40 μM, respectively. GW4064 (1-20 μM) did not show any inhibitory effects on PDE activity with both cGMP or cAMP (Fig 6 C-D) degradation unaffected by treatment with GW4064. Taken together, these data suggest that inhibition of platelet function by GW4064 is regulated by increased synthesis of cGMP and not through increased hydrolysis of this cyclic nucleotide.
Figure 6.
GW4064 modulates platelet cyclic nucleotide signaling. The levels of cGMP (A) and cAMP (B) were measured in platelets on stimulation with CRP-XL (1 μg/mL) in the presence of GW4064 (1-10 μM) that was found to selectively increase cGMP levels. The effects of GW4064 (1-10 μM) on phosphodiesterase activity were measured on the hydrolysis of cGMP (C) and cAMP (D) to establish whether this corresponded to greater cyclic nucleotide production or hydrolysis. While the phosphodiesterase inhibitor IBMX inhibited cGMP and cAMP hydrolysis, GW4064 was without effect. The level of phosphodiesterase activity obtained in the absence of GW4064 was taken as 100%. Data represent mean±SD (n=3), P *** ≤ 0.005, ***P ≤0.001, (ANOVA with Bonferroni post test).
The inhibitory effect of FXR on cyclic nucleotide signalling in platelets
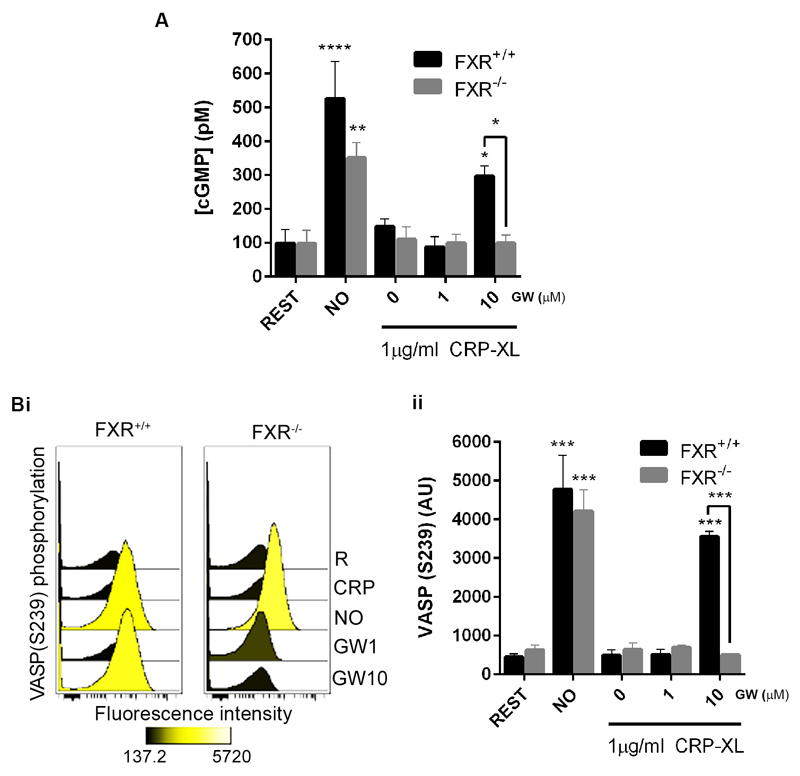
To analyze the potential requirement of FXR for the previously observed ability of GW4064 to modulate platelet cGMP signalling (Fig 6A), the ability of GW4064 to elevate platelet cGMP levels in mice deficient in FXR was explored. The levels of cGMP were increased significantly on addition of the NO-donor PAPA nonoate (10 μM) to both FXR-/- and FXR+/+ platelets. The addition of GW4064 (10 μM) increased cGMP levels in CRP-stimulated FXR+/+, although in FXR-/- platelets intracellular cGMP levels were unchanged (Fig 7 A).
Figure 7.
Platelet FXR mediates cGMP signaling. Platelets derived from FXR+/+ and FXR-/- mice were treated with GW4064 or vehicle containing (DMSO (0.1% (v/v)) for 20 min prior stimulation with CRP-XL (1 μg mL-1). (A) cGMP levels were measured using the Enzyme immunoassay Biotrak (EIA) system and (B) VASP phosphorylation levels (S239) assessed by flow cytometry. R represents untreated resting platelet samples. Data represent FXR-/- mice platelets compared with FXR+/+ mice platelets (control), mean ± SD (n=4), *P ≤ 0.05, **P ≤0.01, ***P ≤0.005, ****P ≤0.001 (ANOVA with Bonferroni post test).
The vasodilator-stimulated phosphoprotein (VASP) is a critical protein involved in cytoskeletal remodelling and regulating adhesive events that are involved in platelet activation. 34 As an established marker of platelet inhibition and antiplatelet drug therapy, 35 VASP is an effector that mediates cGMP-dependent inhibitory mechanisms in platelets. On elevation of cGMP, the cGMP-dependent protein kinase G (PKG) phosphorylates VASP at position S239.
To further investigate whether GW4064 regulates cGMP-mediated inhibitory signalling in mice deficient in FXR, the phosphorylation of VASP at S239 was assessed by flow cytometry using phospho-specific antibodies. 35 On addition of the NO-donor PAPA nonoate (10 μM) VASP phosphorylation at S239 was increased in both FXR+/+ and FXR-/- mouse platelets (Fig 7 B). Treatment with GW4064 (10 μM) was found to increase phosphorylation of VASP at S239 in FXR+/+ platelets, where FXR-/- platelets were unresponsive to GW4064 treatment (Fig 7 B). These data indicate that the inhibitory effects of FXR ligands, mediated through the modulation of cGMP signalling in platelets may be attributed to their actions on FXR.
Discussion
Dyslipidemia is a major risk for cardiovascular disease and is associated with atherosclerosis and thrombotic complications. 36, 37 Inappropriate activation of platelets in the circulation is the major cause of atherothrombosis. 33 Current approaches for suppression of platelet function to prevent thrombosis with aspirin and ADP receptor antagonists are associated with serious bleeding side-effects. Thus, thrombotic disease remains a principal cause of mortality and morbidity worldwide, with increasing rates of incidence of these and underlying obesity-related metabolic disorders. 38 Therefore, more efficacious and safer approaches are required. FXR is a key regulator of lipid and glucose metabolism, and FXR synthetic ligands have been shown to have athero-protective effects, possibly through modulation of combined metabolic and vascular effects. 39. While platelets are anucleate cells, the presence of transcription factors in mammalian platelets have been reported, including PPARs, RXR, LXR, GR and the nuclear factor-κB. 15–20
PPARs are a family of ligand-activated nuclear receptors that, similarly to FXR, bind regulatory elements in responsive genes after the formation of a heterodimeric complex with RXR. 39 We have shown previously that PPARγ interacts with Syk and LAT on the stimulation of platelets with collagen, and PPARγ ligands cause loss of these associations. 30 This is important, since these signaling proteins perform critical roles early in the signalling pathway that is stimulated by the platelet collagen receptor GPVI. More recently, we suggested that the ability of LXR-PPARγ to interact might be based on bidirectional association either with other nuclear receptors or components of the GPVI signaling pathway in the presence or absence of their ligands. 19 In the current study, we therefore evaluated the effects of FXR ligands on platelet function and thrombus formation and characterized potential mechanisms of action.
Our data demonstrate that FXR ligands are able to modulate multiple aspects of platelet function stimulated by adhesion receptors, GPCR agonists and through integrin signaling. The FXR ligand, GW4064 did not cause marked inhibition of CRP-XL-induced tyrosine phosphorylation of Syk or LAT, suggesting that FXR does not serve to modulate the initiation of cell signaling mechanisms that are stimulated by GPVI and that more general downstream mechanisms with the potential to prevent activation through different platelet agonists must be involved.
FXR has been proposed to be a novel and promising therapeutic target to the treatment of atherosclerosis and heart diseases. 42 The implications of FXR activation for vascular function is subject of debate. A previous study suggested that chronic stimulation of FXR with GW4064 impaired endothelium-dependent relaxation due to decreased sensitivity of smooth muscle cells to NO. 23 As FXR ligands are able to inhibit the function of platelets, and they possess the ability to modulate NO signaling in vascular cells 23,43, we sought to determine whether the acute actions of GW4064 may modulate cyclic nucleotide signaling in platelets. Indeed, such signaling, in common with the observed effects of FXR agonists, is known to suppress platelet activation that is stimulated by a range of different platelets agonists, resulting in reduced intracellular calcium mobilisation, secretion, fibrinogen binding and aggregation.
Our study revealed that FXR ligands are able to modulate platelet function by increasing cGMP levels, which is not mediated through the inhibition of PDEs in platelets. GW4064 was found to increase the phosphorylation of VASP at position S239 in FXR+/+ platelets, which is regulated by cGMP-mediated signalling. Consistent with this, the FXR-/- platelets were unresponsive to the actions of GW4064 on cGMP accumulation and VASP phosphorylation at S239. These data indicate that the inhibitory effects of the FXR ligand GW4064, which are associated with increased intracellular accumulation of cGMP, may be attributed to its ability to activate FXR in these cells. In recent years, the presence of endothelial nitric oxide synthase in platelets has become a subject of debate with some studies showing its presence and function, 45, 46 and others its absence 47, 48. We have no evidence for a direct role for FXR in stimulating NO generation, although whether through platelet eNOS, or NO generated by platelets through other means, this remains a potential axis through which the acute effects of FXR in platelets may be mediated.
Further work will be required to explore in detail the mechanism through which FXR modulates cyclic nucleotide signaling in platelets. It is important to note that FXR ligands modulate platelet function in an acute and clearly non-genomic manner. Whether the effects of FXR ligands on platelets are related to their shared abilities to regulate the effects of vascular NO (which have also been attributed to genomic actions of FXR) or other target molecules remains to be established.
Due to its roles in lipid and glucose metabolism, FXR has become a target for drug discovery. Bile acids are the major metabolites of cholesterol that exert genomic and non-genomic effects by activating the FXR. 49 They are produced in the liver and are secreted into the small intestine where they facilitate the absorption of dietary and biliary lipids including cholesterol. Targeting bile acid metabolism and the enterohepatic circulation has, therefore, been considered an attractive mechanism for treating dyslipidemia. 36 Normal levels of bile acids in the systemic circulation were reported to be ≥ 10 µM in posprandial conditions. 50, 51 Bile acids have previously been associated with platelet dysfunction 52 although to date, little is known about the interactions between bile acids and platelet signalling. A previous study reported that Taurocholic acid inhibits platelet activation and promotes fibrinolysis, however, whether the effects of Taurocholic acid on platelet function are related to their shared abilities to regulate bile acid metabolism or other target molecules remains to be established. 53
Whether FXR agonists may be considered in the development of anti-thrombotic agents will require thorough evaluation of their potential to limit thrombosis, while balancing the need for effective hemostasis. Notably, this study demonstrates that 10 µM GW4064 is able to inhibit moderately platelet function in vitro and in vivo, but it is also associated with enhanced bleeding in mice. Emerging evidence suggests that GW4064 is able to decrease plasma triglycerides and insulin resistance in genetic mouse models of obesity. 36 Furthermore, several pre-clinical animal studies have demonstrated that synthetic FXR ligands protect against development of aortic plaque formation in models of atherogenesis. 54 The analysis of FXR deficient mice has demonstrated that despite a pro-atherosclerotic profile these mice did not spontaneously develop plaques even with a high fat diet. Together these lines of evidence, combined with the outcomes of the present study suggest a complex mechanistic role for FXR in the pathogenesis of atherosclerosis that might arise from the combined metabolic and vascular effects. 36, 54, 55
Our study provides evidence that FXR is present in platelets and that its ligands inhibit platelet function and thrombus formation, suggesting a potential new axis for prevention of atherosclerosis and thrombosis based on acute non-genomic actions of this receptor in platelets.
Supplementary Material
Highlights.
The farnesoid X receptor (FXR) acutely regulates platelet function in a non-genomic manner
FXR ligands cause inhibition of platelet activation, secretion, aggregation, thrombus formation, hemostasis and thrombosis
FXR controls platelet function through the modulation of cyclic guanosine monophosphate (cGMP) signaling
Acknowledgments
This work was supported by the British Heart Foundation (grants RG/09/01128094, PG/11/125/29320, RG/15/2/31224, FS/11/86/29/137) and the Biotechnology and Biological Sciences Research Council.
Abbreviations
- FXR
farnesoid X receptor
- cGMP
cyclic guanosine monophosphate
- cAMP
cyclic adenosine monophosphate
- CDCA
chenodeoxycholic acid
- 6-ECDCA
6α-ethyl-chenodeoxycholic acid
- GPVI
glycoprotein VI
- CRP-XL
cross-linked collagen-related peptide
- Syk
spleen tyrosine kinase
- LAT
linker for activation of T-cells
- IBMX
3-isobutyl-1-methylxanthine
- PDE
phosphodiesterase
- PPAR
peroxisome proliferator-activated receptor
- RXR
retinoid X receptor
- LXR
liver X receptor
- GR
glucocorticoid receptor
- eNOS
endothelial nitric oxide synthase
Footnotes
Authorship
Contribution: A.J.U., S.V., P.S., G.F., A.P.B., N.K. O.M-C. and D.D. performed experiments, analyzed results and made figures; M.A. performed experiments and analyzed results; T.S. and E.D. performed experiments; D.B.B. and B.S. designed the research; L.A.M. and J.M.G., designed the research, performed experiments, analyzed results, made figures and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 2.Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA. 2004;101:3668–3673. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang W, Ma K, Zhang J, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Yoyo TY, Karen ES, Thomas GJ, Warner TD, Bishop-bailey D. Farnesoid X Receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Atheroscler Thromb Vasc Biol. 2007;27:2606–2611. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]
- 6.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 7.Willson TM, Jones SA, Moore JT, Kliewer SA. Chemical genomics: functional analysis of orphan nuclear receptors in the regulation of bile acid metabolism. Med Res Rev. 2001;21:513–522. doi: 10.1002/med.1023. [DOI] [PubMed] [Google Scholar]
- 8.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Constantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1 and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 10.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 11.Spinelli SL, Maggirwar SB, Blumberg N, Phipps RP. Nuclear emancipation: a platelet tour de force. Sci Signal. 2010;3(144):pe37. doi: 10.1126/scisignal.3144pe37. [DOI] [PubMed] [Google Scholar]
- 12.Gnatenko DV, Dunn JJ, McCorkle SR, Weissmann D, Perrotta PL, Bahou WF. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood. 2003;101:2285–2293. doi: 10.1182/blood-2002-09-2797. [DOI] [PubMed] [Google Scholar]
- 13.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, et al. Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landry P, Plante I, Ouellet DL, Perron MP, Rousseau G, Provost P. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16:961–966. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104:1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- 16.Ali FY, Davidson SJ, Moraes LA, Traves SL, Paul-Clark MJ, Bishop-bailey D, Warner TD, Mitchell JA. Role of nuclear receptor signaling in platelets: Antithrombotic effects of PPARbeta. Faseb J. 2006;20:326–328. doi: 10.1096/fj.05-4395fje. [DOI] [PubMed] [Google Scholar]
- 17.Moraes LA, Swales KE, Wray JA, Damazo A, Gibbins JM, Warner TD, Bishop-bailey D. Nongenomic signaling of the retinoic x receptor through binding and inhibiting Gq in human platelets. Blood. 2007;109:3741–3744. doi: 10.1182/blood-2006-05-022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moraes LA, Paul-Clark MJ, Rickman A, Flower RJ, Goulding NJ, Perretti M. Ligand-specific glucocorticoid receptor activation in human platelets. Blood. 2005;106:4167–4175. doi: 10.1182/blood-2005-04-1723. [DOI] [PubMed] [Google Scholar]
- 19.Spyridon M, Moraes LA, Jones CI, Sage T, Sasikumar P, Bucci G, Gibbins JM. LXR as a novel antithrombotic target. Blood. 2011;117:5751–5761. doi: 10.1182/blood-2010-09-306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaver E, Romaniuk MA, D’atri LP, Pozner RG, Negrotto S, Benzadon R, Schattner M. NF-κB inhibitors impair platelet activation responses. J Thromb Haemost. 2009;7:1333–1343. doi: 10.1111/j.1538-7836.2009.03492.x. [DOI] [PubMed] [Google Scholar]
- 21.Mencarelli A, Renga B, Distrutti E, Fiorucci S. Antiatherosclerotic effect of farnesoid x receptor. Am J Physiol Heart Circ Physiol. 2009;296:H272–H281. doi: 10.1152/ajpheart.01075.2008. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, Edwards PA. FXR deficiency causes reduced atherosclerosis in Ldlr-/- mice. Atheroscler ThrombVasc Biol. 2006;26:2316–2321. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]
- 23.Kida T, Murata T, Hori M, Ozaki H. Chronic stimulation of farnesoid X receptor impairs nitric oxide sensitivity of vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H195–H201. doi: 10.1152/ajpheart.00679.2008. [DOI] [PubMed] [Google Scholar]
- 24.Vaiyapuri S, Jones CI, Sasikumar P, Moraes LA, Munger SJ, Wright JR, Ali MS, Sage T, Kaiser WJ, Tucker KL, Stain CJ, et al. Gap junctions and Connexin hemichannels underpin hemostasis and thrombosis. Circulation. 2012;125:2479–2491. doi: 10.1161/CIRCULATIONAHA.112.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moraes LA, Vaiyapuri S, Sasikumar P, Ali MS, Kriek N, Sage T, Gibbins JM. Antithrombotic actions of statins involve PECAM-1 signaling. Blood. 2013;122(18):3188–3196. doi: 10.1182/blood-2013-04-491845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shattil SJ, Brass LF. Induction of the fibrinogen receptor on human platelet by intracellular mediators. J Biol Chem. 1987;262:992–1000. [PubMed] [Google Scholar]
- 27.Calderwood DA. Integrin activation. J Cell Sci. 2004;117:657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- 28.Estevez B, Shen Bo, Du X. Targeting integrin and integrin signaling in treating thrombosis. Arterioscler Thromb Vasc Biol. 2015;35:24–29. doi: 10.1161/ATVBAHA.114.303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao C, Boylan B, Bougie D, Gill JC, Birenbaum J, Newman DK, Aster RH, Newman PJ. Eptifibatide-induced thrombocytopenia and thrombosis in humans require FcγRIIa and the integrin β3 cytoplasmic domain. J Clin Invest. 2009;119:504–511. doi: 10.1172/JCI36745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moraes LA, Spyridon M, Kaiser WJ, Jones CI, Sage T, Atherton REL, Gibbins JM. Non-genomic effects of PPARγ ligands: inhibition of GPVI-stimulated platelet activation. J Thromb Haemost. 2010;8:577–587. doi: 10.1111/j.1538-7836.2009.03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989;65:1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 32.De Graaf JC, Banga JD, Moncada S, Palmer RM, de Groot PG, Sixma JJ. Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation. 1992;85:2284–2290. doi: 10.1161/01.cir.85.6.2284. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz UR, Walter U, Eigenthaler M. Taming platelets with cyclic nucleotides. Biochemical Pharm. 2001;62:1153–1161. doi: 10.1016/s0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- 34.Wentworth JK, Pula G, Poole AW. Vasodilator-stimulated phosphoprotein (VASP) is phosphorylated on Ser157 by protein kinase C-dependent and -independent mechanisms in thrombin-stimulated human platelets. Biochem J. 2006;393:555–564. doi: 10.1042/BJ20050796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spurgeon BEJ, Aburima A, Oberprieler NG, Tasken K, Naseem KM. Multiplexed phosphospecific flow cytometry enables large-scale signaling profiling and drug screening in blood platelets. J Thromb Haemost. 2014;12:1733–1743. doi: 10.1111/jth.12670. [DOI] [PubMed] [Google Scholar]
- 36.Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lip Res. 2012;53:1723–1737. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibbins JM. Platelet adhesion signaling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 38.Jackson SP, Schoenwaelder SM. Antiplatelet therapy: in search of the “magic bullet”. Nat Rev Drug Discov. 2003;2:775–789. doi: 10.1038/nrd1198. [DOI] [PubMed] [Google Scholar]
- 39.Mencarelli A, Fiorucci S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J Cell Mol Med. 2010;14:79–92. doi: 10.1111/j.1582-4934.2009.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaiyapuri S, Sage T, Rana RH, Schenk MP, Ali MS, Unsworth AJ, Jones CI, Stainer AR, Kriek N, Moraes LA, Gibbins JM. EphB2 regulates contact-dependent and contact-independent signaling to control platelet function. Blood. 2015;125(4):720–730. doi: 10.1182/blood-2014-06-585083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moritz A, Li Y, Guo A, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3(136):ra64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang R, Ran HH, Zhang XY, Liu P, Lu CY, Xu Q, Huang Y. Farnesoid X Receptor regulates vascular reactivity through nitric oxide mechanism. J Phys and Pharmacol. 2012;63:367–372. [PubMed] [Google Scholar]
- 43.Li J, Wilson A, Kuruba R, Zhang Q, Gao X, He F, Zhang LM, Pitt BR, Xie W, Li S. FXR-mediated regulation of eNOS expression in vascular endothelial cells. Cardiovasc Res. 2008;77:169–177. doi: 10.1093/cvr/cvm016. [DOI] [PubMed] [Google Scholar]
- 44.Pigazzi A, Heydrick S, Folli F, Benoit S, Michelson A, Loscalzo J. Nitric oxide inhibits thrombin receptor-activating peptide-induced phosphoinositide 3-kinase activity in human platelets. J Biol Chem. 1990;265:19028–19034. doi: 10.1074/jbc.274.20.14368. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Xi X, Gu M, Feil R, Ye RD, Eigenthaler M, Hofmann F, Du X. A stimulatory role for cGMP-dependent protein kinase in platelet activation. Cell. 2003;112:77–86. doi: 10.1016/s0092-8674(02)01254-0. [DOI] [PubMed] [Google Scholar]
- 46.Marjanovic JA, Li Z, Stojanovic A, Du X. Stimulatory roles of nitric-oxide synthase 3 and guanylyl cyclase in platelet activation. J Biol Chem. 2005;280:37430–37438. doi: 10.1074/jbc.M506518200. [DOI] [PubMed] [Google Scholar]
- 47.Gambaryan S, Kobsar A, Hartmann S, Birschmann I, Kuhlencordt PJ, Muller-Esterl W, Lohmann SM, Walter U. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J Thromb Haemost. 2008;6:1376–1384. doi: 10.1111/j.1538-7836.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 48.Ozuyaman B, Godecke A, Kusters S, Kirchhoff E, Scharf RE, Schrarder J. Endothelial nitric oxide synthase plays a minor role of inhibition of arterial thrombus formation. Thromb Haemost. 2005;93:1161–1167. doi: 10.1160/TH03-09-0588. [DOI] [PubMed] [Google Scholar]
- 49.Prawitt J, Abdelkarim M, Stroeve JHM, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, Van Kijk TH, Lucas A, Dorchies E, et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861–1871. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shamir R, Johnson WJ, Morlock-fitzpatrick K, Zolfaghari R, Li L, Mas E, Lombardo D, Morel DW, Fisher EA. Pancreatic carboxyl ester lipase: a circulating enzyme that modifies normal and oxidized lipoproteins in vitro. J Clin Invest. 1996;97(7):1696–1704. doi: 10.1172/JCI118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burkard I, Von Eckardstein A, Rentsch KM. Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;826:147–159. doi: 10.1016/j.jchromb.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 52.Shiao YJ, Chen JC, Wang CN, Wang CT. The mode of action of primary bile salts on human platelets. Biochim Biophys Acta. 1993;1146:282–293. doi: 10.1016/0005-2736(93)90367-9. [DOI] [PubMed] [Google Scholar]
- 53.Wiener G, Moore HB, Moore EE, Gonzales E, Diamond S, Zhu S, D’Alessandro A, Banerjee A. Shock releases bile acidinducing platelet inhibition and fibrinolysis. J Surgical Research. 2015;195:390–395. doi: 10.1016/j.jss.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartman HB, Gardell SJ, Petucci CJ, Wang S, Krueger JA, Evans MJ. Activation of Farnesoid X receptor prevents atherosclerotic lesion formation in LDLR-/- and apoE-/- mice. J Lipid Res. 2009;50:1090–1100. doi: 10.1194/jlr.M800619-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.