Drug interactions between antiretroviral therapy and anticoagulant medications are of particular concern given that an estimated 50% of the current HIV population is older than 50 years of age. Moreover, HIV infection itself is characterized by a hypercoaguable state and premature immunologic aging, in which thromboembolic events may be as much as 10 times more prevalent than in the general population across all age spectra.1
Dabigatran was the first direct oral anticoagulant (DOAC) approved by the Food & Drug Administration (FDA), and is the only DOAC with an FDA-approved specific reversal agent, idarucizumab. Unlike warfarin and many other DOACs, dabigatran is not a substrate, inhibitor, or inducer of cytochrome P450 (CYP) metabolic enzymes. However, dabigatran is a substrate of Permeability-glycoprotein (P-gp) and renal multidrug and toxin extrusion-1 (MATE-1) transporters. Cobicistat is a FDA-approved antiretroviral-boosting agent that is co-formulated with numerous fixed-dose combination antiretroviral products due to its inhibitory effects on CYP3A4. Currently, approximately 40% of all treatment-naïve HIV patients in the US are initiated on a cobicistat-boosted antiretroviral regimen. In addition to CYP3A4, cobicistat is also an inhibitor of both P-gp and MATE-1.2 Thus, this study aimed to determine whether the co-administration of cobicistat increases the systemic exposure and anticoagulant effects of dabigatran, and if so, whether separating administration would circumvent this interaction.
This was an open-label, single-sequence drug interaction study conducted in healthy HIV-negative volunteers (Clinical Trial Registration: URL: https://www.clinicaltrials.gov Unique identifier: NCT01896622). All participants gave written informed consent and the study was approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board. Participants first received a single dose of dabigatran 150mg alone (Phase 1). Following a five-day washout period, participants then began cobicistat 150mg daily. After two weeks, a second single dose of dabigatran was given two hours before cobicistat (Phase 2), and then one week later a third single dose of dabigatran was given simultaneously with cobicistat (Phase 3). After each dabigatran dose, blood was collected serially over 24 hours for pharmacokinetic (PK) and thrombin time (TT) analysis (STA®-Thrombin assay, Diagnostica Stago, Inc.) including: area-under-the-concentration-versus-time curve from zero to infinity (AUC0-∞), maximal concentration (Cmax), area-under-the-TT-effect-versus-time curve (AUEC0-24), and TT at 24 hours post-dose (TT24), via Phoenix WinNonlin software (v6.4, Certara, St. Louis, MO). P-values were calculated by paired student t-tests (Microsoft Excel, Redmond, WA).
Sixteen participants completed all phases of the study, and an additional two participants completed Phases 1 and 2 only. Simultaneous administration of cobicistat (Phase 3) resulted in significant increases in dabigatran pharmacokinetic exposure with a 127% increase both in the geometric mean (GM) AUC0-∞ and Cmax (p<0.001). While, there was no alteration in drug elimination, there appeared to be significant increases observed in oral bioavailability (data not shown). Additionally, the anticoagulant effect correspondingly increased with a 33% and 51% increase in GM AUEC0-24 and TT24, respectively (p<0.001). Separation of administration by 2 hours (Phase 2) minimally mitigated this interaction, with increases in GM AUC0-∞, Cmax, AUEC0-24, and TT24 of 110%, 99%, 30%, and 46% (p<0.001 for all comparisons), respectively. (Figure 1A and 1B). In fact, no significant difference in PK or TT parameters was noted between simultaneous (Phase 3) and separated (Phase 2) administration of cobicistat and dabigatran.
Figure 1.
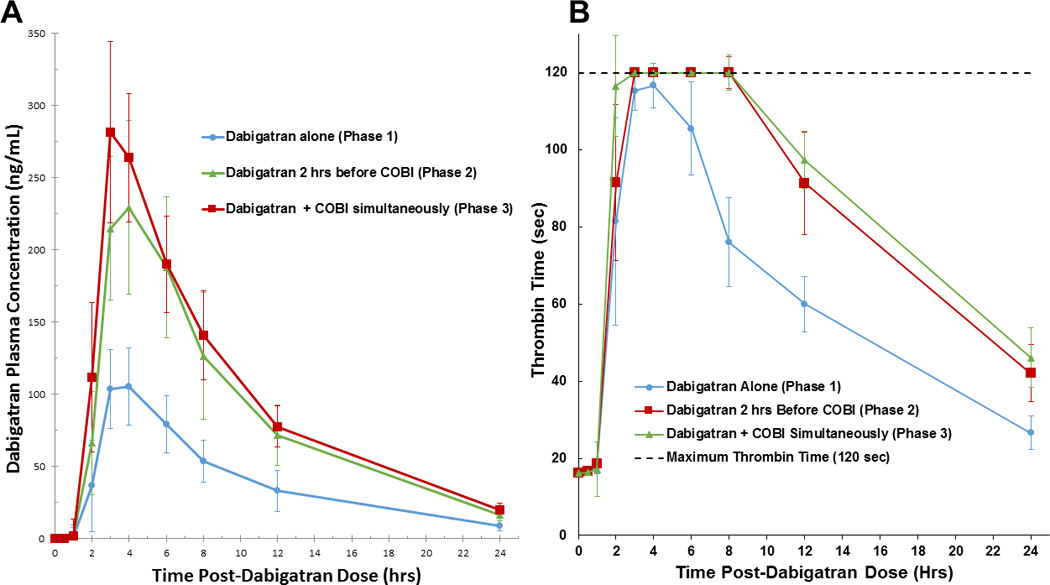
Mean (±SEM) dabigatran plasma concentration (A) and Median (90% CI) thrombin time (B) after administration of dabigatran alone, administration separated by 2 hours, and simultaneously with cobicistat (COBI).
Previous investigations with intestinal P-gp transporter inhibitors have resulted in increased dabigatran drug exposure and corresponding anticoagulation activity. Hartter et al. illustrated that this interaction could potentially be obviated by separating administration by 2 hours.3 In our study, simultaneous cobicistat and dabigatran administration resulted in significant increases in dabigatran exposure and TT measures. However, separated administration by 2 hours was inadequate for circumventing this interaction. Since participants typically experienced maximal dabigatran concentrations 3 – 4 hours after dosing, it is possible that separated administration by ≥ 4 hours may have circumvented the interaction, but this strategy may present a considerable challenge for adherence with standard twice-daily dabigatran administration.
The true clinical impact on dabigatran’s anticoagulant effects may be larger than we measured with the TT assay, STA®-Thrombin, which reports a maximum TT value of 120 seconds. Furthermore, this study was conducted in healthy volunteers, as is standard in most drug interaction studies; it is plausible that HIV-infected patients may have altered susceptibility to this interaction. Kis et al. showed that treatment-experienced HIV patients have increased P-gp gene expression (3.2-fold) as compared to treatment-naïve HIV patients.4 Initiation with 110 mg daily and subsequent titration of dose based on dabigatran concentrations was successfully employed in an HIV-infected patient; however, this patient was receiving a different antiretroviral boosting agent, ritonavir, which is known to demonstrate mixed P-gp inhibition and induction.2,5
Nonetheless, these findings support the need for future investigations conducted in patients receiving concomitant dabigatran and cobicistat, to determine whether dose adjustment is clinically indicated and/or effective. Of note, all currently available alternative DOACs (rivaroxaban, apixaban, and edoxaban) are expected to interact with cobicistat due to CYP3A4 inhibition, leaving subcutaneous enoxaparin or INR-monitored warfarin as remaining chronic anticoagulant options. Fortunately, the availability of idarucizumab for rapid reversal of anticoagulation may be considered as a layer of safety in patients receiving cobicistat-boosted antiretrovirals and dabigatran.
Acknowledgments
Sources of Funding: Funding for this study was provided by the NIH Clinical Center Pharmacy Department, and the National Institute of Allergy and Infectious Diseases (NIAID) intramural research program.
Footnotes
Previous Presentation: Some of the data contained in this manuscript were previously presented in abstract form at the annual Conference on Retroviruses and Opportunistic Infections in February 2016 in Boston, MA.
Disclosures: None.
References
- 1.Saber AA, Aboolian A, LaRaja RD, Baron H, Hanna K. HIV/AIDS and the risk of deep vein thrombosis: a study of 45 patients with lower extremity involvement. Am Surg. 2001;67:645–647. [PubMed] [Google Scholar]
- 2.Marzolini C, Gibbons S, Khoo S, Back D. Cobicistat versus ritonavir boosting and differences in the drug-drug interaction profiles with co-medications. J Antimicrob Chemother. 2016 Mar 5; doi: 10.1093/jac/dkw032. [DOI] [PubMed] [Google Scholar]
- 3.Hartter S, Sennewald R, Nehmiz G, et al. Oral bioavailability of dabigatran etexilate (Pradaxa®) after co-medication with verapamil in healthy participants. British Journal of Clinical Pharmacology. 2013;75:1053–1062. doi: 10.1111/j.1365-2125.2012.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kis O, Sankaran-Walters S, Hoque MT, Walmsley SL, Dandekar S, Bendayan R. HIV-1 alters intestinal expression of drug transporters and metabolic enzymes: implications for antiretroviral drug disposition. Antimicrob Agents Chemother. 2016;60:2771–2781. doi: 10.1128/AAC.02278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perram J, Joseph J, Holloway C. Novel oral anticoagulants and HIV: dabigatran use with antiretrovirals. BMJ Case Rep. 2015 doi: 10.1136/bcr-2015-211651. [DOI] [PMC free article] [PubMed] [Google Scholar]


