Abstract
Phosphorylation on certain Ser/Thr-Pro motifs is a major oncogenic mechanism. The conformation and function of phosphorylated Ser/Thr-Pro motifs are further regulated by the prolyl isomerase Pin1. Pin1 is prevalently overexpressed in human cancers and implicated in oncogenesis. However, the role of Pin1 in oncogenesis in vivo is not known. We have shown that Pin1 ablation is highly effective in preventing oncogenic Neu or Ras from inducing cyclin D1 and breast cancer in mice, although it neither affects transgene expression nor mammary gland development. Moreover, we have developed an ex vivo assay to uncover that a significant fraction of primary mammary epithelial cells from Neu or Ras mice display various malignant properties long before they develop tumors in vivo. Importantly, these early transformed properties are effectively suppressed by Pin1 deletion, which can be fully rescued by overexpression of cyclin D1. Thus, Pin1 is essential for tumorigenesis and is an attractive anticancer target. Our ex vivo assay can be used to study early events of breast cancer development in genetically predisposed mice.
Keywords: Pin1, protein phosphorylation, three-dimensional culture, transgenic mice, tumorigenesis
Introduction
Phosphorylation of proteins on certain serine/threonine residues preceding proline (pSer/Thr-Pro) is a major mechanism in regulating cell proliferation and transformation (Hanahan and Weinberg, 2000; Blume-Jensen and Hunter, 2001; Lu et al, 2002; Lu, 2004). For example, signaling pathways triggered by the oncogenes Neu and Ras lead to the activation of various Pro-directed protein kinases, which eventually enhances transcription of the cyclin D1 gene via multiple transcription factors, including E2F, c-jun/AP-1, β-catenin/TCF and NF-κB (Lee et al, 2000). Furthermore, cyclin D1 stability is regulated by post-translational phosphorylation on the Thr286-Pro site by GSK-3β (Diehl et al, 1997, 1998; Alt et al, 2000). Cyclin D1 plays a pivotal role in the development of breast cancer. Cyclin D1 is overexpressed in 50% of breast cancer patients (Gillett et al, 1994). Importantly, overexpression of cyclin D1 can transform fibroblasts (Hinds et al, 1994; Alt et al, 2000) and it induces mammary tumors (Wang et al, 1994), whereas its inhibition reduces transformed cell growth (Arber et al, 1997). Furthermore, cyclin D1 ablation suppresses the ability of Ha-Ras or c-Neu/HER2/ErbB2 to induce breast cancer in mice (Yu et al, 2001; Bowe et al, 2002). These results indicate that cyclin D1 is an essential downstream target for mammary tumorigenesis induced by oncogenic Neu or Ras, and that a major mechanism in these oncogenic processes is the phosphorylation of Ser/Thr-Pro motifs.
Phosphorylated Ser/Thr-Pro motifs exist in two distinct cis and trans conformations in native proteins, and their conversion is reduced upon phosphorylation (Lu et al, 1996, 1999; Yaffe et al, 1997). Through isomerization of specific pSer/Thr-Pro bonds, Pin1 induces conformational changes in certain proteins following phosphorylation. These conformational changes have profound effects on catalytic activity, protein dephosphorylation, protein–protein interactions, subcellular location and/or turnover of many proteins involved in cell signaling and growth regulation (Yaffe et al, 1997; Zhou et al, 2000; Ryo et al, 2001; Wulf et al, 2001; Liou et al, 2002). Thus, Pin1-dependent phosphorylation-specific prolyl isomerization is an important new signaling mechanism (Lu et al, 2002; Lu, 2004).
We have previously found Pin1 overexpression in human breast cancer tissues (Wulf et al, 2001), which has subsequently been confirmed and expanded to a large number of other tumors (Ryo et al, 2001, 2003a; Ayala et al, 2003; Lu, 2003; Bao et al, 2004). Furthermore, its expression levels closely correlate with cyclin D1 levels in cancer tissues (Ryo et al, 2001; Wulf et al, 2001) and with poor prognosis in prostate cancer (Ayala et al, 2003). Importantly, upregulation of Pin1 has been shown to elevate cyclin D1 gene expression by activating c-jun/AP-1, β-catenin/TCF and NF-κB transcription factors (Ryo et al, 2001, 2003b; Wulf et al, 2001). Pin1 can also directly bind to the phosphorylated Thr286-Pro motif in cyclin D1 and stabilize nuclear cyclin D1 protein by inhibiting its export into the cytoplasm (Liou et al, 2002), where it is normally degraded by ubiquitin-mediated proteolysis (Diehl et al, 1997, 1998; Alt et al, 2000). In addition, the breast epithelial compartment in Pin1−/− adult females failed to undergo the massive proliferative changes associated with pregnancy (Liou et al, 2002), a phenotype resembling the cyclin D1 null phenotype (Fantl et al, 1995; Sicinski et al, 1995). Finally, Pin1 transcription is enhanced by oncogenic Neu or Ras signaling via E2F activation and it enhances the transformed phenotypes of Neu/Ras-transfected mammary epithelial cells (MECs) (Ryo et al, 2002). These results from human tissues and cell cultures suggest that Pin1 is a Neu/Ras downstream target that plays an important role in cell transformation and may be an attractive molecular target for cancer diagnosis and therapy (Lu, 2003). However, it has not been shown whether inhibition of Pin1 function affects tumorigenesis, and where it affects the oncogenic process in vivo. This is further complicated by the findings that Pin1 is also important for activation of the tumor suppressor p53 in response to DNA damage in cell cultures (Wulf et al, 2002; Zacchi et al, 2002; Zheng et al, 2002) and for the degradation of endogenous c-Myc (Yeh et al, 2004).
Genetically modified mice by transgenic overexpression and/or gene ablation have long been used to address the role of cancer-related genes in breast cancer in vivo (Sinn et al, 1987; Muller et al, 1988; Bouchard et al, 1989; Wang et al, 1994; Yu et al, 2001; Bowe et al, 2002). For ex vivo model systems, three-dimensional (3D) basement membrane cultures are increasingly used for modeling the biological activities of cancer genes, especially in breast cancer, because they provide a unique opportunity to model the architecture of epithelium in vitro (O'Brien, 2002). Unlike monolayer cultures, MECs grown in 3D assay will divide and differentiate in a way that recapitulates glandular development in vivo (Barcellos-Hoff et al, 1989; Petersen et al, 1992; Debnath et al, 2002; Gudjonsson et al, 2002). Furthermore, the introduction of oncogenes into these MECs has been shown to result in a distinct and characteristic phenotype with large and atypically formed structure/colonies with clearly disrupted glandular architecture and filled lumen (Muthuswamy et al, 2001; Ryo et al, 2001; Debnath et al, 2002). Thus, 3D cultures provide the appropriate structural and functional context fundamental for modeling the biological activities of cancer genes.
Here we use a combination of these in vivo and in vitro assays to examine the role of Pin1 in breast cancer. Pin1 ablation in mice efficiently abolishes the ability of MMTV-c-Neu or -v-Ha-Ras to induce both cyclin D1 and breast cancers, although it has no effect on MMTV-c-Myc. To examine where Pin1 ablation blocks the oncogenic process, we established 3D cultures of primary MECs derived from these genetically altered mice to study the effects of Pin1 ablation on the growth and differentiation properties of primary MECs directly. In our ex vivo culture system, a significant fraction of primary MECs isolated from Ras or Neu transgenic mice, but not nontransgenic controls, fail to differentiate. These cells instead display various features of malignancy, including forming tumors in nude mice, long before they develop tumors in vivo. Importantly, these early transformed properties are effectively suppressed by Pin1 deletion, which can be fully rescued by overexpression of cyclin D1. These results provide the in vivo and ex vivo evidence for an essential role of Pin1 in early events of tumorigenesis and strongly support Pin1 as an attractive anticancer target. The ex vivo assay can be used to study early events of breast cancer development in genetically predisposed mice.
Results
Pin1 expression in transgenic mice
We have shown that Pin1 is overexpressed in human breast cancer tissues and its expression is increased by activated Neu or Ras (Ryo et al, 2001, 2002; Wulf et al, 2001). To examine the role of Pin1 in breast cancer induced by Neu and Ras, we crossbred Pin1 knockout (Pin1−/−) mice (Liou et al, 2002) and oncogenic transgenic mice overexpressing an activated rat Neu/Her2/ErbB2 kinase (c-Neu) or v-Ha-Ras under the control of the MMTV promoter (Sinn et al, 1987; Muller et al, 1988; Bouchard et al, 1989). As compared with normal controls, Pin1 levels were consistently increased several fold in mammary glands or mammary tumors isolated from Neu/Pin1+/+ or Ras/Pin1+/+ animals (Figure 1A and C). However, no Pin1 protein was detected in mammary gland lysates in all Pin1−/− mice regardless of the transgene (Figure 1A and C), as expected. Interestingly, we found no significant difference in Pin1 levels between Pin1+/+ and Pin1+/− mice (Figure 1B), an observation consistent with our previous findings that Pin1 levels are tightly regulated by multiple mechanisms (Ryo et al, 2002). These results indicate that Pin1 protein is absent in Pin1−/− mice, but remains at wild-type levels in Pin1+/− mice.
Figure 1.
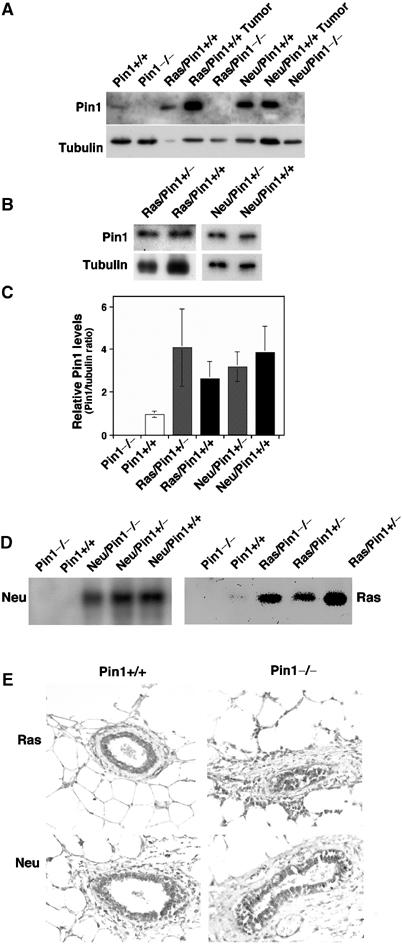
Expression of Pin1 and transgenes in normal mammary glands and cancer tissues derived from the crossbreeding. (A–C) Pin1 protein is absent in Pin1-deficient (Pin1−/−) mice (A), but remains at Pin1+/+ levels in Pin1 heterozygote (Pin1+/−) mice (B). Mammary glands and breast cancer tissues from littermates with indicated genotypes were homogenized and equal amounts of total protein were separated on SDS-containing gels and transferred to membranes. The membranes were cut into two pieces and subjected to immunoblotting analysis with antibodies against Pin1 and tubulin (A, B), followed by semiquantification using Imagequant. The Pin1/tubulin ratio was determined for mammary glands from four different animals and are presented in (C). Note that Pin1 levels in c-Neu or Ha-Ras transgenic mice are variable, but generally higher than in those in nontransgenic mice (A, C). There was no statistically significant difference in Pin1 levels between Pin1+/+ and Pin1+/− mice. (D, E) Pin1 ablation does not affect the expression of the transgenes Ha-Ras or c-Neu. Protein lysates or tissue sections of mammary glands of the specified genotypes were subjected to immunoblotting (D) or were immunostained (E) with anti-c-Neu or anti-Ha-Ras antibodies. Note that out of three to five mice analyzed in each group, there was no statistically significant difference in Neu or Ras levels between Pin1+/+ and Pin1−/− mice.
Pin1 ablation is highly effective in preventing breast cancers induced by oncogenic Neu or Ras, but not Myc in vivo
We next examined the effects of Pin1 function on the incidence of mammary carcinomas by monitoring virgin mice that carried one copy of MMTV-c-Neu or -v-Ha-Ras transgene on either Pin1+/+, Pin1+/− or Pin−/− background over time. As a control for an oncogene that bypasses cyclin D1 in its mechanism of action, we also crossbred Pin1 knockout mice with MMTV-Myc transgenic mice. As shown for cyclin D1 knockout (Yu et al, 2001), Pin1 knockout did not affect breast cancer induced by MMTV-Myc (Figure 2C, Table I). However, it had drastic effects on the ability of Ras or Neu to induce breast cancer (Figure 2, Table I). Almost all MMTV-Ras or -Neu transgenic mice developed breast tumors, with the kinetics similar to those historically observed in these mice, which were slightly variable among different laboratories, likely due to the differences in the genetic background used (Sinn et al, 1987; Muller et al, 1988; Bouchard et al, 1989; Yu et al, 2001; Bowe et al, 2002). However, ∼90% of transgenic littermates in the Pin1−/− background remained breast cancer-free over the same period of time (Figure 2A and B, Table I). Kaplan–Meier analysis confirmed that Pin1−/− transgenic mice had a highly significant advantage in disease-free survival as compared to Pin1+/+ transgenic littermates (log-rank test, P<0.0001 for Neu and P=0.0004 for Ras) (Table I). Interestingly, there was no survival benefit for Pin1+/− mice over their Pin1−/− littermates (Figure 2A and B, Table I). This is consistent with the observation that Pin1 levels in these Pin1+/− animals were not different from the Pin1+/+ mice (Figure 1B and C), indicating that the protective effect is specifically due to the loss of Pin1 function. As compared with those reported by Yu et al (2001), the overall incidence of salivary gland tumors in our population was much lower, precluding statistical analyses, possibly due to different genetic background of our mice. Although further experiments are needed to address the role of Pin1 in other cancers, these results indicate that loss of Pin1 function is highly effective in preventing breast cancer induced by oncogenic Neu or Ras.
Figure 2.
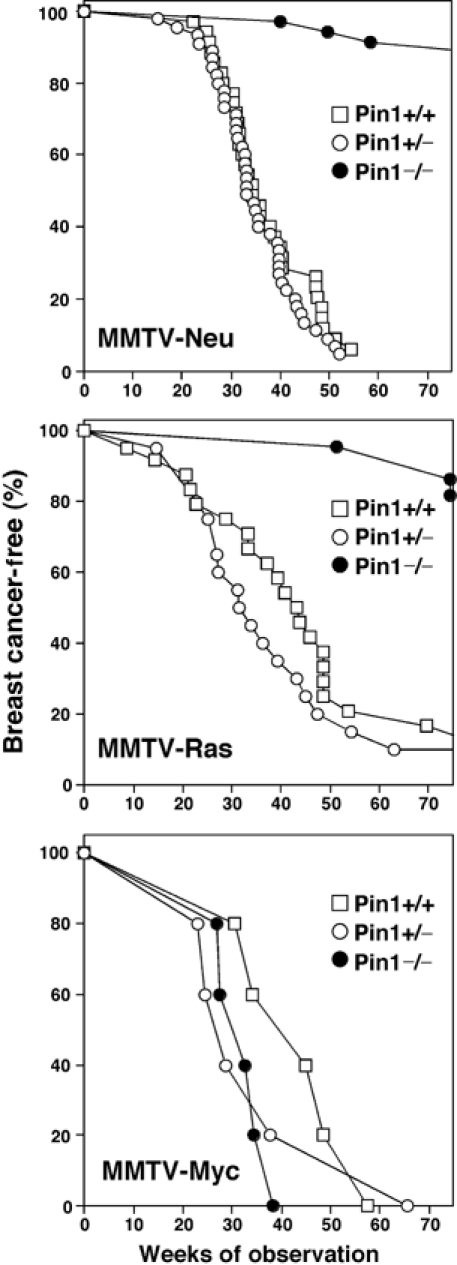
Pin1 ablation is highly effective in preventing breast cancers induced by MMTV-Neu or -Ras, but not -Myc. Transgenic mice overexpressing activated c-Neu, Ras or Myc under the control of the promoter MMTV were crossbred with Pin1−/− mice to generate mice with nine different genotypes. Virgin females were observed for 75 weeks. Breast cancers were recorded at the time of first palpation.
Table 1.
Breast cancer incidence of transgenic mice in different Pin1 backgrounds
| Genotypes | Animals in each group | Animals with breast tumors | Total breast tumor number of disease onset | Median age (weeks) | Kaplan–Meier survival analysis (P-value) |
|---|---|---|---|---|---|
| MMTV-Neu/Pin1+/+ | 32 | 32 | 91 | 33 | NS |
| MMTV-Neu/Pin1+/− | 54 | 52 | 139 | 35 | NS |
| MMTV-Neu/Pin1−/− | 34 | 3 | 4 | >75 | <0.0001 |
| MMTV-Ras/Pin1+/+ | 31 | 30 | 42 | 44 | NS |
| MMTV-Ras/Pin1+/− | 20 | 18 | 26 | 31 | NS |
| MMTV-Ras/Pin1−/− | 23 | 3 | 3 | >75 | 0.0004 |
| MMTV-Myc/Pin1+/+ | 5 | 5 | 11 | 38 | NS |
| MMTV-Myc/Pin1+/− | 5 | 5 | 5 | 27 | NS |
| MMTV-Myc/Pin1−/− | 5 | 5 | 6 | 30 | NS |
| Breast tumors were recorded at the time of first palpation. The animals were usually killed 2–3 weeks later, when the disease was manifest. At the time of autopsy, the number of tumors was recorded. Kaplan–Meier analysis using the log-rank test was performed to analyze the significance of the survival advantage in Pin1−/− in comparison to the Pin1+/− and Pin1+/+ populations. There was no significant difference in disease-free survival between the Pin1+/− and the Pin1+/+ populations or among all the three Myc populations (NS). |
Pin1 ablation affects neither the development of virgin mammary glands nor the expression of the transgenes
Given this striking protection of Pin1 ablation against breast cancer, we were interested in determining the effects of Pin1 ablation on oncogenic processes. It has been reported that mammary glands in Pin1−/− or MMTV-Neu or -Ras transgenic virgin females develop normally (Yu et al, 2001; Liou et al, 2002), although Pin1−/− mammary glands fail to undergo the massive proliferation during pregnancy (Liou et al, 2002). To address the question whether the combination of the transgene with Pin1 deletion affected mammary gland development, we performed whole-mount and histological analyses (Yu et al, 2001; Liou et al, 2002). Morphometric analysis of carmine-stained whole mounts of the virgin mammary glands revealed interindividual variations, but no significant difference in the number of primary ducts, secondary branches or end buds between Pin1+/+ and Pin1−/− mice carrying the Ras or Neu transgene (Table II, Supplementary Figure S1A). All virgin female mice developed proper mammary ducts with an intact lumen, and again there was no detectable difference between Pin1+/+ and Pin1−/− background (Supplementary Figure S1B).
Table 2.
Pin1 ablation does not affect the development of virgin mammary glands
| Genotypes | Primary ducts | Secondary ducts | Tips |
|---|---|---|---|
| MMTV-Neu/Pin1+/+ | 7.0±0.7 | 27.5±3.5 | 162.5±33.0 |
| MMTV-Neu/Pin1−/− | 7.3±1.5 | 31.0±7.6 | 160.5±11.7 |
| MMTV-Ras/Pin1+/+ | 7.2±1.5 | 31.0±4.1 | 160.5±16.3 |
| MMTV-Ras/Pin1−/− | 6.7±1.5 | 30.3±8.5 | 149.3±28.6 |
| Virgin mammary glands from MMTV-Neu or -Ras transgenic mice in Pin1+/+ or Pin1−/− genetic background were stained with carmine red and whole mounts were prepared. Primary ducts, secondary branches and tips and end buds were counted in a representative frame under a dissecting microscope. Three to five animals were analyzed in each cohort. |
We next asked whether Pin1 ablation could affect the expression of the c-Neu or Ha-Ras transgene. It has been shown that expression levels of these transgenes are typically low in non-neoplastic mammary glands, although they tend to be much higher in mammary tumors (Sinn et al, 1987; Muller et al, 1988; Bouchard et al, 1989; Yu et al, 2001). In addition, the transgenes are only expressed in MECs, not in the surrounding architectural and fat pad tissue, which make up for the bulk of the mammary gland in the virgin mouse (Figure 1E). Therefore, we used immunohistochemistry as well as immunoblotting analyses to detect the expression of the c-Neu or Ha-Ras transgene. Both assays showed no detectable difference in transgene expression in mammary glands between Pin1+/+ and Pin1−/− mice (Figure 1E and F). These results indicate that Pin1 ablation does not affect the expression of the transgenes.
Pin1 ablation effectively blocks the induction of cyclin D1 by Neu or Ras
It has been shown that in Neu- or Ras-transgenic mice, cyclin D1 is induced, which is essential for Neu- or Ras-induced breast cancer (Yu et al, 2001). We had previously shown that Pin1 positively regulates cyclin D1 levels by transcriptional activation and post-translation stabilization in response to growth signals in vitro (Ryo et al, 2001; Wulf et al, 2001; Liou et al, 2002). These results suggest that loss of Pin1 might block the induction of cyclin D1 in Neu- or Ras-transgenic mice. Therefore, we analyzed cyclin D1 expression in mammary glands derived from different genetically modified mice by immunoprecipitation, followed by immunoblotting analysis with anti-cyclin D1 antibodies. As shown (Yu et al, 2001; Liou et al, 2002), cyclin D1 was lower in Pin1−/− mice, but induced in Neu or Ras transgenic mice in the Pin1+/+ genetic background (Figure 3A). However, in the Pin1−/− genetic background, cyclin D1 was barely induced in Neu or Ras transgenic mice (Figure 3A). To confirm these results, we performed immunohistochemistry using anti-cyclin D1 antibodies. While cyclin D1 immunostaining signals were readily detected in MECs in Neu/Pin1+/+ or Ras/Pin1+/+, there were barely any cyclin D1 signals in Neu/Pin1−/− or Ras/Pin1−/− mice (Figure 3B). These results indicate that Pin1 ablation effectively blocks the induction of cyclin D1 by Neu or Ras.
Figure 3.
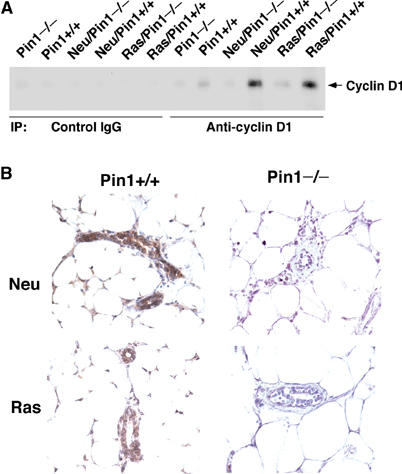
Pin1 ablation effectively blocks the induction of cyclin D1 by Neu or Ras. Protein lysates or tissue sections of mammary glands from virgin littermates of the specified genotypes were subjected to immunoprecipitation with anti-cyclin D1 or control IgG, followed by immunoblotting with anti-cyclin D1 antibodies (A) or immunohistochemistry with anti-cyclin D1 antibodies (B). Note that similar results were obtained in at least four to five mice from each group examined.
Pin1 ablation does not affect the differentiation of primary mouse MECs in 3D cultures
Given that Pin1 ablation is effective in suppressing breast cancer induced by Neu or Ras, we next established ex vivo cultures of primary MECs derived from these mice to determine whether Pin1 deletion affects the growth and differentiation properties of MECs. Our hypothesis was that these ex vivo cultures might allow us to discern growth patterns between MECs that were isolated from Ras or Neu transgenic animals and ‘programmed' to develop into breast cancer from those that were isolated from nontransgenic mice or transgenic mice in Pin1−/− background and ‘programmed' not to develop into breast cancer.
Primary MECs were isolated from morphologically normal mammary glands of wild-type mice or Neu or Ras transgenic mice in Pin1+/+ or Pin1−/− background at ages of 3–4 months. To examine the possibility that small microscopic foci of tumors that were macroscopically not yet detectable might affect the ex vivo culture, we performed histological examinations of the inguinal mammary gland that was contralateral to the mammary gland used for ex vivo cultures and did not find any invasive or in situ carcinoma at these early stages. Furthermore, we did not find any significant difference among these different genetic backgrounds when primary MECs were cultured on collagen-coated dishes (2D cultures) (Supplementary Figure S2). All cells appeared as a rather homogenous population that grew in an anchorage-dependent fashion, required growth factor for survival, and eventually stopped growing within 2 weeks ex vivo. We then plated primary MECs as single-cell suspension in reconstituted basement membrane using modified culture media (3D cultures), as described in Materials and methods. MECs from Pin1+/+ or Pin1−/− mice began to form globular colonies, and the cells in the center started to undergo apoptosis. These globular colonies then developed into organized and polarized acinus-like colonies with an intact lumen by day 10, followed by a stop in cell growth by day 20 of cultures (Figure 4A, data not shown). These orderly differentiated ‘Regular' colonies exhibited polarized expression of E-cadherin (Figure 4A) and showed lost or low-level Ki67 expression (Figure 5E). These in vitro differentiation patterns are similar to those described of human primary MECs and normal MEC cell line MCF10A (Debnath et al, 2002; Gudjonsson et al, 2002). They indicate that the deletion of Pin1 does not affect orderly and terminal differentiation of primary MECs ex vivo.
Figure 4.
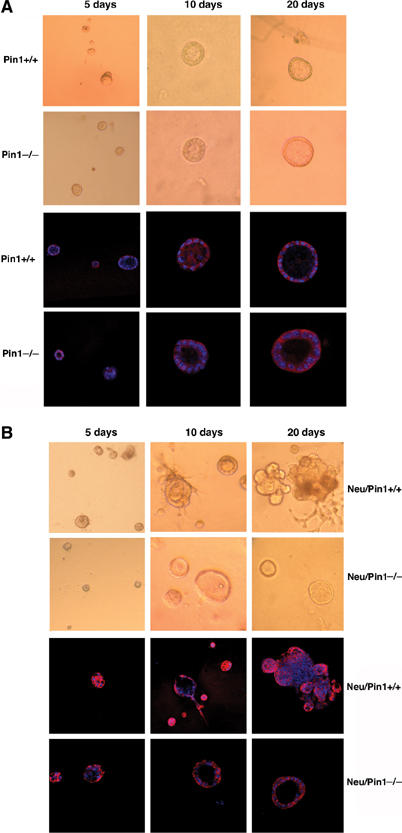
Pin1 ablation does not affect the differentiation of primary MECs in 3D cultures. Primary MECs were isolated from morphologically and histologically normal mammary glands of nontransgenic (A) or Neu transgenic (B) littermates in Pin1+/+ or Pin1−/− background at ages of 3–4 months. After culture in collagen-coated plates for 3–5 days, MECs were plated as single-cell suspension in reconstituted basement membrane (Matrigel) and analyzed at the indicated time points. Phase images were taken at 5, 10 and 20 days in culture, followed by fixation and confocal immunofluorescence staining with anti-E-cadherin antibodies.
Figure 5.
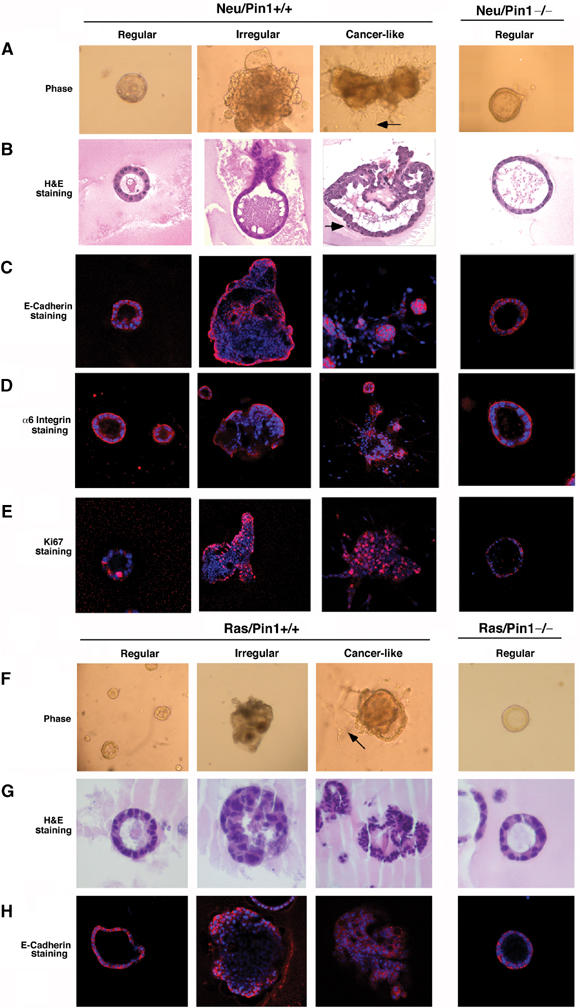
Characterization of abnormal differentiation patterns of MECs derived from Neu or Ras transgenic mice in Pin1+/+ but not Pin1−/− genetic background. Primary MECs were isolated from littermates with different genetic background and subjected to 3D cultures in reconstituted basement membrane for 20 days. Colonies were analyzed by phase-contrast microcopy to reveal the morphology (A, F), fixed and stained with hematoxylin and eosin to reveal the histology (B, G), stained with anti-E-cadherin antibodies to reveal the cell polarity (C, H), with anti-α6 integrin to reveal the base membrane integrity (D), with anti-Ki67 antibodies to reveal cell proliferation (E). Based on these assays, colonies are divided into three categories, namely ‘Regular', ‘Irregular' and ‘Cancer-like'. Arrows in (A, F) point to cell surface spikes protruding into the Matrigel, while arrows in (B) point to a dividing cell. (A, B, F, G) Light microscopy at × 200; (C–E, H), confocal fluorescence microscopy at × 200.
Primary MECs of Neu or Ras mice display various malignant properties, including forming tumors in nude mice, long before they develop tumors in vivo
We found distinct and strikingly different differentiation patterns for MECs derived from Neu or Ras transgenic as opposed to wild-type mice (Figures 4, 5 and Supplementary Figure S4), although there were considerable interindividual variations (Figure 6A–D). Neu and Ras MECs tended to have an overall higher plating efficiency and higher colony counts than nontransgenic cells (Figure 6A), suggesting that Ras and Neu transgenic animals may have an expanded MEC progenitor cell pool. The majority of primary MECs differentiate into well-differentiated round acinar colonies (Figures 5 and 6B), as is the case for almost all cells derived from wild-type mice (Figure 5A and F, first panel, 6B ‘Regular'). However, we observed the stochastic, independent emergence of large, multi-acinar colonies with filled lumen, which were rarely observed in nontransgenic MECs (Figure 5A and F, second panel, 6C ‘Irregular'). More interestingly, we also observed expansive colonies with invading cells emerging from the original acinar colonies (Figure 5A and F, third panel, 6D). These ‘Cancer-like' colonies were reproducibly observed in all primary MEC cultures derived from Neu or Ras transgenic mice, but not from any nontransgenic mice (Figure 5). H&E staining showed that the ‘Regular' colonies were formed by uniform MECs with basally polarized nuclear organization, small nuclei and abundant cytoplasm (Figure 5B and G). ‘Irregular' colonies were large, often had multiple acini and their lumia were characteristically filled (Figure 5B and G). ‘Cancer-like' colonies had disrupted cell polarity, cell surface spikes invading into the Matrigel, persistent mitotic figures, large and irregular nuclei, and high nuclear/cytoplasmic ratio (Figure 5B and G).
Figure 6.
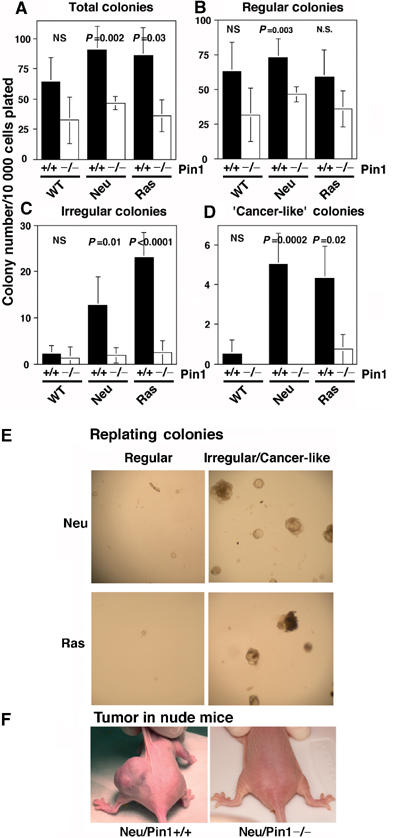
Non-neoplastic primary MECs of Neu or Ras mice in the Pin1+/+, but not Pin1−/−, background exhibit the malignant phenotype, including forming tumors in nude mice. (A–D) Primary MECs were isolated from littermates with different genetic background and subjected to 3D cultures in reconstituted basement membrane for 20 days. Assays were set up for three to five mice of each genotype, plated in quadruples. Colonies were categorized and counted under phase microscopy. The number of colonies in different categories per 10 000 cells plated was plotted as mean±s.d., with P-values being indicated. NS, no significant. (E) Secondary colony formation. ‘Regular' and ‘Irregular' colonies derived from Neu or Ras MECs in Pin1+/+ or Pin1−/− background in 3D cultures were picked separately at 21 days and trypsinized, followed by more rounds of 3D cultures for 20 days. (F) MEC colonies derived from Neu transgenic mice only in Pin1+/+, but not Pin1−/−, background give rise to tumors in nude mice. Day 21 colonies were harvested and resuspended in 100 μl MEGM/4% Matrigel, followed by injecting subcutaneously into female nude mice in duplicates each (right and left flank). Out of six injections of three mice each group, three tumors were derived from Neu/Pin1+/+ colonies, but Neu/Pin1−/− colonies did not generate any tumors.
Loss of E-cadherin expression, breaching of the basement membrane and continuous cell proliferation are some features of invasive breast cancer cells (D'Ardenne et al, 1991; Pavelic et al, 1992; Moll et al, 1993). Therefore, we next performed immunofluorescence staining in situ on these colonies with antibodies against E-cadherin, α6 integrins and Ki67. Consistent with the histological features, we found orderly and mostly basal expression of E-cadherin in the ‘Regular' colonies (Figure 5C and H). E-cadherin expression was lost in those cells that filled the lumen in ‘Irregular' colonies and even more obviously in ‘Cancer-like' colonies (Figure 5C and H). Furthermore, ‘Regular' acini had the orderly, basal α6 integrin expression encircling the acini fully (Figure 5D), a characteristic of normal mammary epithelial acini (D'Ardenne et al, 1991). This was in sharp contrast to disorganized α6 integrin expression in ‘Cancer-like' acini, where basal α6 integrin expression pattern was completely disrupted and epithelial cells broke through and invaded into the basal membrane-containing Matrigel (Figure 5D). Moreover, ‘Irregular' and ‘Cancer-like' colonies continued to express Ki67 beyond day 20 of culture, while ‘Regular' acini tended to be Ki67-negative (Figure 5E). These results together indicate that a significant fraction of primary Neu or Ras MECs fail to differentiate, but continuously grow into invasive colonies. Interestingly, these abnormal properties resemble those of the normal MECs MCF10A transformed with oncogenes in vitro (Muthuswamy et al, 2001; Debnath et al, 2002).
To further confirm that the ‘Regular' colonies are mostly composed of nondividing terminally differentiated cells and the ‘Irregular' colonies contain actively dividing cells, we picked ‘Regular' and ‘Irregular' colonies separately at day 21 and assayed for secondary colony formation. Although cells derived from ‘Regular' colonies gave rise to only very few ‘Regular' secondary acinar colonies, ‘Irregular' colonies gave rise to multiple ‘Irregular' colonies. These results indicate that the cells in these ‘Irregular' colonies retain their proliferative capacity, and that these colonies are indeed the result of clonal expansion of a distinct type of MECs (Figure 6E).
Finally, to confirm that these ‘Cancer-like' colonies indeed contain cancer cells, we surgically transplanted these colonies into nude mice to examine their ability to form tumors. At 1–2 months after the transplantation, tumors were visually identified at 50% of sites that were transplanted with ‘Cancer-like' colonies formed by MECs derived from Neu transgenic mice, but not from control MECs (Figure 6F, data not shown). Taken together, these results indicate that a significant fraction of primary MECs derived from morphologically and histologically normal mammary gland of Neu or Ras mice exhibit the malignant phenotype ex vivo.
Ablation of Pin1 suppresses early transformed properties of Neu or Ras MECs
Given that primary MECs derived from Neu- or Ras-transgenic mice display the transformed phenotype ex vivo long before they produce tumors in vivo, we then asked whether this transformed phenotype is affected by Pin1 ablation. Like wild-type cells, Neu/Pin1−/− MECs and Ras/Pin1−/− MECs tended to have lower colony counts than their Pin1+/+ counterparts (Figure 6A), indicating that loss of Pin1 function may prevent the increase in the MEC progenitor cells seen in Neu or Ras transgenic mice. Importantly, the frequency of ‘Irregular' colonies was greatly reduced in Neu/Pin1−/− or Ras/Pin1−/− MECs, as compared to those from Neu/Pin1+/+ or Ras/Pin1+/+ cells (Figures 5 and 6C). Furthermore, ‘Cancer-like' colonies were absent from Neu/Pin1−/− derived cultures and very rare in Ras/Pin1−/− cultures (Figures 5 and 6D). Moreover, colonies derived from Neu/Pin1−/− MECs failed to form any tumors when transplanted into nude mice (Figure 6F). These data indicate that Pin1 ablation effectively suppresses the early transformed phenotype of Ras or Neu MECs ex vivo.
Overexpression of cyclin D1 in Neu/Pin1−/− primary MECs rescues their malignant phenotype
The above results indicate that in the Pin1−/− genetic background, Neu or Ras fails to transform MEC and to induce breast cancer, as well as to increase cyclin D1 expression. Since cyclin D1 is essential for Neu or Ras to induce breast cancer (Yu et al, 2001; Bowe et al, 2002), we asked whether the failure of Neu or Ras to induce cell transformation and breast cancer in the Pin1−/− genetic background is due to the absence of cyclin D1 induction. To address this question, we used retroviral gene transfer with concomitant GFP expression to deliver cyclin D1 or its T286A mutant to primary MECs derived from Neu/Pin1−/− mice, as described (Debnath et al, 2002). Based on GFP expression, the infection efficiency was over 80% and transgene expression was confirmed by immunoblot (Supplementary Figure S4). Importantly, when infected with cyclin D1 or but not the control vector, Neu/Pin1−/− MECs generated ‘Cancer-like' colonies (Figure 7A), with a similar incidence as that of Neu/Pin1+/+ cells (Figures 7C and 6D). This ‘Cancer-like' phenotype was even more obvious when infected with the cyclin D1T286A mutant (Figure 7B and C), a mutant known to be more stable and potent in transforming cells (Alt et al, 2000). These results further support the idea that the inhibition of tumorigenesis by Pin1 ablation is due to the suppression of cyclin D1.
Figure 7.
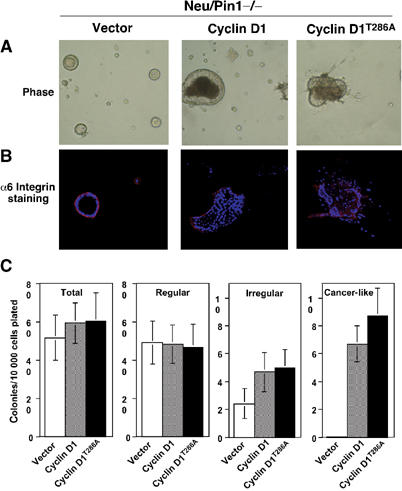
Expression of cyclin D1 or its T286A mutant restores the malignant phenotype of Neu/Pin1−/− primary MECs. Primary MECs derived from Neu/Pin1−/− mice were infected with retroviruses for either control, cyclin D1 or cyclin D1T286A, followed by 3D culture on Matrigel. Expression of cyclin D1 in infected MECs was monitored by Western blotting (Supplementary Figure S4). At day 21, colonies were analyzed by phase-contrast microcopy to reveal the morphology (A), fixed and stained with anti-α6 integrin antibodies to assay basement membrane integrity (B). Colonies were categorized and counted under phase microscopy (C).
Discussion
We have shown that Pin1 ablation is highly effective in preventing oncogenic Neu or Ras from inducing cyclin D1 and breast cancer in mice, although it neither affects transgene expression nor mammary gland development. Moreover, we have developed an ex vivo assay to uncover that a fraction of non-neoplastic primary MECs derived from Ras or Neu mice displays the transformed phenotype long before they develop breast cancer in vivo. These cells fail to differentiate normally, but continuously grow into large, irregular and invasive colonies that display various features of cancer cells, including forming tumors in nude mice. Importantly, this early transformed phenotype is not found in wild-type MECs and effectively suppressed in Neu or Ras transgenic mice by Pin1 ablation, which can be fully rescued by cyclin D1 overexpression. Therefore, the protective effect of Pin1 ablation is inherent to the MECs, which can undergo normal differentiation but are resistant to oncogenic transformation. This study provides the first in vivo and ex vivo evidence for an essential role of Pin1 in early events of tumorigenesis and supports Pin1 as an attractive anticancer target. The ex vivo assay can be used to study early events of breast cancer in genetically predisposed mice.
Our data demonstrate an essential role of Pin1 for tumorigenesis induced by Neu and Ras in vivo. The observations that Pin1 ablation does not affect transgene expression or mammary gland development suggest that it likely affects signaling pathways that are activated by oncogenic Ras or Neu. Indeed, Pin1 deletion suppresses the induction of cyclin D1 by oncogenic Ras or Neu, which are consistent with various previous in vitro studies. Pin1 expression is upregulated by c-Neu or Ha-Ras via E2F (Ryo et al, 2002). Pin1 upregulation in turn enhances signaling downstream from Neu and Ras and also stabilizes cyclin D1 (Ryo et al, 2001, 2003b; Wulf et al, 2001; Liou et al, 2002). These critical roles of Pin1 in transcriptional and post-translational regulation of cyclin D1 may explain why Pin1 deletion blocks the induction of cyclin D1 by Neu or Ras. The lack of cyclin D1 induction in Pin1 null mice is highly significant given that cyclin D1 is an essential downstream target for mammary tumorigenesis. Our findings that overexpression of cyclin D1 or its constitutively active mutant fully rescues the malignant phenotype of Neu/Pin1−/− MECs further underscore the importance of cyclin D1 in Neu-induced breast cancer. Furthermore, this is also consistent with the recently reported protective effect of the cyclin D1 ablation on breast cancers induced by Ha-Ras or c-Neu, but not Myc (Yu et al, 2001; Bowe et al, 2002). Therefore, one major molecular mechanism by which Pin1 ablation protects against breast cancer is the suppression of cyclin D1 induced by Ras or Neu.
To further determine how Pin1 ablation protects against breast cancer, we have established a colony formation assay of non-neoplastic primary MECs based on 3D cultures. Colony formation assays on semi-solid media have been used extensively in the study of hematopoietic progenitor cells (Senn et al, 1967; Lowenberg et al, 1993). The underlying principle is that single stem and progenitor cells can give rise to aggregates of terminally differentiated cells. These assays allow not only the distinction of different types of hematopoietic progenitor cells, but also the distinction between malignant cells (Senn et al, 1967; Lowenberg et al, 1993). In our approach, we have employed this concept to the study of primary MECs derived from mice carrying the oncogenic Neu or Ras transgene. It is based on the emerging evidence that many, if not all, breast cancers may be derived from a pool of MEC progenitor cells (Smalley and Ashworth, 2003). We hypothesized that a colony formation assay, in which only MECs that can divide, giving rise to colonies, might be a tool to distinguish MECs that have the potential to become malignant from those that differentiate normally.
Indeed, the 3D colony cultures can uncover that the growth properties of non-neoplastic primary MECs from Ras/Pin1+/+ or Neu/Pin1+/+ mice differed greatly from those from Ras/Pin1−/− or Neu/Pin1−/− mice. Mouse primary MECs from Pin1+/+ or Pin1−/− mice can differentiate into well-organized round acinar colonies, like primary human MECs and MCF10A (Debnath et al, 2002; Gudjonsson et al, 2002). However, primary MECs derived from Neu/Pin1+/+ or Ras/Pin1+/+ mice revealed a surprising pleomorphism in the 3D cultures. A significant fraction of these colonies derived from morphologically normal MECs ex vivo generated ‘Irregular' colonies that resemble those generated by MEC lines MCF10A transformed with oncogenes (Muthuswamy et al, 2001; Debnath et al, 2002). These colonies bear features of malignancy in that they have filled lumina with epithelial cells that lose E-cadherin expression, retain Ki67 expression and continue to grow into large and bizarre bodies. Furthermore, there are rare ‘Cancer-like' colonies that emerge, with loss of basement membranes and cell–cell junction, and invasion into the reconstituted basement membrane, persistent mitotic figures, large and irregular nuclei, and high nuclear/cytoplasmic ratio. Consistent with the emergence of tumors in vivo, these ‘Cancer-like' colonies emerge in a stochastic fashion. The low frequency of these ex vivo ‘Cancer-like' colonies is not surprising, given that the frequency of ‘in vivo' cancers is even two or more decimals lower. Significantly, the deletion of Pin1 effectively suppresses the ability of c-Neu and Ha-Ras to induce these ‘Cancer-like' colonies in vitro, which correlates with its ability to prevent Ras or Neu-induced breast cancer in vivo. Therefore, the protection of breast cancer by Pin1 deletion is inherent to MECs.
The ex vivo 3D assay of primary mouse MECs has several intriguing features, especially given the availability of a large number of well-established cancer mouse models. As a colony formation assay, it is based on the clonal expansion of single MECs, and therefore assays only those primary cells that have retained the capacity for proliferation. Therefore, this culture system may allow us to identify MECs that have already undergone the ‘programming' towards malignant transformation, but do not yet exhibit the malignant phenotype in vivo or in a 2D culture system. Furthermore, this culture system may afford to study the very early genetic events that precede phenotypic change and in vivo tumor formation. In addition, the ‘ex vivo' tumorigenesis may allow the investigator to study the growth patterns of disorganized, hyperplastic and invasive growth of primary MECs in the absence of other structural cell types of the organ and in the absence of other growth-modulating influences, that are usually present in the organism. Owing to its simplicity, it is a very controllable setting that may allow studying the contribution of the individual cellular and humoral components to the oncogenic process.
Although further studies are required to discern the downstream pathways and genes through which Pin1 regulates tumorigenesis, our study reveals an in vivo function for Pin1 as an essential component of the tumorigenesis pathway initiated by oncogenic activated Neu or Ras. Pin1 may therefore represent an attractive target for developing anticancer agents. Several other factors also support targeting Pin1 in cancer therapy. Pin1 is an enzyme with an extraordinarily high substrate specificity and well-defined active site. Furthermore, Pin1 is broadly overexpressed not only in breast cancer, but also in a number of other cancers such as prostate, lung and colon cancers (Wulf et al, 2001). In prostate cancer, Pin1 overexpression correlates with poor prognosis (Ayala et al, 2003). Given that Pin1 functions as a catalyst for many known oncogenic pathways rather than an oncogene itself (Lu, 2003), one can envision that incorporating a Pin1 inhibitor in classical or targeted anticancer treatment regimen may greatly enhance the efficacy of these agents.
Materials and methods
Animals
MMTV-v-Ha-Ras, MMTV-c-myc (Sinn et al, 1987) or MMTV-c-Neu (Muller et al, 1988; Bouchard et al, 1989) transgenic mice in FVB genetic background were purchased from Charles River Laboratories. Transgenic animals were bred with Pin1−/− mice, which are in mixed genetic background of 129:C57BL6, as described (Liou et al, 2002). Transgenic heterozygous animals were then bred with heterozygous females to obtain the experimental cohort that was followed for the development of tumors. Only virgin females were enrolled in the study and they were examined for the development of tumors twice weekly. For histological sections, the glands were fixed in Bouin's solution, and standard histology sections were stained with hematoxylin/eosin. The slides were reviewed with a rodent histopathologist. For whole-mount preparations, an inguinal gland was removed and stained with carmine red as described (Liou et al, 2002). Primary ducts, side branches and end buds were counted under a dissecting microscope. Immunohistochemistry to detect cyclin D1, Ha-Ras and c-Neu was performed as described (Liou et al, 2002).
Immunoblotting and immunohistochemistry
Immunoblotting and immunohistochemistry were performed as described (Wulf et al, 2001). Briefly, tissue lysates from inguinal mammary glands were prepared and spun, followed by incubation for 10 min at 4°C to allow for solidification of the fat component. The lower liquid phase was aspirated. Immunoprecipitation experiments were carried out using antibody-coupled agarose beads for the c-Neu antigen (sc-7301 AC) and the H-ras antigen (sc-35 AC), while immunoblotting was carried out with antibodies sc-520 for H-Ras, and anti-c-Neu Ab-3 from Oncogene. Polyclonal antibody sc 718 was used for immunoprecipitation and immunoblotting of cyclin D1 (sc 718), all antibodies except for anti-c-Neu were purchased from Santa Cruz Biotechnology. For immunohistochemistry, both tissue sections and matrigel-embedded cultures were fixed with Bouin's solution and paraffin-embedded. The sections were deparaffinized, rehydrated and subjected to antigen retrieval by boiling them for 10 min in 1 × Antigen retrieval solution (Vectra). Slides were blocked with PBS/5% goat serum, and then incubated with antibodies against Ha-Ras, cyclin D1 and c-Neu. They were then processed with biotinylated secondary antibody, and developed using the Vectorstain kit and DAB solution (Vector Labs).
Culture of primary mouse MECs ex vivo
Primary MECs were isolated from the morphologically and histologically normal mammary glands from virgin mice ages 3–4 months. The mammary glands were mechanically disaggregated, and then subjected to collagenase digestion (100 mg/ml) at 37°C with gentle shaking (100 rpm) in a total volume of 10 ml DMEM/F12 per mammary gland for 2 h. The digested material was then washed with HBSS/2% horse serum (Gibco) three times, followed by one wash with HBSS. The pellet was resuspended in trypsin and digested for another 10 min at 37°C, followed by neutralization with 10% horse serum, and a final wash with HBSS. The pellet was resuspended in MEGM and plated on 6 cm culture dishes that had been coated with collagen (50 mcg/ml). After 3–5 days in culture, the MECs were trypsinized, washed with HBSS/10% horse serum, counted and resuspended in DMEM/F12 supplemented with insulin 5 ng/ml, choleratoxin 100 ng/ml and hydrocortisone 500 ng/ml at 100 000 cells/ml. The suspension was then diluted 1:1 with MEGM/4% Matrigel (BD Biosciences 354230) and plated in Falcon Culture slides (BD 354118) that had been coated with Matrigel, at 10 000 cells per chamber. For immunofluorescence, the colonies in Matrigel were fixed with 2% freshly prepared paraformadehyde and analyzed using a BioRad confocal microscope, as described (Debnath et al, 2002; Ryo et al, 2002). For histology, the fixed colonies were paraffin-embedded and processed like tissue blocks. Antibodies used were anti-Ecadherin (Becton), rat anti-Ki67 (Dako) and rat anti-alpha 6 integrin (G0H3, Chemicon).
Retroviral gene transfer
Cyclin D1 and cyclin D1 286A in pBabe were gifts from Drs J Debnath and J Brugge. Murine cyclin D1 and its constitutively active mutant cyclin D1 286A were subcloned into the retroviral vector WIRES from Dr AM Kenney, in which the blasticidin resistance sequence had been replaced with GFP. The constructs were co-transfected with VSV and gag-pol into the packaging cell line 293 EBNA as described (Debnath et al, 2002). The primary MECs were infected on three consecutive days for 6 h each. On day 4, they were subjected to 3D culture assay.
Tumorigenicity assay
In all, 100 000 primary MECs isolated from Neu Pin1+/+ or Neu/Pin−/− mice were subjected to 3D cultures for 21 days. All developing structures were harvested and resuspended in 100 μl MEGM/4% Matrigel. They were injected subcutaneously under the back skin of 5–6-week-old NCr athymic female nude mice (Taconic), in duplicates each (right and left flank). Mice were observed weekly for the visual appearance of tumors at injection sites.
Statistical analysis
Nine cohorts were considered for the analysis of the end point, disease-free survival. The Kaplan–Meier method was used to estimate disease-free survival for each cohort. The significance of the differences in disease-free survival among the cohorts was determined using the log-rank (Mantel–Cox) test.
Supplementary Material
Supplementary Figures
Acknowledgments
We thank Drs J Brugge, T Hunter, B Neel, AM Kenney, S Gil, M Bentires-Alj and J Debnath for constructive advice or constructs. We thank the rodent histopathologist Dr R Bronson for expert review of the histological sections. Y-CL is a Canadian Institute of Health Research Fellow. KPL is a Pew Scholar, a Leukemia and Lymphoma Society Scholar and a consultant to Pintex. This study was supported by NIH Mentored Clinician Scientist Award K08CA093655 to GW and NIH grants R01GM56230 and RO1GM58556 to KPL.
References
- Alt JR, Cleveland JL, Hannink M, Diehl JA (2000) Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev 14: 3102–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber N, Doki Y, Han EK, Sgambato A, Zhou P, Kim NH, Delohery T, Klein MG, Holt PR, Weinstein IB (1997) Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res 57: 1569–1574 [PubMed] [Google Scholar]
- Ayala G, Wang D, Wulf G, Frolov A, Le R, Wheeler T, Sowadski JM, Lu KP, Bao L (2003) Pin1 is a novel prognostic marker in prostate cancer. Cancer Res 63: 6244–6251 [PubMed] [Google Scholar]
- Bao L, Sauter G, Sowadski J, Lu KP, Wang D (2004) Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am J Pathol 164: 1727–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ (1989) Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105: 223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411: 355–365 [DOI] [PubMed] [Google Scholar]
- Bouchard L, Lamarre L, Tremblay PJ, Jolicoeur P (1989) Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell 57: 931–936 [DOI] [PubMed] [Google Scholar]
- Bowe DB, Kenney NJ, Adereth Y, Maroulakou IG (2002) Suppression of Neu-induced mammary tumor growth in cyclin D1 deficient mice is compensated for by cyclin E. Oncogene 21: 291–298 [DOI] [PubMed] [Google Scholar]
- D'Ardenne AJ, Richman PI, Horton MA, McAulay AE, Jordan S (1991) Co-ordinate expression of the alpha-6 integrin laminin receptor sub-unit and laminin in breast cancer. J Pathol 165: 213–220 [DOI] [PubMed] [Google Scholar]
- Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS (2002) The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111: 29–40 [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ (1998) Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Zindy F, Sherr CJ (1997) Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin–proteasome pathway. Genes Dev 11: 957–972 [DOI] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C (1995) Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 9: 2364–2372 [DOI] [PubMed] [Google Scholar]
- Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G (1994) Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res 54: 1812–1817 [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW (2002) Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 115: 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Hinds PW, Dowdy SF, Eaton EN, Arnold A, Weinberg RA (1994) Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci USA 91: 709–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, Albanese C, Fu M, D'Amico M, Lin B, Watanabe G, Haines GK III, Siegel PM, Hung MC, Yarden Y, Horowitz JM, Muller WJ, Pestell RG (2000) Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol 20: 672–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou YC, Ryo R, Huang HK, Lu PJ, Bronson R, Fujimori F, Uchida U, Hunter T, Lu KP (2002) Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc Natl Acad Sci USA 99: 1335–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenberg B, van Putten WL, Touw IP, Delwel R, Santini V (1993) Autonomous proliferation of leukemic cells in vitro as a determinant of prognosis in adult acute myeloid leukemia. N Engl J Med 328: 614–619 [DOI] [PubMed] [Google Scholar]
- Lu KP (2003) Prolyl isomerase Pin1 as a molecular target for cancer diagnostics and therapeutics. Cancer Cell 4: 175–180 [DOI] [PubMed] [Google Scholar]
- Lu KP (2004) Pinning down cell signaling, cancer and Alzheimer's disease. TiBS 29: 200–209 [DOI] [PubMed] [Google Scholar]
- Lu KP, Hanes SD, Hunter T (1996) A human peptidyl–prolyl isomerase essential for regulation of mitosis. Nature 380: 544–547 [DOI] [PubMed] [Google Scholar]
- Lu KP, Liou YC, Zhou XZ (2002) Pinning down the proline-directed phosphorylation signaling. Trends Cell Biol 12: 164–172 [DOI] [PubMed] [Google Scholar]
- Lu PJ, Zhou XZ, Shen M, Lu KP (1999) A function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science 283: 1325–1328 [DOI] [PubMed] [Google Scholar]
- Moll R, Mitze M, Frixen UH, Birchmeier W (1993) Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol 143: 1731–1742 [PMC free article] [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P (1988) Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54: 105–115 [DOI] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS (2001) ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 3: 785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien LE (2002) Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3: 531–537 [DOI] [PubMed] [Google Scholar]
- Pavelic ZP, Pavelic L, Lower EE, Gapany M, Gapany S, Barker EA, Preisler HD (1992) c-myc, c-erbB-2, and Ki-67 expression in normal breast tissue and in invasive and noninvasive breast carcinoma. Cancer Res 52: 2597–2602 [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA 89: 9064–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo A, Liou YC, Lu KP, Wulf G (2003a) Prolyl isomerase Pin1: a catalyst for oncogenesis and a potential therapeutic target in cancer. J Cell Sci 116: 773–783 [DOI] [PubMed] [Google Scholar]
- Ryo A, Liou YC, Wulf G, Nakamura N, Lee SW, Lu KP (2002) Pin1 is an E2F target gene essential for the Neu/Ras-induced transformation of mammary epithelial cells. Mol Cell Biol 22: 5281–5295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo A, Nakamura N, Wulf G, Liou YC, Lu KP (2001) Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat Cell Biol 3: 793–801 [DOI] [PubMed] [Google Scholar]
- Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, Rottapel R, Yamaoka S, Lu KP (2003b) Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell 12: 1413–1426 [DOI] [PubMed] [Google Scholar]
- Senn JS, McCulloch EA, Till JE (1967) Comparison of colony-forming ability of normal and leukaemic human marrow in cell culture. Lancet 2: 597–598 [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA (1995) Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82: 621–630 [DOI] [PubMed] [Google Scholar]
- Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P (1987) Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell 49: 465–475 [DOI] [PubMed] [Google Scholar]
- Smalley M, Ashworth A (2003) Stem cells and breast cancer: a field in transit. Nat Rev Cancer 3: 832–844 [DOI] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV (1994) Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369: 669–671 [DOI] [PubMed] [Google Scholar]
- Wulf GM, Liou YC, Ryo A, Lee SW, Lu KP (2002) Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J Biol Chem 277: 47976–47979 [DOI] [PubMed] [Google Scholar]
- Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Lu KP (2001) Pin1 is overexpressed in breast cancer and potentiates the transcriptional activity of phosphorylated c-Jun towards the cyclin D1 gene. EMBO J 20: 3459–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld J, Xu J, Kuang J, Kirschner MW, Fischer G, Cantley LC, Lu KP (1997) Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 278: 1957–1960 [DOI] [PubMed] [Google Scholar]
- Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T, Counter CM, Nevins JR, Means AR, Sears R (2004) A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol 6: 308–318 [DOI] [PubMed] [Google Scholar]
- Yu Q, Geng Y, Sicinski P (2001) Specific protection against breast cancers by cyclin D1 ablation. Nature 411: 1017–1021 [DOI] [PubMed] [Google Scholar]
- Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio A, Voliniak S, Ronai Z, Blandino G, Schneider C, Del Sal G (2002) The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419: 853–857 [DOI] [PubMed] [Google Scholar]
- Zheng H, You H, Zhou XZ, Murray SA, Uchida T, Wulf G, Gu L, Tang X, Lu KP, Xiao ZXJ (2002) The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature 419: 849–853 [DOI] [PubMed] [Google Scholar]
- Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, Küllertz G, Stark M, Fischer G, Lu KP (2000) Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell 6: 873–883 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures


