Abstract
Protocadherins have homophilic adhesion properties and mediate selective cell–cell adhesion and cell sorting. Knockdown of paraxial protocadherin (PAPC) function in the Xenopus embryo impairs tissue separation, a process that regulates separation of cells of ectodermal and mesodermal origin during gastrulation. We show that PAPC can modulate the activity of the Rho GTPase and c-jun N-terminal kinase, two regulators of the cytoskeletal architecture and effectors of the planar cell polarity pathway. This novel signaling function of PAPC is essential for the regulation of tissue separation. In addition, PAPC can interact with the Xenopus Frizzled 7 receptor, and both proteins contribute to the development of separation behavior by activating Rho and protein kinase Cα.
Keywords: Frizzled 7, paraxial protocadherin, planar cell polarity, tissue separation, Xenopus laevis
Introduction
Morphogenetic processes are important for the establishment of the basic body plan during embryogenesis. Cells have to become motile, change their shape and their adhesive properties. Tissues acquire new positions in the embryo and must stay separated from adjacent cell populations. Such morphogenetic events have been studied extensively during amphibian gastrulation (Keller and Winklbauer, 1992). In the Xenopus embryo, the temporal sequence of defined morphogenetic movements and cell behaviors has been described in detail. In the ectoderm, epiboly movements precede gastrulation (Keller et al, 2000; Davidson et al, 2002). The endoderm moves toward the dorsal ectoderm in a process called vegetal rotation (Winklbauer and Schürfeld, 1999). During gastrulation, involuted mesoderm and overlaying neuroectoderm perform convergent–extension movements, which establish the anteroposterior axis in the embryo (Keller et al, 2000). Meso- and ectoderm also display tissue separation behavior in order to prevent mixing of mesendodermal and ectodermal cells (Wacker et al, 2000). The border that is formed between the neuroectoderm and the mesendoderm is morphologically identifiable as Brachet's cleft. The anterior portion of Brachet's cleft is formed by vegetal rotation and the posterior part is formed during involution of the mesoderm (Winklbauer and Schürfeld, 1999).
Intensive studies have focused on the signaling and structural components that regulate morphogenesis during gastrulation (Narumiya, 2003). The planar cell polarity (PCP) pathway, which was discovered in Drosophila, emerged in vertebrates as the major player in the regulation of convergent–extension movements (Huelsken and Birchmeier, 2001; Mlodzik, 2002). The PCP pathway in vertebrates is triggered by secreted glycoproteins of the Wnt-5a class, which bind to seven-transmembrane receptors of the Frizzled family. Downstream of the receptor, small GTPases such as Rho, Rac and Cdc42 as well as c-jun N-terminal kinase (JNK) are activated (Veeman et al, 2003). These molecules control rearrangements of the actin cytoskeleton and induce changes in cell shape in vertebrates as well as in invertebrates (Nobes and Hall, 1995). Perturbation of this noncanonical Wnt signaling cascade abolishes the polarization of mesodermal and ectodermal cells in Xenopus and Zebrafish thereby preventing convergent–extension movements (Heisenberg et al, 2000; Tada and Smith, 2000; Wallingford et al, 2000). A useful assay system to study PCP signaling in Xenopus is the animal cap explant culture. Prospective ectoderm from the animal pole of a blastula can be induced to form mesoderm and neuroectoderm under the influence of activin-type TGF-β growth factors (Tiedemann and Tiedemann, 1956; Green and Smith, 1990). Such induced animal caps also display convergent–extension movements, which results in a dramatic elongation of the explants.
Separation behavior, which is developed by the mesendoderm during involution, is also regulated through noncanonical Wnt signaling and involves in Xenopus Fz7 receptor function and the activation of protein kinase Cα (PKCα; Winklbauer et al, 2001). The regulation of tissue separation can easily be studied in Xenopus either by analyzing Brachet's cleft formation in the embryo or by using an in vitro separation assay (Wacker et al, 2000; Winklbauer et al, 2001). This test relies on the observation that cells of the dorsal mesoderm do not integrate into the ectodermal tissue, when placed on the inner layer of an animal cap explant. Dorsal mesoderm induced by activin or BVg1 also separates from ectoderm, whereas ventral mesoderm induced by FGF does not develop separation behavior (Wacker et al, 2000).
Morphogenetic processes also involve structural elements that directly modulate the adhesive properties of cells. Such molecules include fibronectin, integrins, cadherins and protocadherins (Hadeball et al, 1998; Kim et al, 1998; Reintsch and Hausen, 2001; Davidson et al, 2002; Frank and Kemler, 2002; Kuroda et al, 2002). At the beginning of gastrulation, paraxial protocadherin (PAPC) in Xenopus is expressed in the dorsal mesoderm and at later developmental stages in the somites (Kim et al, 1998). Axial protocadherin (AXPC) expression starts at the end of gastrulation and persists in the notochord (Kuroda et al, 2002). Both protocadherins are involved in homophilic cell–cell aggregation and cell sorting. PAPC is also involved in regulating gastrulation movements. A dominant-negative (dn) form of PAPC inhibits the elongation of animal cap explants without altering mesodermal patterning, implying a role in convergent–extension movements (Kim et al, 1998).
In this study, we present evidence that PAPC function is required for tissue separation during gastrulation in Xenopus. PAPC directly interacts with the Xfz7 receptor, which has been shown to regulate tissue separation through modulation of PKCα activity (Winklbauer et al, 2001). PAPC, however, cannot compensate for the loss of Xfz7 receptor function, indicating that these molecules have nonredundant functions in tissue separation. In addition to its adhesive properties, PAPC can exert signaling functions. It can modulate Rho and JNK activity, two intracellular targets of the PCP signaling cascade, and can thereby regulate morphogenesis during Xenopus gastrulation. We also show that PAPC and Xfz7 are sufficient to induce tissue separation behavior in ectodermal explants, independent of mesoderm induction.
Results
PAPC is expressed in the dorsal mesendoderm at gastrula stages
During Xenopus gastrulation, the involuting mesoderm develops the ability to separate from the ectoderm (Wacker et al, 2000). Recent studies demonstrated that Xfz7 receptor function is required in the mesoderm for this morphogenetic process (Winklbauer et al, 2001). Xfz7 mRNA, which is found preferentially on the dorsal side, however, is not restricted to the mesoderm (Figure 1B; Medina et al, 2000). One could therefore postulate that additional factors are localized in the mesoderm layer when tissue separation develops that contribute to this phenomenon. Such a component could be PAPC (Kim et al, 1998). Whole-mount in situ hybridizations on sagittally sectioned gastrulae demonstrated that in contrast to Xfz7, which is expressed in all three germ layers (Figure 1B), PAPC mRNA can be detected in the dorsal mesendoderm (Figure 1A).
Figure 1.
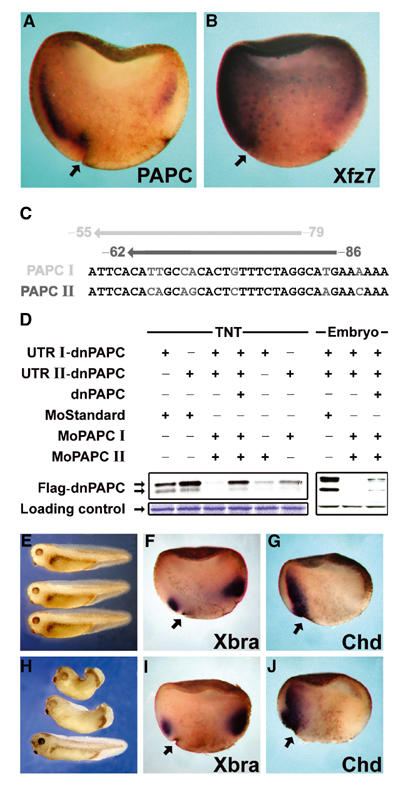
PAPC is expressed in the dorsal mesoderm and regulates morphogenesis during gastrulation. In situ hybridization of sagittally fractured gastrula-stage embryos showing, (A) expression of PAPC mRNA and (B) expression of Xfz7 mRNA. (C, D) Antisense morpholino oligonucleotides block translation of PAPC mRNA. (C) Morpholino oligonucleotides were designed to target the 5′UTRs of both PAPC alleles at the positions indicated. (D) MoPAPC I and II block translation of PAPC mRNA both in an in vitro coupled transcription/translation reaction (TNT) and in Xenopus embryos. (E–J) Knockdown of PAPC by microinjection of MoPAPC impairs morphogenesis without affecting mesoderm patterning. Xenopus embryos were injected at the 4–8 cell stage with a combination of both MoPAPC I and II (MoPAPC) into the DMZ. The embryos were analyzed by either in situ hybridization at early and midgastrula stages or were grown until the tailbud stage, at which time the phenotype was observed. (E) Uninjected embryos. (H) Embryos injected with 80 ng of MoPAPC exhibiting a range of phenotypic defects. Whole-mount in situ hybridizations for brachyury (Xbra; F, I) and chordin (chd; G, J) in control morpholino (MoCo)-injected embryos (F, G) and embryos injected with MoPAPC (I, J) are shown. Arrows indicate the dorsal blastopore lip.
PAPC knockdown does not affect mesoderm patterning
In order to analyze the function of PAPC during gastrulation, we employed a knockdown strategy. Using database research (http://www.ncbi.nih.gov/blast), we identified two different mRNAs coding for PAPC in Xenopus laevis and designated them as PAPC I and II. PAPC I was reported by Kim et al (1998) and PAPC II was reported as an EST entry (GenBank accession number BU911425 or IMAGp998L0914214Q3 RZPD; see also Materials and methods). We designed antisense morpholino oligonucleotides against each PAPC copy (MoPAPC I and II) and tested their ability to block the translation of the respective mRNAs (Figure 1C and D). The effect of MoPAPC I and II on translation of PAPC mRNA was demonstrated in an in vitro transcription/translation system as well as in the embryo. Translation of mRNAs derived from expression constructs that represent the two PAPC gene copies (UTR I-dnPAPC, UTR II-dnPAPC) was blocked by the respective antisense morpholino oligonucleotides. We noticed that each individual oligonucleotide was able to inhibit translation of both PAPC I and PAPC II mRNAs despite the fact that both oligonucleotides have only 21 out of 25 nucleotides in common. Embryos injected on the dorsal side with both PAPC morpholino oligos (MoPAPC I and II, henceforth referred to as MoPAPC) developed only mildly aberrant phenotypes. The closure of the blastopore was delayed, and in tadpole stages the trunk was slightly shortened. In rare cases, ‘spina bifida' was observed, but head structures formed normally (Figure 1H). The phenotypes indicate that early pattern formation was not affected, but morphogenetic processes were perturbed. This interpretation was supported by the use of marker genes expressed at gastrula stages. In early gastrulae, MoPAPC did not alter the expression of the pan-mesodermal marker brachyury (Xbra) and of chordin (chd), a marker for dorsal mesoderm (Figure 1F and 6). At midgastrula stages, Xbra expression was still not altered in MoPAPC-injected samples (Figure 1I). Chordin-expressing cells, however, remained on the outside in MoPAPC-injected embryos but were found in the involuted mesoderm in siblings injected with an unspecific antisense control morpholino oligonucleotide (MoCo; Figure 1J). This strongly suggested that gastrulation movements rather than patterning processes are affected in such embryos.
Figure 6.
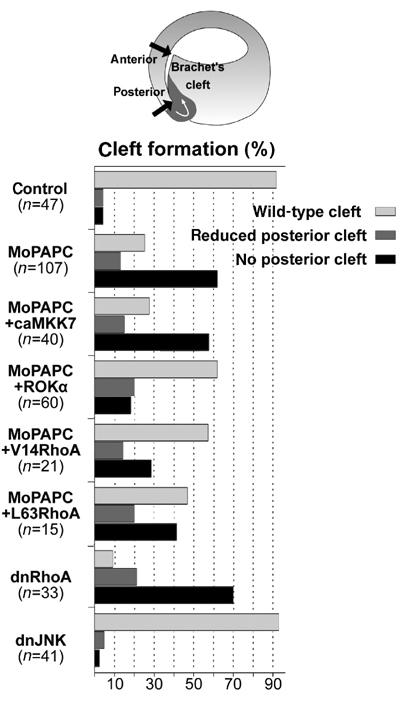
PAPC-mediated regulation of Brachet's cleft formation requires RhoA. MoPAPC (40 ng) injection impairs formation of posterior Brachet's cleft. This effect of MoPAPC could be rescued by coinjection of Rho-associated protein kinase alpha mRNA (ROKα, 100 pg), constitutively active RhoA mRNA (V14RhoA, 50 pg), or by constitutively active human RhoA DNA (L63RhoA, 15 pg). Conversely, inhibition of RhoA signaling by injection of dominant-negative RhoA mRNA (N19RhoA, 1 ng) impaired posterior cleft formation. Activation of JNK signaling by microinjection of 200 pg of constitutively active MKK7 mRNA did not rescue the effect of MoPAPC and inhibition of JNK signaling by injection of 1 ng of dominant-negative JNK mRNA did not affect Brachet's cleft formation.
Tissue separation depends on PAPC function
In the next step of the analysis, we asked whether a morpholino-based knockdown of PAPC function could impair tissue separation. This was tested by examining the formation of Brachet's cleft, the border between ectoderm and mesendoderm (Figure 2A). Embryos were injected at the 4–8 cell stage on the dorsal side with MoPAPC, grown until gastrula stages (NF stage 10.5), fixed, sagittally sectioned through the dorsal midline and cleft formation examined. Injection of MoPAPC, but not MoCo, caused defects in the posterior cleft, whereas the anterior part, which is formed during vegetal rotation, was never impaired (Figure 2B and C). The effect of MoPAPC was specific because the cleft defects could be rescued by injection of synthetic PAPC mRNA (Figure 2D). Knockdown of Xfz7 receptor function resulted in downregulation of PKCα activity and a loss of tissue separation (Winklbauer et al, 2001). MoXfz7-induced defects could be rescued by the overexpression of PKCα. Hence, we investigated whether Xfz7/PKCα and PAPC have overlapping functions during tissue separation. We performed experiments to rescue the effect of MoPAPC on posterior cleft formation by expression of Xfz7 or PKCα. Defects in cleft formation caused by MoPAPC could not be restored by overexpression of Xfz7 or PKCα (Figure 2E). Cleft inhibition was even exacerbated in MoPAPC- and Xfz7-injected embryos. Inhibition of posterior cleft formation was enhanced when PAPC and Xfz7 functions were knocked down simultaneously. Low amounts of MoPAPC or MoXfz7 alone had no effect on Brachet's cleft. When injected in combination however, posterior cleft formation was inhibited (Figure 2E). The synergistic inhibiting effect of MoPAPC and MoXfz7 on cleft formation as well as the inability of Xfz7 and PKCα to rescue Brachet's cleft in MoPAPC-injected embryos suggests that PAPC and the Xfz7/PKCα cassette do not have redundant functions in tissue separation. Knockdown of Xfz7 and PAPC affected only the posterior domain of the cleft, indicating that the anterior part is regulated in a different, as yet unknown manner.
Figure 2.
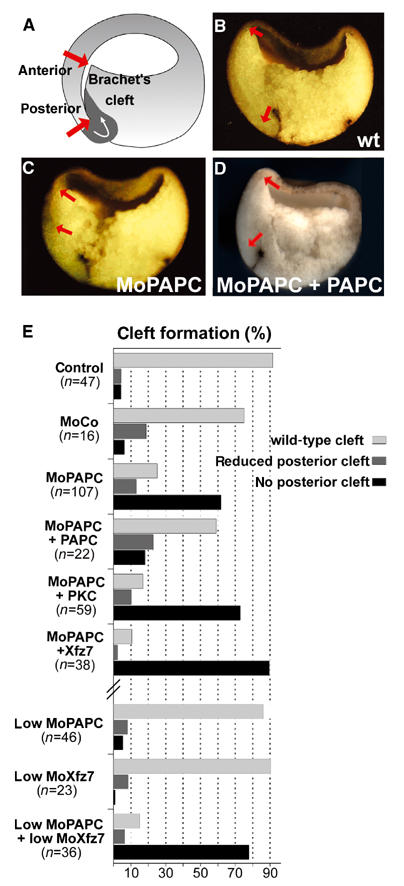
Knockdown of PAPC by microinjection of MoPAPC perturbs formation of Brachet's cleft. MoPAPC (40 ng) was injected into two dorso-vegetal blastomeres at the eight-cell stage. Formation of Brachet's cleft was analyzed in embryos at stage 10.5 that were fractured sagittally through the dorsal midline. (A) Scheme describing the formation of Brachet's cleft at stage 10.5. The length of Brachet's cleft from the anterior to the posterior end, indicated by red arrows, was analyzed in control morpholino (MoCo) as well as MoPAPC-injected embryos. (B) Brachet's cleft develops normally in MoCo-injected embryos. (C) Development of posterior cleft is abolished in the MoPAPC-injected embryos. (D) Injection of 1 ng of PAPC mRNA rescues the effect of MoPAPC. (E) Summary of the experiments showing the effect of MoPAPC injection on formation of Brachet's cleft. Formation of posterior part of Brachet's cleft was impaired by knockdown of PAPC and the effect of MoPAPC was rescued by coexpression of 1 ng of PAPC mRNA. Injection of MoCo had no effect on Brachet's cleft formation. Microinjection of either 300 pg of Xfz7 or 500 pg of XPKCα mRNA did not compensate for the loss of PAPC function. PAPC and Xfz7 act synergistically to control Brachet's cleft formation. Injection of low doses of MoPAPC (20 ng) or MoXfz7 (40 ng) did not affect cleft formation. These morpholinos when injected together abolished the posterior part of Brachet's cleft.
The role of PAPC in tissue separation was also demonstrated in animal cap assays (Figure 3A). When animal cap cells were injected with synthetic mRNA for BVg1, dorsal mesoderm was induced. This induction was reflected in the expression of the mesodermal marker genes Xbra and PAPC (Figure 3B). BVg1-induced animal cap tissue separated from ectoderm, but when PAPC function was knocked down by injection of MoPAPC, separation behavior was lost (Figure 3C and D). The effect of MoPAPC in this assay was specific because tissue separation could be restored by overexpression of PAPC (Figure 3E). Knockdown of PAPC function also impaired convergent–extension movements. BVg1-induced animal caps elongated dramatically due to convergent–extension movements. This elongation of animal caps was blocked by MoPAPC and the effect of MoPAPC was rescued by injection of synthetic PAPC mRNA (Figure 3F–I). The ability of MoPAPC to inhibit convergent–extension movements, which are controlled by PCP signaling, suggests that PAPC can modulate this signaling cascade.
Figure 3.
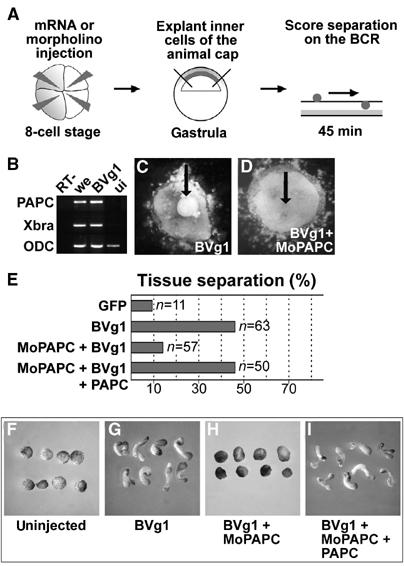
PAPC function is necessary for BVg1-induced separation behavior and elongation of animal caps. (A) Scheme describing the blastocoel roof assay for analysis of separation behavior. (B) RT–PCR analysis of Xbra and PAPC expression in BVg1 mRNA (50 pg)-injected animal caps. (C) BVg1-injected animal cap cells show separation behavior. (D) Coinjection of 80 ng MoPAPC abolishes BVg1-induced separation behavior. (E) Compilation of the in vitro separation assays. (F–I) BVg1-induced elongation of animal caps requires PAPC function. (G) Injection of 50 pg of BVg1 mRNA induces elongation of animal caps. (H) MoPAPC (80 ng) injection abolishes BVg1-induced elongation of animal caps. (I) Injection of 800 pg of mRNA for PAPC rescues the effect of MoPAPC.
Gain of Xfz7 and PAPC function is sufficient to induce tissue separation
Previously, we demonstrated that gain of Xfz7 function elicited tissue separation in a mesodermal background and knockdown of Xfz7 impaired Brachet's cleft formation (Winklbauer et al, 2001). Knockdown experiments demonstrated a requirement for PAPC in Brachet's cleft formation, as well. We therefore investigated whether Xfz7 and PAPC were sufficient to induce separation behavior. This was tested in an in vitro separation assay using animal cap explants. Embryos were injected into the animal pole with Xfz7 and PAPC mRNA, cells were removed from the inner layer of the animal cap at early gastrula stages and groups of cells were positioned on the inner surface of uninjected animal cap explants. After 45 min, uninjected or PAPC-injected ectodermal tissue had sunk into the blastocoel roof. Injection of Xfz7 alone induced separation behavior in only 30% of the explants (Winklbauer et al, 2001). In contrast, cells expressing PAPC in combination with Xfz7 stayed separated from the ectoderm in 80% of the explants, demonstrating that the combination of PAPC and Xfz7 functions is sufficient to induce separation behavior (Figure 4). Remarkably, mesoderm was not induced in PAPC- and Xfz7-injected animal cap cells (data not shown), indicating that separation behavior can be elicited in a nonmesodermal background. Coexpression of Xfz7 and another member of the protocadherin family, AXPC, did not result in tissue separation, demonstrating the specificity of PAPC in this process.
Figure 4.
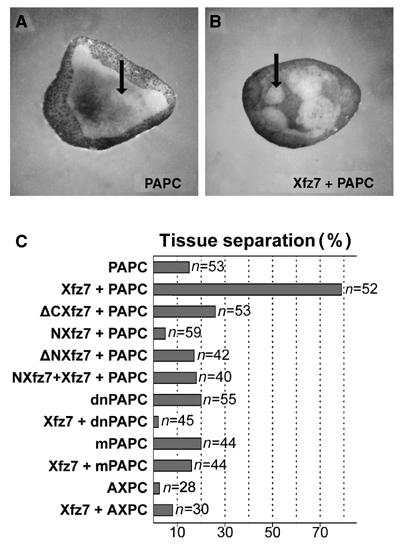
Xenopus PAPC and Xfz7 induce separation behavior in animal cap cells. (A) PAPC does not induce separation behavior when injected alone. (B) When Xenopus PAPC and Xfz7 are expressed together, they induce separation behavior of the animal cap cells. (C) Compilation of the experiments. RNAs for the following proteins were injected: Xenopus PAPC (400 pg), mPAPC (400 pg), dnPAPC (400 pg), Xfz7 (300 pg), ΔCXfz7 (300 pg), NXfz7 (300 pg), ΔNXfz7 (300 pg) and AXPC (600 pg).
Induction of separation behavior required full-length PAPC and Xfz7 proteins. Deletion of the N-terminus, the C-terminus or the C-terminus together with the transmembrane domains of Xfz7 (ΔN-Xfz7, ΔC-Xfz7 and NXfz7) abolished tissue separation. When Xfz7 and PAPC were coexpressed with the secreted ectodomain of Xfz7 (NXfz7), tissue separation was inhibited. Based on this dominant-negative effect of NXfz7, we hypothesized that PAPC and Xfz7 can interact. A membrane-tethered PAPC construct (mPAPC), which lacks most of the intracellular C-terminus but still possesses the adhesive properties of the wild-type molecule (Kim et al, 1998), as well as the secreted extracellular domain of PAPC (dnPAPC) is inactive in tissue separation (Figure 4C). This finding indicated that PAPC has signaling functions in addition to its adhesive properties.
PAPC can modulate the activities of JNK and Rho
The notion that PAPC is involved in signaling processes is supported by experiments demonstrating that in embryos in which lim-1 function is knocked down, PAPC expression is downregulated and Rho activation is blocked. Overexpression of PAPC rescues Rho activation in these embryos (Hukriede et al, 2003). Knockdown of PAPC function in BVg1-induced animal caps inhibited convergent–extension movements, which are controlled by the PCP pathway (Figure 3F–I). Furthermore, it has been shown that other members of the protocadherin family can contribute to PCP signaling in Drosophila as well as in vertebrates (Peifer and McEwen, 2002). Intracellular effectors of this noncanonical Wnt signaling cascade are Rho and JNK. We therefore investigated whether PAPC is able to modulate the activities of these signaling components.
First, we tested whether PAPC could modulate JNK activity. Synthetic mRNAs for HA-tagged JNK (HA-JNK) and PAPC were injected into animal blastomeres at the four-cell stage. HA-JNK was immunoprecipitated from embryo extracts and the activated form of JNK was detected with a monoclonal antibody recognizing the phosphorylated form of JNK (Phospho-JNK). Expression of HA-JNK alone did not result in its phosphorylation, but when PAPC was coexpressed, Phospho-JNK was detected. Knockdown of PAPC function in BVg1-induced animal cap tissue resulted in a reduction of Phospho-JNK (Figure 5A).
Figure 5.
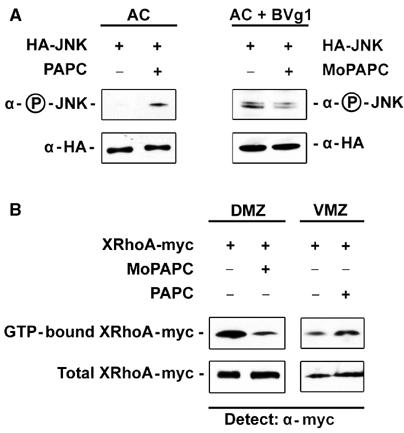
PAPC activates JNK and RhoA. For the JNK activity assay, 200 pg of HA epitope-tagged JNK mRNA was injected either alone or in combination with 500 pg of PAPC mRNA into the animal blastomeres at the four-cell stage. HA-JNK was precipitated from extracts of 20 embryos at stage 11 and activation of JNK was monitored by its phosphorylation using a phosphospecific antibody. (A) JNK is activated by PAPC mRNA expression as measured by JNK phosphorylation. Equal amounts of immunoprecipitated HA-JNK were loaded in each lane. Injection of 100 pg of BVg1 mRNA activates JNK and injection of MoPAPC inhibits BVg1-induced activation of JNK. (B) PAPC expression enhances RhoA activity in the VMZ and PAPC function is required for RhoA activity in the DMZ. For gain of PAPC function assays, myc epitope-tagged RhoA DNA was injected into the VMZ either alone or in combination with 1 ng of PAPC mRNA. Activated RhoA was precipitated from 30 early gastrulae by RBD-GST, blotted onto a nitrocellulose membrane and detected using anti-myc Ab1 monoclonal antibody. Whole embryo extract was used as a loading control. Overexpression of PAPC causes activation of RhoA in the VMZ as monitored by precipitation of active RhoA by RBD-GST. Loss of PAPC function by MoPAPC (80 ng) injection greatly reduced RhoA activity in the DMZ.
Recently, it was reported that Rho activity is lower in the ventral marginal zone (VMZ) than in the dorsal marginal zone (DMZ; Habas et al, 2003; Hukriede et al, 2003) in gastrulating Xenopus embryos. Hence, we injected PAPC mRNA into the VMZ and asked whether it could increase Rho activity. Myc-tagged Xenopus RhoA (RhoA-myc) was expressed in the VMZ either alone or in combination with PAPC. The activated, GTP-bound form of RhoA-myc was then precipitated from gastrula extracts using the GST-tagged Rho-binding domain of rhotekin (RBD-GST; Ren et al, 1999) and detected by an anti-myc antibody. PAPC expression increased Rho activity in the VMZ (Figure 5B). Consistent with this observation, Rho activity was reduced on the dorsal side when PAPC function was knocked down by the injection of MoPAPC (Figure 5B).
These experiments demonstrate that PAPC is able to modulate the activity of JNK and Rho and reveal novel signaling properties of the PAPC protein.
PAPC-mediated Rho activity is essential for tissue separation
Rho and JNK are regulators of the cytoskeleton and are involved in many aspects of morphogenesis. To test a plausible role for Rho and JNK in tissue separation, we expressed dominant-negative forms of RhoA and JNK on the dorsal side of embryos. Microinjection of dnRhoA inhibited the formation of Brachet's cleft, but dnJNK did not (Figure 6). We then tested whether activation of Rho or JNK could rescue the MoPAPC-induced defects in the embryos. Expression of constitutively active mitogen-activated protein kinase kinase 7 (caMKK7), a kinase acting directly upstream of JNK (Davis, 2000; Yamanaka et al, 2002), failed to restore cleft formation in MoPAPC-injected embryos. In contrast, an activated form of Rho-associated kinase (ROKα; Ohan et al, 1999), an effector of Rho, a constitutively active Xenopus RhoA (V14RhoA) and a constitutively active human Rho (L63RhoA) were able to rescue the MoPAPC-induced cleft defect (Figure 6).
These experiments demonstrate that PAPC-dependent activation of Rho is essential for the development of tissue separation. It also indicates that JNK and Rho, which both can be activated by PAPC, have nonredundant functions in the gastrulating Xenopus embryo.
The extracellular domains of Xfz7 and PAPC can interact
Experiments in animal cap explants demonstrated that gain of PAPC function in combination with Xfz7 is able to induce tissue separation, and that the extracellular as well as the intracellular domains of both proteins are required for this morphogenetic process. We therefore investigated whether PAPC and Xfz7 could interact biochemically with each other. Constructs were generated in which the extracellular domain of Xfz7 was tagged with the myc epitope (NXfz7-myc) and the ectodomain of PAPC was tagged with the flag epitope (flag-dnPAPC; Kim et al, 1998). Synthetic mRNA for both proteins was injected at the four-cell stage. Flag-dnPAPC was immunoprecipitated from gastrula-stage homogenates with an anti-flag antibody, separated on SDS–PAGE gels and a Western blot was probed with rabbit anti-myc antibody. NXfz7-myc co-precipitated with flag-dnPAPC, indicating that the Xfz7 and PAPC ectodomains interact (Figure 7). Neither NXfz7-myc nor flag-dnPAPC was precipitated by nonspecific mouse IgG. NXfz7-myc was not precipitated in the presence of a secreted extracellular domain of the closely related AXPC (flag-NAXPC), indicating that the Xfz7 and PAPC interaction is specific (Figure 7). This experiment shows that a biochemical interaction between Xfz7 and PAPC can occur in vivo (Figure 8).
Figure 7.
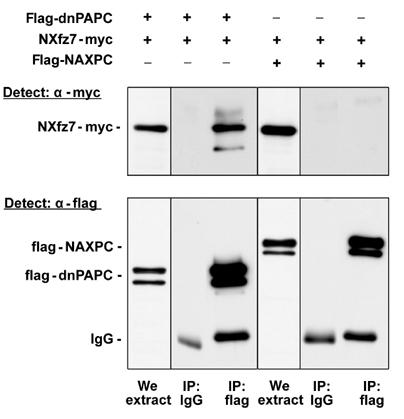
Xenopus PAPC and Fz7 interact. A flag epitope-tagged secreted extracellular domain of PAPC (flag-dnPAPC, 1 ng mRNA) or a flag epitope-tagged secreted extracellular domain of AXPC (flag-NAXPC, 1 ng mRNA) was injected with a myc epitope-tagged ectodomain of Fz7 (NXfz7-myc, 500 pg mRNA) into the animal blastomeres at the 2–4 cell stage. dnPAPC and NAXPC were immunoprecipitated at the gastrula stage using mouse anti-flag antibody and co-immunoprecipitated NXfz7 was detected using rabbit anti-myc antibody. NXfz7 and dnPAPC co-immunoprecipitated, indicating that they interact. The secreted ectodomain of Xfz7 does not precipitate with NAXPC, demonstrating the specificity of the PAPC and Fz7 interaction.
Figure 8.
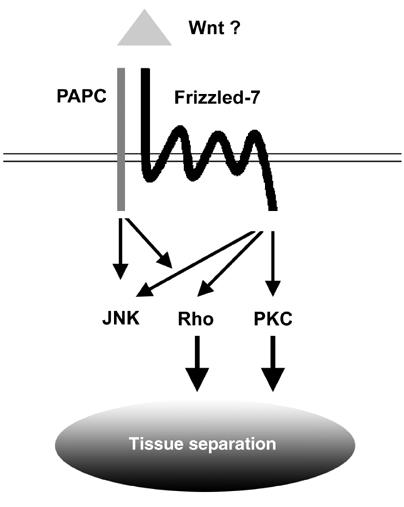
Xenopus Fz7 and PAPC regulate tissue separation. Xfz7 modulates PKCα activity and PAPC activates PCP signaling. The combined and balanced activities of PKCα and Rho are essential for tissue separation behavior in the dorsal mesoderm.
Discussion
PAPC is involved in tissue separation
During gastrulation, a virtual structure called Brachet's cleft is formed between meso- and ectoderm to prevent cell mixing. A well-documented mechanism for separating groups of cells or tissues during embryonic development is the differential expression of specific adhesion molecules. Cadherins and protocadherins display homophilic adhesion properties and contribute to cell and tissue sorting (Takeichi, 1991; Leckband and Sivasankar, 2000). To date, the expression of three adhesion molecules of the protocadherin family with distinct adhesive properties has been described in the early Xenopus embryo. NF-protocadherin is expressed in the embryonic ectoderm and mediates cell adhesion (Bradley et al, 1998). AXPC is expressed in the axial mesoderm and mediates the sorting of cells with notochord fate (Kim et al, 1998; Kuroda et al, 2002). PAPC is expressed in the presomitic mesoderm and mediates the establishment of the boundary between anterior and posterior parts of the somites (Kim et al, 2000).
Experimental evidence presented here supports the idea that PAPC is involved in tissue separation during Xenopus gastrulation. During the early- and midgastrula stages, PAPC mRNA can be detected in the involuting mesendoderm (Figure 1). Knockdown of PAPC function in the DMZ using antisense morpholino oligonucleotides blocks the formation of the posterior part of Brachet's cleft (Figure 2). These data agree with the finding that Xenopus embryos in which lim-1 function is inhibited show blocked PAPC expression and impaired tissue separation (Hukriede et al, 2003).
Further evidence for a role for PAPC in tissue separation comes from animal cap experiments. The ability of BVg1-induced animal cap tissue to separate from ectoderm is lost when PAPC function is knocked down by MoPAPC (Figure 3). Gain of combined function of PAPC and Xfz7 can induce tissue separation in animal cap explants. AXPC, a closely related protocadherin, fails to do so when combined with Xfz7, demonstrating the specificity of PAPC in this process (Figure 4). Taken together, these experiments demonstrate that PAPC function is required during gastrulation for the development of tissue separation behavior.
Mode of PAPC function: adhesion or signaling
Animal cap cells ectopically expressing PAPC separate from those overexpressing AXPC, and such selective adhesion could explain the role of PAPC in tissue separation (Kim et al, 1998). Here we present evidence that the adhesive properties of PAPC alone are not sufficient to explain its function in tissue separation. PAPC in combination with Xfz7 can elicit separation behavior in animal caps. In contrast, mPAPC, a C-terminal deletion that still exhibits full adhesive properties (Kim et al, 1998), is inactive in this assay, demonstrating the importance of the cytoplasmic domain and arguing for a role in signaling events (Figure 4). It has been demonstrated that the C-termini of various protocadherins can interact with components of signaling pathways such as Fyn tyrosine kinase (Kohmura et al, 1998), protein phosphatase 1 (PP1; Yoshida et al, 1999) and the adapter molecule disabled-1 (Homayouni et al, 2001). Xenopus NF-protocadherin, which lacks the entire C-terminus, however, acts as a dominant-negative molecule and perturbs cell–cell adhesion in the ectodermal layer (Bradley et al, 1998; Heggem and Bradley, 2003).
For the development of separation behavior in the mesoderm during Xenopus gastrulation, the activities of full-length PAPC as well as the full-length Xfz7 receptor are essential and the two molecules synergize in the process of tissue separation (this work and Winklbauer et al, 2001). Immunoprecipitation experiments demonstrated that the extracellular domains of Xfz7 and PAPC can interact specifically (Figure 7). When the interaction of PAPC and Xfz7 in animal caps was perturbed by introducing a secreted ectodomain of Xfz7 (NXfz7), separation behavior was lost (Figure 4).
During tissue separation, Xfz7 modulates PKCα (Winklbauer et al, 2001) but reduced PAPC levels do not influence PKCα activity (Hukriede et al, 2003). Gain of PAPC function increases Rho and JNK activities in the VMZ or animal cap explants (Figure 5). Knockdown of PAPC function on the dorsal side of the embryo reduces the amount of active Rho. JNK activation is inhibited in BVg1-induced animal caps by MoPAPC (Figure 5).
These experiments indicate that the signaling activity of PAPC is required for the development of separation behavior. Since MoPAPC-induced cleft defects can be rescued neither by Xfz7 nor PKCα, one can conclude that PAPC and Xfz7 have nonredundant signaling functions in the process of tissue separation.
PAPC signaling and regulators of the cytoskeleton
Rearrangements of the cytoskeleton are the basis of changes in cell shape and morphogenetic processes during embryogenesis. RhoA and JNK are two proteins that can mediate such changes in the cytoskeleton. These effector molecules are utilized by several signal transduction pathways including the PCP, which regulates convergent–extension movements in Xenopus and Zebrafish. PAPC can modulate Rho and JNK activity, which then results in changes morphogenetic cell behavior. Knockdown of PAPC in BVg1-stimulated animal caps blocks convergent–extension movements, indicating that PCP signaling is perturbed. Further evidence that protocadherins can regulate PCP in vertebrates comes from the finding that defective protocadherin 15 causes deafness due to malformations of the hair cell stereocilia in the inner ear in mice and humans (Alagramam et al, 2001a, 2001b). A connection between protocadherins and PCP signaling has also been described in Drosophila. Frizzled receptors and the protocadherins Fat, Dachsous and Flamingo interact genetically in the establishment of PCP (Clark et al, 1995; Das et al, 2002; Peifer and McEwen, 2002; Yang et al, 2002; Ma et al, 2003). Future experiments will have to clarify whether PAPC is a component of the PCP signaling cascade in Xenopus.
Modulation of PAPC-mediated Rho activity also affects tissue separation, another morphogenetic cell behavior in the gastrulating Xenopus embryo. Brachet's cleft formation is impaired by MoPAPC or dnRho, and this defect can be rescued by overexpression of constitutively active RhoA. dnJNK does not inhibit cleft formation and the MoPAPC-induced separation defects cannot be rescued by caMKK7, a kinase acting directly upstream of JNK (Figure 6). This indicates that Rho and JNK exert nonredundant functions in the gastrulating embryo. Recent experiments describing overlapping as well as nonredundant functions of Rho, Rac and Cdc42 in the Xenopus mesoderm support this interpretation (Tahinci and Symes, 2003).
Based on the results presented here, the following model for tissue separation can be proposed. In the dorsal mesoderm, Xfz7 activates PKCα, and PAPC activates Rho and JNK. The combined and balanced activities of PKCα and Rho are essential for tissue separation.
Materials and methods
Xenopus embryo manipulations
Xenopus eggs were obtained from females injected with 300 IU of human chorionic gonadotrophin (Sigma), and were fertilized in vitro. Eggs were dejellied with 2% cysteine hydrochloride pH 8 and embryos were microinjected in 1 × MBS-H (88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, 0.41 mM CaCl2, 0.33 mM Ca(NO3)2, 10 mM HEPES pH 7.4, 10 μg/ml streptomycin sulfate and 10 μg/ml penicillin). The embryos were cultured in 0.1 × MBS-H and staged according to Nieuwkoop and Faber (1967).
In vitro separation assay and analysis of Brachet's cleft formation
Blastocoel roof assay for separation behavior of animal cap cells was performed as described (Wacker et al, 2000). Embryos were injected into the animal pole with synthetic mRNA or morpholino antisense oligonucleotide at the four-cell stage. At stage 10.5, the inner animal cap cells derived from injected embryos were placed on uninjected caps and the separation behavior was analyzed 45 min later. GFP mRNA was coinjected as a tracer.
For analysis of Brachet's cleft formation, embryos were injected into two dorso-vegetal blastomeres with morpholino antisense oligonucleotide or synthetic mRNA at the eight-cell stage. The length of Brachet's cleft from the anterior to the posterior end was analyzed in embryos at stage 10.5 that were fractured sagittally through the dorsal midline.
DNA constructs and mRNA synthesis
Flag-tagged constructs containing the antisense morpholino oligonucleotide consensus sequences (UTR I/II-dnPAPC) were generated by partially amplifying the 5′UTR of pCS2+·FL-PAPC (Kim et al, 1998) and of IMAGp998L0914214Q3 (RZPD) with UTR I-F (5′-GAATTCCCAGAGATGAACTC-3′), UTR I-R (5′-CTTGAAGAATCAAAGTTGCACC-3′), UTR II-F (5′-CTATTTAGGTGACACTATAGAAC-3′), UTR II-R (5′-CTTGTGACTTTGAAGTAAAGTAG-3′) and cloning of these fragments into pCRII-TOPO® (Invitrogen). Subsequently, the EcoRI fragments were directionally subcloned into the EcoRI site of pCS2+·flag-DN-PAPC (Kim et al, 1998). For rescue experiments, a full-length PAPC construct (PAPC) lacking a 5′UTR was constructed by internal replacement of the StyI–NotI fragment of pCS2+·FL-PAPC and pCS2+·M-PAPC (Kim et al, 1998). For immunoprecipitation experiments, a secreted flag-tagged form of AXPC (flag-NAXPC) was generated by amplifying the entire extracellular domain of AXPC (amino acids 20–813, lacking the signal peptide) using pBSK·AXPC (Kuroda et al, 2002) as template and the following primers: forward 5′-TAATCCTCGAGGGATCTTCCACCAAGGTG-3′ and reverse 5′-TACCATCTAGACCGTTGTTTGCTTCGCTC-3′. The PCR fragment was then subcloned into the XhoI/XbaI sites of pCS2+-chd SP/flag (Kim et al, 1998) resulting in pCS2+·flag-NAXPC. Myc epitope-tagged Xenopus NXfz7 was constructed by amplifying the extracellular domain of Xfz7 and the fun domain of Frzb (Medina et al, 2000) and cloning them into pCS2+MT. Myc epitope-tagged Xenopus RhoA was constructed by amplifying the coding sequence of RhoA (Wunnenberg-Stapleton et al, 1999) by PCR and cloning it into pCS2+MT. Both NXfz7-myc and RhoA-myc have the myc epitope tag at their C-terminus. All of the constructs were confirmed by sequencing.
Capped mRNAs were synthesized from linearized plasmids using the mMessage mMachine Kit (Ambion). Xfz7, NXfz7, NXfz7-myc, ΔNXfz7, ΔCXfz7 and Myc epitope-tagged EGFP constructs were linearized with Asp718 and mRNA was synthesized using the SP6 mMessage mMachine Kit. PAPC, flag-dnPAPC, mPAPC, flag-NAXPC, PKCα, HA epitope-tagged JNK and dominant-negative JNK constructs were linearized with NotI and mRNA was synthesized using the SP6 mMessage mMachine Kit. Xenopus RhoA, constitutively active V14RhoA and dominant-negative N19RhoA constructs were linearized with XhoI and mRNA was synthesized using the T3 mMessage mMachine Kit. The other constructs that were linearized and mRNAs synthesized were as follows: constitutively active MKK7 (linearized with XbaI and transcribed with SP6), BVg1 (linearized with EcoRI and transcribed with SP6), Xenopus ROKα (linearized with ApaI and transcribed with SP6) and AXPC (linearized with XhoI and transcribed with the T3 mMessage mMachine Kit).
Morpholino antisense oligonucleotide design and synthesis
By database research, we identified two PAPC alleles: PAPC I (Kim et al, 1998) and PAPC II (IMAGp998L0914214Q3 from RZPD Deutsches Resourcenzentrum fuer Genomforschung GmbH, GenBank accession number BU911425). Consequently, two morpholino oligonucleotides were designed to complement the 5′UTR regions of both alleles between −55 and −79 (PAPC I) and between −62 and −86 (PAPC II) upstream of the corresponding AUGs. Morpholino antisense oligonucleotides were synthesized by Gene Tools LLC.
In vitro translation and Western blot analysis
For in vitro transcription and translation, the TNT® Coupled Reticulocyte Lysate System (Promega) was used according to the manufacturer's instructions and proteins were detected by incorporating [35S]methionine. The use of equal amounts of protein was verified by Coomassie® Brilliant Blue R-250 staining. For Western blot analysis, Xenopus embryos were extracted in 20 mM HEPES pH 7.4, 130 mM NaCl, 2 mM EDTA, 1 mM EGTA, 1% NP-40 supplemented with a cocktail of protease inhibitors (Calbiochem). Proteins were separated by denaturing SDS–PAGE using a 10% polyacrylamide gel. Subsequently, proteins were transferred to a nitrocellulose membrane and detected with monoclonal mouse anti-flag antibody (M2, Sigma).
RT–PCR
Total RNA was prepared from embryos or animal cap explants with Trizol® reagent (Invitrogen). First strand cDNA primed with random hexamers was synthesized with H minus M-MuLV reverse transcriptase (Fermentas) and PCR was performed using standard conditions and the following sets of primers: PAPC-F (5′-CACCTGGACAATTGTGC-3′) and PAPC-R (5′-ATGGTTCGGTATGTGCAGG-3′), 55°C, 23 cycles; Xbra-F (5′-CACAGTTCATAGCAGTGACCG-3′) and Xbra-R (5′-TTCTGTGAGTGTACGGACTGG-3′), 65°C, 28 cycles; ODC-F (5′-GTCAATGATGGAGTGTATGGATC-3′) and ODC-R (5′-TCCATTCCGCTCTCCTGAGCAC-3′), 65°C, 25 cycles.
In situ hybridization
Hemi-section in situ hybridization and antisense probe preparation were carried out as described (Epstein et al, 1997). Digoxigenin-labeled antisense RNA was synthesized by linearizing plasmids containing Chd (Sasai et al, 1994) and PAPC (Kim et al, 1998) with EcoRI and XbaI respectively, and transcribed using T7 RNA polymerase. The plasmid containing Xbra (Smith and Harland, 1991) was linearized with SalI and transcribed with SP6 polymerase. The Xfz7 (Medina et al, 2000) construct was linearized with NotI and transcribed with T3 RNA polymerase.
JNK activity assay
Activation of JNK was determined by monitoring its phosphorylation. In all, 20 Xenopus embryos were injected with 200 pg of JNK-HA mRNA into the animal pole at the four-cell stage. The embryos were grown until stage 11, at which time protein was extracted in 10 mM HEPES pH 7.4, 150 mM NaCl, 2 mM EDTA, 1 mM EGTA, 1% NP-40, supplemented with a cocktail of protease inhibitors (Calbiochem), a cocktail of phosphatase inhibitors (phosphatase inhibitor cocktail I and II, Sigma) and 100 nM okadaic acid (Sigma). JNK-HA was immunoprecipitated using 1 μg of anti-HA high-affinity antibody (clone 3F10, Roche). Immunoprecipitated JNK-HA was loaded on SDS–PAGE, blotted and probed with an anti-phospho-JNK (G9, Cell Signaling) or anti-HA antibody. For the detection of phosphorylated JNK, SuperSignal West Femto reagent (Pierce) was used.
RhoA activity assay
For Rho activity assays, Xenopus embryos were injected either into the VMZ (for gain of function) or DMZ (for loss of function) at the four-cell stage. Myc epitope-tagged XRhoA DNA (50 pg) was either injected alone or in combination with 1 ng of PAPC mRNA or 80 ng MoPAPC. The embryos were grown until stage 10.5 at which time embryo extract was prepared in the Rho lysis buffer (50 mM Tris pH 7.2, 1% Triton X-100, 500 mM NaCl, 10 mM MgCl2 and a cocktail of proteinase inhibitors). GST-RBD binding assays were performed as described (Ren et al, 1999) and samples were resolved using 12% SDS–PAGE and immunoblotted with mouse anti-myc (Ab1, Calbiochem) antibody. Whole embryo extracts were used as loading controls.
Co-immunoprecipitation
Xenopus embryos were injected with 500 pg NXfz7-myc, 1 ng flag-dnPAPC or 1 ng flag-NAXPC mRNA at 2–4 cells stage. The embryos were grown until gastrula stage and protein was extracted in NP-40 lysis buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% NP-40, 5% glycerol with a cocktail of proteinase inhibitors). The embryo extract was incubated for 2 h with 5 μg of anti-flag (M2, Sigma) or 5 μg of mouse IgG (Sigma). The samples were centrifuged and 30 μl of protein G beads (Pierce) was added to the supernatant. The beads were incubated with the protein extract for 2 h, centrifuged and washed four times with NP-40 lysis buffer (containing 0.5% NP-40). The immunoprecipitates were separated on 10% SDS–PAGE. For Western blotting, a rabbit anti-myc antibody (kind gift of François Fagotto) was used.
Acknowledgments
We thank A Alonso, M Asashima, K Cho, E De Robertis, F Fagotto, A Hall, M Kühl, J Liu, A Marchini, D Melton, R Moon, E Nishida, M Schwarz, J Smith and A Yamamoto for providing plasmids and reagents, U Müller and K Linsmeier for technical support and S Cramton for critically reading the manuscript. We thank A Schambony and D Wedlich for sharing unpublished data, R Winklbauer for stimulating discussions and P Hausen for support. RKS is supported by a postdoctoral fellowship from the Faculty of Medicine, University of Heidelberg.
References
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP (2001a) The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet 27: 99–102 [DOI] [PubMed] [Google Scholar]
- Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CR, Lowry RB, Knaus R, Van Laer L, Bernier FP, Schwartz S, Lee C, Morton CC, Mullins RF, Ramesh A, Van Camp G, Hageman GS, Woychik RP, Smith RJ, Hageman GS (2001b) Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet 10: 1709–1718 [DOI] [PubMed] [Google Scholar]
- Bradley RS, Espeseth A, Kintner C (1998) NF-protocadherin, a novel member of the cadherin superfamily, is required for Xenopus ectodermal differentiation. Curr Biol 8: 325–334 [DOI] [PubMed] [Google Scholar]
- Clark HF, Brentrup D, Schneitz K, Bieber A, Goodman C, Noll M (1995) Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev 9: 1530–1542 [DOI] [PubMed] [Google Scholar]
- Das G, Reynolds-Kenneally J, Mlodzik M (2002) The atypical cadherin Flamingo links Frizzled and Notch signaling in planar polarity establishment in the Drosophila eye. Dev Cell 2: 655–666 [DOI] [PubMed] [Google Scholar]
- Davidson LA, Hoffstrom BG, Keller R, DeSimone DW (2002) Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin alpha(5)beta(1), fibronectin, and tissue geometry. Dev Biol 242: 109–129 [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- Epstein M, Pillemer G, Yelin R, Yisraeli JK, Fainsod A (1997) Patterning of the embryo along the anterior–posterior axis: the role of the caudal genes. Development 124: 3805–3814 [DOI] [PubMed] [Google Scholar]
- Frank M, Kemler R (2002) Protocadherins. Curr Opin Cell Biol 14: 557–562 [DOI] [PubMed] [Google Scholar]
- Green JB, Smith JC (1990) Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature 347: 391–394 [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X (2003) Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 17: 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeball B, Borchers A, Wedlich D (1998) Xenopus cadherin-11 (Xcadherin-11) expression requires the Wg/Wnt signal. Mech Dev 72: 101–113 [DOI] [PubMed] [Google Scholar]
- Heggem MA, Bradley RS (2003) The cytoplasmic domain of Xenopus NF-protocadherin interacts with TAF1/set. Dev Cell 4: 419–429 [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW (2000) Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405: 76–81 [DOI] [PubMed] [Google Scholar]
- Homayouni R, Rice DS, Curran T (2001) Disabled-1 interacts with a novel developmentally regulated protocadherin. Biochem Biophys Res Commun 289: 539–547 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W (2001) New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 11: 547–553 [DOI] [PubMed] [Google Scholar]
- Hukriede NA, Tsang TE, Habas R, Khoo PL, Steiner K, Weeks DL, Tam PP, Dawid IB (2003) Conserved requirement of Lim1 function for cell movements during gastrulation. Dev Cell 4: 83–94 [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P (2000) Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond [Biol] 355: 897–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Winklbauer R (1992) Cellular basis of amphibian gastrulation. Curr Top Dev Biol 27: 39–89 [DOI] [PubMed] [Google Scholar]
- Kim SH, Jen WC, De Robertis EM, Kintner C (2000) The protocadherin PAPC establishes segmental boundaries during somitogenesis in Xenopus embryos. Curr Biol 10: 821–830 [DOI] [PubMed] [Google Scholar]
- Kim SH, Yamamoto A, Bouwmeester T, Agius E, De Robertis EM (1998) The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development 125: 4681–4690 [DOI] [PubMed] [Google Scholar]
- Kohmura N, Senzaki K, Hamada S, Kai N, Yasuda R, Watanabe M, Ishii H, Yasuda M, Mishina M, Yagi T (1998) Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron 20: 1137–1151 [DOI] [PubMed] [Google Scholar]
- Kuroda H, Inui M, Sugimoto K, Hayata T, Asashima M (2002) Axial protocadherin is a mediator of prenotochord cell sorting in Xenopus. Dev Biol 244: 267–277 [DOI] [PubMed] [Google Scholar]
- Leckband D, Sivasankar S (2000) Mechanism of homophilic cadherin adhesion. Curr Opin Cell Biol 12: 587–592 [DOI] [PubMed] [Google Scholar]
- Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD (2003) Fidelity in planar cell polarity signalling. Nature 421: 543–547 [DOI] [PubMed] [Google Scholar]
- Medina A, Reintsch W, Steinbeisser H (2000) Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech Dev 92: 227–237 [DOI] [PubMed] [Google Scholar]
- Mlodzik M (2002) Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet 18: 564–571 [DOI] [PubMed] [Google Scholar]
- Narumiya S (2003) Signal transduction underlying cell morphogenesis. J Biochem (Tokyo) 134: 305–307 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J (1967) Normal Table of Xenopus laevis (Daudin). Amsterdam: North Holland [Google Scholar]
- Nobes CD, Hall A (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81: 53–62 [DOI] [PubMed] [Google Scholar]
- Ohan N, Agazie Y, Cummings C, Booth R, Bayaa M, Liu XJ (1999) RHO-associated protein kinase alpha potentiates insulin-induced MAP kinase activation in Xenopus oocytes. J Cell Sci 112: 2177–2184 [DOI] [PubMed] [Google Scholar]
- Peifer M, McEwen DG (2002) The ballet of morphogenesis: unveiling the hidden choreographers. Cell 109: 271–274 [DOI] [PubMed] [Google Scholar]
- Reintsch WE, Hausen P (2001) Dorsoventral differences in cell–cell interactions modulate the motile behaviour of cells from the Xenopus gastrula. Dev Biol 240: 387–403 [DOI] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 18: 578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79: 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM (1991) Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 82: 37–46 [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC (2000) Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127: 2227–2238 [DOI] [PubMed] [Google Scholar]
- Tahinci E, Symes K (2003) Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol 259: 318–335 [DOI] [PubMed] [Google Scholar]
- Takeichi M (1991) Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251: 1451–1455 [DOI] [PubMed] [Google Scholar]
- Tiedemann H, Tiedemann H (1956) Experiments on the chemical identification of embryonal induction substances. Hoppe Seylers Z Physiol Chem 306: 7–32 [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Wacker S, Grimm K, Joos T, Winklbauer R (2000) Development and control of tissue separation at gastrulation in Xenopus. Dev Biol 224: 428–439 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM (2000) Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405: 81–85 [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Medina A, Swain RK, Steinbeisser H (2001) Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature 413: 856–860 [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Schürfeld M (1999) Vegetal rotation, a new gastrulation movement involved in the internalization of the mesoderm and endoderm in Xenopus. Development 126: 3703–3713 [DOI] [PubMed] [Google Scholar]
- Wunnenberg-Stapleton K, Blitz IL, Hashimoto C, Cho KW (1999) Involvement of the small GTPases XRhoA and XRnd1 in cell adhesion and head formation in early Xenopus development. Development 126: 5339–5351 [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E (2002) JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 3: 69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Axelrod JD, Simon MA (2002) Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell 108: 675–688 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe M, Kato H, Dutta A, Sugano S (1999) BH-protocadherin-c, a member of the cadherin superfamily, interacts with protein phosphatase 1 alpha through its intracellular domain. FEBS Lett 460: 93–98 [DOI] [PubMed] [Google Scholar]


