Abstract
AIM
To investigate the role of microRNA-34a (miR-34a) in the induction of apoptosis of human lens epithelial (HLE-B3) cells.
METHODS
The apoptosis of HLE-B3 cells was detected by Annexin V-PE apoptosis detection kit after the treatment with 200 µmol/L H2O2 for 24h and lentiviral miR-34a vector transfection. The expression of miR-34a in the cells was quantified by quantitative real time polymerase chain reaction (qRT-PCR) in response to H2O2 exposure and the vector transfection. The effects of overexpression of miR-34a on the expression of B-cell lymphoma-2 (Bcl-2) and silent information regulator 1 (SIRT1) was determined by qRT-PCR and Western blot.
RESULTS
The expression of miR-34a was up-regulated by the treatment of H2O2 in HLE-B3 cells. The increased expression of miR-34a is accompanied with the cell apoptosis. Consistence with the H2O2 exposure, ectopic overexpression of miR-34a in HLE-B3 cells promoted cells apoptosis. Importantly the anti-apoptosis factors Bcl-2 and SIRT1 were reduced significantly by up-regulation of miR-34a in HLE-B3 cells.
CONCLUSION
MiR-34a promotes the apoptosis of HLE-B3 cells by down-regulating Bcl-2 and SIRT1, suggesting that miR-34a may involve in the pathogenesis of cataract formation and targeting miR-34a may be a potentially therapeutic approach for treatment of cataract.
Keywords: human lens epithelial cells, microRNA-34a, apoptosis
INTRODUCTION
It is well recognized that the lens epithelial cells (LECs) are essential for maintaining the homeostasis and transparency of the entire lens[1]. Damages, oxidative stress in particular, to the epithelial cells have been regarded as the major cause of age-related cataract (ACR)[2]. Exposing to the oxidative stress from reactive oxygen species (ROS), such as H2O2 and the free radicals superoxide (O22−) may eventually lead to lens opacity[3]. Analysis of lens and aqueous humor taken from the cataract patients demonstrated a significant elevated H2O2 levels compared to normal controls[4]. It is known that one of major early changes in cataract is LECs apoptosis induced by oxidative stress. Therefore, LECs apoptosis induced by oxidative stress appears to be a common cellular basis in development of non-congenital cataract[5]. The effect of oxidative stress on growth factors, cytokines, extracellular matrix (ECM) in LECs apoptosis have been extensively studied, but unfortunately the detailed molecular mechanisms controlling the process of apoptosis in the LECs remain unclear.
MicroRNAs (miRNAs) is an abundant class of nonprotein-coding 22 nucleotide RNAs, that regulate gene expression post-transcriptionally[6]. MiRNAs regulate mRNA degradation or translational interference by combining with their target mRNAs[7]. They have been implicated in numerous cell processes such as proliferation, migration, and especially apoptosis[8]. So far, more than 1000 human miRNAs have been recognized, and hundreds of them are known to contribute to ophthalmic diseases[9]. In particular, the relevance of microRNA-34a (miR-34a) to ARC has been reported[10]–[11]. It was indicated that miR-34a was increased in the cataractous human lenses[11].
Previous study demonstrated that miR-34a plays an important role in the regulation of various cellular processes, such as proliferation, migration and tumor invasion. Importantly, miR-34a is also a pro-apoptosis regulator, so inhibition of miR-34a expression implicates reduction of cell apoptosis. One of mechanisms of the function of promoting apoptosis by miR-34a is through targeting on and repression of B-cell lymphoma-2 (Bcl-2)
Besides Bcl-2, miR-34a can also repress the expression of silent information regulator 1 (SIRT1) that has a critical function in protecting cells from apoptosis induced by H2O2. The reduced SIRT1 expression is associated with severity of cataract[12]. However, the relevance of miR-34a and the down stream target genes Bcl-2 and SIRT1 in the pathogenesis of ARC has not been studied. In the current study, the following research was conducted 1) the expression level of miR-34a induced by H2O2 in human lens epithelial (HLE-B3) cells and its relevance to apoptosis; 2) the effects of over-expression of miR-34a on LEC apoptosis; 3) the correlation of LEC apoptosis with the expression of Bcl-2 and SIRT1.
MATERIALS AND METHODS
Cell Culture with H2O2
HLE-B3 cells were obtained from American Type Culture Collection (ATCC; Rockville, MD, USA). Cells were cultured in minimum essential medium (EME; GIBCO, Carlsbad, CA, USA) mixed with 10% fetal bovine serum (FBS; GIBCO, Carlsbad, CA, USA) at 37°C in an atmosphere of 5% CO2 with humidity. Previous study showed that the apoptosis of HLE-B3 cells increased with 200 µmol/L H2O2, which was widely used as a model of cataract formation[13]. In present study, apoptosis induced by H2O2 in the HLE-B3 cells was adapted according to the previous publication, in brief, HLE-B3 cells were grown in 6 wells plates up to 70% confluence and then were treated with 200 µmol/L H2O2 (Sigma-Aldrich, St. Louis, MO, USA) for 24h.
Lentivirus Packaging and Transfection
The oligonucleotides of miR-34a and nonspecific control were amplified and cloned into pCDHCMV-MCS-EF1-mCherry constructs to construct miR-34a vector (miR-34a) and non-specific miRNA vector (miR-NC), which were purchased from Genecopoeia (Guangzhou, China). The lentiviral vectors were co-transfected with pPACK packaging plasmid into 293 T cells. Four to eight hours after transfection, virus supernatant was harvested, and then it was used to transfect HLE-B3 cells for 6h. For maintaining the stable expression, the transfected HLE-B3 cells were selected in the medium containing 2 µg/mL puromycin (GIBCO, Carlsbad, CA, USA) for 10d after stable expression was achieved. The transfection efficiency was confirmed by fluorescence microscope and quantitative real time polymerase chain reaction (qRT-PCR). After stably expressing miR-34a and miR-NC, the cells were harvested for apoptosis detection and Western blot analysis.
Apoptosis Detection
The apoptotic cells were detected by flow cytometry using the PE Annexin V Apoptosis Detection kit (BectonDickinson, MountainView, CA, USA). The cells were harvested after H2O2 treatment. The staining was performed according to the manufacture's instruction. Briefly, 5×104 cells were collected with trypsinization and washed in ice-cold phosphate buffer saline (PBS), then resuspended in 500 µL binding buffer and incubated with 5 µL Annexin V and 5 µL 7-amino-actinomycin D (7-AAD) for 15min at room temperature without light, and then it was washed again and suspended in 500µL PBS. Flow cytometric analysis was promptly performed using a FACS calibur instrument (BectonDickinson, MountainView, CA, USA).
Quantitative Real-time Polymerase Chain Reaction
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The total RNA isolated from cells was subsequently reverse transcribed to cDNA using PrimeScript RT reagent Kit (TaKaRa, Dalian, China). The synthesized cDNA was then used to amplify genes of Bcl-2 and SIRT1 by quantitative PCR using SYBR Green qPCR Super Mix (TaKaRa, Dalian, China) on an ABI PRISM® 7500 Sequence Detection System. The U6 small nuclear RNA and 18s rRNA were used as internal controls for miR-34a and Bcl-2, SIRT1 mRNA, respectively. For mature miR-34a expression analysis, specific reverse transcription was detected by a specific stem-loop real-time PCR miRNA kit (RiboBio, Shanghai, China). Sequences of all primers were listed in Table 1. Relative mRNA expression was calculated by the 2−ΔΔCt method.
Table 1. Gene-specific primers and sequences used in the present study.
| Gene | Primer sequence 5′-3′ |
| BCL2 | F: TCATGTGTGTGGAGAGCGTC |
| R: AGCCTCCGTTATCCTGGATC | |
| SIRT1 | F: ACTTCAGGTCAAGGGATGG |
| R: GTTCTGGGTATAGTTGCGAAG | |
| 18s rRNA | F: CCTGGATACCGCAGCTAGGA |
| R: GCGGCGCAATACGAATGCCCC | |
| hsa-miR-34a | F: ACACTCCAGCTGGGTGGCAGTGTCTTAGCTGGT |
| R: CTCAACTGGTGTCGTGGA | |
| U6 | F: CTCGCTTCGGCAGCACA |
| R: AACGCTTCACGAATTTGCGT |
Western Blot Analysis
The 20 µg protein lysate from the cultured cells underwent 10% SDS-PAGE and was transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membrane was incubated with rabbit monoclonal antibody against human Bcl-2 (CST, MA, USA) and SIRT1 (Abcam, Cambridge, UK) followed by horseradish peroxidase (HRP )-labeled goat anti-rabbit IgG (Southern Biotech, AL, USA) and examined by chemiluminescence. GAPDH was used as a protein-loading control. The specific protein bands were detected with the electro-chemi-luminescence (ECL) chemiluminescence kit (Pierce, Rockford, IL, USA), then exposured to Kodak X-ray films (Eastman Kodak Co.). The intensity and areas of the gel images from Western blots were scanned and quantified using the Image J software (National Institutes of Health).
Statistical Analysis
Results were expressed as means±SD. SPSS 13.0 software was used for statistical analysis. Differences were performed using an independent sample t-test. Statistical differences were considered significant at P<0.05. All of the experiment was repeated at least 3 times.
RESULTS
H2O2 Induced Apoptosis and Up-regulated MicroRNA-34a in Human Lens Epithelial Cells
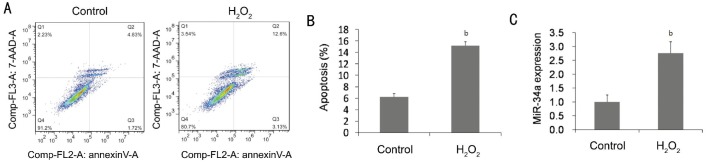
To investigate the effects of oxidative stress on HLE-B3 cell apoptosis, 200 µmol/L H2O2 was used for the treatment of HLE-B3 for 24h. A significant increase of the cell numbers of apoptosis was seen in H2O2-treated HLE-B3 cells compared with the control (Figure 1A and 1B), importantly the increased cell death was accompanied with a significant up-regulation of miR-34a expression (Figure 1C).
Figure 1. H2O2 induced apoptosis and up-regulated miR-34a expression in HLE-B3 cells.
A, B: Flow cytometry analysis of the apoptosis induced by H2O2. Early apoptotic cells were shown in Q3: lower right quadrant and late apoptotic; Necrotic states were in Q2: upper right quadrant. The numbers of apoptotic cells induced by H202 were significantly increased. C: Quantitative RT-PCR showed that miR-34a was up-regulated significantly in H2O2-treated group. Data were expressed as mean±SD, n=3, bP<0.01.
Overexpression of MicroRNA-34a Induced Apoptosis in Human Lens Epithelial Cells
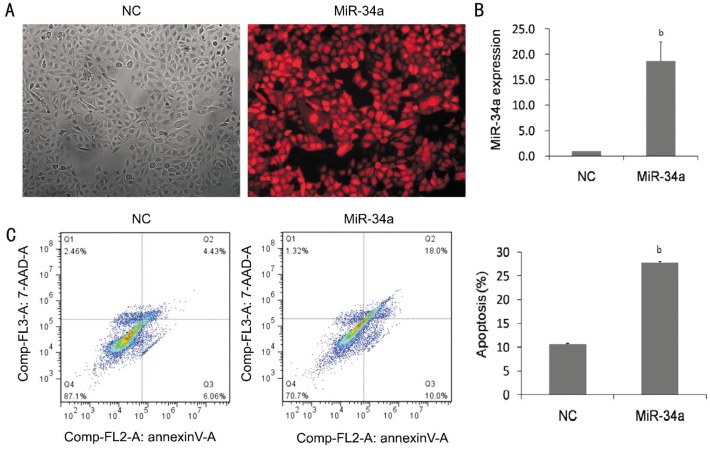
To identify the role of miR-34a in modulating apoptosis in HLE-B3 cells, a lentiviral vector with overexpressing miR-34a was established in HLE-B3 cells. The transfection efficiency was 90%, which was indicated by expressing red fluorescence protein (RFP) in the cells (Figure 2A). Real-time PCR analysis indicated that the expression of miR-34a was 18.51±2.8 folds increased in the miR-34a group compared with that in the miR-NC group (Figure 2B). The numbers of apoptotic cells were increased to 27.83% by the overexpression of miR-34a, whereas 10.64% apoptotic cells were revealed in the cells without miR-34a transfection (Figure 2C).
Figure 2. Overexpression of miR-34a induced apoptosis in HLE-B3 cells.
A: The phase contrast and fluorescent images of HLE-B3 cells transfected with lentiviruses containing miR-34a or miR-NC. The transfection efficiency is shown by red fluorescence protein (RFP). B: Quantitative RT-PCR showed that miR-34a increased significantly in miR-34a group. C: Flow cytometry analysis showed the effects of overexpression of miR-34a on the induction of apoptosis, and the numbers of apoptotic cells were increased 3 folds compared with non-specific miRNA vector. NC: negative control group in which the cells were transfected with non-specific miRNA vector. Data were expressed as mean±SD, n=3, bP<0.01.
Overexpression of MicroRNA-34a Suppressed B-cell Lymphoma-2 and Silent Information Regulator 1 Expression
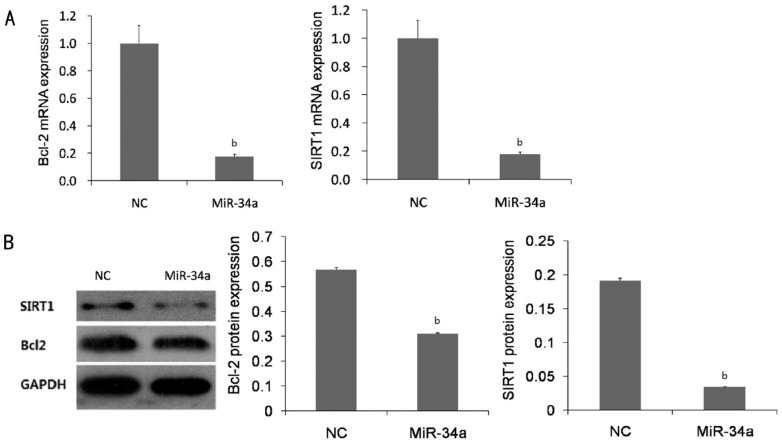
To explore the mechanism of miR-34a induced apoptosis in the cells, we investigated whether overexpression of miR-34a affected Bcl-2 and SIRT1 expression in HLE-B3 cells. Our research showed that transfection the cell with lentivirus with insertion of miR-34a resulted in a remarkable decrease Bcl2 and SIRT1 mRNA expression compared with the miR-NC (Figure 3A). Consistence with founding of mRNA, the protein levels of Bcl2 and SIRT1 were also significantly reduced in the cells with overexpression of miR-34a compared with the miR-NC (Figure 3B).
Figure 3. Bcl2 and SIRT1 expressions were inhibited by overexpression of miR-34a in HLE-B3 cells.
A: Quantitative RT-PCR showed that overexpression of miR-34a leads to a remarkable inhibition of the expression of Bcl-2 and SIRT1 mRNA. B: Western blot demonstrated a significant decrease in protein levels of Bcl-2 and SIRT1 in the cells with overexpression of miR-34a. NC: Negative control group in which the cells were transfected with non-specific miRNA vector. Data were expressed as mean±SD, n=3, bP<0.01.
DISCUSSION
ARC is one of the leading causes of blindness on earth. The formation of the cataract has been extensively studied. It is recognized that oxidative stress may be the most important risk factor in the pathogenesis of ARC, and the LEC is the most vulnerable cells in lens to oxygen species damage, which results in the cell apoptosis and the consequence is loss of lens transparency. Along with LEC apoptosis, it is unable to maintain a stable environment and osmotic pressure in the lens, and resist to harmful external factors, leading to lens opacity. LEC apoptosis was considered as a common cellular basis in development of non-congenital cataract.
Evidences have ascertained the effect of miR-34a as an apoptosis promoter in lens and retinal disease[11],[14]; however, the regulation of miR-34a expression and its role with LEC apoptosis induced by oxidative stress and the relevance to Bcl-2 and SIRT1 have not been studied in vitro. Our research identified, for the first time, that oxidative stress increased the miR-34a expression; furthermore, the up-regulating of miR-34a connected to increasing apoptosis of human LECs; most importantly, Bcl-2 and SIRT1, the downstream genes of miR-34a were down-regulated by overexpression of miR-34a. These observations support the suggestion that miR-34a is an apoptosis regulator and plays an important role in the pathogenesis of the cataract formation.
It is known that the oxidants trigger apoptosis pathway and contribute to initiate cataract development[15]. Peroxidative damage mediated by toxic metabolites of oxygen such as OH, H2O2 is a common method to induce LECs apoptosis in vitro. In our research, we found that H2O2 induced a significantly increased apoptosis in HLE-B3 cells, which was agreed with the previous report showing that the apoptosis was highly increased when HLE-B3 cells were treated with 200 µmol/L H2O2[13]. Notably, the increased apoptosis in the LECs was consistent with the up-regulation of miR-34a after H2O2 treatment. It indicated that miR-34a expression was able to induce apoptosis of HLE-B3 cells upon exposure to oxidative stress. The result supported the notion that oxidative stress induced apoptosis of LECs though miR-34a, which may contribute to the ARC. Our in vitro result further supported the role of miR-34a in the formation of ARC reported by Chien et al[11] which showed that miR-34a was up-regulated in the specimen of human ARC.
To confirm the effect of miR-34a in the induction of apoptosis in the HLE-B3 cell, overexpression of miR-34a was established in the cell line. The increase of the gene expression of miR-34a by transfection of the lentivirus with miR-34a was confirmed by qRT-PCR. Our study clearly indicated that the number of apoptosis in HLE-B3 cells was significantly increased in the condition of overexpression of miR-34a.
Previous report showed that miR-34a increased apoptosis in neuroblastoma cell lines[16]. The pro-apoptotic functions of miR-34a were further demonstrated in numerous cancer tissues[17]–[19]. Suppression of expression of miR-34a was able to inhibit cell apoptosis[18]. Our result, further confirmed the notion that miR-34a was an apoptosis inducer in LECs. Taking together, the findings suggested that miR-34a may play a crucial role in cataract progression and identification of its role on the ARC may shed light on the mechanism of lens opacification.
It is confirmed that Bcl-2 and SIRT1 were regulated by miR-34a. MiR-34a can directly combine and regulate Bcl-2 in SH-SY5Y cells[20]. As an important anti-apoptosis protein, Bcl-2 can protect lens epithelial cells from oxidative stress and decrease LEC apoptosis, which eventually prevent developing cataract. Bcl-2 expression was decreased in the LECs when apoptosis was induced[13]. Cell apoptosis was highly correlated to the reduction of the expression of Bcl-2 in LECs[21]. In addition, Bcl-2 expression was also significantly decreased in the lens epithelium from elderly individuals compared with those in fetus and children[22]. In the current study, we found that overexpression of miR-34a not only promoted the LECs apoptosis, but also inhabited the expression of Bcl-2 and SIRT1. Therefore, those results demeonstrated that miR-34a mediated LECs apoptosis may be through reduction of Bcl-2.
Besides Bcl-2, the expression of SIRT1 was inhibited by the overexpression of miR-34a in our study. Previous studies found that SIRT1 upregulated the expressions of Nrf2 and activated the Nrf2/antioxidant response element (ARE) pathway to defend cells from oxidative stress[23]. Upon binding to the 3′-untranslated regions (UTR) of SIRT1, miR-34 down-regulation of SIRT1 led to an increase in acetylated p53 that mediated the cell cycle and apoptosis. And a positive feedback loop was suggested between p53 and miR-34a[24]. The decreased expression of SIRT1 in the lens epithelium corresponded to higher cataract scores as well as the age of patients[12]. Our study further showed that reduced SIRT1 expression was associated with the overexpression of miR-34a. It indicated that the one way of miR-34a induced LECs apoptosis was achieved by inhibition of SIRT1 expression, and decreased expression of SIRT1 in LEC may be a risk factor for the initiation of ARC.
In summary, our results shows that the oxidative stress up-regulated miR-34a expression in LEC, and overexpression of miR-34a promotes the apoptosis in the HLE-B3 cells by suppressing the Bcl-2 and SIRT1 expression (Figure 4). However, the study is limited by not presenting the parental HLE-B3 control. In further studies, down-regulation of miR34a is conducted to observe the cell apoptosis and downstream signal expression such as Bcl-2 and SIRT1 in HLE-B3 cells. The present study provides a new insight into the role of miRNAs in the pathogenesis of ARC and might represent a potential approach for therapy of ARC.
Figure 4. Network of miR-34a, Bcl-2, SIRT1 and apoptosis in HLE-B3 cells.
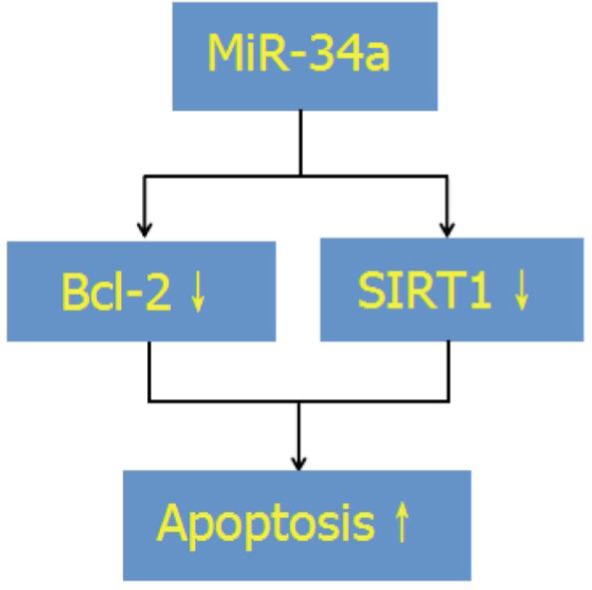
↓: Decrease; ↑: Increase.
Acknowledgments
Conflicts of Interest: Li QL, None; Zhang HY, None; Qin YJ, None; Meng QL, None; Yao XL, None; Guo HK, None.
REFERENCES
- 1.Mathias RT, White TW, Gong X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev. 2010;90(1):179–206. doi: 10.1152/physrev.00034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010;44(3):155–165. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dairou J, Malecaze F, Dupret JM, Rodrigues-Lima F. The xenobiotic-metabolizing enzymes arylamine N-acetyltransferases in human lens epithelial cells: inactivation by cellular oxidants and UVB-induced oxidative stress. Mol Pharmacol. 2005;67(4):1299–1306. doi: 10.1124/mol.104.009738. [DOI] [PubMed] [Google Scholar]
- 4.Spector A. Oxidative stress-induced cataract: mechanism of action. Faseb J. 1995;9(12):1173–1182. [PubMed] [Google Scholar]
- 5.Zhang ZF, Zhang J, Hui YN, Zheng MH, Liu XP, Kador PF, Wang YS, Yao LB, Zhou J. Up-regulation of NDRG2 in senescent lens epithelial cells contributes to age-related cataract in human. PLoS One. 2011;6(10):e26102. doi: 10.1371/journal.pone.0026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng CH, Liu JH, Woung LC, Lin TJ, Chiou SH, Tseng PC, Du WY, Cheng CK, Hu CC, Chien KH, Chen SJ. MicroRNAs and cataracts: correlation among let-7 expression, age and the severity of lens opacity. Br J Ophthalmol. 2012;96(5):747–751. doi: 10.1136/bjophthalmol-2011-300585. [DOI] [PubMed] [Google Scholar]
- 11.Chien KH, Chen SJ, Liu JH, Chang HM, Woung LC, Liang CM, Chen JT, Lin TJ, Chiou SH, Peng CH. Correlation between microRNA-34a levels and lens opacity severity in age-related cataracts. Eye (Lond) 2013;27(7):883–888. doi: 10.1038/eye.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin TJ, Peng CH, Chiou SH, Liu JH, Lin-Chung-Woung, Tsai CY, Chuang JH, Chen SJ. Severity of lens opacity, age, and correlation of the level of silent information regulator T1 expression in age-related cataract. J Cataract Refract Surg. 2011;37(7):1270–1274. doi: 10.1016/j.jcrs.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Xing K, Badamas R, Kuszynski CA, Wu H, Lou MF. Overexpression of thioredoxin-binding protein 2 increases oxidation sensitivity and apoptosis in human lens epithelial cells. Free Radic Biol Med. 2013;57:92–104. doi: 10.1016/j.freeradbiomed.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smit-McBride Z, Forward KI, Nguyen AT, Bordbari MH, Oltjen SL, Hjelmeland LM. Age-dependent increase in miRNA-34a expression in the posterior pole of the mouse eye. Mol Vis. 2014;20:1569–1578. [PMC free article] [PubMed] [Google Scholar]
- 15.Li WC, Kuszak JR, Dunn K, Wang RR, Ma W, Wang GM, Spector A, Leib M, Cotliar AM, Weiss M, Et A. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130(1):169–181. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26(34):5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 17.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26(5):745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26(5):731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Cao W, Fan R, Wang L, Cheng S, Li H, Jiang J, Geng M, Jin Y, Wu Y. Expression and regulatory function of miRNA-34a in targeting survivin in gastric cancer cells. Tumour Biol. 2013;34(2):963–971. doi: 10.1007/s13277-012-0632-8. [DOI] [PubMed] [Google Scholar]
- 20.Alural B, Ozerdem A, Allmer J, Genc K, Genc S. Lithium protects against paraquat neurotoxicity by NRF2 activation and miR-34a inhibition in SH-SY5Y cells. Front Cell Neurosci. 2015;9:209. doi: 10.3389/fncel.2015.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji Y, Cai L, Zheng T, Ye H, Rong X, Rao J, Lu Y. The mechanism of UVB irradiation induced-apoptosis in cataract. Mol Cell Biochem. 2015;401(1–2):87–95. doi: 10.1007/s11010-014-2294-x. [DOI] [PubMed] [Google Scholar]
- 22.Weng J, Zhang H. the characteristics of bcl-2 and PCNA expression in the lens epithelium of human being. Zhonghua Yan Ke Za Zhi. 2001;37(3):197–199. [PubMed] [Google Scholar]
- 23.Huang K, Huang J, Xie X, Wang S, Chen C, Shen X, Liu P, Huang H. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2013;65:528–540. doi: 10.1016/j.freeradbiomed.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008;105(36):13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]