Abstract
AIM
To evaluate retinal nerve fiber layer (RNFL) thickness profile in patients of thyroid ophthalmopathy with no clinical signs of optic nerve dysfunction.
METHODS
A prospective, case-control, observational study conducted at a tertiary care centre. Inclusion criteria consisted of patients with eyelid retraction in association with any one of: biochemical thyroid dysfunction, exophthalmos, or extraocular muscle involvement; or thyroid dysfunction in association with either exophthalmos or extra-ocular muscle involvement; or a clinical activity score (CAS)>3/7. Two measurements of RNFL thickness were done for each eye, by Cirrus HD-optical coherence tomography 6mo apart.
RESULTS
Mean age of the sample was 38.75y (range 13-70y) with 18 males and 22 females. Average RNFL thickness at first visit was 92.06±12.44 µm, significantly lower than control group (101.28±6.64 µm) (P=0.0001). Thickness of inferior quadrant decreased from 118.2±21.27 µm to 115.0±22.27 µm after 6mo (P=0.02). There was no correlation between the change in CAS and RNFL thickness.
CONCLUSION
Decreased RNFL thickness is an important feature of thyroid orbitopathy, which is an inherent outcome of compressive optic neuropathy of any etiology. Subclinical RNFL damage continues in the absence of clinical activity of the disease. RNFL evaluation is essential in Grave's disease and active intervention may be warranted in the presence of significant damage.
Keywords: thyroid ophthalmopathy, retinal nerve fibre layer, optical coherence tomography
INTRODUCTION
Thyroid orbitopathy (TO) is characterized by an increase in volume of orbital contents, viz. extraocular muscles and orbital fat, which is a result of mucopolysachharide infiltration of these tissues. Increased intra-orbital pressure leads to optic nerve compression at the narrow orbital apex. Compression causes ischemia and nerve damage. Danesh-Meyer et al[1] have reported significant retinal nerve fiber layer (RNFL) changes in optical coherence tomography (OCT) of patients with chiasmal compression. This emphasizes that RNFL is implicated in compressive neuropathy of the optic pathway. Bartelena et al[2] have shown that subclinical optic neuropathy occurs even in mild degrees of soft tissue involvement in TO. This damage may reflect in OCT evaluation of RNFL of the affected eyes.
OCT has been harnessed to detect RNFL changes at a pre-clinical stage in glaucoma and diabetic retinopathy[3]–[4]. The authors propose to extend its use for the detection of RNFL changes in TO. This study aims to detect changes in the RNFL by OCT, much before the clinical appreciation of optic nerve damage is possible.
SUBJECTS AND METHODS
A tertiary care centre based prospective longitudinal case-control observational study was conducted in the Department of Ophthalmology, King George's Medical University, Lucknow, India, over a duration of one year from August 2010 to July 2011.
A written informed consent based on Declaration of Helsinki was taken from all the patients. The patient evaluation protocol has been adopted from the European Group of Graves' Orbitopathy (EUGOGO) guidelines[5]. A proforma based detailed history and examination was done and recorded in a computer based data analysis program.
Evaluation of patients of thyroid ophthalmopathy included details like symptoms and signs of TO, clinical activity score (CAS), best corrected visual acuity (BCVA), refractive errors, intraocular pressure (IOP), axial length, fundus examination and assessment of optic nerve function. Age and gender matched healthy controls were selected from the outpatient facility of the department.
Formulation of inclusion criteria, exclusion criteria and RNFL score (RNFLS) was done for the purpose of this study. The clinically worse eye of each case was included for the study purpose. If both the eyes behaved similarly, a simple random selection was done to decide upon the inclusive eye. The inclusive eye from the controls was randomly selected.
Inclusion criteria: patients with eyelid retraction (upper eyelid margin at or above the superior limbus in primary gaze without frontalis muscle overaction, or lower lid margin below the inferior limbus) in association with any one of: biochemical thyroid dysfunction (hyperthyroid or hypothyroid), exophthalmos (a measurement of >18 mm on Hertel's exophthalmometer), or extraocular muscle involvement (restrictive myopathy or objective radiological evidence of enlarged muscles on orbital ultrasonography, contrast enhanced CT or MRI scans); or, thyroid dysfunction in association with either exophthalmos or extra-ocular muscle involvement; or, a CAS>3/7.
Exclusion criteria (for cases and controls): 1) eyes with BCVA less than 20/30 on Snellen's chart (we aim to detect early changes in RNFL much before the vision is affected); 2) presence of clinical signs of optic nerve dysfunction (as evidenced by abnormal pupillary reaction, abnormal color vision or contrast sensitivity, or visual field defects on 120 degrees full field charting by Humphrey's Visual Field Analyzer); 3) patients with conditions known to be associated with RNFL abnormalities (e.g. diabetic or hypertensive retinopathy[6], IOP>18 mm Hg[7]–[8], myopia more than -1.5 spherical equivalent[9], axial length >23 mm[10]–[11]; 4) scans with signal strength of less than 5/10 in OCT.
A RNFLS was devised as an index representing the diseased state of the RNFL. A score of 1 was given to an eye for each quadrant of abnormal (thin or thick) RNFL present (minimum score: 0, maximum score: 4).
RNFL thickness analysis (average and quandrantic thickness-temporal, nasal, superior and inferior) of the selected eyes (cases and controls) was done using Cirrus HD-OCT (Carl Zeiss, Dublin, California, USA) with internal focus fixation adjustment. Two sets of observations were obtained, one at the time of induction into the study and second, after a 6mo follow-up period.
Statistical Analysis
Statistical analysis was done using paired and unpaired t-tests, ANOVA and Pearson Correlation. P<0.05 were taken as significant and P<0.01 as highly significant.
RESULTS
Forty eyes with TO and an equal number of control eyes were studied. Mean age of the sample was 38.75y (range 13-70y) with 18 males and 22 females. Normative RNFL thickness data, with regard to age and quadrant, was derived from the control data. Control data was grouped with class intervals of 10y each from 10 to 70 years of age. Adjacent groups which did not show statistically significant difference in average RNFL thickness values (on post hoc tests after ANOVA), were clubbed to form the final age group classification for this study, as represented in Tables 1, 2. Values upto two standard deviations on either side of the mean values have been taken as the reference range.
Table 1. Comparison of average RNFL thickness between age groups in controls.
| Age groups (a) | Average RNFL thickness (µm) | 1P (between adjacent groups) |
| 10-19 (n=3) | 109.75±2.25 | |
| 20-30 (n=9) | 102.17±4.11 | 0.005 |
| 31-40 (n=11) | 102.41±4.39 | 0.9 |
| 41-50 (n=10) | 101.95±4.42 | 0.8 |
| 51-60 (n=4) | 98.56±3.58 | 0.2 |
| 61-70 (n=3) | 85.58±5.21 | 0.003 |
1Post hoc test after ANOVA, significant P<0.05.
Table 2. Normative RNFL thickness-age-wise distribution using ANOVA.
| Parameters | Age (a) |
1P | ||
| <20 (n=3) | 20-60 (n=34) | >60 (n=3) | ||
| Temporal | 68.33±6.66 | 70.17±5.52 | 50.33±1.15 | <0.01 |
| Nasal | 99.33±9.29 | 85.18±9.06 | 62.33±4.93 | <0.01 |
| Superior | 113±2.65 | 123.41±8.95 | 109.67±15.14 | 0.01 |
| Inferior | 138.33±12.66 | 128.26±10.62 | 120±7 | 0.1 |
| Average | 109.75±2.25 | 101.76±4.23 | 85.58±5.28 | <0.01 |
1Post hoc test after ANOVA, significant P<0.05.
n=40, µm
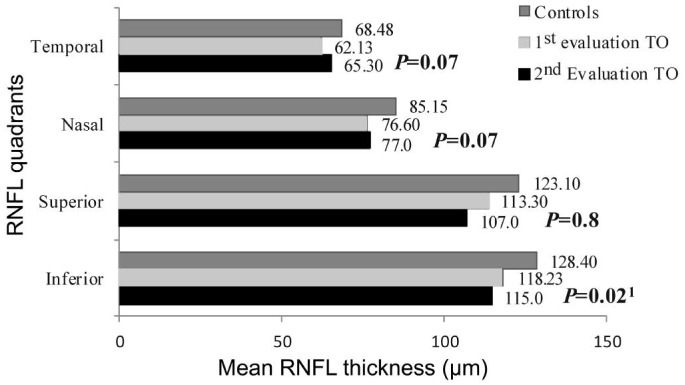
Analysis of patient data at the first evaluation (n=40) revealed significant RNFL thinning in all the quadrants (P<0.01) as compared to the controls, as depicted in Table 3. Change in quadrantic RNFL thickness of these eyes after a 6mo period was not statistically significant (P>0.05), except in the inferior quadrant, which underwent a significant thinning (P=0.02), as shown in Figure 1.
Table 3. Comparison of RNFL thickness between cases and controls at 1st evaluation.
| RNFL quadrant | Controls | Cases (1st evaluation) | 1P |
| Temporal | 68.48±7.49 | 62.13±12.45 | 0.007 |
| Nasal | 85.15±12.26 | 76.60±11.66 | 0.002 |
| Superior | 123.10±10.07 | 113.30±20.48 | 0.003 |
| Inferior | 128.40±10.92 | 118.23±21.27 | 0.009 |
| Average | 101.28±6.64 | 92.06±12.44 | 0.0001 |
1Unpaired t-test (significant P<0.05, highly significant P<0.01).
µm
Figure 1. Change in mean RNFL thickness after 6mo.
1Paired t-test, significant P<0.05.
Mean CAS of the patients at the two visits were 1.95 and 0.68, respectively. This decrease in the CAS was statistically significant (P<0.001). On the contrary, mean RNFLS increased from 1.43 at first evaluation, to 1.53 six months later. This change was not statistically significant (P=0.52). No correlation was observed between CAS and RNFLS at either of the visits. The mean changes in CAS and RNFLS did not show any correlation either, as shown in Table 4.
Table 4. Correlation between CAS and RNFLS.
| Scores | CAS | RNFLS | 1Pearson correlation (r) |
| 1st evaluation (a) | 1.95 | 1.43 | -0.13 |
| 2nd evaluation (b) | 0.68 | 1.53 | -0.02 |
| Change (Δ=a-b) | 1.27 | -0.1 | +0.14 |
1No correlation: r=-4 to +4; moderate correlation: r=+5 to +7, and r=-5 to -7; high correlation: r<-8, and r>+8.
DISCUSSION
Optic nerve function is evaluated clinically in terms of visual acuity, color vision, contrast sensitivity, pupillary reaction, and visual field assessment. However, irreversible structural damage occurs much earlier than functional impairment, as is well known and repeatedly emphasized through RNFL thickness evaluation by OCT in glaucoma patients[12]–[14]. This study, by excluding clinically detectable functional impairment of optic nerve, further underlines the specificity of the results.
OCT is a non-invasive, non-contact modality that has been used for measurement of peripapillary RNFL thickness. The OCT measured RNFL thickness is not affected by corneal and lenticular birefringence. No additional reference plane is required to calculate RNFL thickness because Cirrus OCT provides an absolute cross-sectional measurement of retina from which RNFL thickness is calculated[15]. An excellent intra-session and inter-session reproducibility of these measurements has been documented[16]–[17].
Normal RNFL is known to show age and ethnicity related variations[18]. Due to very few publications on quantification of nerve fiber layer thickness in normal Indian eyes[19]–[20], we derived our own reference range from the 40 healthy, age and gender matched controls. This data shows significant difference between the average RNFL thickness of normal eyes <20 years old, 20-60 years old and those >60 years old. An age related thinning was observed. In all the three age groups, RNFL was thickest in the inferior quadrant, followed by superior, nasal and temporal quadrants (ISNT rule of RNFL). This reflects the observations of previously published data, indicating the representativeness of our control group[20]–[22].
Compressive optic neuropathy is characterized by an increase in resistance to orthograde axonal transport, leading to axonal injury[23]. This injury is reversible in the initial period, whereas a severe involvement (in the form of secondary optic atrophy) causes changes in the RNFL in the form of thinning, which can be objectively documented on OCT evaluation of the RNFL[24]–[25].
Optic nerve pathology in thyroid ophthalmopathy is compressive neuropathy. RNFL in patients with TO in our study shows significant thinning in all quadrants, signifying the presence of irreversible damage, even with a clinically normal optic nerve. This is in contradiction to the single published study in this regard by Neudorfer et al[26] in 2013, which showed an increased peripapillary RNFL thickness in patients with TO. Initial RNFL edema caused by optic nerve compression may account for this increase in thickness. Subsidence of the edema, ultimately, leads to irreversible RNFL damage, which is evidenced by further thinning at six months' follow-up in our study. Moreover, we found no correlation between the RNFL status and clinical activity of TO. After a six month follow up period, the CAS of our patients improved, whereas the RNFL damage did not. Hence, a clinically evident improvement is not paralleled in RNFL recovery. A further thinning was apparent in the inferior quadrant after six months. This can be explained by the fact that the inferior quadrant being the thickest, was able to demonstrate even subtle changes in six months' time. A similar progressive trend might be found in other quadrants as well after a longer duration of follow up.
The current EUGOGO guidelines for the management of Grave's orbitopathy are based on clinical activity and clinical severity of the disease, which are determined by a set of pre-defined criteria[5]. The only criterion defined for optic nerve assessment is in terms of presence or absence of relative afferent pupillary defect (RAPD). However, as demonstrated by our study, significant RNFL damage was present even in the absence of an afferent pupillary defect. Therefore, OCT evaluation of the RNFL thickness ought to be an integral part of patient evaluation in TO, which may detect significant damage even in the absence of an afferent pupillary defect.
Continued RNFL thinning was observed in our study even in mild disease. The EUGOGO guidelines indicate active management only for moderate and severe sight threatening disease, while a wait and watch with local measures is advocated for clinically mild disease. Although, steroids and orbital radiation have been documented to have potential value in mild disease, they are not usually recommended[27]–[28]. However, low dose steroids may have a role in averting the compressive RNFL changes. This requires further randomized controlled studies to weigh the benefits against the risks.
In conclusion, TO is characterized by a decreased RNFL thickness, which is an inherent outcome of compressive optic neuropathy of any etiology. Subclinical RNFL damage continues in the absence of clinical activity of the disease. OCT is a convenient non-invasive tool for the evaluation and follow up of RNFL status. RNFL evaluation is essential in Grave's disease and significant changes may require active intervention.
Acknowledgments
Conflicts of Interest: Mugdha K, None; Kaur A, None; Sinha N, None; Saxena S, None.
REFERENCES
- 1.Danesh-Meyer HV, Carroll SC, Foroozan R, Savino PJ, Fan J, Jiang Y, Vander Hoorn S. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci. 2006;47(11):4827–4835. doi: 10.1167/iovs.06-0327. [DOI] [PubMed] [Google Scholar]
- 2.Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy: reality and perspective. Endocr Rev. 2000;21(2):168–199. doi: 10.1210/edrv.21.2.0393. [DOI] [PubMed] [Google Scholar]
- 3.Falsini B, Marangoni D, Salgarello T, Stifano G, Montrone L, Campagna F, Aliberti S, Balestrazzi E, Colotto A. Structure-function relationship in ocular hypertension and glaucoma: interindividual and interocular analysis by OCT and pattern ERG. Graefes Arch Clin Exp Ophthalmol. 2008;246(8):1153–1162. doi: 10.1007/s00417-008-0808-5. [DOI] [PubMed] [Google Scholar]
- 4.Peng PH, Lin HS, Lin S. Nerve fiber layer thinning in patients with preclinical retinopathy. Can J Ophthalmology. 2009;44(4):417–421. doi: 10.3129/i09-112. [DOI] [PubMed] [Google Scholar]
- 5.Bartalena L, Baldeschi L, Dickinson A, et al. Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158(3):273–285. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 6.Chihara E, Matsuoka T, Ogura Y, Matsumura M. Retinal nerve fiber layer defect as an early manifestation of diabetic retinopathy. Ophthalmology. 1993;100(8):1147–1151. doi: 10.1016/s0161-6420(93)31513-7. [DOI] [PubMed] [Google Scholar]
- 7.Gyatsho J, Kaushik S, Gupta A, Pandav SS, Ram J. Retinal nerve fiber layer thickness in normal, ocular hypertensive, and glaucomatous Indian eyes: an optical coherence tomography study. J Glaucoma. 2008;17(2):122–127. doi: 10.1097/IJG.0b013e31814b9817. [DOI] [PubMed] [Google Scholar]
- 8.Tavares IM, Medeiros FA, Weinreb RN. Inconsistency of the published definition of ocular hypertension. J Glaucoma. 2006;15(6):529–533. doi: 10.1097/01.ijg.0000212279.03595.70. [DOI] [PubMed] [Google Scholar]
- 9.Choi SW, Lee SJ. Thickness changes in the fovea and peripapillary retinal nerve fiber layer depend on the degree of myopia. Korean J Ophthalmol. 2006;20(4):215–219. doi: 10.3341/kjo.2006.20.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akram MAR, Malik IQ, Ahmad I, Sarwar S, Hussain M. Correlation between axial length and retinal nerve fiber layer thickness in myopic eyes. Pak J Ophthalmol. 2013;29(3):169–172. [Google Scholar]
- 11.Oner V, Aykut V, Tas M, Alakus MF, Iscan Y. Effect of refractive status on peripapillary retinal nerve fibre layer thickness: a study by RTVue spectral domain optical coherence tomography. Br J Ophthalmol. 2013;97(1):75–79. doi: 10.1136/bjophthalmol-2012-301865. [DOI] [PubMed] [Google Scholar]
- 12.Mokbel TH, Elbendary AM, El Sharkawy HT, El Desouky WM. Retinal nerve fiber layer versus optic nerve head evaluation in the diagnosis of glaucoma and glaucoma suspect patients. J Egypt Ophthalmol Soc. 2013;106(4):253–258. [Google Scholar]
- 13.Quiglet HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107(5):453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 14.Sahli E, Tekeli O. Evaluation of retinal nerve fiber layer thickness with spectral domain OCT in primary open angle glaucoma and ocular hypertension. J Clin Exp Ophthalmol. 2012;3:247. [Google Scholar]
- 15.Sowmya V, Venkataramanan VR, Vishnu Prasad KP. Analysis of retinal nerve fiber layer thickness using optical coherence tomography in normal South Indian population. Indian J Ophthalmol. 2014;5(1):5–10. [Google Scholar]
- 16.Mwanza JC, Chang RT, Budenz DL, Durbin MK, Gendy MG, Shi W, Feuer WJ. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51(11):5724–5730. doi: 10.1167/iovs.10-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansoori T, Viswanath K, Balakrishna N. Reproducibility of peripapillary retinal nerve fiber layer thickness measurements with spectral domain optical coherence tomography in normal and glaucomatous eyes. Br J Ophthalmol. 2011;95(5):685–688. doi: 10.1136/bjo.2010.183020. [DOI] [PubMed] [Google Scholar]
- 18.Alasil T, Wang K, Keane PA, Lee H, Baniasadi N, de Boer JF, Chen TC. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2013;22(7):532–541. doi: 10.1097/IJG.0b013e318255bb4a. [DOI] [PubMed] [Google Scholar]
- 19.Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fiber layer thickness in normal latinos. Invest Ophthalmol Vis Sci. 2003;44(8):3369–3373. doi: 10.1167/iovs.02-0975. [DOI] [PubMed] [Google Scholar]
- 20.Sony P, Sihota R, Tewari HK, Venkatesh P, Singh R. Quantification of the retinal nerve fiber layer thickness in normal Indian eyes with optical coherence tomography. Indian J Ophthalmol. 2004;52(4):303–309. [PubMed] [Google Scholar]
- 21.Ramakrishnan R, Mittal S, Ambatkar S, Kader MA. Retinal nerve fibre layer thickness measurements in normal Indian population by optical coherence tomography. Indian J Ophthalmol. 2006;54(1):11–15. doi: 10.4103/0301-4738.21608. [DOI] [PubMed] [Google Scholar]
- 22.Malik A, Singh M, Arya SK, Sood S, Ichhpujani P. Retinal nerve fiber layer thickness in Indian eyes with optical coherence tomography. Nepal J Ophthalmol. 2012;4(1):59–63. doi: 10.3126/nepjoph.v4i1.5852. [DOI] [PubMed] [Google Scholar]
- 23.Clifford-Jones RE, McDonald WI, Landon DN. Chronic optic nerve compression: An experimental study. Brain. 1985;108(Pt 1):241–262. doi: 10.1093/brain/108.1.241. [DOI] [PubMed] [Google Scholar]
- 24.Clifford-Jones RE, Landon DN, McDonald WI. Remyelination during optic nerve compression. J Neurol Sci. 1980;46(2):239–243. doi: 10.1016/0022-510x(80)90082-9. [DOI] [PubMed] [Google Scholar]
- 25.Groth SL, Harrison A, Grajewski AL, Lee MS. Retinal nerve fiber layer thickness using spectral-domain optical coherence tomography in patients with no light perception secondary to optic atrophy. J Neuroophthalmol. 2013;33(1):37–39. doi: 10.1097/WNO.0b013e318272c7cd. [DOI] [PubMed] [Google Scholar]
- 26.Neudorfer M, Blum S, Kesler A, Varssano D, Leibovitch I. Retinal and peripapillary nerve fiber layer thickness in eyes with thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2013;54(15):1436. [Google Scholar]
- 27.Prummel MF, Mourits MP, Blank L, Berghout L, Koornneef L, Wiersinga WM. Randomised double-blind trial of prednisone versus radiotherapy in Graves' ophthalmopathy. Lancet. 1993;342(8877):949–954. doi: 10.1016/0140-6736(93)92001-a. [DOI] [PubMed] [Google Scholar]
- 28.Prummel MF, Terwee CB, Gerding MN, Baldeschi L, Mourits MP, Blank L, Dekker FW, Wiersinga WM. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves' ophthalmopathy. J Clin Endocrinol Metab. 2004;89(1):15–20. doi: 10.1210/jc.2003-030809. [DOI] [PubMed] [Google Scholar]