Abstract
P. vivax and P. falciparum parasites display different tropism for host cells and induce very different clinical symptoms and pathology, suggesting that the immune responses required for protection may differ between these two species. However, no study has qualitatively compared the immune responses to P. falciparum or P. vivax in humans following primary exposure and infection. Here, we show that the two species differ in terms of the cellular immune responses elicited following primary infection. Specifically, P. vivax induced the expansion of a subset of CD8+ T cells expressing the activation marker CD38, whereas P. falciparum induced the expansion of CD38+ CD4+ T cells. The CD38+ CD8+ T cell population that expanded following P. vivax infection displayed greater cytotoxic potential compared to CD38- CD8+ T cells, and compared to CD38+ CD8+ T cells circulating during P. falciparum infection. We hypothesize that P. vivax infection leads to a stronger CD38+ CD8+ T cell activation because of its preferred tropism for MHC-I-expressing reticulocytes that, unlike mature red blood cells, can present antigen directly to CD8+ T cells. This study provides the first line of evidence to suggest an effector role for CD8+ T cells in P. vivax blood-stage immunity. It is also the first report of species-specific differences in the subset of T cells that are expanded following primary Plasmodium infection, suggesting that malaria vaccine development may require optimization according to the target parasite.
Trial Registration
anzctr.org.au ACTRN12612000814875; anzctr.org.au ACTRN12613000565741; anzctr.org.au ACTRN12613001040752; ClinicalTrials.gov NCT02281344; anzctr.org.au ACTRN12612001096842; anzctr.org.au ACTRN12613001008718
Author Summary
The specific immune responses that contribute to protective immunity in humans following Plasmodium infection are yet to be fully characterized. The species P. vivax and P. falciparum account for most human infections, yet little is known about P. vivax specific immune responses and whether they are similar to or distinct from P. falciparum. Here, we establish that P. vivax and P. falciparum elicit distinct cellular immune responses following primary infection, with the expansion of a subset of CD38+ CD8+ T cells with a cytotoxic potential in P. vivax but not in P. falciparum infection. This study provides the first evidence for the activation of CD8+ T cells in P. vivax blood-stage infection and demonstrates the existence of species-dependent host immune responses to malaria. These findings have important implications for P. vivax vaccine development, and suggest that future malaria vaccine studies should be adapted according to the target Plasmodium spp.
Introduction
Malaria vaccine research efforts have been directed predominantly at P. falciparum, since globally it is the major cause of malaria-related mortality [1]. However, it is now recognized that P. vivax is poised to become the dominant species in areas where it is endemic [2] and can be associated with severe pathology [2,3]. Yet, compared to what is known about responses to P. falciparum, little is known about immune responses to P. vivax infection. This lack in knowledge is due in part to confounders that are present in samples from naturally-infected individuals living in malaria-endemic regions where parasitic co-infections and cross-species immunity are present; and technical difficulties associated with experimental infection of humans due to a lack of a method for the continuous in vitro culture of P. vivax [4]. It has been generally assumed that P. vivax would elicit similar immune responses compared to P. falciparum. However, the two parasites display very different features in terms of life cycle, invasion mechanism and immunopathology [2,3,5] and thus may generate distinct host specific immune responses. A few studies have compared global frequencies of circulating lymphocyte populations during P. falciparum or P. vivax infection in naturally infected humans [6,7], but have not investigated their activated or effector phenotype.
The recent establishment of different models of Controlled Human Malaria Infection (CHMI) provides the opportunity to obtain samples from malaria-naive healthy volunteers following first exposure to Plasmodium blood-stage parasites, thereby greatly enhancing our understanding of the host-parasite immune response [8,9]. Until recently, such experimental infection studies could be done only with P. falciparum due to the lack of a continuous in vitro culture system of P. vivax as a source of parasitized red blood cells [8]. Recently, however, a cell bank of cryopreserved P. vivax infected erythrocytes was successfully derived from a naturally-infected individual and used to experimentally infect malaria-naive healthy adult volunteers, establishing for the first time a CHMI model with P. vivax [10].
Here, we have taken advantage of this novel resource to compare cellular immune responses generated following experimental blood-stage infection of naive volunteers with P. vivax or P. falciparum. Overall, we found marked differences in the immune profiles generated following infection with the two species. Specifically, P. vivax but not P. falciparum infection led to the expansion of a specific subset of CD8+ T cells which were associated with an activated phenotype and cytotoxic potential. This study enhances our understanding of P. vivax associated immunity and Plasmodium species-specific immunity, identifying for the first time components of the immune response to blood-stage infection that are species-specific.
Methods
Ethics
Experimental infection of malaria-naive healthy adult volunteers was undertaken at QPharm Pty Ltd (Brisbane, Australia); all clinical studies were registered on the Australian and New Zealand Clinical Trials Registry (ANZCTR): P. falciparum clinical trial ID numbers ACTRN12612000814875, ACTRN12613000565741, ACTRN12613001040752 and NCT02281344; and P. vivax clinical trial numbers ACTRN12612001096842 and ACTRN12613001008718, with written informed consent and approval of the QIMR Berghofer Medical Research Institute Human Research Ethics Committee (QIMRB-HREC) and the Western Institutional Review Board (ethics board for the trial sponsor, Program for Appropriate Technology in Health, PATH).
Sample collection and processing
Inoculum preparation, volunteer recruitment, infection, monitoring and treatment were performed as described previously for P. falciparum [11] or P. vivax [10]. In brief, healthy malaria-naive individuals were intravenously inoculated with freshly thawed P. falciparum 3D7 or P. vivax parasitized erythrocytes and treated with anti-malarial drugs when the parasitemia exceeded the approximate threshold of 10,000 parasites/mL, at day 7–8 post-infection or day 14 post-infection for P. falciparum or P. vivax, respectively. The infecting dose for P. falciparum was 1,800 viable parasitized red blood cells. Parasite growth modeling using in silico analysis estimated that the infecting dose for P. vivax was 15 fold lower compared to P. falciparum (Khoury D & McCarthy JS, in preparation). Blood samples were collected prior to infection, at day 7 post-infection for P. falciparum infected volunteers, and day 14 post-infection for P. vivax infected volunteers. Peripheral blood collected in Lithium Heparin Vacutainers (Becton Dickinson) was either used directly for flow cytometry analysis, or peripheral blood mononuclear cells (PBMC) isolated using standard Ficoll density gradient centrifugation.
Determination of parasitemia and kinetics
Parasitemia was determined using a consensus P. falciparum or P. vivax species-specific quantitative PCR assay as previously described [12]. Parasite levels were assessed once daily until day four post-infection and then twice daily until treatment. All samples were batch tested in triplicate together after each study completion. Limit of detection was 64 parasites/ml [12]. Exponential growth equation fitting parasitemia kinetics for P. falciparum or P. vivax infected volunteers was calculated with GraphPad Prism (version 6.0).
Ex vivo phenotyping of lymphocytes by flow cytometry on whole blood
Staining buffer was PBS supplemented with 0.5% FCS and 4 mM EDTA. Whole blood collected in Lithium Heparin vacutainers was lysed and fixed with BD FACS lysing solution (Becton Dickinson) and lymphocytes permeabilized with BD FACS permeabilising solution 2 (Becton Dickinson) according to the manufacturer’s instructions. Cells were then resuspended in 50 μl of staining buffer containing anti-human CD4-BV510 (Becton Dickinson, 1:200 dilution), anti-human CD8-APC-H7 (Becton Dickinson, 1:400 dilution), anti-human CD19-PE-Cy7 (Biolegend, 1:200 dilution), anti-human CD38-APC (Biolegend, 1:400 dilution), anti-human Perforin-PE (Biolegend, 1:400 dilution), anti-human Granzyme B-Pacific Blue (Biolegend, 1:400 dilution) and 1 μl of human Fc receptor blocking solution (Human TruStain FcX, Biolegend) for 30 minutes at room temperature, washed and resuspended in staining buffer before acquisition on LSR Fortessa 4 (Becton Dickinson) with Diva software. FlowJo software version 6.0 was used for gating.
Cell sorting
CD38+ CD8+ T cells, CD38- CD8+ T cells as well as CD8- cells were sorted from freshly isolated PBMC. Approximately 10x106 cells were resuspended in 50 μl of staining buffer containing anti-human CD4-BV510 (Biolegend, 1:200 dilution), anti-human CD8-APC-H7 (Becton Dickinson, 1:400 dilution) and anti-human CD38-PerCpCy5.5 (Biolegend, 1:400 dilution) for 20 minutes at 4°C, washed and resuspended in staining buffer. Just before the sorting, 1 μg/mL of propidium iodide (Sigma-Aldrich) was added to allow for assessment of viability. Pi-CD8+CD4-CD38+, Pi-CD8+CD4-CD38-, and Pi-CD8- cells were sorted using a BD Aria III cell sorter (Becton Dickinson) directly in staining buffer and kept on ice until further use for in vitro assays.
CD107a-based degranulation assay
Sorted CD38+ and CD38- CD8+ T cells were plated at 50,000 cells/well in RPMI 1640 containing 25 mM Hepes, 2 mM L-glutamine (Invitrogen), and supplemented with 10 units/mL of Penicillin (Life Technologies), 10 μg/mL of Streptomycin (Life Technologies) and 10% fetal bovine serum (Life Technologies) in a 96-well plate pre-coated overnight with 10 μg/mL of anti-human CD3 OKT3 antibody (Biolegend) together with 0.75x106 cells/mL autologous CD8- cells, anti-human CD107a-FITC (Biolegend, 1:200 dilution) and 1 μg/mL of co-stimulatory antibodies anti-human CD28 and anti-human CD49d (Becton Dickinson) for 5 hours at 37°C in an atmosphere of 5% C02. Following stimulation, cells were resuspended in 20 μl of staining buffer containing anti-human CD4-BV510 (Biolegend, 1:200 dilution), anti-human CD8-APC-H7 (Becton Dickinson, 1:400 dilution) for 20 minutes at 4°C, washed and resuspended in staining buffer before acquisition on LSR Fortessa 4 (Becton Dickinson) with Diva software. FlowJo version 6.0 was used for gating.
Results
P. falciparum and P. vivax primary blood-stage infection in humans generated similar parasitemia curves
P. falciparum and P. vivax experimental infection of malaria-naive volunteers was performed under similar procedures [10,11]. However, due to logistical reasons associated with parasite density in the inoculum stock, and a lack of a continuous in vitro culture system for P. vivax [4], the infecting dose for P. vivax was estimated to be 15-fold lower than that used in the P. falciparum studies (Khoury D & McCarthy JS, in preparation). Demographics of P. falciparum and P. vivax infected volunteers were comparable in term of age, gender, BMI and ethnicity (S1 Table).
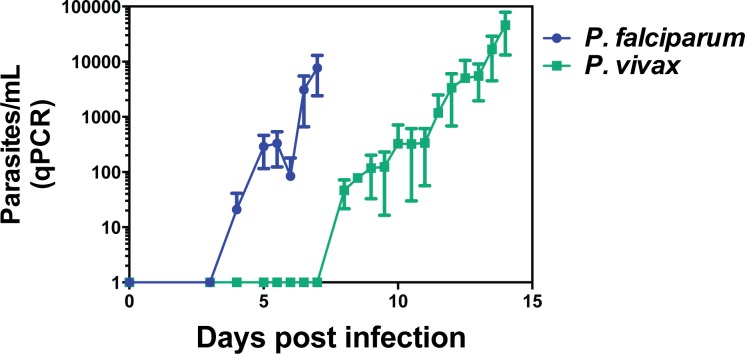
Parasitemia growth curves determined by quantitative PCR (qPCR) from P. falciparum and P. vivax experimental infection studies were similar for both parasites (Fig 1 and Table 1) except for the delayed onset of detectable blood-stage parasitemia with P. vivax. Specifically, P. falciparum parasites were detected as early as day 4 of infection whereas P. vivax parasites were detected at day 8 of infection, consistent with the differences in the size of the starting inocula. Interestingly, all individuals infected with P. vivax developed symptoms of malaria before the time of treatment while more than 40% of P. falciparum infected individuals were asymptomatic until anti-malarial drug administration (S2 Table).
Fig 1. Kinetics of parasite levels during experimental blood-stage infection with P. falciparum or P. vivax.
Malaria-naive volunteers were infected intravenously at day 0 with freshly thawed cryopreserved P. falciparum or P. vivax infected erythrocytes. Parasite growth modeling using in silico analysis estimated that the P. vivax infecting dose was 15 times lower compared to the P. falciparum infecting dose (Khoury D & McCarthy JS, in preparation). Parasite levels in peripheral blood of infected volunteers were determined using consensus Plasmodium species-specific qPCR as previously described [12]. Blood samples used to assess immune responses were collected prior to infection. Graphs show mean data from 19 volunteers from three independent cohorts (P. falciparum) and 8 volunteers from four independent cohorts (P. vivax); error bars indicate SD.
Table 1. Statistics of exponential growth equation fitting the parasitemia curves of P. falciparum and P. vivax infected naïve volunteers.
| P. falciparum | P. vivax | |
|---|---|---|
| Growth rate (days-1) | 2.2 [1.5–2.9] | 1.9 [1.3–2.5] |
| Doubling time (days) | 0.31 [0.24–0.46] | 0.36 [0.27–0.54] |
| R square | 0.60 | 0.63 |
P. falciparum and P. vivax primary blood-stage infection in humans elicited qualitatively different immune responses
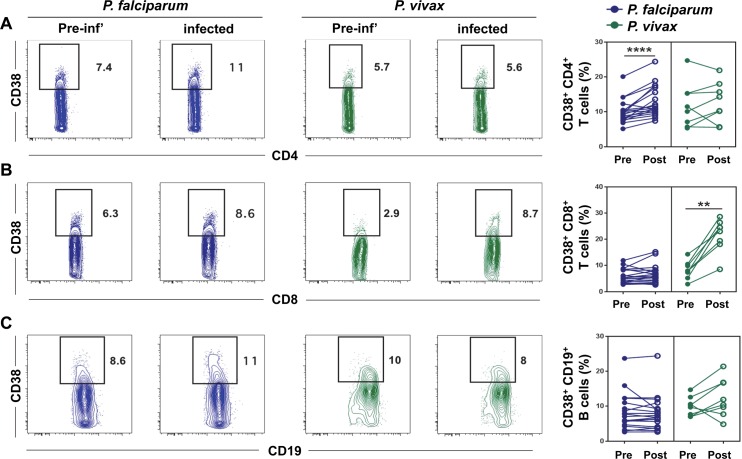
In order to compare cellular immune responses to infection between P. falciparum and P. vivax infected volunteers, we determined the phenotype of lymphocytes ex vivo from whole blood samples obtained prior to infection and during infection once the parasitemia exceeded 10,000 parasites/mL (which corresponded to day 7 and day 14 for P. falciparum and P. vivax infected volunteers, respectively). CD38 is a surface glycoprotein that modulates cell adhesion, signal transduction and intracellular Ca2+ levels, and is specifically upregulated on lymphocytes following activation [13]. We have recently shown that the frequency of CD38+ T cells and B cells circulating in the peripheral blood of test volunteers was dynamically regulated during experimental blood-stage infection with P. falciparum and that the expansion of CD38+ CD4+ T cells following infection was inversely correlated with parasite burden [14]. Thus, we compared the frequencies of CD38+ T cells and B cells circulating before and after infection in P. falciparum or P. vivax infected volunteers. There was a higher frequency of CD38+ CD4+ T cells circulating following infection in P. falciparum but not P. vivax infected volunteers (Fig 2A). Conversely, P. vivax infection but not P. falciparum elicited a higher frequency of circulating CD38+ CD8+ T cells (Fig 2B). No significant differences were observed in the frequency of CD38+ B cells circulating following infection with P. falciparum or P. vivax (Fig 2C). There was no correlation between the expansion of CD38+ CD8+ T cells following infection and parasite burden (S1 Fig). Overall, these data suggest that the quality of the immune response generated following primary blood-stage infection in humans is Plasmodium species-dependent.
Fig 2. The frequency of CD38+ T cells and B cells circulating during experimental blood-stage infection with P. falciparum or P. vivax.
Blood samples were collected from malaria-naive volunteers prior to (pre) and post-infection (post) with P. falciparum or P. vivax infected erythrocytes, as described in legend to Fig 1. The frequency of CD38+ cells was determined by flow cytometry in (A) CD4+ T cells, (B) CD8+ T cells, or (C) CD19+ B cells. Differences in cell frequencies between pre- and post-infection were determined using the non-parametric paired Wilcoxon test. Plots show representative staining of CD38 from one P. falciparum infected volunteer and one P. vivax infected volunteer. Graphs show paired data from 19 volunteers from three independent cohorts (P. falciparum) and 8 volunteers from four independent cohorts (P. vivax); ****, p < 0.0001; **, p < 0.01.
CD38+ CD8+ T cells expanded in P. vivax infection are associated with a cytotoxic function
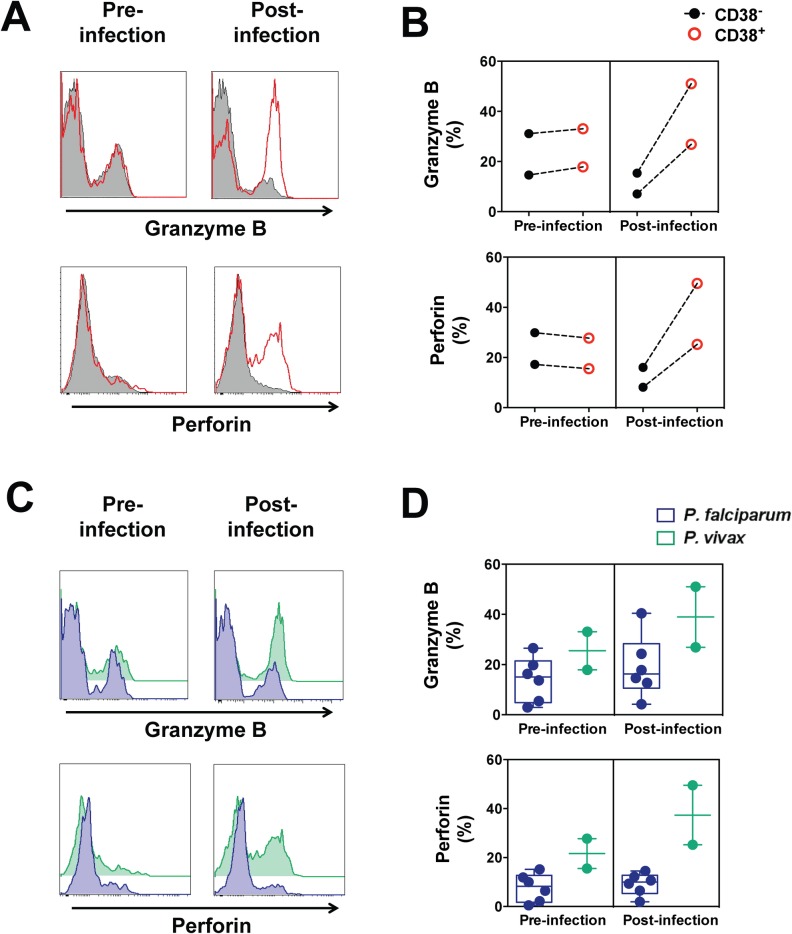
Since very little information on P. vivax protective immune responses is available, we aimed to further understand the contribution of CD38+ CD8+ T cells to P. vivax blood-stage immunity. Effector CD8+ T cells perform classical cytotoxic functions by killing infected cells through perforin-mediated dependent mechanisms. To determine the cytotoxic potential of CD38+ CD8+ T cells generated during P. vivax infection, we measured their intracellular expression of granzyme B and perforin by flow cytometry, in an independent cohort (n = 2 because of logistics associated with vivax experimental infection). We found similar expression of granzyme B and perforin in CD38+ and CD38- CD8+ T cells before infection (Fig 3A and 3B). However, post-infection, CD38+ CD8+ T cells had a greater expression of granzyme B and perforin compared to CD38- CD8+ T cells (Fig 3A and 3B). Additionally, CD38+ CD8+ T cells circulating during infection contained a higher amount of granzyme B and perforin in P. vivax infected volunteers compared to P. falciparum infected volunteers (Fig 3C and 3D) whereas no differences were observed prior to infection.
Fig 3. Granzyme B and perforin expression in circulating CD38+ CD8+ T cells following P. vivax blood-stage infection.
Blood samples were collected from malaria-naive volunteers prior to and post-infection with P. falciparum or P. vivax infected erythrocytes, as described in legend to Fig 1. The expression of granzyme B and perforin within CD38+ CD8+ T cells and CD38- CD8+ T cells was determined by flow cytometry. (A) Representative staining of granzyme B and perforin in CD38+ and CD38- CD8+ T cells from one P. vivax infected volunteer. (B) The frequency of cells expressing granzyme B and perforin in CD38+ or CD38- CD8+ T cells circulating pre- and post-infection with P. vivax. (C) Representative staining of granzyme B and perforin in circulating CD38+ CD8+ T cells from one P. falciparum infected volunteer and one P. vivax infected volunteer. (D) The frequency of cells expressing granzyme B and perforin in circulating CD38+ CD8+ T cells pre- or post-infection in P. vivax or P. falciparum infected volunteers. Graphs show mean data from 6 volunteers (P. falciparum) or 2 volunteers (P. vivax); error bars indicate SD.
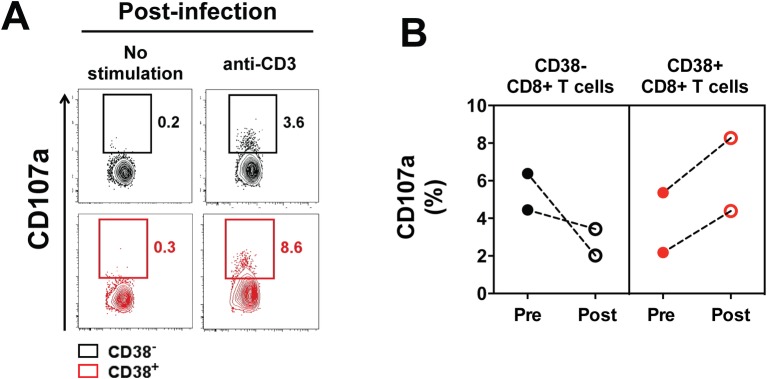
In order to further investigate the cytotoxic potential of CD38+ CD8+ T cells and CD38- CD8+ T cells circulating during P. vivax infection, we tested their ability to degranulate in vitro following TCR stimulation. Prior to infection, CD38+ and CD38- CD8+ T cells had the same ability to degranulate, whereas post-infection CD38+ CD8+ T cells had a higher degranulation compared to CD38- CD8+ T cells (Fig 4).
Fig 4. Degranulation in circulating CD38+ and CD38- CD8+ T cells following P. vivax blood-stage infection.
Blood samples were collected from malaria-naive volunteers prior to and post-infection with P. falciparum or P. vivax infected erythrocytes, as described in legend to Fig 1. Degranulation of CD38+ and CD38- CD8+ T cells was determined by flow cytometry using a CD107a-based degranulation assay on sorted cells after five hours stimulation with plate-bound anti-human CD3 (10 μg/mL). (A) Representative staining of CD107a in circulating CD38+ and CD38- CD8+ T cells from one P. vivax infected volunteer. (B) Frequency of CD107a+ cells after stimulation (background subtracted) in circulating CD38+ and CD38- CD8+ T cells pre- or post-infection in P. vivax infected volunteers. Graphs show mean data from 2 volunteers.
Overall, these findings suggest that CD38+ CD8+ T cells have a greater cytotoxic potential compared to CD38- CD8+ T cells and that this cytotoxic function is specifically activated in the CD38+ CD8+ T cells circulating during P. vivax infection but not P. falciparum infection.
Discussion
Herein we report on the first study to compare the quality of cellular immune responses elicited in humans by experimental blood-stage infection with P. vivax or P. falciparum. In this study we utilized a model of controlled infection of malaria-naive human volunteers, thereby avoiding potential confounders of pre-existing immunity and cross-species immunity. We found marked differences between responses to the two Plasmodium species in terms of the phenotype of T cells that expanded during infection. P. falciparum infection elicited a significant expansion of CD38+ CD4+ T cells whereas P. vivax infection led to the expansion of CD38+ CD8+ T cells. We have recently shown that the frequency of circulating CD38+ CD4+ T cells was significantly increased following experimental infection with P. falciparum and inversely correlated with parasite levels [14]. Here we show that P. vivax blood-stage infection elicited a substantially different type of immune response compared to P. falciparum, with significant changes in the CD8+ T cell compartment rather than in the CD4+ T cell compartment. There was no significant association between the expansion of CD38+ CD8+ T cells and parasite burden in P. vivax infected volunteers, suggesting that the expansion of CD38+ CD4+ T cells in P. falciparum infection and the expansion of CD38+ CD8+ T cells in P. vivax infection might have distinct contributions to the immune response to blood-stage infection.
A possible explanation for the qualitative differences observed between P. falciparum and P. vivax associated immune responses may relate to their distinct tropism during blood-stage infection. P. vivax merozoites preferentially invade reticulocytes [15] which, although they are enucleated, still express MHC-I molecules which remain from the nucleated reticuloblast stage. This is in contrast to mature erythrocytes that have completely lost expression of MHC molecules. Previous work using a genetically engineered mouse model of murine malaria has shown that CD8+ T cells can be activated by parasitized erythroblasts but not parasitized mature red blood cells through MHC-I dependent mechanisms [16]. Thus, we hypothesize that the higher proportion of infected reticulocytes in P. vivax infection leads to the activation of a higher proportion of CD38+ CD8+ T cells in an MHC-I dependent manner in comparison to P. falciparum infection.
While it is established that CD4+ T cells and parasite-specific antibodies are critical for protective immune responses to blood-stage malaria [17], the contribution of CD8+ T cells is less clear. Studies in mice have shown that CD8+ T cells were activated and associated with protective function in lethal [18] or chronic [19] blood-stage malaria. However, no association between CD8+ T cells and protective immunity to primary blood-stage infection in humans has been reported yet. Our data suggest that the CD38+ CD8+ T cells that specifically expand during P. vivax infection display increased cytotoxic function compared to other CD8+ T cells. Hence their function might be to kill parasitized reticulocytes through MHC-I dependent and perforin-mediated mechanisms. This proposal is further supported by the enhanced expression of cytotoxic molecules observed in CD38+ CD8+ T cells circulating during P. vivax compared to P. falciparum infection. In contrast, we could not identify an expansion of cytotoxic CD8+ T cell population following P. falciparum infection of malaria-naïve volunteers.
These findings have important implications in regard to P. vivax vaccine development. Indeed, most efforts so far have been directed toward the direct translation of the findings associated with P. falciparum vaccine development to P. vivax [20] (e.g. the use of ortholog antigens that were shown to be protective against P. falciparum). Here we show that P. falciparum and P. vivax elicit qualitatively different immune responses and are likely to require distinct vaccine-induced immune responses for protection. Thus, the immunization strategy may need to be adapted for each Plasmodium species to mount optimal protective immune responses, and the design of a universal vaccine conferring protection against multiple Plasmodium species might be conceptually more challenging than initially thought.
In conclusion, in this study we report that primary exposure of humans to different Plasmodium species elicited qualitatively distinct immune responses: P. falciparum infection generated changes in CD4+ T cells whereas P. vivax preferentially activated a subset of CD8+ T cells expressing the activation marker CD38 and associated with a cytotoxic function. The specific expansion of CD38+ CD8+ T cells following P. vivax infection might be due to the preference for P. vivax merozoites to infect reticulocytes which can activate CD8+ T cells through MHC-I dependent mechanisms. Overall our data are consistent with the proposal that protective immune responses to Plasmodium are species-dependent. These findings have important implications for malaria vaccine development strategies.
Supporting Information
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank the Q-pharm staff who conducted the human infection studies, in particular Suzanne Elliot, Nannette Douglas and Gem Mackenroth; Paul Griffin; Katharine Trenholme and Silvana Sekuloski for their critical role in the study management, and Fiona Amante for coordinating the substudy. We also thank Paula Hall and the flow cytometry facility of QIMR Berghofer Medical Research Institute for technical assistance with cell sorting.
Data Availability
All relevant data are within the paper.
Funding Statement
JGB was supported by an International Research Tuition Award from the University of Queensland. DLD is supported by a National Health and Medical Research Council of Australia (NHMRC) Principal Research Fellowship. JSM is supported by a Government of Queensland Government Clinical Research Fellowship. The clinical studies were funded predominantly through funding provided by the Program for Appropriate Technology in Health (PATH) Malaria Vaccine Initiative (MVI) and Medicines for Malaria Venture (MMV). The laboratory research was supported by NHMRC Program Grant #1037304. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. World Malaria Report (2015) Available: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/. [Google Scholar]
- 2.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, et al. (2007) Vivax malaria: neglected and not benign. Am J Trop Med Hyg 77: 79–87. [PMC free article] [PubMed] [Google Scholar]
- 3.Hemmer CJ, Holst FG, Kern P, Chiwakata CB, Dietrich M, et al. (2006) Stronger host response per parasitized erythrocyte in Plasmodium vivax or ovale than in Plasmodium falciparum malaria. Trop Med Int Health 11: 817–823. 10.1111/j.1365-3156.2006.01635.x [DOI] [PubMed] [Google Scholar]
- 4.Noulin F, Borlon C, Van Den Abbeele J, D'Alessandro U, Erhart A (2013) 1912–2012: a century of research on Plasmodium vivax in vitro culture. Trends Parasitol 29: 286–294. 10.1016/j.pt.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 5.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, et al. (2009) Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 9: 555–566. 10.1016/S1473-3099(09)70177-X [DOI] [PubMed] [Google Scholar]
- 6.Worku S, Bjorkman A, Troye-Blomberg M, Jemaneh L, Farnert A, et al. (1997) Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: distinct gammadelta+ T cell patterns in Plasmodium falciparum and P. vivax infections. Clin Exp Immunol 108: 34–41. 10.1046/j.1365-2249.1997.d01-981.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goncalves RM, Salmazi KC, Santos BA, Bastos MS, Rocha SC, et al. (2010) CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: do different parasite species elicit similar host responses? Infect Immun 78: 4763–4772. 10.1128/IAI.00578-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauerwein RW, Roestenberg M, Moorthy VS (2011) Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol 11: 57–64. 10.1038/nri2902 [DOI] [PubMed] [Google Scholar]
- 9.Engwerda CR, Minigo G, Amante FH, McCarthy JS (2012) Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol 28: 515–521. 10.1016/j.pt.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 10.McCarthy JS, Griffin PM, Sekuloski S, Bright AT, Rockett R, et al. (2013) Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis 208: 1688–1694. 10.1093/infdis/jit394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy JS, Sekuloski S, Griffin PM, Elliott S, Douglas N, et al. (2011) A pilot randomised trial of induced blood-stage Plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS One 6: e21914 10.1371/journal.pone.0021914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockett RJ, Tozer SJ, Peatey C, Bialasiewicz S, Whiley DM, et al. (2011) A real-time, quantitative PCR method using hydrolysis probes for the monitoring of Plasmodium falciparum load in experimentally infected human volunteers. Malar J 10: 48 10.1186/1475-2875-10-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funaro A, Spagnoli GC, Ausiello CM, Alessio M, Roggero S, et al. (1990) Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol 145: 2390–2396. [PubMed] [Google Scholar]
- 14.Burel JG, Apte SH, Groves PL, Klein K, McCarthy JS, Doolan DL (2016) Reduced Plasmodium parasite burden associates with cd38+ cd4+ t cells displaying cytolytic potential and impaired IFN-γ production. PLoS Pathog 12(9): e1005839 10.1371/journal.ppat.1005839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galinski MR, Barnwell JW (1996) Plasmodium vivax: Merozoites, invasion of reticulocytes and considerations for malaria vaccine development. Parasitol Today 12: 20–29. [DOI] [PubMed] [Google Scholar]
- 16.Imai T, Ishida H, Suzue K, Hirai M, Taniguchi T, et al. (2013) CD8(+) T cell activation by murine erythroblasts infected with malaria parasites. Sci Rep 3: 1572 10.1038/srep01572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wipasa J, Elliott S, Xu H, Good MF (2002) Immunity to asexual blood stage malaria and vaccine approaches. Immunol Cell Biol 80: 401–414. 10.1046/j.1440-1711.2002.01107.x [DOI] [PubMed] [Google Scholar]
- 18.Imai T, Shen J, Chou B, Duan X, Tu L, et al. (2010) Involvement of CD8+ T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain. Eur J Immunol 40: 1053–1061. 10.1002/eji.200939525 [DOI] [PubMed] [Google Scholar]
- 19.Horne-Debets JM, Faleiro R, Karunarathne DS, Liu XQ, Lineburg KE, et al. (2013) PD-1 dependent exhaustion of CD8+ T cells drives chronic malaria. Cell Rep 5: 1204–1213. 10.1016/j.celrep.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 20.Arevalo-Herrera M, Chitnis C, Herrera S (2010) Current status of Plasmodium vivax vaccine. Hum Vaccin 6: 124–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper.