Abstract
Comparison of amino acid sequence similarity is the fundamental concept behind the protein phylogenetic tree formation. By virtue of this method, we can explain the evolutionary relationships, but further explanations are not possible unless sequences are studied through the chemical nature of individual amino acids. Here we develop a new methodology to characterize the protein sequences on the basis of the chemical nature of the amino acids. We design various algorithms for studying the variation of chemical group transitions and various chemical group combinations as patterns in the protein sequences. The amino acid sequence of conventional myosin II head domain of 14 family members are taken to illustrate this new approach. We find two blocks of maximum length 6 aa as ‘FPKATD’ and ‘Y/FTNEKL’ without repeating the same chemical nature and one block of maximum length 20 aa with the repetition of chemical nature which are common among all 14 members. We also check commonality with another motor protein sub-family kinesin, KIF1A. Based on our analysis we find a common block of length 8 aa both in myosin II and KIF1A. This motif is located in the neck linker region which could be responsible for the generation of mechanical force, enabling us to find the unique blocks which remain chemically conserved across the family. We also validate our methodology with different protein families such as MYOI, Myosin light chain kinase (MLCK) and Rho-associated protein kinase (ROCK), Na+/K+-ATPase and Ca2+-ATPase. Altogether, our studies provide a new methodology for investigating the conserved amino acids’ pattern in different proteins.
Introduction
All living organisms are made up of proteins. The biochemical information that resides in the protein primary sequence maintain their structure, function, and even its own stability. This biochemical information is instructed/governed by the amino acid sequences. The versatility of amino acid sequences provide the different outcomes [1]. In fact, the chemical properties of amino acids which are embedded in the primary protein sequences take the key role to determine the biological activity of the protein. In-silico techniques to analyze this information in terms of their chemical nature or structure are yet to be explored completely. In literature, the phylogenetic analyses are done from various angles and different perspectives such as multiple alignments for the selection of conserved block [2], Randomized Accelerated Maximum Likelihood (RAXML) [3], conditional Lempel-Ziv (LZ) complexity [4], tree alignment graph [5] etc. A phylogeny or evolutionary tree represents the evolutionary relationships among a group of protein sequences. The longer the branch in the horizontal dimensions of the tree, the larger is the amount of change. Trees are useful in bioinformatics, system biology and various phylogenetic comparative methods [3, 6–10]. Despite its great implication, phylogenetic tree can find only similarity analysis of amino acid sequences but not the chemical nature of amino acids in a protein. Also multiple sequence alignment of several hundred sequences always produces a log jam in respect of time and biasness [11]. Several groups have worked with reduced amino acid alphabets to tackle to the above stated problems by reducing the sample size which can perform at the same level as the full alphabets in correct pair wise alignment of sequences with regards to structure similarity but low sequence identity [12, 13]. Recently XIE et al. [14] proposed a new method by using hydropathy group of amino acids to analyze the similarity/dissimilarity of protein sequence based on the conditional probability of the protein sequence [14]. Studies are also done on using the substitution matrices from several protein blocks of aligned sequence segments resulting the characterization of related proteins [15]. However, studying only the amino acid sequences of proteins limits our scope of understanding of the similarities and differences among proteins with regard to their biochemical nature. Further analyses of the sequences considering chemical nature are required and it can be done if the sequences can be studied through the chemical nature of individual amino acid.
In this report, we propose a mathematical model where the twenty amino acids are segregated into eight groups on the basis of their chemical nature. Further, we study the transition among the eight groups of distinct chemical properties of the amino acids of conventional myosin’s head domains. Their transitions in the sequences are calculated to demonstrate the unique chemical transition pattern of amino acid clustering in each sequence of conventional myosin II family. Various patterns with repetition and without repetition of amino acids’ chemical group are enumerated. We also expand our study to another inter sub-family of Kinesin, KIF1A and find a unique common block in the neck linker region. This evolutionary conserved block is hypothesized to reveal the functional role in the conversion of chemical energy to mechanical energy. Further, our studies are expanded to different protein families and results demonstrate various common blocks in the family members have been highlighted.
Materials and Methods
Amino acid categorization
Twenty amino acids are categorized into eight chemical groups according to their side chain shown in Table 1.
Table 1. Amino acid categorization based on their chemical nature.
| Chemical Nature of the Group | Amino Acids |
|---|---|
| Acidic | Aspartate (D), Glutamate (E) |
| Basic | Arginine (R), Histidine (H), Lysine (K) |
| Aromatic side chain | Tyrosine (Y), Phenylalanine (F), Tryptophan (W) |
| Aliphatic side chain | Isoleucine (I), Leucine (L), Valine (V), Alanine (A), Glycine (G) |
| Cyclic | Proline (P) |
| Sulfur containing | Methionine (M), Cysteine (C) |
| Hydroxyl containing | Serine (S), Threonine (T) |
| Acidic amide | Glutamine (Q), Asparagine (N) |
In order to characterize the amino acid sequence, we transform these groups into numerical sequence (mapping 20 distinct amino acids into eight groups as integer 1-8 only), each amino acid from respective chemical groups are replaced by corresponding group numbers shown in Table 2. These eight chemical groups of amino acids are used to characterize the chemical nature of the sequences in various ways.
Table 2. Mapping Twenty standard amino acids to eight chemical groups of amino acids.
| Amino Acids | D | E | R | H | K | Y | F | W | I | L | V | A | G | P | S | T | M | C | Q | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numerical Value | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||||
| Group Name | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | ||||||||||||
Myosin super family
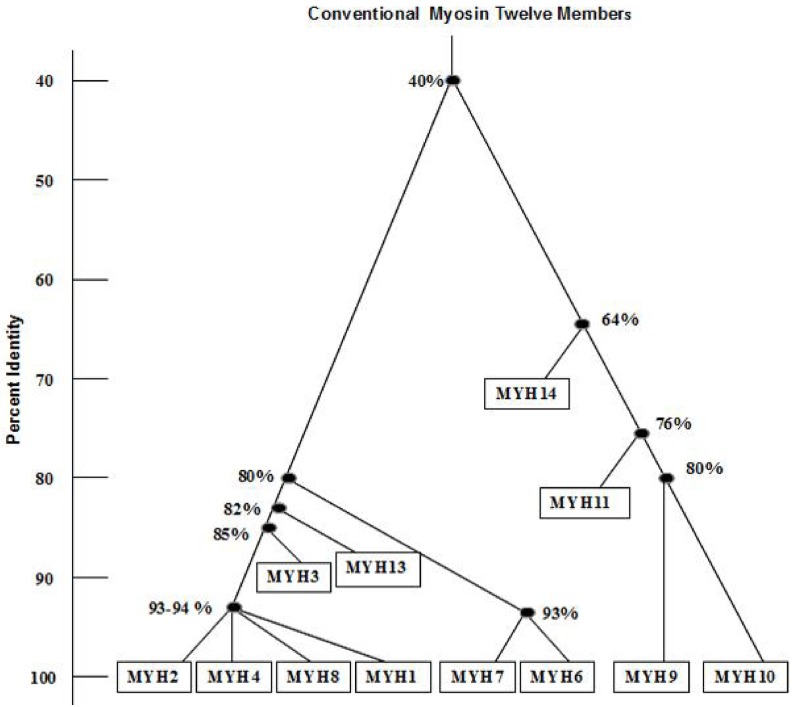
To analyze the effectiveness of our mathematical model we select human actin based conventional motor protein myosin II family [16–18] whose phylogenetic tree is shown in Fig 1.
Fig 1. Conventional Myosin II Family as previously described in [16–18].
Based on the heavy chain amino acid sequence similarities myosin II is broadly divided into two groups; (a) non-muscle myosin group and (b) muscle myosin group as detailed in Table 3.
Myosin II, the conventional two headed myosin protein, is composed of one pair each of heavy chain (220kDa), essential light chain (ELC, 17kDa) and regulatory light chain (RLC, 20kDa). Each heavy chain has a globular head domain containing the Actin and ATP binding domain, required for their motor activity, an intermediate domain that forms an α-helical coiled coil and a C-terminal non-helical tailpiece [19]. We extend the list of the human NMIIs, by adding the sequences annotated as human NMIIs sequences in uniport database. All together we collect a total of 15 candidate sequences. Most of these proteins have been assigned to a particular group by previous studies. From the new sequences, we discard MYH16 gene, as a pseudo gene [20–22]. Table 3 lists with their names, length of head domain, accession number, protein names and groups (Data is collected from www.uniport.org).
Table 3. Details of conventional myosin II family members in human.
| Seq. Nos. | Gene Name | Length of the Head Domain (aa) | Accession Number | Protein Names and Remarks |
|---|---|---|---|---|
| 1 | MYH14 | 860 | Q7Z406 | Myosin-14(NMHC II-C) |
| 2 | MYH 11 | 843 | P35749 | Myosin-11 (SM MyHc) |
| 3 | MYH9 | 836 | P35579 | Myosin-9 (NMHC II-A) |
| 4 | MYH10 | 843 | P35580 | Myosin-10 (NMHC II-B) |
| 5 | MYH15 | 850 | Q9Y2K3 | Myosion-15 |
| 6 | MYH7B | 845 | A7E2Y1 | Myosion-7B (Cardiac β MyHC) |
| 7 | MYH2 | 844 | Q9UKX2 | Myosin-2 (IIa MYH2) |
| 8 | MYH4 | 842 | Q9Y623 | Myosin-4 (IIb MYH4) |
| 9 | MYH8 | 841 | P13535 | Myosin-8 (Perinatal MyHC) |
| 10 | MYH1 | 842 | P12882 | Myosin-1 (Ix/d MYH1) |
| 11 | MYH3 | 829 | P11055 | Myosin-3 (Embryonic MyHC) |
| 12 | MYH13 | 842 | Q9UKX3 | Myosin-13 (Extraocular MyHC) |
| 13 | MYH7 | 838 | P12883 | Mysion-7 (Cardiac β MyHC) |
| 14 | MYH6 | 840 | P13533 | Mysion-6 (Cardiac α MyHC) |
This myosin family is having two sub groups: a) Seq.nos. 1-4 is the non-muscle myosin group, and b) Seq nos. 5-14 is the muscle myosin group. (aa-amino acid)
We chose the motor domain because these core sequences are very much conserved in all myosin classes. They consist of 7-stranded β-sheet encircled by several α-helixes. Myosin motor domain has an Actin binding region which can show open and close conformations in response to ATP binding and has a neck linker region that contains IQ motifs (IQxxxRGxxxR) responsible for myosin light chain binding [23–28].
Myosin I family
To validate our methods with other myosin family, we chose to study MYOI class of unconventional myosin (the second biggest sub-family of myosin). Myosin Is are single headed myosins that lack the heptapeptide motif responsible for coiled-coil association in the C-terminal like myosin II [29]. There are 8 myosin I isoforms found in vertebrates, and generally referred as MYO1A-H using nomenclature adopted from Human Genome organization [30, 31]. In Table 4 shows their names, length, accession number, protein names and remarks.
Table 4. Details of unconventional myosin sub-family MYOI class members in human.
| Seq. Nos. | Gene Name | Length of the Head Domain (aa) | Accession Number | Protein Names and Remarks |
|---|---|---|---|---|
| 1 | MYO1A | 694 | Q9UBC5 | Unconventional myosin-Ia |
| 2 | MYO1B | 701 | O43795 | Unconventional myosin-Ib |
| 3 | MYO1D | 695 | O94832 | Unconventional myosin-Id |
| 4 | MYO1G | 707 | B0I1T2 | Unconventional myosin-Ig |
| 5 | MYO1C | 731 | O00159 | Unconventional myosin-Ic |
| 6 | MYO1H | 690 | Q8N1T3 | Unconventional myosin-Ih |
| 7 | MYO1E | 692 | Q12965 | Unconventional myosin-Ie |
| 8 | MYO1F | 690 | O00160 | Unconventional myosin-If |
KIF1A class of kinesin family
To extend and validate our methods from intra-protein group to inter-protein group, we chose to study KIF1A class of kinesin protein family. Both the KIF1A class of kinesin and myosin II motor domain sequences are evolved from same ancestor [32]. Table 5 lists their names, length, accession number, protein names and remarks.
Table 5. Details of kinesin sub-family KIF 1A class members in human.
Other protein family
Further, study is also directed to by choosing different protein families such as Myosin light chin kinase (MYLK/MLCK) in Table 6 [33–35], Rho-associated protein kinase (ROCK) in Table 7 [36, 37], Na+/K+-ATPase or sodium-potassium pump enzyme in Table 8 [38, 39] and Ca2+-ATPase transport protein in Table 9 [40–42]. The enzymes MLCK and Rho are known for phosphorylation of the regulatory light chain of myosin II.
Table 6. Details of MLCK protein family members in human.
Table 7. Details of Rho-associated protein kinase family members in human.
Table 8. Details of Na+/K+-ATPase family members in human.
| Seq. Nos. | Gene Name | Length | Accession Number | Protein Names and Remarks |
|---|---|---|---|---|
| 1 | ATP1A1 | 1023 | P05023 | Sodium/potassium-transporting ATPase subunit alpha-1 |
| 2 | ATP1A2 | 1020 | P50993 | Sodium/potassium-transporting ATPase subunit alpha-2 |
| 3 | ATP1A3 | 1013 | P13637 | Sodium/potassium-transporting ATPase subunit alpha-3 |
| 4 | ATP1A4 | 1029 | Q13733 | Sodium/potassium-transporting ATPase subunit alpha-4 |
| 5 | ATP1B1 | 303 | P05026 | Sodium/potassium-transporting ATPase subunit beta-1 |
| 6 | ATP1B2 | 290 | P14415 | Sodium/potassium-transporting ATPase subunit beta-2 |
| 7 | ATP1B3 | 279 | P54709 | Sodium/potassium-transporting ATPase subunit beta-3 |
| 8 | ATP1B4 | 357 | B7ZKV8 | Sodium/potassium-transporting ATPase subunit beta-4 |
Table 9. Details of Ca2+-ATPase family members in human.
| Seq. Nos. | Gene Name | Length (aa) | Accession Number | Protein Names and Remarks |
|---|---|---|---|---|
| 1 | ATP2A1 | 1001 | O14983 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 |
| 2 | ATP2A2 | 1042 | P16615 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 |
| 3 | ATP2A3 | 1043 | Q93084 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 3 |
| 4 | ATP2B1 | 1258 | P20020 | Plasma membrane calcium-transporting ATPase 1 |
| 5 | ATP2B2 | 1243 | Q01814 | Plasma membrane calcium-transporting ATPase 2 |
| 6 | ATP2B3 | 1220 | Q16720 | Plasma membrane calcium-transporting ATPase 3 |
| 7 | ATP2B4 | 1241 | P23634 | Plasma membrane calcium-transporting ATPase 4 |
Computational algorithms
We use different algorithms for studying chemical group transitions count, pattern analysis with repetition and without repetition of chemical groups, conserved block findings etc. For all the cases separate programs are designed on using Matlab software 2013a. All the algorithms are enumerated below:
Algorithm 1: Chemical group transitions and ordered pair analysis
This algorithm is developed to list the total number of amino acids of certian groups (say G1) followed by the listing of the number of amino acid ordered pairs having first amino acid fixed (belonging to G1 group) and the second amino acid from every other possible groups for a given sequence.
Input: Set of primary protein sequences.
Output: Number count of chemical group transitions and lists the total number of amino acids from each group.
Firstly, amino acid sequence is transformed into numerical value (called numerical sequence having 1-8 numerals using Table 2). Initially a null matrix (G) of size 8 by 8 is generated for each sequence. Pair wise (say (x, y)) numerical sequence is read from left to right till the end of the sequence and the corresponding (x, y) cell value (ith row and jth column) of the matrix (G) is incremented by one. The cell (i, j) value of the final G matrix represents the number count from i to j chemical group transitions (or number count of (i, j) or (Gi, Gj) ordered pairs) for a particular sequence. On the other hand, every row value (a particular group) of the matrix tells about the number of transitions at which one amino acid (of one group) in a sequence changes as ordered pair to other possible groups including the self group. Every row value count (aggregate) from the matrix G is stored in column wise into another matrix (F) listing the total number of amino acids of certian groups followed by the listing of the number of amino acid ordered pairs having first amino acid fixed and the second amino acid from every other possible groups for a given sequence providing a hyphen (-) between them. Finally, we get the F matrix of size m × n; where n is the total number of sequences and m is the number of various chemical groups (for our analysis m = 8).
Algorithm 2: Common pattern finding without repetition of chemical groups
This algorithm is developed to find the various lengths of chemical patterns without repetition of chemical groups and their location in the sequence.
Input: Set of primary protein sequences and pattern length (L).
Output: Common pattern with amino acid sequence and location.
Firstly, as in Algorithm 1 amino acid sequence is transformed into numerical sequence (using Table 2). Given an input of pattern length L (2 ≤ L ≤ 8), all possible combination of pattern of length L using the numerical numbers 1-8 are generated without repetition of same numerals. Every possible pattern is investigated among the sequences, and if the pattern is found for all the sequences, the pattern is selected and stored along with the locations, otherwise the pattern is discarded. By varying pattern length L, we can search for different lengths of patterns discussed above. Finally, based on the selected patterns and their locations, corresponding primary sequence is obtained.
Algorithm 3: Common pattern finding with the repetition of chemical groups
This algorithm is developed to find the various lengths of chemical patterns like Algorithm 2 but with the repetition of chemical groups and their location in the sequence. To reduce the time complexity for generating the pattern of bigger length, here the concatenation technique is used starting with 4 length patterns.
Input: Set of primary protein sequences, common motif /pattern (L ≥ 4).
Output: Common motif/pattern with amino acid sequence and location.
Firstly, amino acid sequence set is transformed into numerical sequence like Algorithm 1. The required length of the common motifs (L) is then divided by 4 and the quotient (q) and remainder (r) are noted. All possible tetrameric permutations of 1-8 (with repetitions) (e.g. 1111, 1112… 8887, 8888) are generated, and searched in the transformed sequences. The tetramers present in all the sequences are stored, and the rest are discarded. In the next iteration, all possible tetramers of 1-8 (generated at the start) are concatenated to the stored tetrameric sequences and the resulting motif is found out.
Algorithm 4: Similarity analysis of pattern/block
This algorithm is developed to find out the highest percentage of similarity (with regards to chemical group) of a pattern/block from the given set of sequences and the corresponding location in the sequence.
Input: Set of primary protein sequences, inputted pattern/block sequence.
Output: Highest percentage similarity of the block with the given set of primary sequences along with multiple occurrences on each of the patterns and their locations.
Firstly, the inputted pattern/ block of length L and the set of amino acid sequences are taken out and transformed into the numerical sequences (using Table 2). L length pattern is considered as window of size L. Every numerical sequence is read from left to right equal to the inputted block of window size L and records the pairwise number of matching count m (say). Window is sliding to the next L length block right shifted by one; update the current record count (m) if the similarity matching is found more than the previous value. This is done for the last block of window. The records for the highest similarity matches and their positions in the sequences are noted. Finally, the percentage of similarity matching = (m/L) × 100) is calculated. Based on the inputted block and their locations, primary protein sequence of the block is obtained.
Illustration: Two amino acids sequence blocks “DRSMYI” and “EKTCWV” are transformed into the same numerical sequence “127634” as D/E, R/K, S/T, M/C, Y/W, I/V coming from same chemical group though they are different amino acids. Similarity matching of these two blocks is done based on alignment of two blocks. For an example, the above two blocks “DRSMYI” and “EKTCWV” are 100% similar (6 positions) with regards to chemical groups as position wise they are from same chemical group. Now if the first amino acid ‘D’ from the first block is replaced by Q i.e. “QRSMYI” then two blocks are approximately 84% similar ((6-1/6)*100) or by the alignment of these blocks 5 positions amino acids are from same chemical group and another one (first amino acid) is from different chemical groups.
Results
Percent identity matrix of a NMII family
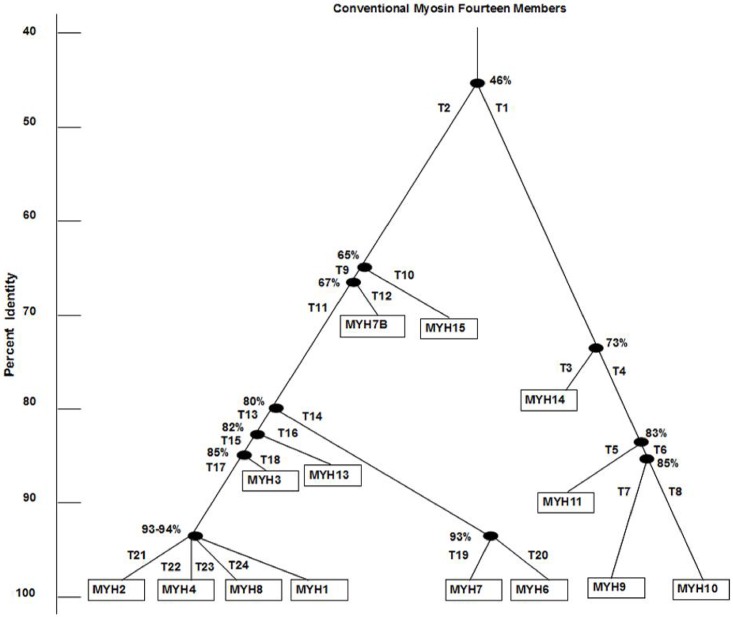
We use www.uniport.org database to find myosin II sequences. MYH15 and MYH7B are not listed in the previous phylogenetic tree. To find a correct position of two newly sequenced myosin II (MYH15 and MYH7B) in the existing phylogenetic tree, we deploy pair wise percent identity matrix shown in Table 10 of the myosin head domain (839-860 aa) for every pair of sequences of the myosin II fourteen members (sequence number in serial from Table 3). The rooted phylogenetic tree (Fig 2) of the myosin fourteen members is obtained from http://www.ebi.ac.uk/Tools/msa/clustalw2/.
Table 10. Percentage identity matrix of every pair sequences of myosin II head domain by using the site www.ebi.ac.uk/Tools/msa/clustalw2/.
| Seq. Vs. Seq. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 1 | 100.0 | 73.21 | 76.47 | 78.57 | 45.47 | 49.04 | 47.29 | 46.56 | 47.83 | 47.17 | 48.61 | 48.25 | 47.34 | 46.02 |
| 2 | 73.21 | 100.0 | 84.33 | 83.51 | 46.86 | 49.88 | 49.57 | 49.57 | 50.37 | 49.82 | 50.31 | 50.06 | 49.88 | 48.66 |
| 3 | 76.47 | 84.33 | 100.0 | 85.77 | 48.01 | 49.08 | 48.77 | 48.77 | 49.20 | 48.89 | 50.37 | 50.12 | 48.95 | 48.21 |
| 4 | 78.57 | 83.51 | 85.77 | 100.0 | 47.72 | 49.39 | 49.08 | 48.35 | 49.63 | 48.71 | 50.55 | 49.57 | 50.12 | 47.80 |
| 5 | 45.47 | 46.86 | 48.01 | 47.72 | 100.0 | 68.95 | 63.15 | 64.14 | 63.34 | 62.70 | 66.18 | 66.27 | 65.06 | 64.30 |
| 6 | 49.04 | 49.88 | 49.08 | 49.39 | 68.95 | 100.0 | 65.76 | 65.91 | 66.99 | 65.79 | 69.26 | 68.38 | 66.75 | 65.55 |
| 7 | 47.29 | 49.57 | 48.77 | 49.08 | 63.15 | 65.76 | 100.0 | 92.28 | 93.92 | 93.59 | 79.47 | 79.52 | 85.94 | 85.75 |
| 8 | 46.56 | 49.57 | 48.77 | 48.35 | 64.14 | 65.91 | 92.28 | 100.0 | 92.36 | 95.49 | 79.95 | 80.45 | 85.08 | 87.02 |
| 9 | 47.83 | 50.37 | 49.20 | 49.63 | 63.34 | 66.99 | 93.92 | 92.36 | 100.0 | 93.32 | 80.62 | 80.55 | 86.17 | 86.16 |
| 10 | 47.17 | 49.82 | 48.89 | 48.71 | 62.70 | 65.79 | 93.59 | 95.49 | 93.32 | 100.0 | 80.31 | 80.21 | 84.84 | 85.95 |
| 11 | 48.61 | 50.31 | 50.37 | 50.55 | 66.18 | 69.26 | 79.47 | 79.95 | 80.62 | 80.31 | 100.0 | 90.69 | 79.07 | 79.19 |
| 12 | 48.25 | 50.06 | 50.12 | 49.57 | 66.27 | 68.38 | 79.52 | 80.45 | 80.55 | 80.21 | 90.69 | 100.0 | 79.24 | 79.83 |
| 13 | 47.34 | 49.88 | 48.95 | 50.12 | 65.06 | 66.75 | 85.94 | 85.08 | 86.17 | 84.84 | 79.07 | 79.24 | 100.0 | 82.46 |
| 14 | 46.02 | 48.66 | 48.21 | 47.80 | 64.30 | 65.55 | 85.75 | 87.02 | 86.16 | 85.95 | 79.19 | 79.83 | 82.46 | 100.0 |
Fig 2. A rooted phylogenetic tree based on percent sequence similarity analysis of myosin heavy chain II head domain of humans.
Based on the head domain amino acid sequence, MYH15 has almost 65% identity with other nine members of the left sub-tree and MYH7B has almost 67% identity with other eight members of the left sub-tree. Positions of these two members in the phylogenetic tree are shown in Fig 2. At the branching point, left sub-tree and right sub-tree symbolized with T1, T2…T24 where T1 implies sequence no. 1-4 and T2 implies sequence no. 6-14 from Table 3 and so on. Our current analyses from the percent identity matrix (Table 10) for the fourteen members of the myosin head domain suggest that the first branching point (T) is at 46%, almost close to the previous reports [16, 17], which is 40%. Furthermore deviations are found for the right branch of the phylogenetic tree at the breaking point at T3 (73% vs. 64%), at T5 (83% vs. 76%) and at T7 (85% vs. 80%). It is interesting to note that there is not much deviation for any breaking point found for the left branch of the phylogenetic tree i.e. among the sequences 5-14.
Chemical group transitions for the fourteen members of myosin II family head domain
In line with the conventional term “Substitution Matrix”used in Bioinformatics and evolutionary Biology, our Algorithm 1 describes the number of transitions at which one amino acid (Say G1) in a sequence changes to other possible groups (G1,G2,…,G8) including the self-group as ordered pairs. This is done while looking for the similarity of the protein sequences. We hereby describe the distinct chemical group transitions for every protein sequences of myosin II family. Group transition is the transition of one chemical group to other possible chemical groups; like from G1 (acidic) to G2 or others. Theses transitions or ordered pairs are counted and recorded in numbers (S1 Table) for every sequence and aggregate transition for one particular group to all other groups are shown in Table 11.
Table 11. List the total number of amino acids from a particular chemical group followed by the order pairs count except self order pair for each sequence of myosin head domain.
| Seq. Nos. | #G1-#X1 | #G2-#X2 | #G3-#X3 | #G4-#X4 | #G5-#X5 | #G6-#X6 | #G7-#X7 | #G8-#X8 |
|---|---|---|---|---|---|---|---|---|
| 1 | 102-90 | 129-114 | 76-71 | 311-192 | 48-43 | 31-31 | 77-70 | 85-81 |
| 2 | 106-89 | 129-114 | 81-75 | 290-191 | 27-24 | 38-37 | 83-74 | 88-83 |
| 3 | 106-91 | 132-118 | 81-75 | 290-184 | 30-27 | 38-36 | 67-64 | 91-85 |
| 4 | 107-93 | 138-121 | 84-78 | 285-191 | 29-26 | 38-37 | 72-68 | 89-84 |
| 5 | 103-88 | 127-108 | 92-83 | 298-199 | 25-23 | 42-41 | 82-75 | 80-74 |
| 6 | 99-88 | 129-113 | 88-82 | 292-186 | 34-31 | 37-34 | 81-78 | 84-78 |
| 7 | 102-89 | 130-114 | 89-83 | 290-192 | 29-27 | 33-31 | 93-83 | 77-72 |
| 8 | 102-89 | 129-112 | 91-85 | 281-189 | 31-29 | 38-35 | 93-85 | 76-69 |
| 9 | 100-87 | 129-113 | 91-85 | 280-192 | 30-28 | 36-34 | 98-86 | 76-70 |
| 10 | 101-88 | 128-112 | 90-84 | 283-190 | 30-28 | 38-35 | 93-83 | 78-70 |
| 11 | 103-89 | 134-116 | 90-85 | 274-187 | 29-27 | 35-34 | 98-87 | 75-68 |
| 12 | 102-90 | 126-109 | 91-85 | 272-180 | 31-29 | 45-41 | 89-78 | 85-76 |
| 13 | 102-88 | 131-116 | 89-83 | 285-187 | 32-30 | 36-35 | 82-74 | 80-72 |
| 14 | 103-88 | 131-115 | 89-82 | 284-190 | 27-25 | 37-36 | 83-76 | 85-77 |
Here, #Gi is the number count of amino acids from Gi chemical group and #Xi is the the number count of order pairs except the pair (Gi,Gi).
Phylogenetic tree analysis for the fourteen members of Myosin II head domain
It is observed that based on one or more chemical group transitions with their distinct ranges in numbers, every branches of the phylogenetic tree can be explained clearly as shown in Table 12. For illustration, at branching point 46% identity (Fig 2) where left sub-tree T2 having sequences 5-14 and right sub-tree (T1) having sequences 1-4. For example, if we look on the Table 11 in column no. 4, G3 group to other group transitions number ranges for T2 are a) 88-92 with self-transition and b) 82-85 without self-transition, and ranges for T1 are a) 76-84 with self-transition and b) 71-78 without self-transition. Therefore, we found the distinct ranges value of T2 branch which is higher than T1 branch i.e. muscle group are more aromatic than the non-muscle group. One can study the significance of amino acids for this aromatic side group of myosin II family.
Table 12. Distinct ranges of every branch of the phylogenetic tree obtained from Table 11, percentage (%) identity of each branching point from Fig 2.
| Percent identity of original sequence (%) | Hitting Groups | Distinct Range in Respective Sub-Tree | Sequences Comparison | ||||
|---|---|---|---|---|---|---|---|
| From Fig 1 | From Fig 2 | Left sub-tree | Right sub-tree | ||||
| 40% | 46% | G3 | T2 | a) 88-92 b) 82-85 |
T1 | a) 76-84 b) 71-78 |
(1-4) Vs. (5-14) |
| 64% | 73% | G3, G4, G5, G6 | T3 | a) 48 b) 43 |
T4 | a) 27-30 b) 24-26 |
(1) Vs. (2-4) |
| 76% | 83% | G2, G5, G7, G8 | T5 | a) 83 b) 74 |
T6 | a) 67-72 b) 64-68 |
(2) Vs. (3-4) |
| 80% | 85% | All, G4 | T7 | a) 290 b) 184 |
T8 | a) 285 b) 191 |
(3) Vs. (4) |
| - | 65% | G2, G3, G4, G5 | T9 | a) 25 b) 23 |
T10 | a) 27-34 b) 25-31 |
(5) Vs. (6-14) |
| - | 67% | G1, G5 | T11 | a) 34 b) 31 |
T10 | a) 27-29 b) 25-29 |
(6) Vs. (7-14) |
| 80% | 80% | G4 | T13 | a) 89-93 b) 78-87 |
T14 | a) 82-83 b) 74-76 |
(7-12) Vs. (13-14) |
| 82% | 82% | G2, G4, G6, G7, G8 | T15 | a) 129-134 b) 112-116 |
T16 | a) 126 b) 109 |
(7-11) Vs. (12) |
| 85% | 85% | G2, G4, G8 | T17 | a) 128-130 b) 134 |
T18 | a) 112-114 b) 116 |
(7-10) Vs. (11) |
| 93% | 93% | G4, G5, G6, G7, G8 | T19 | a) 32 b) 30 |
T20 | a) 27 b) 25 |
(13) Vs. (14) |
| 93-94% | 93-94% | G4 | T21-T22-T23-T24 a) 290, 281, 280, 283 b) 192, 189, 192, 190 |
(7-10) | |||
If a particular branching point can be explained through the more than one chemical group transitions, then maximum hitting group is given the higher priority which is listed and highlighted. Say, for the branching point at 76% (Table 12) which can be explained through the hitting groups G2, G5, G7 and G8 where G7 is highlighted as it contains the maximum number of distinct range with respect to others i.e. a) 83 and b) 74 for left sub-tree (T5) and a) 67-72 and b) 64-68 for right sub-tree (T6). Therefore, we find among the non-muscle group, SM MyHc is more hydroxyl containing than other two members (NMHC II-A and NMHC II-B) of this group. So in similar fashion, other branches can also be explained.
Commonality among NMII family members
We are interested to search new blocks of similar chemical nature which may remain same across the myosin family. We are able to detect only a maximum six (6) length patterns without repetitions in all members of conventional myosin. Table 13 shows the consecutive distinct chemical patterns of various lengths (especially for length 5 aa and length 6 aa) for conventional myosin. The two unique patterns of lengths 6 aa are shown in Table 14. Our algorithms can also predict unique patterns of lower lengths (< 6 aa) without repetitions. We have also examined the higher length patterns, with repetition of chemical groups, by using Algorithm 3, which are common to all the members of conventional myosin as well as distinct for nonmuscle or muscle groups. It is to be noted that if we consider the pattern of higher length (> 6 aa) with repetition of chemical pattern in a particular block, then the pattern having the length less than or equal to six (≤ 6 aa) in that block must be sub pattern of that block. We find the maximum of 20 aa length pattern considering the repetition of the chemical groups as shown in Table 14. Our algorithm reveals the presence of few patterns which are conserved throughout the myosin family or either the non-muscle or the muscle groups (Table 13).
Table 13. Conserved chemical patterns in myosin II all members and comparison between Non-Muscle Vs. Muscle Group.
| Length (Number Count) | Pattern of Length 5 aa and 6 aa | Existence of Pattern in Non-Muscle Group (Seq. Nos. 1-4) | Existence of Pattern in Muscle Group (Seq. Nos. 5-14) |
|---|---|---|---|
| 6 (2) | 352471 378124 | Yes | Yes |
| 5 (8) | 37812 52471 74281 78124 35247 48532 43827 64837 | Yes | Yes |
| 6 (11) | 524361 361428 286154 731846 184765 476518 651874 847651 628435 438276 827634 | Yes | No |
| 5 (49) | 16324 24361 36142 41632 12734 24731 27341 37241 47312 73412 24831 38214 82314 84321 45821 18426 61428 42718 48721 72184 28615 34571 54371 31846 48731 73184 84173 47651 14856 61548 85641 86154 51874 18476 76481 65187 76518 52436 28435 35824 43582 27634 46327 63274 62843 87243 38276 82763 84765 | Yes | No |
| 6 (3) | 813472 635247 748532 | No | Yes |
| 5 (9) | 13472 24318 43182 81347 63524 24678 67842 82467 74853 | No | Yes |
Given an input of pattern length (L), all possible combination of pattern of length L using the numerical number 1-8 are generated without repetition using Algorithm 2.
Table 14. Two unique patterns of length 6 aa and one pattern of length 20 aa common to myosin 14 members, their position and corresponding amino acids.
| Seq. Nos. | Pattern of length 6 aa | Pattern of length 20 aa | |
|---|---|---|---|
| 352471 | 378124 | 34842742818772342342 | |
| Position-Sequence | Position-Sequence | Position-Sequence | |
| 1 | 560-FPKATD | 497-YTNEKL | 248-FGNAKTVKNDNSSRFGKFIR |
| 2 | 541-FPKATD | 478-YTNEKL | 228-FGNAKTVKNDNSSRFGKFIR |
| 3 | 534-FPKATD | 471-YTNEKL | 221-FGNAKTVKNDNSSRFGKFIR |
| 4 | 541-FPKATD | 478-YTNEKL | 228-FGNAKTVKNDNSSRFGKFIR |
| 5 | 552-FPKATD | 492-FTNEKL | 243-FGNAKTLRNDNSSRFGKFIR |
| 6 | 543-FPKASD | 483-FTNEKL | 233-FGNAKTLRNDNSSRFGKFIR |
| 7 | 543-FPKATD | 483-FTNEKL | 233-FGNAKTVRNDNSSRFGKFIR |
| 8 | 543-FPKATD | 483-FTNEKL | 233-FGNAKTVRNDNSSRFGKFIR |
| 9 | 543-FPKATD | 483-FTNEKL | 233-FGNAKTVRNDNSSRFGKFIR |
| 10 | 543-FPKATD | 483-FTNEKL | 233-FGNAKTVRNDNSSRFGKFIR |
| 11 | 541-FPKATD | 481-FTNEKL | 231-FGNAKTVRNDNSSRFGKFIR |
| 12 | 542-FPKATD | 482-FTNEKL | 232-FGNAKTVRNDNSSRFGKFIR |
| 13 | 540-FPKATD | 480-FTNEKL | 230-FGNAKTVRNDNSSRFGKFIR |
| 14 | 541-FPKATD | 481-FTNEKL | 231-FGNAKTVRNDNSSRFGKFIR |
First two patterns of length 6 aa are without repeating of chemical groups (using Algorithm 2) and last pattern of length 20 aa are with the repeating of chemical groups (using Algorithm 3).
The interesting point is to note that the patterns which are conserved throughout the myosin family are mostly located near the ATP binding region. This finding pushes us to hypothesize that these patterns of amino acids must play some important role during ATP binding process. The other different patterns which are specific for any particular group (either non-muscle or muscle) may be responsible for their groups’ specific functions. The entire 5 length patterns (Table 13) with their location and corresponding amino acids sequence of myosin II fourteen members are listed in S2 Table.
Our methods are also able to detect few homologous commonalities in the sequence patterns of myosins, like serine (S) changed to threonine (T). In our conventional sequence matching program, we describe these as different amino acids whereas chemically both are hydroxyl containing and therefore having the same chemical property. We can say that these patterns are chemically conserved through evolutions and may be true that they are doing the same function in different proteins. Head domain of myosin II have 3 broad sub domains: i) ATP Binding Site- It has two sub domains, a) Switch-1 and b) Switch-2 [27], ii) Actin Binding Site and iii) IQ domain. Various unique patterns from Table 13 are shown in Table 15 with their respective positions.
Table 15. Specific patterns of the amino acids and their location into different sub-domains of the myosin II head domain.
| Seq. Nos. | Length (aa) | Pattern | ATP Domain | Switch-1 | Switch-2 | Actin Domain |
|---|---|---|---|---|---|---|
| 1-14 | 5 | 74281 | Yes | Yes | ||
| 5 | 48532 | Yes | ||||
| 1-4 | 6 | 651874, 847651, 476518 | Yes | |||
| 5 | 16324, 41632, 24731, 37241, 47312 | |||||
| 5 | 63274 | Yes | ||||
| 5-14 | 6 | 813472, 748532 | Yes | |||
| 5 | 74853, 81347, 13472 | |||||
| 5 | 24678, 67824 | Yes |
Commonality among MYOI family members
Similar to myosin II, applying Algorithm 2 and Algorithm 3 among the MYOI family members, we are able to detect a block of maximum length 10 aa and three blocks of second maximum of length 9 aa with the repeating of chemical groups and two blocks of maximum length 5 aa without repeating the chemical groups as shown in Table 16. Among the biggest four blocks with the repetition of the chemical groups, the first, second and fourth blocks are located in the ATP binding region and the third block is located in converter sub-domain. And among the last two blocks without repetition the chemical groups, first one is located in Actin binding site (region of interest) and last one is located in purine loop.
Table 16. Conserved chemical patterns in MYOI family members, their position and original amino acids sequences.
| Seq. Nos. | Pattern of length 10 aa and 9 aa | Patterns of length 6 aa | ||||
|---|---|---|---|---|---|---|
| 444143431 | 424224434 | 344127244 | 64258 | 74853 | ||
| Pos.-Seq. | Pos.-Seq. | Pos.-Seq. | Pos.-Seq. | Pos.-Seq. | Pos.-Seq. | |
| 1 | 141-VLEAFGNAKT | 377-GVLDIYGFE | 618-VRVRRAGYA | 181-YLLEKSRLV | 588-CIKPN | 47-SVNPY |
| 2 | 148-VLEAFGNAKT | 384-GVLDIYGFE | 625-VRVRRAGYA | 188-YLLEKSRVV | 595-CIKPN | 54-SVNPY |
| 3 | 144-VLEAFGNAKT | 380-GVLDIYGFE | 619-VRVRRAGFA | 184-YLLEKSRVI | 589-CIKPN | 48-SVNPY |
| 4 | 144-VLEAFGNART | 392-GVLDIYGFE | 631-VRVRRAGFA | 184-YLLEKSRVL | 601-CIKPN | 48-SVNPY |
| 5 | 180-VLEAFGNAKT | 418-GLLDIYGFE | 655-LRVRRAGFA | 220-YLLEKSRVV | 625-CIKPN | 86-SVNPY |
| 6 | 145-VLEAFGNART | 388-GLLDIYGFE | 625-LRVRRAGFA | 185-YLIEKSRVV | 595-CIKPN | 51-SVNPY |
| 7 | 152-LLEAFGNAKT | 385-GVLDIYGFE | 616-IRVRRAGYA | 192-FLLEKSRVV | 586-CIKPN | 58-SVNPF |
| 8 | 150-LLEAFGNAKT | 383-GVLDIYGFE | 614-IRVRRAGFA | 190-FLLEKSRVV | 584-CIKPN | 56-SVNPF |
Common motif between myosin II and KIF1A class of Kinesin
To further illustrate and validate our method beyond the same protein group, we compare the protein sequences of the head domain of Kinesin family and myosin II family. Kinesin motor domain is much smaller than the myosin’s (340 vs. 860 amino acids). Although these two proteins share no amino acid identity, as determined by the computational algorithm programs, research on their crystal structure reveals a striking similarity between them. The structural overlap points out some short stretches of sequence conservations [32, 43]. Our algorithm reveals the presence of an octamer with repetition (“84268444”) in both conventional myosin II and the KIF1A class of kinesins. In myosins, this sequence is present in the converter domain, where as in kinesins, it is present in the neck linker region [44]. These sequences and their corresponding positions are shown in Table 17. When sequences of other classes of kinesin are compared with these myosins, this motif is not found suggesting that this common feature is unique to the conventional myosin and KIF1A class of Kinesins.
Table 17. Common motifs Myosin II and KIF 1A class of Kinesin, their position and sequences.
| Protein | Position-Sequence | Protein | Position-Sequence |
|---|---|---|---|
| KIF1A | 353-QIRCNAVI | MYH2 | 698-QLRCNGVL |
| KIF1B | 347-QIKCNAVI | MYH4 | 696-QLRCNGVL |
| KIF1C | 347-QIRCNAII | MYH8 | 695-QLRCNGVL |
| MYH14 | 715-QLRCNGVL | MYH1 | 696-QLRCNGVL |
| MYH11 | 698-QLRCNGVL | MYH3 | 693-QLRCNGVL |
| MYH9 | 691-QLRCNGVL | MYH13 | 696-QLRCNGVL |
| MYH10 | 698-QLRCNGVL | MYH7 | 692-QLRCNGVL |
| MYH15 | 704-QLRCNGVL | MYH6 | 694-QLRCNGVL |
| MYH7B | 699-QLRCNGVL |
Common motif between myosin II and MYOI
Here, we compare the protein sequences between two protein families-the head domain of conventional myosin II family and the unconventional myosin MYOI family. We have shown a block/pattern of length 20 aa (Table 14) which have 100% similarity among the myosin II members, but 80–90% similarity among MYOI members (Table 18). There are blocks of length 9 aa which have 100% similarity in MYOI members, but 89–100% similarity among myosin II members (S3 Table).
Table 18. Comparison of common motifs from Myosin II with MYOI class of myosin, their position and sequences.
| Seq. Nos. | Similarity (%) | Pattern of length 20 aa |
|---|---|---|
| “34842742818772342342” | ||
| Position-Sequence | ||
| MYH14 | 248-FGNAKTVKNDNSSRFGKFIR | |
| 1 | 85 | 145-FGNAKTIRNNNSSRFGKYMD |
| 2 | 90 | 152-FGNAKTVRNDNSSRFGKYMD |
| 3 | 85 | 148-FGNAKTNRNDNSSRFGKYMD |
| 4 | 80 | 148-FGNARTNRNHNSSRFGKYMD |
| 5 | 90 | 184-FGNAKTLRNDNSSRFGKYMD |
| 6 | 90 | 149-FGNARTLRNDNSSRFGKYMD |
| 7 | 85 | 156-FGNAKTVRNNNSSRFGKYFE |
| 8 | 85 | 154-FGNAKTVRNNNSSRFGKYFE |
Here, MYH14 (Seq. No. 1, Table 14) is taken as reference sequence from Myosin II family and Seq. Nos. 1-8 are MYOI family members.
We also check the commonality between the MLCK and ROCK protein families, and Na+/K+-ATPase and Ca2+-ATPase families. Although the MLCK and Rho sequences are highly dissimilar, we find three significant blocks, one block of length 7 aa and two blocks of length 6 aa common between them with 100% similarity (S4 Table). They are located in ATP binding site (starting site of activation loop), start of proton acceptor site and ending site of activation loop. Among the Na+/K+-ATPase family members, four alpha members are highly similar. We are able to detect the maximum of length 78 aa block located in E1-E2_ATPase region (S5 Table). And the maximum of length 8 aa block is detected among the four beta members of Na+/K+-ATPase family (S5 Table). Similarly, we find three biggest blocks among the Ca2+-ATPase family of length 15 aa, 12 aa and 12 aa (S6 Table). All the blocks from Ca2+-ATPase family are almost 80–90% similar while comparing with Na+/K+-ATPase family and vice versa.
Discussion
We have documented that our algorithm can detect the chemical commonality among proteins. This is surely beyond the capacity of the existing conventional programs. We validate our method by rerunning it in other group of proteins which have same functional role. Results from these experiments suggest that our algorithm can find many common chemical patterns as a block sequence throughout the family. Our method proves its commonality by identifying the common patterns between myosin II and KIF1A which is the first report as per our knowledge. We extend our study between Myosin I and Myosin II, Rho and MLCK, and Na+/K+-ATPase and Ca2+-ATPase.
For the analysis of any newly determined sequences or their evolutionary ancestry, sequence similarity searching is one of the first and foremost informative steps. By the virtue of modern protein sequence databases and searching programs, like BLAST (units 3.4) [45], PSI-BLAST [45], SSEARCH (unit 3.10) [46, 47], HMMER3 [8, 48] and FASTA (unit 3.9) [49], one can produce a very accurate and comprehensive statistical estimates and can predict more than 80% protein sequence samples that share significant sequence similarity. While searching for the sequence similarity is an effective and reliable strategy for identifying sequences, which share a common evolutionary ancestor and the formation of phylogenetic tree, it also possess a significant limitation. When we find some excess sequence similarity, we imply that they are from common ancestry and homologous to each other, which is not entirely true. Homologous sequences do not always share significant entire sequence similarity, but has significant intermediate sequence and structural similarity. Search tools like BLAST, HMMER or FASTA cannot detect this false negatives (i.e.; homologous sequences with non-significant similarity scores), although they can detect and minimize the false positive [8]. Therefore, the emergence of the study on the alignment free analysis is obvious. Using alignment free methods, the biological sequence was supposed to be transformed into an object for which statistical or algebraic theory may be useful as analytical tools. For analyzing the DNA sequences based on the above mentioned basics, there had been significant number of works for the last three decades. These allow to analyze DNA sequences qualitatively and offer a way of viewing, comparing and sorting various genomic sequences. Although, both DNA and protein sequences belong to a symbolic sequence, there are very few methods for analyzing protein sequences qualitatively. This is mainly because of the extension of protein sequences having increased number of possible alternatives for 20 amino acids. These amino acids are the keys to understand the proteins existing in the cell. So, analysis of these amino acid sequences is an important part for the post-genomic study. Recently, several schemes have been proposed to plot amino acid sequences. They categorized the 20 amino acids into different types, including as a word with three to five different letters. Their processes not only describe amino acid sequence, but also determine the similarity/dissimilarity of different protein sequences [14, 47, 50–57]. However, their methods only consider the physical information of the sequences that are conserved in a particular amino acid sequences. Neither their physicochemical properties nor the properties of the adjacent amino acids are considered in those works. Few groups have used the reduced amino acids alphabets to reduce the sample size. They are able to design native-like protein structure from those reduced amino acids alphabets and they hypothesize that their approach might be useful for structurally related proteins weak sequence identity. But one distinguished limitation on their methods is the limited sample of sequences which can only determine a fraction of total sequences. Further, few group works with the converted amino acid sequences of proteins into three letter sequence based on the hydropathy profile of amino acid and algor a conditional probability as a new invariant for the protein sequences [14]. Their method can also show the distribution of amino acids at different positions in the sequences. But, they cannot predict the pattern of conserved chemical nature in the sequences. Therefore, grouping the amino acids based on their chemical properties may offer a better insight into the comparative study of proteins.
All the designed algorithms follow our categorization of various groups based on chemical properties of individual amino acid. Our method gives the clear explanation of the breaking point of every branch of the phylogenetic tree using the number of distinct chemical group transitions. We are also able to place two new myosins (MYH15 and 7B) in the updated phylogenetic tree (Fig 2) by using percent identity matrix. Based on our designed algorithm we can easily find out the conserved domain/common motif with and without repetition of amino acids. By using the results of Algorithm 2, we have provided several conserved patterns that are common to the NMII family. We find another two significant blocks, one block of length 20 aa maximum of 40% similarity in between non-muscle myosin and muscle myosin group located in ATP binding region and another one block of length 17 aa maximum of 94% similarity located nearer to the ATP binding region (S7 Table, by using Algorithm 3 and 4). This may also suggest that the ATP binding region is more chemically conserved compared to Actin binding region. This commonality of chemical patterns may be useful for predicting the structures of proteins, designing the drugs, inhibitors etc. We have shown a longest block of length 20 aa (34842742818772342342, Table 14) among myosin II members in human. The two sub-blocks from the above block “348427”/FGNAKT and “8772342”/NSSRFGK are fixed amino acids sequence among all the members. There is no-chemical group variation of the sequences of those two sub-blocks. These two sub-blocks are previously described in [58] which is located in the ending of HH (helix H). They have worked with 8 representative sequences (among three from myosin class-II, and two from myosin class-I) from different classes of myosin. As per our hypothesis considering the invariant chemical group, we find that 7th and 8th positions of 20 aa length block are replaced by V/L and K/R respectively where V or L and K or R respectively from same chemical group. Combining these two positions along with others 5 fixed positions and two sub-blocks, we find the block of length of 20 aa which is chemically conserved across the myosin II 14 members in human and that is new compared to the earlier works. The conserved block of length 10 aa (4414348427, Table 16) from MyoI members, last 6 residues are the first sub-block “348427” as described above and also matched with the previous findings [58]. The first position of the 10 aa length block is varying either V or L, and keeping next three positions fixed, combining the 10 aa length chemically conserved block which is also new observation using our algorithm. Two small sub blocks as “414”/LDI and “431”/GFE are located in between HO and HP reported in [58]. This finding is also matched combining with the biggest conserved block of length 9 aa (“444143431”, Table 16) from MyoI members. Similar is the case for the block of length 9 aa (344127244, Table 16) among MyoI members found by our algorithm, a sub-block is previously reported as “4127244”/LLEKSR in [58] located in between HH and HI among various classes of myosin. Further, we have shown another block of length 9 aa (424224434) which is highly chemically conserved among Myo1 family members but not the conserved as well among various classes of myosin except the position 7th which is ‘4’/G as observed in previous report located in between HA′ and HB′ [58]. Therefore our findings of various conserved blocks are bigger in length (aa) and could be responsible for group/family specific functions.
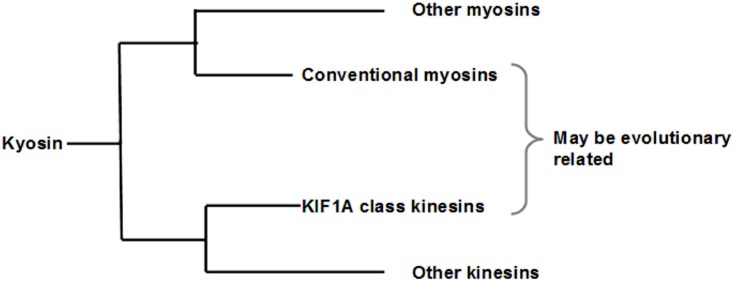
We have also found that NMII with KIF1A class of Kinesin having a specific pattern which can be studied further for the explanation of biological significance. The neck linker regions of KIF1A class of kinesins are known to be conserved among the family members, but not among the entire super family of kinesins [44]. This would explain the absence of this motif in other kinesins. In kinesin, the neck linker sequence has been shown to be responsible for the generation of mechanical force, in a manner analogous to the converter domains of myosins [59, 60]. This suggests that the KIF1A class of kinesins and conventional myosins may be more closely related than other kinesins and myosins as they share a common motif even though they are from different motor protein families. We hypothesize that this motif must play a functional role in the conversion of chemical energy to mechanical energy. This is probably the reason of the said evolutionarily conserved status (Fig 3).
Fig 3. Proposed phylogenetic tree of KIF1A and Conventional myosins.
This result proves the validity of our method in highlighting similarities among proteins with dissimilar amino acid sequences and from different families. The motif shows only 50–62.5% similarity if one goes by amino acid sequence alone, yet the differences in amino acids are minor (leucine and isoleucine, or alanine and glycine) in terms of their chemical natures. Thus the overall chemical nature of the motif is more similar than what alignment of amino acids would imply. It has been already documented that ATP turnover of myosin I follow the same pathway like other myosins [30]. Our current results of having 80–90% similarity in the ATP binding site of myosin I and myosin II also supports that previous finding. Our algorithms also can find the repetition of same pattern in different positions of particular sequence which is quite harder to see by the sequence alignment method. One can easily modify our algorithm based on requirements like changing the number of groups, weight assigning of the groups etc. and this is also applicable even if one needs twenty standard amino acids as twenty groups. Also application can be directed for the pattern analysis among the protein families of various evolutionary species where protein functioning is same. Further, our block finding algorithm (Algorithm 4) can also be used for identifying the highly similar/dissimilar block in the unknown sequences especially for cancer gene or protein family based on neighborhood pattern analysis which is our further investigation. Our confidence relies on this method for assessing the commonalities among various motor proteins and will be quite useful particularly for investigating the function of amino acids during its structural conformation in pathological conditions.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank to group members of Dr. S S Jana and Prof. P Pal Choudhury for their helpful discussion. J. K. D and P. D thank ISI and IACS respectively for supporting their fellowships.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1. Gromiha MM, Selvaraj S. Important amino acid properties for determining the transition state structures of two-state protein mutants. FEBS Lett. 2002;526(1–3):129–34. Epub 2002/09/05. 10.1016/S0014-5793(02)03122-8 [DOI] [PubMed] [Google Scholar]
- 2. Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17(4):540–52. Epub 2000/03/31. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 3. Rokas A. Phylogenetic analysis of protein sequence data using the Randomized Axelerated Maximum Likelihood (RAXML) Program. Curr Protoc Mol Biol. 2011;Chapter 19:Unit19.1 Epub 2011/10/12. 10.1002/0471142727.mb1911s96 [DOI] [PubMed] [Google Scholar]
- 4. Zhang S, Wang T. Phylogenetic analysis of protein sequences based on conditional LZ complexity. MATCH Commun Math Comput Chem. 2010;63(3):701–16. [Google Scholar]
- 5. Smith SA, Brown JW, Hinchliff CE. Analyzing and synthesizing phylogenies using tree alignment graphs. PLoS Comput Biol. 2013;9(9):e1003223 Epub 2013/10/03. PubMed Central PMCID: PMC3784503. 10.1371/journal.pcbi.1003223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Felsenstein J. Phylogenies and the Comparative Method. The American Naturalist. 1985;125(1):1–15. 10.1086/284325 [DOI] [PubMed] [Google Scholar]
- 7. Feng DF, Doolittle RF. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–60. Epub 1987/01/01. 10.1007/BF02603120 [DOI] [PubMed] [Google Scholar]
- 8. Pearson WR. An introduction to sequence similarity (“homology”) searching. Curr Protoc Bioinformatics. 2013;Chapter 3:Unit3.1 Epub 2013/06/12. PubMed Central PMCID: PMC3820096. 10.1002/0471250953.bi0301s42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penny D. The comparative method in evolutionary biology. Journal of Classification. 1992;9(1):169–72. 10.1007/BF02618482 [DOI] [Google Scholar]
- 10. Zhang S, Yang L, Wang T. Use of information discrepancy measure to compare protein secondary structures. Journal of Molecular Structure: THEOCHEM. 2009;909(1–3):102–6. 10.1016/j.theochem.2009.05.031. [DOI] [Google Scholar]
- 11. Pham TD, Zuegg J. A probabilistic measure for alignment-free sequence comparison. Bioinformatics. 2004;20(18):3455–61. Epub 2004/07/24. 10.1093/bioinformatics/bth426 [DOI] [PubMed] [Google Scholar]
- 12. Li T, Fan K, Wang J, Wang W. Reduction of protein sequence complexity by residue grouping. Protein Eng.2003;16(5): 323–330. 10.1093/protein/gzg044 [DOI] [PubMed] [Google Scholar]
- 13. Peterson EL, Kondev J, Pheriot JA, Phillips R. Reduced amino acid alphabets exhibit an improved sensitivity and selectivity in fold assignment. Bioinformatics. 2009;25(11): 1356–1362. 10.1093/bioinformatics/btp164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xie X-l, Zheng L-f, Yu Y, Liang L-p, Guo M-c, Song J, et al. Protein sequence analysis based on hydropathy profile of amino acids. Journal of Zhejiang University Science B. 2012;13(2):152–8. 10.1631/jzus.B1100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA. 1992;89(22): 10915–10919. 10.1073/pnas.89.22.10915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheney RE, Riley MA, Mooseker MS. Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskeleton. 1993;24(4):215–23. Epub 1993/01/01. 10.1002/cm.970240402 [DOI] [PubMed] [Google Scholar]
- 17. Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, et al. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem. 2004;279(4):2800–8. Epub 2003/11/05. 10.1074/jbc.M309981200 [DOI] [PubMed] [Google Scholar]
- 18. Sellers JR. Myosins: a diverse superfamily. Biochim Biophys Acta. 2000; 1496(1):3–22. 10.1016/S0167-4889(00)00005-7 [DOI] [PubMed] [Google Scholar]
- 19. Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol. 1999;11(1):26–33. Epub 1999/02/27. 10.1016/S0955-0674(99)80004-0 [DOI] [PubMed] [Google Scholar]
- 20. Stedman HH, Kozyak BW, Nelson A, Thesier DM, Su LT, Low DW, et al. Myosin gene mutation correlates with anatomical changes in the human lineage. Nature. 2004;428(6981):415–8. Epub 2004/03/26. 10.1038/nature02358 [DOI] [PubMed] [Google Scholar]
- 21. Pereira TV, Salzano FM, Mostowska A, Trzeciak WH, Ruiz-Linares A, Chies JA, et al. Natural selection and molecular evolution in primate PAX9 gene, a major determinant of tooth development. Proc Natl Acad Sci U S A. 2006;103(15):5676–81. Epub 2006/04/06. PubMed Central PMCID: PMC1458632. 10.1073/pnas.0509562103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perry GH, Verrelli BC, Stone AC. Comparative analyses reveal a complex history of molecular evolution for human MYH16. Mol Biol Evol. 2005;22(3):379–82. Epub 2004/10/08. 10.1093/molbev/msi004 [DOI] [PubMed] [Google Scholar]
- 23. Dominguez R, Freyzon Y, Trybus KM, Cohen C. Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualization of the pre-power stroke state. Cell. 1998;94(5):559–71. Epub 1998/09/19. 10.1016/S0092-8674(00)81598-6 [DOI] [PubMed] [Google Scholar]
- 24. Heintzelman MB, Schwartzman JD. A novel class of unconventional myosins from Toxoplasma gondii. J Mol Biol. 1997;271(1):139–46. Epub 1997/08/08. 10.1006/jmbi.1997.1167 [DOI] [PubMed] [Google Scholar]
- 25. Houdusse A, Kalabokis VN, Himmel D, Szent-Gyorgyi AG, Cohen C. Atomic structure of scallop myosin subfragment S1 complexed with MgADP: a novel conformation of the myosin head. Cell. 1999;97(4):459–70. Epub 1999/05/25. 10.1016/S0092-8674(00)80756-4 [DOI] [PubMed] [Google Scholar]
- 26. Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261(5117):50–8. Epub 1993/07/02. 10.1126/science.8316857 [DOI] [PubMed] [Google Scholar]
- 27. Smith CA, Rayment I. Active site comparisons highlight structural similarities between myosin and other P-loop proteins. Biophys J. 1996;70(4):1590–602. Epub 1996/04/01. PubMed Central PMCID: PMC1225128. 10.1016/S0006-3495(96)79745-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288(5463):88–95. Epub 2001/02/07. 10.1126/science.288.5463.88 [DOI] [PubMed] [Google Scholar]
- 29. Coluccio LM. Myosin I. Am. J. Physiol.1997;273:C347–359. [DOI] [PubMed] [Google Scholar]
- 30. Gillespie PG et al. Myosin-I nomenclature. J Cell Biol. 2001;155(5):703–4. 10.1083/jcb.200110032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lewis JH, Lin T, Hokanson DE, Ostap EM. Temperature Dependence of Nucleotide Association and Kinetic Characterization of Myo1b. Biochemistry, 2006;45(38):11589–11597. 10.1021/bi0611917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kull FJ, Vale RD, Fletterick RJ. The case for a common ancestor: kinesin and myosin motor proteins and G proteins. J Muscle Res Cell Motil. 1998;19(8):877–86. Epub 1999/02/27. 10.1023/A:1005489907021 [DOI] [PubMed] [Google Scholar]
- 33. Connell LE, Helfman DM. Myosin light chain kinase plays a role in the regulation of epithelial cell survival. Journal of Cell Science 2006;119: 2269–2281; 10.1242/jcs.02926 [DOI] [PubMed] [Google Scholar]
- 34. Gao Y, Ye LH, Kishi H, Okagaki T, Samizo K, Nakamura A, Kohama K. Myosin light chain kinase as a multifunctional regulatory protein of smooth muscle contraction. IUBMB Life. 2001;51 (6): 337–44. 10.1080/152165401753366087 [DOI] [PubMed] [Google Scholar]
- 35. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002; 298 (5600): 1912–34. 10.1126/science.1075762 [DOI] [PubMed] [Google Scholar]
- 36. Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell Mol Life Sci. 2010;67 (2): 171–7. 10.1007/s00018-009-0189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviours. Nat Rev Mol Cell Biol. 2003;4 (6): 446–56. 10.1038/nrm1128 [DOI] [PubMed] [Google Scholar]
- 38. Forrest MD. The sodium-potassium pump is an information processing element in brain computation. Frontiers in Physiology. 5 (472). 10.3389/fphys.2014.00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forrest MD, Wall MJ, Press DA, Feng J. The Sodium-Potassium Pump Controls the Intrinsic Firing of the Cerebellar Purkinje Neuron. PLoS ONE. 2012; 7 (12): e51169 10.1371/journal.pone.0051169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jensen TP, Filoteo AG, Knopfel T, Empson RM. Pre-synaptic plasma membrane Ca2+ ATPase isoform 2a regulates excitatory synaptic transmission in rat hippocampal CA3 (PDF). J. Physiol. 2007;579(1):85–99. 10.1113/jphysiol.2006.123901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dode L, Van Baelen K, Wuytack F, Dean WL. Low temperature molecular adaptation of the skeletal muscle sarco(endo)plasmic reticulum Ca2+-ATPase 1 (SERCA 1) in the wood frog (Rana sylvatica). Journal of Biological Chemistry. 2001;276 (6): 3911–9. 10.1074/jbc.M007719200 [DOI] [PubMed] [Google Scholar]
- 42. Brini M, and Carafoli E. The Plasma Membrane Ca2+- ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium. Cold Spring Harb Perspect Biol. 2011; 3(2):a004168 10.1101/cshperspect.a004168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vale RD, Fletterick RJ. The design plan of kinesin motors. Annu Rev Cell Dev Biol. 1997;13:745–77. Epub 1997/01/01. 10.1146/annurev.cellbio.13.1.745 [DOI] [PubMed] [Google Scholar]
- 44. Al-Bassam J, Cui Y, Klopfenstein D, Carragher BO, Vale RD, Milligan RA. Distinct conformations of the kinesin Unc104 neck regulate a monomer to dimer motor transition. J Cell Biol. 2003;163(4):743–53. Epub 2003/11/26. PubMed Central PMCID: PMC2173678. 10.1083/jcb.200308020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. Epub 1997/09/01. PubMed Central PMCID: PMC146917. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith TF, Waterman MS. IIdentification of common molecular subsequences. J Mol Biol. 1981;147(1):195–7. Epub 1981/03/25. 10.1016/0022-2836(81)90087-5 [DOI] [PubMed] [Google Scholar]
- 47. Pearson WR. Searching protein sequence libraries: comparison of the sensitivity and selectivity of the Smith-Waterman and FASTA algorithms. Genomics. 1991;11(3):635–50. Epub 1991/11/01. 10.1016/0888-7543(91)90071-L [DOI] [PubMed] [Google Scholar]
- 48. Johnson LS, Eddy SR, Portugaly E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinformatics. 2010;11:431 Epub 2010/08/20. PubMed Central PMCID: PMC2931519. 10.1186/1471-2105-11-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988;85(8):2444–8. Epub 1988/04/01. PubMed Central PMCID: PMC280013. 10.1073/pnas.85.8.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li C, Xing L, Wang X. 2-D graphical representation of protein sequences and its application to coronavirus phylogeny. BMB Rep. 2008;41(3):217–22. Epub 2008/04/02. 10.5483/BMBRep.2008.41.3.217 [DOI] [PubMed] [Google Scholar]
- 51. Li J, Li F, Wang W. Simplification of protein sequence and alignment-free sequence analysis. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Jin Zhan. 2006;33(12):1215–22. [Google Scholar]
- 52. Munteanu CR, Gonzalez-Diaz H, Magalhaes AL. Enzymes/non-enzymes classification model complexity based on composition, sequence, 3D and topological indices. J Theor Biol. 2008;254(2):476–82. Epub 2008/07/09. 10.1016/j.jtbi.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 53. Randić M, Krilov G. Characterization of 3-D sequences of proteins. Chemical Physics Letters. 1997;272(1):115–9. 10.1016/S0009-2614(97)00447-8. [DOI] [Google Scholar]
- 54. Wen J, Zhang Y. A 2D graphical representation of protein sequence and its numerical characterization. Chemical Physics Letters. 2009;476(4–6):281–6. 10.1016/j.cplett.2009.06.017. [DOI] [Google Scholar]
- 55. Yao YH, Dai Q, Li C, He PA, Nan XY, Zhang YZ. Analysis of similarity/dissimilarity of protein sequences. Proteins. 2008;73(4):864–71. Epub 2008/06/07. 10.1002/prot.22110 [DOI] [PubMed] [Google Scholar]
- 56. Yao YH, Dai Q, Li L, Nan XY, He PA, Zhang YZ. Similarity/dissimilarity studies of protein sequences based on a new 2D graphical representation. J Comput Chem. 2010;31(5):1045–52. Epub 2009/09/25. [DOI] [PubMed] [Google Scholar]
- 57. Yau SS, Yu C, He R. A protein map and its application. DNA Cell Biol. 2008;27(5):241–50. Epub 2008/03/20. 10.1089/dna.2007.0676 [DOI] [PubMed] [Google Scholar]
- 58. Cope MJ, Whissstock J, Rayment J, Kendrick-Jones J. Conservation within the myosin motor domain: implications for structure and function. Structure. 1996; 4(8):969–987. 10.1016/S0969-2126(96)00103-7 [DOI] [PubMed] [Google Scholar]
- 59. Hariharan V, Hancock WO. Insights into the Mechanical Properties of the Kinesin Neck Linker Domain from Sequence Analysis and Molecular Dynamics Simulations. Cell Mol Bioeng. 2009;2(2):177–89. Epub 2009/06/01. PubMed Central PMCID: PMC3085455. 10.1007/s12195-009-0059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, et al. A structural change in the kinesin motor protein that drives motility. Nature. 1999;402(6763):778–84. Epub 2000/01/05. 10.1038/45483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.