Abstract
In this study 308 ticks (Ixodes ariadnae: 26 larvae, 14 nymphs, five females; I. vespertilionis: 89 larvae, 27 nymphs, eight females; I. simplex: 80 larvae, 50 nymphs, nine females) have been collected from 200 individuals of 17 bat species in two countries, Hungary and Romania. After DNA extraction these ticks were molecularly analysed for the presence of piroplasm DNA. In Hungary I. ariadnae was most frequently identified from bat species in the family Vespertilionidae, whereas I. vespertilionis was associated with Rhinolophidae. Ixodes ariadnae was not found in Romania. Four, four and one new bat host species of I. ariadnae, I. vespertilionis and I. simplex were identified, respectively. DNA sequences of piroplasms were detected in 20 bat ticks (15 larvae, four nymphs and one female). I. simplex carried piroplasm DNA sequences significantly more frequently than I. vespertilionis. In I. ariadnae only Babesia vesperuginis DNA was detected, whereas in I. vespertilionis sequences of both B. vesperuginis and B. crassa. From I. simplex the DNA of B. canis, Theileria capreoli, T. orientalis and Theileria sp. OT3 were amplified, as well as a shorter sequence of the zoonotic B. venatorum. Bat ticks are not known to infest dogs or ruminants, i.e. typical hosts and reservoirs of piroplasms molecularly identified in I. vespertilionis and I. simplex. Therefore, DNA sequences of piroplasms detected in these bat ticks most likely originated from the blood of their respective bat hosts. This may indicate either that bats are susceptible to a broader range of piroplasms than previously thought, or at least the DNA of piroplasms may pass through the gut barrier of bats during digestion of relevant arthropod vectors. In light of these findings, the role of bats in the epidemiology of piroplasmoses deserves further investigation.
Introduction
Bats (Chiroptera) are the second largest order of mammals, with more than 1200 species. Owing to their flying habit, feeding preference and high level of adaptability, bats are widely distributed and present on all continents except the Antarctica [1]. In this scenario, invasion of men into bat habitats and adaptation of bats to urban areas increased the chances for contact between humans, domestic animals and bats [2], thus promoting the chances of pathogen transmission.
Accordingly, during the past decades, the epidemiological significance of bats has become increasingly recognized. Bats can be reservoirs or carriers of numerous species of viruses, bacteria and parasites, among them many with zoonotic potential to infect humans [3]. This is especially important in the context of synanthropic life of several bat species, which tend to roost or breed in human settlements, even in man-made buildings such as steeples, attics, cellars and barns [4]. In comparison with bat species that prefer forested habitats, urbanized populations of chiropterans may form larger, more stable and aggregated colonies [1,4]. Thus, urban bats may reach the highest individual number in the local mammalian fauna, further increasing their epidemiological significance. In addition, bat species that are indigenous in Europe feed predominantly on arthropods, and this makes it possible for them to get into contact with vector-borne pathogens not only from their ectoparasites, but also from their food [5].
Blood-sucking ectoparasites of bats may be potential vectors of a broad range of pathogens [2]. Depending on their taxonomic group, these ectoparasites can be highly (e.g. bat flies) or less specialized to bats (e.g. soft ticks, bugs), and exceptionally can settle on other mammals, even on humans [6]. Interestingly, while ixodid ticks of bats are not known to feed on other mammals, except Ixodes vespertilionis on humans [7], ixodid ticks that frequently infest domestic animals (e.g. Ixodes ricinus, Dermacentor reticulatus, Haemaphysalis spp.) have also been collected from bats [8, 9, 10].
Three species of ixodid bat ticks (Acari: Ixodidae) occur in Europe, i.e. I. vespertilionis, I. ariadnae and I. simplex [11]. These are specialized for bats, i.e. all three developmental stages that need a blood meal (larvae and nymphs for moulting, females for oviposition) will typically suck blood on bats, as exemplified by I. ariadnae [11]. While these three tick species and their genotypes appear to be widely distributed across the Palaearctic [12], only few data are available on their vector potential. Concerning molecular investigations of vector-borne pathogens in ixodid bat ticks, bartonellae were reported from I. vespertilionis [13], but to the best of our knowledge none from I. simplex or I. ariadnae. Therefore the present study was undertaken to ameliorate this lack of data on pathogens and/or pathogen DNA carried by ixodid bat ticks. Recently, DNA of Babesia canis has been detected in bat faeces [5], therefore piroplasms were chosen as the target group of analyses. It was also within the scope of this study to examine the geographical range and host spectrum of these tick species in Hungary and Romania, by including 17 species of bats (from three families and six genera) from these two countries.
Materials and Methods
Tick collection and identification
Ixodid ticks were collected from bats, caught for monitoring purposes, on 24 locations in Hungary in 2008–2015, and on seven locations in Romania in 2015 (Fig 1; data in S1 Table). These bats were caught (as part of a monitoring program) at the entrance of caves between sunset and dawn, using standard Ecotone mist-nets (Gdynia, Poland) with 12 m length, 2.5 m height and 14 × 14 mm mesh. Bats were handled without anesthesia, but released immediately after tick removal to alleviate suffering. Data (species, sex) of bats, from which the ticks were removed, were recorded. The ticks were immediately put into and stored in 96% ethanol. Morphological identification was done with a stereo microscope (SMZ-2T, Nikon Instruments, Japan) using standard morphological keys (subadults: [14]; females: [15]).
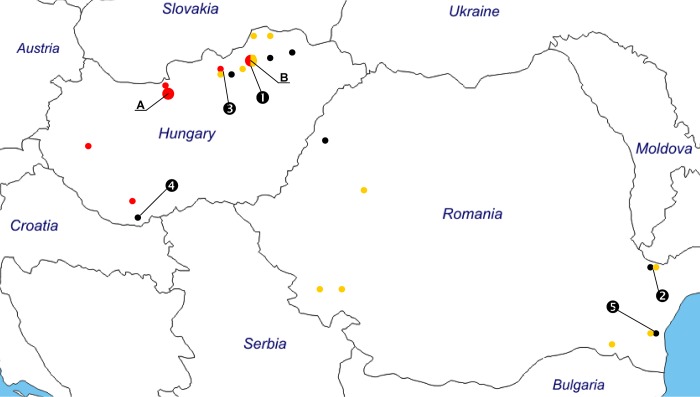
Fig 1. Sampling sites of the present study.
Color of collection sites for Ixodes ariadnae are marked with red dots, for I. vespertilionis with yellow dots, and for I. simplex with black dots. Letters: A—Ariadne Cave System and caves in the Pilis Mountains (bats sampled at three caves), B—Bükk Highlands Cave system (bats sampled at nine caves). Numbers in black circles indicate places, where piroplasm-carrier bat ticks were collected (Table 3). Small and unseparated dots with different colour indicate the same place with two tick species. Two places close to each other in northeast Hungary (Baradla and Béke Caves) are marked with one dot. Coordinates for the individual places are shown in S1 Table.
DNA extraction and molecular analyses
DNA was extracted (except for one I. simplex larva from Barbastella barbastellus) with the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions and including extraction controls. Ticks were dried, then washed three times (in detergent containing water, in tap water and in distilled water) and minced at the bottom of 1.5 ml Eppendorf tubes in 100 μl PBS with pointed scissors. Between each sample the scissors were washed and burned for decontamination. Samples were then incubated overnight at 56°C in tissue lysis buffer containing proteinase-K.
DNA samples were molecularly screened with a conventional PCR that amplifies an approx. 500 bp long part of the 18S rDNA gene of piroplasms, modified from Casati et al. [16]. The primers BJ1 (forward: 5'-GTC TTG TAA TTG GAA TGA TGG-3') and BN2 (reverse: 5'-TAG TTT ATG GTT AGG ACT ACG-3') were used in a reaction volume of 25 μl, which included 5 μl of extracted DNA, and 20 μl of reaction mixture containing 0.5 unit HotStarTaq Plus DNA polymerase (5U/ μl), 200 μM PCR nucleotid mix, 1 μM of each primer and 2.5 μl of 10× Coral Load PCR buffer (15 mM MgCl2 included). For amplification an initial denaturation step at 95°C for 10 min was followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 54°C for 30 s and extension at 72°C for 40 s. Final extension was performed at 72°C for 5 min.
All PCRs were run with appropriate positive and negative controls. During all tests positive controls showed positivity, whereas negative (non-template) controls and extraction controls remained negative (the latter indicating absence of sample contamination). PCR products were electrophoresed in a 1.5% agarose gel, stained with ethidium-bromide and visualized under ultra-violet light. Purification and sequencing (twice) were done from all PCR positive samples by Biomi Inc. (Gödöllő, Hungary). Representative sequences were submitted to GenBank (accession numbers KU958544-53). Phylogenetic analyses were conducted according to the Tamura-Nei model [17] and Maximum Composite Likelihood method by using MEGA version 5.2 [18].
Statistical analyses
Association of tick species with bat families was assessed by Fisher's exact test. Intensities of tick infestation (i.e. number of ticks on a bat individual) were compared between bat species by using Mann-Whitney U-test and Kruskal Wallis H-test in R program. The following bat species (in Table 1, Table 2: harboring different stages of the same tick species) were analysed: Myotis bechsteinii (n = 7), My. daubentonii (n = 28), My. emarginatus (n = 9), Miniopterus schreibersii (n = 95), Rhinolophus ferrumequinum (n = 16), R. hipposideros (n = 12). Bat species with small sample size (n<5) were excluded from the latter analysis. The COIN (Conditional Inference Procedures in a Permutation Test Framework) package was used to correct P values of linked parameters. Differences were considered significant when P<0.05.
Table 1. Tick species and stages collected in Hungary, shown according to their bat hosts.
Five females and two nymphs of I. ariadnae, which were collected from cave walls (Ariadne Cave System), are not included.
| Tick | Bat (number of ticks per number of bats) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vespertilionidae | Rhinolophidae | Miniopteridae | |||||||||||
| Species | Stage | MALC | MBEC | MNAT | MEMA | MDAU | MDAS | MMYO | PAUR | BBAR | RHIP | REUR | MSCH |
| Ixodes ariadnae | larva | 4/2 | 3/3 | 1/1 | 6/6 | 5/3 | 5/2 | - | 1/1 | - | 1/1 | - | - |
| nymph | - | 4/4 | - | 4/3 | - | - | 1/1 | 3/3 | - | - | - | - | |
| female | - | - | - | - | - | - | - | - | - | - | - | - | |
| Ixodes vespertilionis | larva | 1/1 | - | - | - | - | - | 1/1 | - | 8/7 | - | - | |
| nymph | - | - | - | - | - | - | - | - | - | 6/4 | - | - | |
| female | - | - | - | - | - | - | - | - | - | 2/1 | 1/1 | - | |
| Ixodes simplex | larva | - | - | - | - | - | - | - | - | 1/1 | - | - | 23/10 |
| nymph | - | - | - | - | - | - | - | - | - | - | - | 11/10 | |
| female | - | - | - | - | - | - | - | - | - | - | - | 4/4 | |
Abbreviations: MALC- Myotis alcathoe, MBEC—My. bechsteinii, MNAT—My. nattereri, MEMA—My. emarginatus, MDAU—My. daubentonii, MDAS—My. dasycneme, MMYO—My. myotis, PAUR—Plecotus auritus, BBAR—Barbastella barbastellus, RHIP—Rhinolophus hipposideros, REUR—R. euryale, MSCH—Miniopterus schreibersii.
Table 2. Tick species and stages collected in Romania, shown according to their bat hosts.
| Tick | Bat (number of ticks per number of bats) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vespertilionidae | Rhinolophidae | Miniopteridae | ||||||||
| Species | Stage | MNAT | MCAP | MDAU | MBLY | ESER | REUR | RFER | RMEH | MSCH |
| Ixodes vespertilionis | larva | 1/1 | 9/1 | 28/16 | 4/1 | 2/2 | - | 26/9 | 7/4 | 2/2 |
| nymph | - | 4/2 | 9/8 | 2/2 | - | - | 6/6 | - | - | |
| female | - | 1/1 | 1/1 | - | 1/1 | 1/1 | 1/1 | - | - | |
| Ixodes simplex | larva | - | - | - | - | - | - | - | 56/33 | |
| nymph | - | - | - | - | - | - | - | 39/33 | ||
| female | - | - | - | - | - | - | - | 5/5 | ||
Abbreviations: MNAT—Myotis nattereri, MCAP—My. capaccinii, MDAU—My. daubentonii, MBLY—My. blythii, ESER—Eptesicus serotinus, REUR—Rhinolophus euryale, RFER—R. ferrumequinum, RMEH—R. mehelyi, MSCH—Miniopterus schreibersii.
Ethical approval
Authorization for bat capture was provided by the National Inspectorate for Environment and Nature in Hungary (No. 14/2138-7/2011). Bat banding license numbers are TMF-14/32/2010 (DK), 59/2003 (PE) and TMF-493/3/2005 (TG), 65/2003 (SAB). Bats were handled according to the current law of animal welfare regulation (1998. XXVIII.). Permission from the Institutional Animal Care and Use Committee (IACUC) was not necessary, because bats were released in the field after tick removal (none taken to participating Institutes).
Results
Tick infestations of bats
In the two countries 308 ixodid ticks have been collected from 200 individuals of 17 bat species (Table 1, Table 2). Ixodes ariadnae was represented by 45, I. vespertilionis by 124 and I. simplex by 139 specimens (larvae, nymphs and females). In Hungary I. ariadnae was significantly more frequently found on bat species in the family Vespertilionidae, whereas I. vespertilionis was associated with Rhinolophidae (P<0.00001). Ixodes ariadnae was not collected in Romania, where I. vespertilionis occurred usually on representatives of both Vespertilionidae and Rhinolophidae (Table 2). Discounting one larva collected from Barbastella barbastellus, I. simplex was exclusively found on Mi. schreibersii.
In general, there was no significant difference between the intensity of tick infestation between bat species (p = 0.4279, df = 5, χ2 = 4.9026), but there was a significant difference in the intensity of infestation of bats with different tick stages (larva: n = 155; nymph: n = 79; female: n = 13; p = 0.0005, df = 2, χ2 = 14.924), i.e. ixodid tick larvae occurred in highest individual number on their hosts. In the case of I. ariadnae or I. vespertilionis there was no significant difference in the intensity of infestation between bat species, i.e. between My. bechsteinii and My. emarginatus (p = 0.4497, W = 28) or between My. daubentonii, R. ferrumequinum and R. hipposideros (p = 0.8719, df = 2, χ2 = 0.27423), respectively (Table 1, Table 2). Similarly, concerning these five bat species, there was no significant difference between intensities of their infestations with different tick stages, except for My. daubentonii and R. ferrumequinum on which larvae occurred significantly more frequently than nymphs/females (p = 0.02013, df = 2, χ2 = 7.8106). Similarly, infestation of Mi. schreibersii with I. simplex had the highest intensity when larvae were present on bats (larva: n = 79; nymph: n = 50; female: n = 9; p = 0.001674, df = 2, χ2 = 12.786).
DNA of piroplasms in bat ticks
DNA sequences of piroplasms were detected in 20 bat ticks (Table 3). Ixodes simplex carried piroplasm DNA significantly more frequently (13 of 138 specimens), than I. vespertilionis (3 of 124 specimens) (P = 0.02). The largest variety of Babesia and Theileria DNA sequences was also shown to be present in I. simplex (Table 3).
Table 3. Results of molecular analyses of bat ticks for the presence of piroplasms.
| Ixodesspecies | Tick stage or sex | PCR positive / all analysed ticks | Results of sequencing(length, % identity, sample number) | Bat host of PCR positive ticks# | Location(s) of PCR positive ticks in Fig 1 | Reference sequence | Accession number of sequence in this study (name of isolate) |
|---|---|---|---|---|---|---|---|
| I. ariadnae | larva | 4/26 | Babesia vesperuginis (448 bp, 100%, 4×) | MDAS | 1 | AJ871610 | KU958544 (Ia-Bv-1) |
| nymph | 0/14 | - | - | - | - | - | |
| female | 0/5 | - | - | - | - | - | |
| I. vespertilionis | larva | 3/89 | Babesia vesperuginis (448 bp, 100%, 2×) | ESER, MDAU | 2 | AJ871610 | KU958544 (Ia-Bv-1) |
| Babesia crassa (410 bp, 98.5%, 1×) | RHIP | 3 | KF791205 | KU958546 (Iv-Bcr-1) | |||
| nymph | 0/27 | - | - | - | - | ||
| female | 0/8 | - | - | - | - | ||
| I. simplex | larva | 8/79 | Babesia crassa (410 bp, 98.3%, 1×) | MSCH | 4 | KF791205 | KU958545 (Is-Bcr-1) |
| Babesia venatorum-like (105 bp, 100%, 1×) | MSCH | 2 | KC007118 | KU958553 (Is-Bv-1) | |||
| Babesia canis (420 bp, 100%, 1×) | MSCH | 2 | JF461253 | KU958552 (Is-Bca-2) | |||
| Theileria capreoli (425 bp, 99.5%, 1×) | MSCH | 4 | KJ188219 | KU958547 (Is-Tc-1) | |||
| Theileria orientalis (432 bp, 100%, 4×) | MSCH | 2, 4, 5 | AB668373 | KU958549 (Is-To-1) | |||
| nymph | 4/50 | Babesia crassa (410 bp, 98.5%, 1×) | MSCH | 2 | KF791205 | KU958546 (Iv-Bcr-1) | |
| Babesia canis (420 bp, 100%, 1×) | MSCH | 2 | KC902833 | KU958551 (Is-Bca-1) | |||
| Babesia canis (420 bp, 100%, 2×) | MSCH | 2 | JF461253 | KU958552 (Is-Bca-2) | |||
| female | 1/9 | Theileria sp. OT3 (432 bp, 100%, 1×) | MSCH | 4 | DQ866839 | KU958550 (Is-TOT3-1) |
#Abbreviations: MDAS—Myotis dasycneme, ESER—Eptesicus serotinus, MDAU—M. daubentonii, RHIP—Rhinolophus hipposideros, MSCH—Miniopterus schreibersi
In I. ariadnae only a DNA sequence of B. vesperuginis (identity: 448/448 bp = 100%) was shown to be present. All four PCR-positive larvae were removed from the same bat. In I. vespertilionis larvae sequences of B. vesperuginis (identity: 448/448 bp = 100%) and B. crassa (identity: 404/410 bp = 98.5%) were detected (Table 3).
Adding to the presence of the latter sequence in I. simplex nymphs, in a larva of this tick species the sequence of another genotype of B. crassa (identity: 403/410 = 98.3%) was demonstrated (Table 3), which was not detected before in Hungary. From I. simplex a shorter sequence of the zoonotic B. venatorum (identity: 105/105 bp = 100%) was also amplified, showing less identity with other piroplasms (second closest to B. occultans and T. equi, with 103/105 bp = 98.1% identity). In I. simplex larvae/nymphs two sequences of B. canis (both identities: 420/420 bp = 100%) were also detected.
Results of sequencing demonstrated DNA of two Theileria spp. exclusively in I. simplex larvae. These were T. capreoli (identity: 423/425 bp = 99.5%) and T. orientalis (identity: 432/432 bp = 100%). In addition, one female I. simplex carried the sequence of Theileria sp. OT3 (identity: 432/432 bp = 100%) (Table 3).
In the phylogenetic analysis, all sequences of Babesia and Theileria spp. amplified from bat ticks in the present study clustered together with relevant genotypes available in GenBank (and published from previously known "type" hosts of these piroplasms) (Fig 2). Their separation from other piroplasms was confirmed by high bootstrap values (Fig 2). Taken together, piroplasm sequences were demonstrated in bat ticks from three places of Hungary and two places of Romania (Table 3, Fig 1); three sequences of piroplasms were detected only in samples from Hungary, three of them only in Romania and three in both countries (Fig 2).
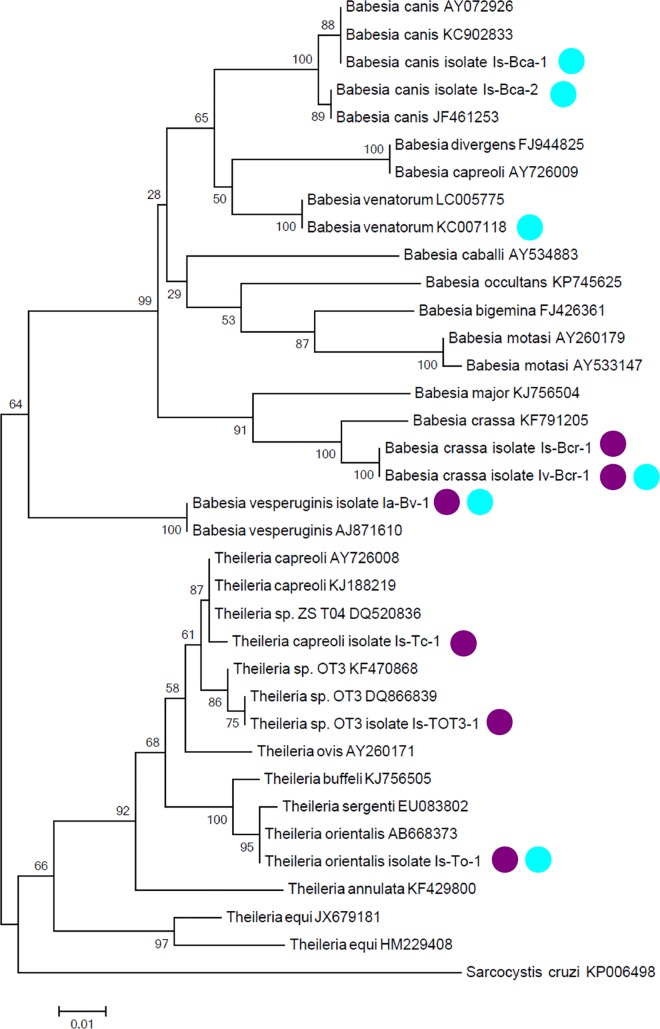
Fig 2. Phylogenetic relationships of 18S rDNA sequences of piroplasms identified in this study and relevant sequences previously deposited in GenBank.
Sequences identified in the present study in Hungary and in Romania are highlighted with purple or turquoise dots, respectively. The shorter sequence of Babesia venatorum from this study is not included, therefore its reference sequence (to which it showed 100% identity) is marked. Branch lengths correlate to the number of substitutions inferred according to the scale shown.
Discussion
In this study all three ixodid tick species have been collected, which are specialized to bat hosts in Europe. Among them, I. ariadnae is known to occur in three countries of Central and Western Europe (Hungary, Germany and Belgium: [19]), whereas I. vespertilionis and I. simplex are more widespread on the continent (the latter predominantly south of latitude 49° N: [20]). In the present study I. ariadnae was not found in Romania, suggesting that the bat tick fauna in this country is similar to that in the Balkans, with the predominance of I. simplex [21]. A plausible explanation for this phenomenon is the absence of regular bat migration between the mountainous regions of Hungary and Romania, which otherwise could have caused the spread of I. ariadnae towards the southeast (the main route of long distance bat migration in the region is in the southwestern-northeastern direction: [22]).
The present results confirmed that the preferred hosts of I. ariadnae belong to Vespertilionidae [11, 19], those of I. vespertilionis to Rhinolophidae, while I. simplex is adapted to parasitize Mi. schreibersii (Miniopteridae) [23]. Nevertheless, to the best of our knowledge, several new host associations of ixodid bat ticks are reported here for the first time. In particular, I. ariadnae was found newly on four Myotis spp., I. vespertilionis on two Myotis spp., as well as on Eptesicus serotinus and Rhinolophus mehelyi, finally I. simplex on Barbastella barbastellus (Table 4).
Table 4. Host associations of ixodid bat ticks reported previously and in this study.
| Host species reported previously | References | New host species in this study | |
|---|---|---|---|
| Ixodes ariadnae | Myotis alcathoe | [11, 19] | Myotis dasycneme |
| Myotis bechsteinii | Myotis daubentonii | ||
| Myotis blythii | Myotis emarginatus | ||
| Myotis myotis | Myotis nattererii | ||
| Plecotus auritus | |||
| Ixodes vespertilionis | Miniopterus schreibersii | [9, 21, 23, 24, 25, 26, 27, 28] | Eptesicus serotinus |
| Myotis bechsteinii | Myotis capaccinii | ||
| Myotis blythii | Myotis dasycneme | ||
| Myotis brandtii | Rhinolophus mehelyi | ||
| Myotis daubentonii | |||
| Myotis emarginatus | |||
| Myotis myotis | |||
| Myotis mystacinus | |||
| Myotis nattererii | |||
| Plecotus auritus | |||
| Rhinolophus euryale | |||
| Rhinolophus ferrumequinum | |||
| Rhinolophus hipposideros | |||
| Ixodes simplex | Miniopterus schreibersii | [21, 23, 25, 29] | Barbastella barbastellus |
| Myotis alcathoe | |||
| Rhinolophus euryale | |||
| Rhinolophus ferrumequinum |
The intensity of tick infestation was not significantly different between small and large size bat species (in the case of My. daubentonii vs. R. ferrumequinum, respectively), suggesting that factors depending on body size (such as the body surface area, interrelated with metabolic rate, heat emission: [30]) may not be crucial for host finding by bat ticks. Similarly, it has been reported that body size of passeriform bird species did not significantly influence the intensity of their tick infestation [31]. On the other hand, the intensity of infestation with bat tick larvae was significantly higher, than with later stages in the life cycle. This finding is consistent with the significant decrease of individual number of tick stages with the advance of tick developmental cycle [32].
Taking into account the considerable lack of data in literature on the vector potential of ixodid bat ticks, and the recent finding of B. canis DNA in bat faeces [5], DNA extracts of 307 specimens were molecularly analysed for the presence of piroplasms (Apicomplexa: Piroplasmida). Among piroplasms, Babesia species are known to be transmitted transovarially by female ticks to the next generation (i.e. to larvae prior to their blood meal), whereas Theileria species are transmitted transstadially [33]. The latter implies that there is no other way for tick larvae to harbor theileriae or to contain theileria DNA, than to ingest these with the blood meal from a host/reservoir which is either theileria-infected or at least theileria DNA is present in its blood stream.
Babesia vesperuginis DNA was molecularly identified here in I. ariadnae and I. vespertilionis. This piroplasm is pathogenic to bats, and was reported to infect Pipistrellus pipistrellus [34], several Myotis spp. (including My. daubentonii, on which bat species a PCR positive tick was collected in the present study) as well as Plecotus auritus [35]. Taking into account that soft ticks (Argas vespertilionis) have been incriminated as vectors of B. vesperuginis [34], the present results suggest that bat ticks carrying this piroplasm (or its DNA) ingested it with the blood meal, i.e. further Myotis spp. (exemplified by My. dasycneme) and Eptesicus serotinus might also be susceptible to B. vesperuginis.
Babesia crassa has low pathogenicity in small ruminants and its vector is unknown [36]. This piroplasm (or closely related genotypes) were reported to occur only in the Middle-East, but recently one genotype has also been identified in Haemaphysalis inermis ticks in Central Europe, Hungary [37]. In the present study two different DNA sequences of B. crassa were detected in bat ticks (I. vespertilionis, I. simplex) in both Hungary and Romania. These two bat tick species have never been reported from small ruminants, and therefore their PCR positivity can be explained by ingesting B. crassa DNA-containing blood meal from bats. In this context it may be epidemiologically relevant that B. crassa was reported to be present in H. sulcata [38], and this tick species was reported to infest bats in the larval stage and small ruminants in the adult stage [39].
Babesia canis is an important parasite of dogs. Wild canids are also susceptible [40]. The known vector of this piroplasm is Dermacentor reticulatus, which is a tick species seldom infesting bats, including Mi. schreibersii [8]. Recently, bats were reported to pass the DNA of B. canis in their faeces [5]. Taking into account that it is very unlikely that relevant lineages of I. simplex (found to be PCR positive here) had become infected from canids (from which hosts I. simplex has never been reported) in a previous stage or generation, B. canis or its DNA might have been present in the blood of relevant bats. This possibility is supported by recent finding of B. canis DNA in bat tissues [41].
Interestingly, the DNA of B. venatorum was amplified from one larva of I. simplex in Romania. Although the sequence was 100% identical with B. venatorum and differed from other piroplasms, because of its shortness no final conclusion can be drawn on the occurrence of B. venatorum DNA in bat ticks. This piroplasm (associated with cervids as hosts) is zoonotic, with I. ricinus as its vector. It is noteworthy that I. ricinus occurs on bats (e.g. [9]), and the present results suggest that this may allow bats to become carriers of B. venatorum or its DNA (taking into account that I. simplex has never been reported from cervids or from humans). On the other hand, the host of I. simplex, Mi. schreibersii may live in large colonies in the human environment (e.g. mines, man made tunnels, ruins: [20]). Therefore, this preliminary finding deserves further molecular epidemiological investigation.
Among Theileria spp. and genotypes, the DNA of Theileria sp. OT3 has been detected here in a female I. simplex. This piroplasms (with unknown pathogenicity) was formerly reported to infect small ruminants in Italy [42], but recently its DNA has also been reported from Haemaphysalis punctata in northern Hungary [37], and this tick species is known to infest bats [10].
In addition, the DNA of two Theileria spp. have been shown here to be present in larvae of I. simplex from Mi. schreibersii. Among them, T. capreoli is a mildly pathogenic parasite of cervids. The tick species H. concinna, in which the DNA of T. capreoli has been recently demonstrated in Hungary[37], is also known to occasionally infest bats [43].
Members of the T. orientalis complex (T. orientalis, T. buffeli) infect cattle in the tropical-subtropical regions of the globe, usually with low pathogenicity. Recently, T. orientalis has been shown to emerge in Central Europe [37] and Australia [44], sometimes severely affecting cattle [45]. Vectors of the T. orientalis complex are Haemaphysalis spp. [33]. Haemaphysalis spp. may accidentally infest bats, and in particular H. punctata, the most likely vector of T. orientalis in Europe, was synonymously called "H. rhinolophi" [10]. Furthermore, in South-East Asia (where species of the T. orientalis complex are widespread) at least one Haemaphysalis sp. has bats as preferred hosts [46]. These literature data attest a possible connection between large ruminants and bats via Haemaphysalis sp. ticks. In the present study only bat tick (I. simplex) larvae were PCR positive for T. capreoli and T. orientalis. This means that relevant piroplasms (or their DNA) could have been acquired by the larvae exclusively from the blood of bat hosts, because there is no transovarial, only transstadial transmission in the case of theileriae [33]. The significance of the potential epidemiological role of bats in bovine theileriosis deserves further attention, as several bat species may use cattle stables for roosting [47].
In summary, competent vectors of the above piroplasms (that have been hitherto reported from hosts other than bats) are D. reticulatus, I. ricinus and Haemaphysalis spp. These tick species are rarely found on bats, most likely attaching to bats when roosting in nests of small mammals (e.g. in tree holes) [5], or when gleaning bat species feed on insects from the lower vegetation in meadows or forests. However, Mi. schreibersii, associated with most of the piroplasms identified in the present study, is not known to forage on the ground level [48]. Alternatively, blood-sucking flies have the potential to carry and transmit Babesia spp. [49] and Theileria spp. [50], and flies (Insecta: Diptera) are among the frequent food items of e.g. Mi. schreibersii [51]. This implies that bats may get into contact with or may have access to piroplasms or piroplasm DNA from their food.
Thus, there are two plausible explanations for the above, unexpected findings. The first is that bats get into frequent contact with the DNA of vector-borne pathogens contained in their food. During digestion this DNA may pass through the gut wall (barrier) un- or only partly digested, thus appearing in the circulation (or perhaps other tissues) from where bat ticks can take it up with their blood meal. In support of this possibility, it has recently been verified that meal-derived DNA fragments (even long ones) can avoid degradation and through not-yet-known mechanisms enter the circulation, at least in humans [52].
Another, although less likely explanation is that bats are susceptible to a broader range of piroplasms than previously thought. The phylogeny of piroplasms has recently been shown to reflect considerable host diversity and limited host specificity [53], suggesting that these tick-borne protozoa have undergone frequent host switches during their evolution. In this context the present results may imply that bats may share piroplasms with a broad range of mammals (from various orders). Similarly, several Babesia and Theileria spp. are known to infect hosts from different mammalian orders (e.g. B. caballi, B. canis, B. divergens, B. microti, T. equi: [53]).
Conclusions
Bat ticks are not known to infest dogs or ruminants, i.e. typical hosts and reservoirs of piroplasms molecularly identified in I. vespertilionis and I. simplex. Therefore, DNA sequences of piroplasms detected in these bat ticks most likely originated from the blood of their respective bat hosts. This may indicate that either bats are susceptible to a broader range of piroplasms than previously thought, or at least the DNA of piroplasms may pass through the gut barrier of bats during digestion of relevant insect vectors. In light of these findings, the role of bats in the epidemiology of piroplasmoses deserves further investigation.
Supporting Information
Letters: A—Ariadne Cave System and caves in the Pilis Mountains, B—Bükk Highlands Cave System (see Fig 1).
(DOC)
Acknowledgments
The survey was organized in the framework of the EurNegVec COST Action TD1303. Financial resources were provided by OTKA 115854. The research was also supported by the 11475-4/2016/FEKUT grant of the Hungarian Ministry of Human Resources. GF was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. PCE 236/2011 grant and the framework of Domus Hungarica, Hungarian Academy of Science provided financial help to ADS. Jére, Cs., Csősz, I., Bücs, Sz., Varga, Á., D’Amico, G. and Ionică, A. provided help in different stages of sample collection in Romania.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by OTKA 115854 (http://www.otka.hu/en), 11475-4/2016/FEKUT (http://2010-2014.kormany.hu/en/ministry-of-human-resources), and PCE 236/2011 (http://www.osztondijak.ro/scholarship/Palyazati-felhivas-magyarorszagi-osztondijak-elnyeresere-MTA-Domus-Hungarica). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dietz C, Nill D, von Helversen O. Bats of Britain, Europe and Northwest Africa. A & C Black, London, 2009. 406 pp. [Google Scholar]
- 2.Szőke K, Hornok S. Epidemiological importance of bats (Chiroptera) in Europe, with emphasis on their blood-sucking ectoparasites as potential transmitters of vector-borne pathogens. Magy Állatorvos. 2016;138:15–29. [Google Scholar]
- 3.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19:531–545. 10.1128/CMR.00017-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunz TH, Lumsden LF. Ecology of cavity and foliage roosting bats In: Kunz TH, Feneon MB (eds): Bat ecology. The University of Chicago Press, Chicago, 2003;3–89. [Google Scholar]
- 5.Hornok S, Estók P, Kováts D, Flaisz B, Takács N, Szőke K et al. Screening of bat faeces for arthropod-borne apicomplexan protozoa: Babesia canis and Besnoitia besnoiti-like sequences from Chiroptera. Parasit Vectors. 2015;8:441 10.1186/s13071-015-1052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estrada-Peña A, Jongejan F. Ticks feeding on humans: a review of records on human- biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol. 1999;23:685–715 [DOI] [PubMed] [Google Scholar]
- 7.Piksa K, Nowak-Chmura M, Siuda K. First case of human infestation by the tick Ixodes vespertilionis (Acari: Ixodidae). Int J Acarol. 2013;38:1–2. [Google Scholar]
- 8.Neumann LG. Ixodidae: Dermacentor reticulatus. Das Tierreich. 1911;26:98–100. [Google Scholar]
- 9.Ševčík M, Krištofík J, Uhrin M, Benda P. New records of ticks (Acari: Ixodidae) parasitising on bats in Slovakia. Vespertilio 2010;13–14:139–147. [Google Scholar]
- 10.Estrada-Peña A. Las garrapatas (Acari: Ixodoidea) parásitas de murciélagos (Mammalia, Chiroptera). (II). Rev Ibér Parasitol. 1989;49:165–175. [Google Scholar]
- 11.Hornok S, Kontschán J, Kováts D, Kovács R, Angyal D, Görföl T et al. Bat ticks revisited: Ixodes ariadnae sp. nov. and allopatric genotypes of I. vespertilionis in caves of Hungary. Parasit Vectors. 2014;7:202 10.1186/1756-3305-7-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornok S, Estrada-Peña A, Kontschán J, Plantard O, Kunz B, Mihalca AD et al. High degree of mitochondrial gene heterogeneity in the bat tick species Ixodes vespertilionis, I. ariadnae and I. simplex from Eurasia. Parasit Vectors. 2015;8:457 10.1186/s13071-015-1056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornok S, Kovács R, Meli ML, Kontschán J, Gönczi E, Gyuranecz M et al. First detection of bartonellae in a broad range of bat ectoparasites. Vet Microbiol. 2012;159:541–543. 10.1016/j.vetmic.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 14.Feider Z. Ixodoidea In: Fauna of the Popular Republic of Romania. 1965, volume 5/2 Published by Academiei Republicii Populare Romane, Bucuresti. [in Romanian]. [Google Scholar]
- 15.Hornok S, Kontschán J, Estrada-Peña A, Fernández de Mera IG, Tomanović S, de la Fuente J. Contributions to the morphology and phylogeny of the newly discovered bat tick species, Ixodes ariadnae in comparison with I. vespertilionis and I. simplex. Parasit Vectors. 2015; 8:47 10.1186/s13071-015-0665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. 2006;13:65–70. [PubMed] [Google Scholar]
- 17.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–26. [DOI] [PubMed] [Google Scholar]
- 18.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5:molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornok S, Krawczyk A. First record of Ixodes ariadnae in Western Europe, Belgium. Acta Vet Hung. 2016;64:467–471. [DOI] [PubMed] [Google Scholar]
- 20.Hutson AM, Aulagnier S, Benda P, Karataş A, Palmeirim J, Paunović M. Miniopterus schreibersii. The IUCN Red List of Threatened Species 2008: e.T13561A4160556. [Google Scholar]
- 21.Burazerović J, Ćakić S, Mihaljica D, Sukara R, Ćirović D, Tomanović S. Ticks (Acari: Argasidae, Ixodidae) parasitizing bats in the central Balkans. Exp Appl Acarol. 2015;66:281–291. 10.1007/s10493-015-9891-6 [DOI] [PubMed] [Google Scholar]
- 22.Hutterer R, Ivanova T, Meyer-Cords C, Rodrigues L. Bat Migrations in Europe. A Review of Banding Data and Literature. Naturschutz und BiologischeVielfalt 28. Federal Agency for Nature Conservation, 2005, Bonn. [Google Scholar]
- 23.Arthur DR. The Ixodes ticks of Chiroptera (Ixodoidea, Ixodidae). J Parasitol. 1956;42:180–196. [PubMed] [Google Scholar]
- 24.Rupp D, Zahn A, Ludwig P. Actual records of bat ectoparasites in Bavaria (Germany). Spixana 2004;27:185–190. [Google Scholar]
- 25.Siuda K, Stanko M, Piksa K, Górz A. Ticks (Acari: Ixodida) parasitizing bats in Poland and Slovakia. Wiad Parazytol. 2009;55:39–45. [PubMed] [Google Scholar]
- 26.Mihalca AD, Dumitrache MO, Magdaş C, Gherman CM, Domşa C, Mircean V et al. Synopsis of the hard ticks (Acari: Ixodidae) of Romania with update on host associations and geographical distribution. Exp Appl Acarol. 2012;58:183–206. 10.1007/s10493-012-9566-5 [DOI] [PubMed] [Google Scholar]
- 27.Frank R, Kuhn T, Werblow A, Liston A, Kochmann J, Klimpel S. Parasite diversity of European Myotis species with special emphasis on Myotis myotis (Microchiroptera, Vespertilionidae) from a typical nursery roost. Parasit Vectors. 2015;8:101 10.1186/s13071-015-0707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piksa K, Stańczak J, Biernat B, Górz A, Nowak-Chmura M, Siuda K. Detection of Borrelia burgdorferi sensu lato and spotted fever group rickettsiae in hard ticks (Acari, Ixodidae) parasitizing bats in Poland. Parasitol Res. 2016;115:1727–31. 10.1007/s00436-016-4936-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krištofík J, Danko Š. Arthropod ectoparasites (Acarina, Heteroptera, Diptera, Siphonaptera) of bats in Slovakia. Vespertilio 2012;16:167–189 [Google Scholar]
- 30.Hock RJ. The metabolic rates and body temperatures of bats. Biol Bull. 1951;101:289–299. [Google Scholar]
- 31.Hornok S, Flaisz B, Takács N, Kontschán J, Csörgő T, Csipak Á et al. Bird ticks in Hungary reflect western, southern, eastern flyway connections and two genetic lineages of Ixodes frontalis and Haemaphysalis concinna. Parasit Vectors. 2016;9:101 10.1186/s13071-016-1365-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korotkov IuS. Methods for estimating the demographic structure of the taiga tick (Ixodidae) based on results of standard parasitological observations. Parazitologiia 2004;38:492–502. [in Russian] [PubMed] [Google Scholar]
- 33.Fujisaki K, Kawazu S, Kamio T. The taxonomy of the bovine Theileria spp. Parasitol Today. 1994;10:31–33. [DOI] [PubMed] [Google Scholar]
- 34.Gardner RA, Molyneux DH. Babesia vesperuginis: natural and experimental infections in British bats (Microchiroptera). Parasitology 1987;95 (Pt 3):461–9. [DOI] [PubMed] [Google Scholar]
- 35.Sebek Z, Sixl W, Rosicky B. Ein Beitrag zur Charakteristik der Naturherde der Piroplasmose und zur Kenntnis der Wirtstiere mit Daten zur Rinderplasmose in der Steiermark und von Kleinsäugeruntersuchungen in der CSSR (Sporozoa, Haemosporina). Mitteilungen der Abteilung für Zoologie am Landesmuseum Joanneum Graz 1975;4:67–80. [Google Scholar]
- 36.Hashemi-Fesharki R, Uilenberg G. Babesia crassa n.sp. (Sporozoa, Babesiidae) of domestic sheep in Iran. Vet Q. 1981;3:1–8. 10.1080/01652176.1981.9693787 [DOI] [PubMed] [Google Scholar]
- 37.Hornok S, Takács N, Kontschán J, György Zs, Micsutka A, Iceton S et al. Diversity of Haemaphysalis-associated piroplasms of ruminants in Central-Eastern Europe, Hungary. Parasit Vectors. 2015;8:627 10.1186/s13071-015-1236-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aktas M. A survey of ixodid tick species and molecular identification of tick-borne pathogens. Vet Parasitol. 2014;200:276–283. 10.1016/j.vetpar.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 39.Filippova NA, Neronov V M, Farhang-Azad A. Data on the ixodid fauna (Acarina, Ixodidae) of small mammals in Iran. Entomol Obozrenie. 1976;55:467–479. [in Russian] [Google Scholar]
- 40.Kuttler KL. Canine babesiosis In: Ristic M (ed.): Babesiosis of domestic animals and man. CRC Press, Boca Raton, Florida; 1988. pp. 12–13. [Google Scholar]
- 41.Corduneanu A, Sándor AD, Mihalca AD, Hrazdilová K, Modry D, Hornok S: Molecular evidence of canine pathogens in tissues of European bats–Proceedings of the 17th International Bat Research Conference, 2016, Durban, South Africa, pp. 50–51. Available: http://www.harrison-institute.org/IBRC2016_FullProgram.pdf, Accessed November 16, 2016
- 42.Giangaspero A, Marangi M, Papini R, Paoletti B, Wijnveld M, Jongejan F. Theileria sp. OT3 and other tick-borne pathogens in sheep and ticks in Italy: molecular characterization and phylogeny. Ticks Tick Borne Dis. 2015;6:75–83. 10.1016/j.ttbdis.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 43.Lebedeva NN, Korenberg EI. Distribution of Haemaphysalis concinna Koch in the Soviet Union and some general features of its ecology. Folia Parasitol. 1981;28:249–261. [PubMed] [Google Scholar]
- 44.Kamau J, de Vos AJ, Playford M, Salim B, Kinyanjui P, Sugimoto C: Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasit Vectors. 2011;4:22 10.1186/1756-3305-4-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Izzo MM, Poe I, Horadagoda NJdVA, House. Haemolytic anaemia in cattle in NSW associated with Theileria infections. Aust Vet J. 2010;88:45–51. 10.1111/j.1751-0813.2009.00540.x [DOI] [PubMed] [Google Scholar]
- 46.Hoogstraal H: Studies on Southeast Asian Haemaphysalis ticks (Ixodoidea, Ixodidae). Redescription, hosts, and distribution of H. traguli Oudemans. The larva and nymph of H. vidua W. and N. Identity of H. papuana toxopei Warburton (n. comb.). J Parasitol. 1964;50:765–782. [PubMed] [Google Scholar]
- 47.Dekker JJA, Regelink JR, Janssen EA, Brinkmann R, Limpens HJGA. Habitat use of female Geoffroy’s bats (Myotis emarginatus) at it’s two northernmost maternity roosts and the implications for their conservation. Lutra 2013;56:111–120. [Google Scholar]
- 48.Vincent S, Nemoz M, Aulagnier S. Activity and foraging habitats of Miniopterus schreibersii (Chiroptera, Miniopteridae) in southern France: implications for its conservation. Hystrix It J Mammal. 2011;22:57–72. [Google Scholar]
- 49.Friedhoff KT. Transmission of Babesia: Other means of transmission In: Ristic M (ed.): Babesiosis of domestic animals and man. CRC Press, Boca Raton, Florida; 1988. p. 45. [Google Scholar]
- 50.Hammer JF, Jenkins C, Bogema D, Emery D. Mechanical transfer of Theileria orientalis: possible roles of biting arthropods, colostrum and husbandry practices in disease transmission. Parasit Vectors. 2016;9:34 10.1186/s13071-016-1323-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Presetnik P, Aulagnier S. Diet of Miniopterus schreibersii in northeastern Slovenia (Central Europe). Mammalia 2013;77:297–305. [Google Scholar]
- 52.Spisák S, Solymosi N, Ittzés P, Bodor A, Kondor D, Vattay G, et al. Complete Genes May Pass from Food to Human Blood. PLoS ONE 2013;8(7):e69805 10.1371/journal.pone.0069805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lack JB, Reichard MV, Van Den Bussche RA. Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int J Parasitol. 2012;42:353–363. 10.1016/j.ijpara.2012.02.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Letters: A—Ariadne Cave System and caves in the Pilis Mountains, B—Bükk Highlands Cave System (see Fig 1).
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.