Abstract
The paired-box transcription factor Pax7 has been claimed to specify the muscle stem cell lineage since inactivation of Pax7 led to a failure to detect muscle satellite cells. Here we show that muscles of juvenile Pax7(−/−) mice at P11 contain a reduced but substantial number of satellite cells. Neither juvenile nor adult Pax7(−/−) mice displayed a significant reduction in the number and size of myotubes, indicating that the remaining number of satellite cells sufficed to allow normal postnatal muscle growth. The number of satellite cells in Pax7 mutant mice declined strongly during postnatal development, although single satellite cells were readily identified in adult Pax7 mutant mice. Muscle regeneration was impaired in adult Pax7 mutant mice. Our results clearly indicate an essential function of Pax7 for renewal and maintenance of muscle stem cells and exclude an exclusive role of Pax7 in satellite cell specification.
Keywords: muscle cell proliferation, muscle regeneration, muscle satellite cells, Pax7, stem cells
Introduction
The ability of skeletal muscles of adult mammals for postnatal growth and regeneration is attributed to a small population of cells situated within the basal lamina that engulfs myofibers. Upon injury or muscle growth, satellite cells become activated and turn into proliferating myoblasts, which eventually fuse to pre-existing myotubes or to each other to form new myotubes (Bischoff, 1994). The satellite cell pool is constantly replenished during lifetime, although there is a decline in satellite cell numbers and a reduced proliferative capacity in aged individuals (Bischoff, 1994; Seale and Rudnicki, 2000). Myotubes in mammals, in contrast, are postmitotic and cannot re-enter the cell cycle. Although there is a wide agreement that satellite cells represent the main source of muscle stem cells and are required for efficient muscle regeneration, it has been questioned whether they represent the sole source of muscle precursor cells. Recently, other cell populations collectively called adult stem cells have been proposed to contribute to muscle regeneration (Ferrari et al, 1998; Gussoni et al, 1999; Jackson et al, 1999; Polesskaya et al, 2003). However, the view that noncommitted bone marrow- or muscle-derived stem cells participate in muscle regeneration after (trans)-differentiating into muscle precursor cells (Seale et al, 2000; LaBarge and Blau, 2002) is not commonly accepted (Wagers et al, 2002; Camargo et al, 2003). At present, the situation is far from clear and requires a careful analysis of the molecules and mechanisms that determine the identity of muscle stem cells as well as their renewal and contribution to the regeneration process.
Currently, we do not know whether satellite cells represent a separate myogenic lineage either of somitic or nonsomitic origin that is established early during embryogenesis and laid aside for future usage or whether satellite cells originate from embryonic or fetal myoblasts, which encounter a temporary differentiation block and are therefore prevented to be used up for myotube formation during development. Despite their disputed origin, satellite cells seem to rely on the same network of transcription factors for determination and differentiation than myogenic precursor cells during embryonic development. Like their embryonic cousins, satellite cells express MyoD and Myf5, and combined disruption of these genes leads to the ablation of satellite cells similar to the annihilation of other myogenic cells (Braun et al, 1992; Rudnicki et al, 1993). Although satellite cells can be characterized by expression of characteristic marker genes such as m-Cadherin, CD34, Msx1, cMet, MNF or Foxk1 (Garry et al, 2000) and Pax7 (Cornelison and Wold, 1997; Beauchamp et al, 2000; Cornelison et al, 2000; Seale et al, 2000) and by their typical morphological appearance, it has been proposed that they do not represent a unique cell type but a rather heterogenous population of muscle precursor cells.
The paired-box gene Pax7 has attracted particular attention in this context since it has been reported that homozygous Pax7 mutant mice completely lack muscle satellite cells, suggesting that Pax7 is at the top of the molecular hierarchy controlling satellite cell specification (Seale et al, 2000). Pax7 has been originally described as a member of the paired-homeobox gene family, which is expressed during somite development (Jostes et al, 1990). Pax7(−/−) mice fail to thrive and most of them subsequently die within 2–3 weeks probably because of dysgenesis of neural crest derivatives (Mansouri et al, 1996). It has been shown that the function of Pax7 partially overlaps with the paralogous Pax3 gene during development (Mansouri and Gruss, 1998), which might explain the lack of a discernable phenotype in Pax7 mutant mice during somite formation. Pax3 and Pax7 have been proposed to keep cells in a deregulated undifferentiated and proliferative state facilitating for example formation of alveolar rhabdomyosarcomas. Both genes strongly stimulate cell proliferation in various tissues (Mansouri, 1998; Mennerich and Braun, 2001).
Here, we show that juvenile Pax7(−/−) mice at P11 contain a reduced but substantial number of muscle satellite cells, which mostly disappear during postnatal life. We found a correlation of the number of satellite cells with the ability of muscle regeneration, which challenges the recent claims of an alternative muscle regeneration pathway based on adult stem cells. Based on the postnatal decline in the number of satellite cells, we propose that Pax7 has a crucial function for renewal and propagation or maintenance of muscle stem cells and rule out the possibility that Pax7 is essential for foundation of the satellite cell lineage.
Results
Essentially normal postnatal muscle growth in juvenile and adult Pax7(−/−) mice
To analyze the physiological role of Pax7 in the control of proliferation and differentiation of muscle satellite and precursor cells, we took advantage of two available Pax7 mutant strains, which both lack expression of Pax7. In both strains, the Pax7 gene was inactivated by insertion of the neomycin gene into the first exon of the paired box (the paired box is encoded by three exons), which abolishes DNA-binding activity of the paired domain (Chalepakis and Gruss, 1995). The Pax7 lacZ strain contains an additional β-galactosidase reporter gene in front of the neomycin gene to track the fate of Pax7-expressing cells in heterozygous and homozygous mutant animals (Mansouri et al, 1996).
Macroscopical and histological examination of homozygous Pax7 mutant mice at P11 revealed no significant reduction of the thickness of the diaphragm or of other muscles (Musculas pectoralis major, M. tibialis anterior, M. gastrocnemius, M. erector spinae, Musculi interossei and Mm. intercostales), which could not be attributed to the reduced bodyweight of Pax7(−/−) mice. We were also unable to observe a significantly reduced diameter of myofibers at P11 beyond statistical variations (Supplementary Figure I). While most of the Pax7(−/−) deceased by weaning on a mixed C57/BL6/129Sv genetic background, between 5 and 10% of mutant animals survived until adulthood and were amendable to further analysis. Based on the rather normal histological appearance of the musculature in juvenile mutant animals, it seems unlikely that the cause of death of deceased Pax7 mutants is due to malfunctions of the skeletal musculature. Although adult Pax7(−/−) never reached the size of their wild-type and heterozygous littermates, the thickness of individual muscles was only moderately affected (Figure 1A and B). Yet, muscles of adult Pax7(−/−) mice contained more small-sized myofibers than heterozygous or wild-type muscles (Figure 1B).
Figure 1.

(A) Macroscopic view of Pax7(+/−) and Pax7(−/−) mice at P8 and P60. The growth retardation of homozygous mutant animals is evident. (B) Hematoxylin- and eosin-stained paraffin sections of the M. gastrocnemius (GC) at P11 (a, e), the M. tibialis anterior (TA) (b, f), the diaphragm (c, g) and the body wall musculature (d, h) of Pax7(+/−) (a–d) and Pax7(−/−) (e–h) mice at P60. Note that the thickness of individual muscles in Pax7(−/−) mice was only moderately affected although muscles of Pax7(−/−) mice contained more small-sized myofibers.
During postnatal life, myofibers are constantly replenished by fusion of satellite cells with existing myotubes. Freshly regenerated myotubes can be easily identified by the presence of centrally located nuclei derived from fused satellite cells. We therefore screened for the presence of centrally located nuclei at different postnatal stages in hetero- and homozygous Pax7 mutant animals. Newly formed myotubes were found in hetero- and homozygous mutant animals at nearly equal rates at P11, while the number of centrally located nuclei was significantly reduced at P60. The formation of newly formed myotubes clearly indicated that residual muscle stem cells were present in P60 Pax7(−/−) mice (Supplementary Figure I).
Expression of Pax7 is upregulated in proliferating, satellite cell-derived myoblasts
To study the expression of Pax7 in situ in resting and regenerating skeletal muscles, we analyzed the expression of the Pax7-lacZ allele by histochemical staining for β-galactosidase activity on cryosections from various muscles. A comparatively weak Pax7-lacZ activity was detected in satellite cells of nonregenerating skeletal muscles (M. tibialis anterior) taken from Pax7(+/−) mice. After freeze-crush or cardiotoxin-induced skeletal muscle damage, both the number and the staining intensity of Pax7-lacZ-positive cells increased reflecting an expansion of satellite cell-derived myoblasts in the injured muscle and an upregulation of the Pax7 gene expression in proliferating myoblasts (Figure 2). Surprisingly, we also found Pax7-lacZ-positive satellite cells in adult Pax7(−/−) (P60) mice, which previously have been claimed to be completely devoid of satellite cells. Although the number of Pax7-lacZ-positive satellite cells was severely reduced in adult Pax7(−/−) (P60) mice, a careful examination proved the presence of several Pax7-lacZ-positive satellite cells in different muscle groups. In addition, the number of Pax7-lacZ-positive satellite cells increased significantly in muscles of adult Pax7(−/−) (P60) mice after cardiotoxin-induced muscle damage (Figure 2), indicating that satellite cells, which lack Pax7, can divide and are able to respond to physiological cues driving satellite cell expansion.
Figure 2.
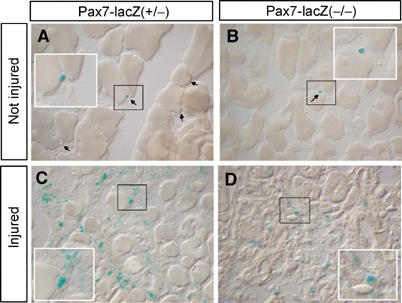
Increased expression of Pax7-lacZ in activated muscle precursor cells of Pax7(−/−) and Pax7(−/−) mice. LacZ staining of cryostat sections from Mm. tibialis anteriores of Pax7-lacZ(+/−) (A, C) and Pax7-lacZ(−/−) (B, D) mice without prior damage (A, B) and 10 days after cardiotoxin-induced muscle injury (C, D). Pax7(+/−) mice do contain more LacZ-positive satellite cells both in uninjured and injured muscles than Pax7(−/−) mutants. Note the increase of LacZ-positive satellite cells and the compromised regeneration in Pax7(−/−) mutant animals.
Severe loss of muscle satellite cells during postnatal development
The normal postnatal growth of skeletal muscles and the presence of Pax7-lacZ-positive cells in juvenile and adult Pax7(−/−) mice prompted us to scrutinize vigorously the number of satellite cells in Pax7(+/−) and Pax7(−/−) mutant animals at different postnatal stages (P11 and P60) using various established techniques. At P11, we detected a reduced but substantial number of muscle satellite cells by electron microscopy (EM) in various muscles (see Materials and methods) of Pax7(−/−) mice (Figure 3 and Table I). In addition, we isolated myofibers from muscles of Pax7(+/−) and Pax7(−/−) mice and stained for CD34 expression, a characteristic marker of satellite cells. Similar to the results obtained by EM counting, we observed a high number of CD34-positive satellite cells in Pax7(−/−) mice at P11, although the amount of satellite cells was clearly lower compared to Pax7(+/−) littermates (Table I and Figure 4; see also Supplementary Figure II). Finally, Pax7-lacZ staining was employed to identify satellite cells both on isolated myofibers (Figure 4) and on tissue sections (Figure 2). Since a single Pax7-lacZ allele yielded a comparatively low β-Gal activity, we probably systematically under-rated the number of Pax7-lacZ-positive cells in Pax7(+/−) mice, which might explain the relative high number of Pax7-lacZ-positive satellite cells in Pax7(−/−) mice (Table I). Double staining with antibodies against Pax7-lacZ and CD34 revealed that both in Pax7(+/−) and Pax7(−/−) mice most Pax7-lacZ-positive cells expressed CD34 and vice versa (Supplementary Figure IIIA).
Figure 3.
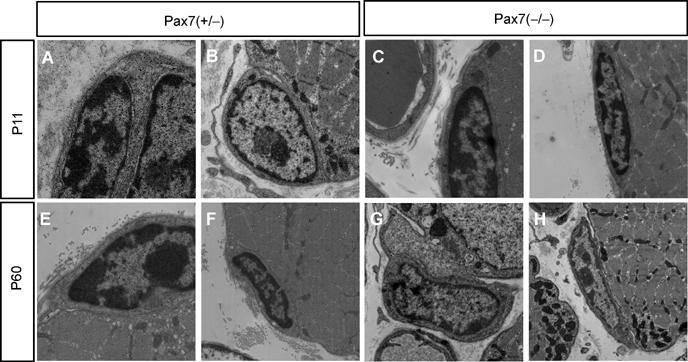
EM microphotographs of satellite cells from interossei muscles of Pax7(+/−) and Pax7(−/−) mice at different postnatal stages. Satellite cells were present in Pax7(+/−) (A, B, E, F) and Pax7(−/−) (C, D, G, H) mice both at P11 (A–D) and P60 (E–H). No morphological abnormalities were noted in satellite cells from Pax7(−/−) mice. The plasma membrane that separates satellite cells from adjacent myofibers, the basal lamina that engulfs satellite cells and myofibers and the heterochromatic state of the nuclei of satellite cells are clearly visible both in Pax7(+/−) and Pax7(−/−) muscles.
Table 1.
Severe loss of muscle satellite cells during postnatal development
| EM | CD34 | Pax7-LacZ | |
|---|---|---|---|
| Pax7(−/−), P60 | 0.57% (1045) | No specific CD34 staining of satellite cells | 0.25% (2047) |
| Pax7(+/−), P60 | 3.5% (922) | 2.44% (4024) | 2.05% (4181) |
| Pax7(−/−), P11 | 3.25% (677) | 2.0% (1169) | 6.8% (1775) |
| Pax7(+/−), P11 | 8.25% (449) | 10.5% (1957) | 4.45% (2067) |
| The number of satellite cells was estimated at P11 and P60 using either electron microscopy counting (EM), CD34 antibody staining or Pax7-lacZ staining. The number of Pax7-lacZ-positive cells in Pax7(+/−) is probably under-rated since a single Pax7-lacZ allele yields a comparatively low β-Gal activity. Numbers in parentheses indicate counted nuclei (myonuclei+satellite cells nuclei=100%). | |||
Figure 4.
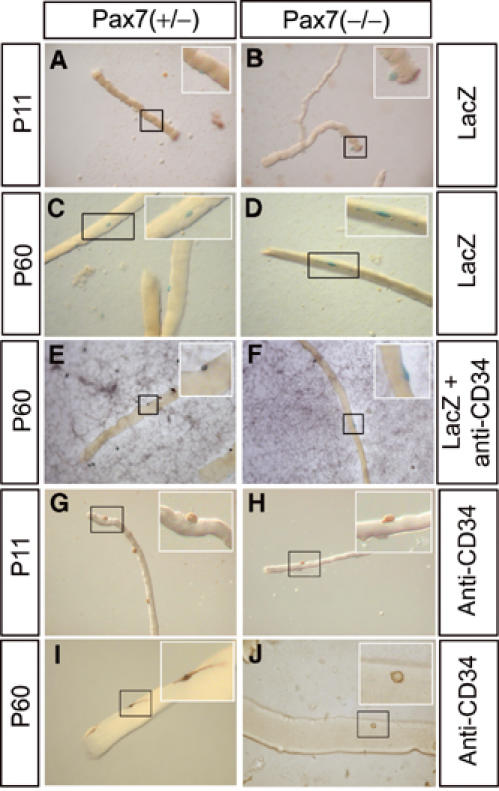
Expression of Pax7-lacZ and CD34 in satellite cells from Pax7(−/−) and Pax7(−/−) mice at different postnatal stages. Myotubes were isolated from muscles of Pax7(+/−) (A, C, E, G, I) and Pax7(−/−) (B, D, F, H, J) mice at P11 (A, B, G, H) and P60 (C–F, I, J) and stained for LacZ activity (A–D), reacted with an antibody against CD34 (G, H) or both (E, F). LacZ-positive cells on myotubes from Pax7(−/−) mice show the characteristic morphology of satellite cells (D). Note the abnormal morphology of CD34-positive cells on myotubes from Pax7(−/−) mice at P60 (J). Such cells were not counted as satellite cells. No costaining was observed for Pax7-lacZ and CD34 in Pax7(−/−) mutants at P60 (F).
In adult Pax7(−/−) mice at P60 the number of satellite cells was very low, indicating a severe loss of satellite cells during postnatal development. Based on EM counting, the number of satellite cells dropped to 0.57% from 3.25% at P11. Similarly, Pax7-lacZ staining revealed the presence of 0.25% satellite cells down from 6.8% at P11. In addition, we did not detect CD34-positive cells with typical satellite cell morphology at P60 (Figure 4J). We cannot exclude that residual CD34-positive cells present at this developmental stage were either abnormal satellite cells or contaminating mesenchymal stem cells. It should also be pointed out that even in a wild-type situation not all satellite express Pax7 and CD34 simultaneously (Morgan and Partridge, 2003). Hence, the low number of satellite cells in muscles of adult Pax7 (−/−) mice might lead to failure in detecting coexpression of both genes in these cells. In any case, the remaining CD34-positive cells of adult Pax7(−/−) mice were clearly discernible from CD34- and Pax7-lacZ-positive satellite cells, which could be easily identified in young Pax7(−/−) mice or in Pax7(+/−) mice (Supplementary Figures IA and 4E). In contrast to the reduced number of satellite cells, we did not detect a reduction of mesenchymal Sca-1+ cells, which adhere to isolated myotubes either in Pax7(+/−) or in Pax7(−/−) mice irrespective of their age (Supplementary Figure IIIB).
Reduced number, impaired maintenance but presumably normal differentiation of satellite cell-derived myoblasts from juvenile and adult Pax7 mutant mice
To further prove the presence of satellite cells in muscles of Pax7(−/−) mice at various stages of postnatal development and to evaluate the differentiation capability of Pax7(−/−) satellite cells in vitro, we cultured primary satellite cells from myofibers of P8 and P60 Pax7(+/−) and Pax7(−/−) mice (Figure 5A). We readily identified satellite cells in cultures of Pax7(+/−) and Pax7(−/−) mice based on Pax7-lacZ staining (data not shown). In addition, these cells expressed the myogenic bHLH gene MyoD, which is characteristic of activated satellite cells (Figure 5A). The absolute number of satellite cell-derived MyoD-positive myoblasts was significantly lower when myotubes from Pax7(−/−) mice were used, although at P8 in some areas of the plates similar densities of satellite cell-derived MyoD myoblasts were found (Figure 5Af). This finding probably reflected the reduced number of satellite cells at this developmental stage in Pax7(−/−) mice, which resulted in the absence of satellite cells on some myotubes. At P60, the number of satellite cell-derived MyoD-positive myoblasts further declined paralleling the decrease of satellite cells observed in vivo. The reduced number of satellite cell-derived myoblasts from Pax7(−/−) mice closely matched the reduced formation of myosin heavy chain (MyHC)-expressing myotubes in vitro at P8 and P60, which might indicate that the ability of Pax7(−/−) myoblasts to differentiate was not reduced. Figure 5Ab and d shows representative examples of differentiated myotubes from Pax7(−/−) myoblasts.
Figure 5.
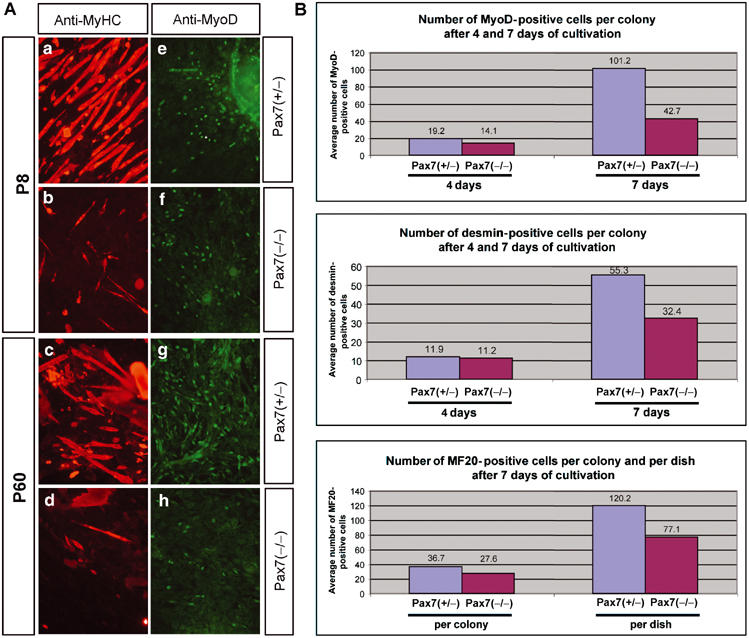
(A) Expression of MyoD and differentiation of satellite cell-derived myoblasts from Pax7(−/−) and Pax7(−/−) mice at different postnatal stages. Primary satellite cell cultures were obtained from isolated myotubes of Pax7(+/−) (a, c, e, g) and Pax7(−/−) (b, d, f, h) mutant mice at P8 (a, b, e, f) and P60 (c, d, g, h) and cultured for 6 days. Cells were reacted with antibodies against MyHC (a–d) and MyoD (e–h). The number of satellite cell-derived MyoD-positive myoblasts was significantly lower in Pax7(−/−) cultures (f, h) when compared to Pax7(+/−) controls (e, g) giving rise to fewer MyHC-positive myotubes (b, d). (B) Clonal analysis of satellite cell-derived myoblasts from pooled hind limb muscles of P11 Pax7(+/−) and Pax7(−/−) mice. After 4 days of culture, the average number of MyoD- (upper panel) and desmin- (middle panel) positive cells per colony was not dramatically decreased in cultures derived from Pax7(−/−) mice. After 7 days, a considerable decline in the average number of MyoD- and desmin-positive cells per colony was apparent in Pax7(−/−) cultures. Similarly, the average number of differentiated, MF20-positive myocytes (lower panel) at day 7 derived from Pax7(−/−) mice was considerably lower per single colony and per dish compared to Pax7(+/−) cultures.
To further access the specification and the proliferation potential of satellite cells lacking Pax7, we plated equal numbers of satellite cells derived from pooled hind limb muscles of P11 Pax7(+/−) and Pax7(−/−) mice at clonal densities and analyzed the expression of several markers over a time course of proliferation and differentiation. As shown in Figure 5B (see also Supplementary Figure IV), the number of MyoD- and desmin-positive cells derived from Pax7(−/−) mice was not dramatically decreased after 4 days in culture when compared to heterozygous mice. However, continued cultivation revealed that the number of MyoD- and desmin-positive cells per colony was significantly reduced in Pax7 (−/−) cultures after 7 days (Figure 5B). Likewise, the number of differentiated myocytes at day 7, as indicated by MyHC staining, was considerably lower in Pax7(−/−) colonies. In addition to the mere reduction of differentiated myocytes, Pax7(−/−) myotubes were smaller and the MyHC staining intensity was lower (Supplementary Figure IVH). Taken together, our results strongly suggest that Pax7 is not required for initial proliferation of satellite cells but for their maintenance and the extended proliferation potential of satellite cells.
The severe reduction of satellite cells in adult Pax7 mutant mice does not seem to initiate compensatory or alternate muscle repair mechanisms
To further characterize the musculature of adult Pax7(−/−) mice at the molecular level, we performed Northern blot analyses using various muscle-specific probes. As shown in Figure 6A, the expression of most muscle-specific genes that we studied was slightly and unspecifically downregulated. More interestingly, we found a clear decrease of the expression of myogenic determination genes Myf5 and MyoD by quantitative RT—PCR, while the expression of the differentiation genes Myogenin and MRF4 (Myf6) increased to some extent (Figure 6B). Since Myf5 and MyoD are expressed in satellite cells and myoblasts derived thereof, respectively, the downregulation of Myf5 and MyoD suggests a postnatal decline of myoblasts in Pax7(−/−) mice. In addition, it illustrated that a major contribution of nonsatellite muscle stem cells to postnatal muscle growth seems unlikely because the determination and differentiation of all cells would also depend on Myf5 and MyoD. It should be mentioned, however, that our RT–PCR data represent the average expression in all cells present in skeletal muscle and hence cannot be used as a precise marker for distinct cell populations.
Figure 6.
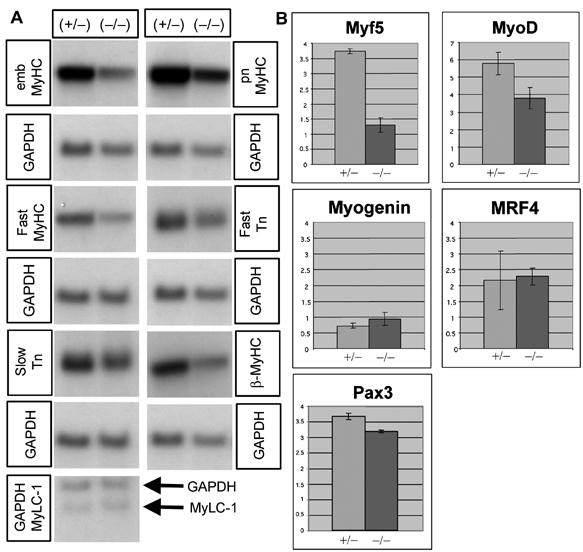
Reduced expression of Myf5 and MyoD but normal expression of various skeletal muscle markers and of Pax3 in Pax7(−/−) mice. (A) Northern blot analysis of various muscle-specific mRNAs isolated from adult (P60) Pax7(+/−) and Pax7(−/−) mice. (B) Quantitative real-time RT–PCR of Myf5, MyoD, Myogenin, MRF4 and Pax3 mRNAs from muscles of adult (P60) Pax7(+/−) and Pax7(−/−) mice. Values on the Y-axis represent the ratio between the SQ of the mRNA of interest and the SQ of GAPDH. Due to different amplification efficiencies, it is not possible to compare between different mRNAs. Note the significant reduction of the expression of Myf5 and MyoD but not Pax3 in mutant animals.
Since it has been shown that the function of Pax7 partially overlaps with the paralogous Pax3 gene during development, we also analyzed the expression of Pax3 gene using quantitative RT–PCR. Surprisingly, we did not find a compensatory increase of Pax3 expression but a minor reduction compared to Pax7(+/−) animals, which argues against a decisive compensatory function of Pax3 in Pax7(−/−) mutant mice. The low quality of available Pax3 antibodies prevented a specific detection of Pax3 in the remaining satellite cells by immunofluorescence (data not shown).
Impaired muscle regeneration in adult Pax7 mutant mice
To determine the regenerative potential of Pax7-deficient skeletal muscle in response to acute damage, we induced regeneration by freeze-crush injury or by injection of cardiotoxin into the M. tibialis anterior. Histological analysis of Pax7(−/−) mice 10 days after injury revealed a high number of mononuclear cells in the muscles, differences in caliber size, and numerous necrotic fibers, while muscles of Pax7(+/−) mice in the regenerating area were characterized by a high content of myotubes with centrally located nuclei indicating successful regeneration (Figure 7C and D). However, we also found numerous regenerating myofibers in Pax7(−/−) mice, indicating that the low number of remaining satellite cells in mice lacking Pax7 was sufficient to mediate an albeit comprised regenerative response.
Figure 7.
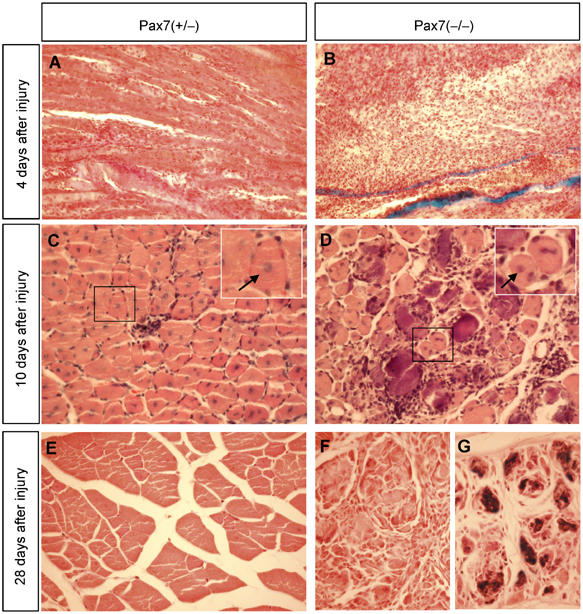
Impaired skeletal muscle regeneration of adult Pax7(−/−) mice. Paraffin sections from Pax7(−/−) (A, C, E) and Pax7(+/−) (B, D, F, G) were stained with hematoxylin and eosin 4 days (A, B), 10 days (C, D) and 28 days (E–G) following cardiotoxin injection into the M. tibialis anterior. Note the efficient muscle regeneration in Pax7(+/−) mice as indicated by the presence of centrally located nuclei in virtually all myotubes (C). By contrast, Pax7(−/−) lesions contain myotubes of different caliber size and numerous necrotic fibers (D). At 1 month after injury, the tissue architecture of damaged muscles is virtually restored in Pax7(+/−) mice while Pax7(−/−) mutant muscles still contain numerous necrotic fibers (G) and show a disarranged tissue morphology (F).
At 4 weeks after injury, the lesion was no longer detectable in heterozygous mice (Figure 7E). In Pax7(−/−) mutants, the histological appearance was heterogeneous. Some regions showed a comparatively good regeneration, while in other areas disarranged myotubes and hyaline deposits characteristic of necrotic material were evident (Figure 7F and G). Taken together, our data clearly show a severe but not overwhelming regeneration deficit in Pax7(−/−) mice, which parallels the postnatal decline of satellite cells. The remaining Pax7-deficient satellite cells, however, seem to be capable of expanding and completing a partial regeneration of damaged skeletal muscles.
Discussion
Our analysis of satellite cells in Pax7(−/−) mice has unambiguously established that Pax7 is dispensable for the specification of the satellite cell lineage since we detected a large number of satellite cells in juvenile Pax7(−/−) mutant mice using five different methods (EM, Pax7-lacZ staining, CD34 staining, in vitro cultivation of satellite cells from isolated myofibers and clonal satellite cell analysis). However, we detected a continuous decrease in the number of satellite cells in Pax7(−/−) mutants, which strongly suggests a role of Pax7 in the renewal and propagation of satellite cells. In addition, we found an essentially normal degree of muscle formation in adult Pax7 mutant animals, which cannot be explained without the contribution of the highly proliferative satellite cell population to the growth of immature muscles (reviewed in Bischoff, 1994; Morgan and Partridge, 2003). Moreover, adult Pax7(−/−) mice still possess a certain potential for skeletal muscle regeneration, although the efficiency of regeneration is severely hampered. The compromised regenerative response of Pax7(−/−) mice came along with an expansion of the remaining Pax7-lacZ satellite cells and resulted in numerous regenerated muscle fibers, although faulty regeneration was evident and a complete repair was never achieved.
Satellite cells give rise to both myoblasts, which proliferate extensively and repair damaged myofibers, and new satellite cells, which replenish the stem cell pool (Hawke and Garry, 2001). Although it has been suggested that these functions might be attributed to different subsets of satellite cells (Morgan and Partridge, 2003), it is not unlikely that satellite cells possess enough plasticity to respond specifically to different environmental stimuli thereby enabling them to fulfill different tasks. The continuous decline of satellite cells in Pax7 mutant mice makes Pax7 an attractive candidate to control renewal of the satellite cell pool. The expression of Pax7 in the majority if not all satellite cells and the enhanced expression of Pax7 in regenerating muscle tissue do not necessarily exclude such a concept. The presence of Pax7 in activated satellite cells might permit a subset of these cells either by cell autonomous decisions or by local signaling events to ‘fall back' and refill the original stem cell pool. Similar examples of transcription factors that direct expansion and propagation of cell lineages without specifying them include GATA-2, which is required for proliferation/survival of early hematopoietic cells, but not for erythroid and myeloid terminal differentiation (Tsai and Orkin, 1997), and SOX10, which maintains multipotency and inhibits neuronal differentiation of neural crest stem cells (Kim et al, 2003).
The minor reduction of MyoD- and desmin-positive cells in satellite cell-derived colonies of Pax7(−/−) after 4 days of cultivation seems to exclude a simple proliferation defect of satellite cells. Similarly, the ratio between satellite cells and myoblasts derived thereof was not changed drastically in myotube cultures grown in matrigel, although the total number of satellite cells was severely decreased in adult Pax7 mutant mice. Instead, continued cultivation of equal numbers of satellite cells from P11 Pax7(+/−) and Pax7 (−/−) mice at clonal densities revealed a decline in the number of satellite-derived myoblasts isolated from Pax7(−/−) mutant mice, although the initial amplification of these cells occurred normally. Similarly, in regenerating skeletal muscles of adult homozygous Pax7 mutant mice, Pax7-lacZ cells expanded noticeably suggesting a rather normal response of Pax7 mutant cells to proliferative stimuli. Hence, Pax7 might be required neither for the specification nor the initial proliferation of satellite cells but for their maintenance and extended proliferation.
Our hypothesis that Pax7 plays a major role in the renewal and propagation of satellite cells but not their specification is in line with previous reports that Pax3 and possibly also Pax7 maintain cells in a deregulated, undifferentiated and proliferative state (Mansouri, 1998). Murine Pax genes have been shown to promote oncogenesis in tissue culture cells and in mice (Maulbecker and Gruss, 1993), and PAX3/7-FKHR fusion genes are present in primary rhabdomyosarcoma tumors where they might inappropriately activate transcription of genes with PAX DNA-binding sites and thereby induce tumorigenic behavior (Barr, 1999). It is tempting to speculate that the role of Pax genes in the pathogenesis of primary muscle tumors represents an exaggerated reflection of the normal function of Pax genes to keep muscle cells in a proliferative state that enables maintenance of the muscle stem cell pool.
The strong reduction of satellite cells in Pax7 mutant mice during postnatal development represents an interesting model to access the contribution of satellite cells and the hypothesized alternative muscle stem cells for skeletal muscle regeneration. Interestingly, we found a clear correlation of the decreased number of satellite cells with impaired muscle regeneration in Pax7 mutant mice. In our view, this argues against a significant contribution of an alternative muscle regeneration pathway based on adult stem cells. Although it cannot be excluded that Pax7 might also play a role in adult stem cell-mediated muscle regeneration, this possibility does not seem plausible for several reasons: (i) the formation of muscle satellite cells and differentiation of muscle precursor cells were not dependent on Pax7, hence a putative alternate stem cell population would probably also require Pax7 for propagation and renewal of committed stem cells but not their specification. However, no clear evidence for the presence of nonsatellite stem cells responsible for muscle repair was found in Pax7 mutants. (ii) We detected a clear reduction in the expression of Myf5 and MyoD in Pax7 mutant muscles concomitant with the reduction of satellite cells. Since both genes are required for muscle cell determination, a putative alternative adult stem cell population would also require an upregulation of either of these genes, which was not the case. (iii) The relative increase in the number of Pax7-lacZ cells in regenerating muscle of homozygous Pax7 mutant mice compared to heterozygous Pax7-lacZ mice correlated with the number of resting Pax7-lacZ cells, which remained in homozygous Pax7 mutants and not with a proposed nonsatellite muscle stem cell population.
In the current study, we did observe a virtually unchanged expression of the Pax3 gene in postnatal muscles of Pax7 mutant mice, although the paralogous Pax3 gene shows an overlap with the functions of Pax7 during somitic development. Together with the continuous elimination of satellite cells during postnatal life, this finding makes an important postnatal compensation of Pax7 by Pax3 less likely, although this conclusion has to be confirmed by combined postnatal knockouts of both genes.
Previously, Pax7(−/−) mice have been described to be completely devoid of satellite cells probably due to the inability of mutant animals to specify the satellite cell lineage (Seale et al, 2000). These findings are difficult to explain in the light of our current investigations in particular since the same mutant strain on the same genetic background has been used for the investigation. However, some differences in the analyses are evident and should be pointed out: (i) We have analyzed different muscle groups from several different animals, whereas Seale et al apparently only used muscles derived from the hind limb. (ii) In the current study, several mice between P8 and P11 were studied. Although Seale et al also analyzed mice within this range, the low number of mice analyzed and the analysis of single individuals that were not exactly age-matched might have inappropriately amplified minor differences. (iii) We have counted a large number of myonuclei (several thousands) to avoid statistical deviations and used a number of different techniques to corroborate our results. Finally, (iv) in addition to the Pax7 strain without lacZ we have utilized a Pax7-lacZ strain that allowed the easy identification of cells that normally express Pax7 (i.e. satellite cells). Taken together, these differences might culminate in substantial differences in the perception of the Pax7 phenotype and in a rather different interpretation. It should also be emphasized that we did not phenotypically select Pax7(−/−) mice prior to analysis and that all Pax7(−/−) mice showed craniofacial abnormalities and retarded growth as described originally (Mansouri et al, 1996). Only adult Pax7(−/−) mice represented a ‘selected' population of individuals since only approximately 5–10% of all Pax7(−/−) mice survived into adulthood. Nevertheless, the rather normal growth and morphological appearance of the musculature of juvenile Pax7(−/−) mice seem to exclude a selection based on malfunctions of the musculo-skeletal system. It is clear that neither the generation of satellite cells nor their ability to regenerate damaged myotubes relies strictly on Pax7 since satellite cells develop normally in juvenile Pax7 mutant mice and satellite cells derived from adult Pax7 mutant mice express MyoD and differentiate normally in culture.
It is of major importance to understand the mechanisms and pathways that govern muscle repair processes to develop applied therapeutic and clinical tools that will ultimately lead to an improved treatment of muscle disorders. In the current study, we have re-defined the function of Pax7 as a major regulator of satellite cell renewal and propagation. This knowledge will probably help us to manipulate the availability of muscle stem cells for therapeutic purposes, in particular in aged and diseased skeletal muscles.
Materials and methods
Origin of mouse mutants and induction of muscle regeneration
The generation of Pax7 and Pax7-lacZ mutant mice has been published (Mansouri et al, 1996). Mutant mice were maintained on a mixed 129Sv/C57/BL6 background and genotyped by Southern blot analysis. Induced damage of skeletal muscle using freeze-crush injury and cardiotoxin injection was performed as described previously (Floss et al, 1997; Seale et al, 2000). Identification of satellite cells by EM was performed according to routine procedures and has been outlined before (Bancroft and Stevens, 1990; Braun et al, 1992). Muscles for EM analysis were prepared from the diaphragm, Mm. interossei dorsales, Mm. intercostales and M. tibialis anterior. Tissues were prepared either for paraffin or cryostat sectioning and subsequent hematoxylin and eosin staining using established techniques (Bancroft and Stevens, 1990).
Satellite cell cultures, immunohistochemical analysis and LacZ staining
Isolation of myotubes, culture and staining of satellite cells with an antibody against CD34 were accomplished as described (Neuhaus et al, 2003). Myotube cultures were prepared from Mm. interossei dorsales and Mm. intercostales. Positive cells were counted in relation to all myotube nuclei. For Pax7 lacZ staining, myotubes or 10 μM muscle cryosections (muscles frozen without fixation) were fixed for 5 min at room temperature in 0.2% glutaraldehyde (in PBS buffer with 5 mM EGTA and 2 mM MgCl2). After washing three times for 10 min in PBS buffer containing 0.01% Na desoxycholate, 0.02% Nonidet P40, 5 mM EGTA and 2 mM MgCl2, the samples were incubated at 37° in PBS buffer containing 0.01% Na desoxycholate, 0.02% Nonidet P40, 5 mM EGTA, 2 mM MgCl2, 10 mM K3[Fe(CN)6], 10 mM K4[Fe(CN)6] and 1.5–2 mg/ml X-Gal. Histochemical stainings for Pax3 expression were attempted by using antibodies obtained from Active Motif Inc., catalog number 39335 and from Dr Charles Ordahl, UCSF, USA. For LacZ staining of tissue sections, muscles were dissected at different time points after crush injury and sectioned on a cryostat. MyoD was detected with a rabbit polyclonal anti-MyoD IgG antibody from Santa Cruz Biotech. Inc. (SC-304). MyHC was detected with the MF20 mouse monoclonal antibody. The CD34 antigen was detected with a rat anti-mouse CD34 antibody from BD Biosciences Pharmingen.
For clonal analysis of satellite cell-derived myoblasts, muscles from both hind limbs were minced using scissors and incubated for 1 h at 37°C with 10 ml of 1.25 mg/ml protease from Streptomyces griseus (Sigma, catalog number P 8811) in PBS. After incubation, muscles were triturated 50 times with a glass pipette to release satellite cells attached to muscle fibers and the digestion was stopped by adding 10–20 ml of DMEM with 20% FCS. The suspension was filtered through a 70 μm nylon cell strainer (BD Labware, catalog number 352350) to remove muscle debris. The cell suspension was concentrated after filtration by centrifugation for 5 min at 200 g and resuspended in cultivation medium (DMEM with 20% FCS, 10% horse serum, 1% chicken serum and penicillin/streptomycin) and plated on a collagen-coated 3.5 cm dish at different densities. The medium was first changed 2 days after plating followed by daily changes of the medium.
Quantitative RT–PCR and Northern blot analysis
Isolation of RNA and Northern blot analysis were performed using established procedures that have been described previously (Neuhaus et al, 2003). RT–PCR analysis was essentially carried out as described by Neuhaus et al (2003). RNA was treated with RNase free DNase and reverse transcribed using Expand Reverse Transcriptase (Roche). Quantitative RT–PCR was performed in a reaction mix containing 1 × TaqPol buffer (Eppendorf), 1 × Enhancer (Eppendorf), 1.5 mM MgCl2, deoxynucleoside triphosphates at 200 μM each, primers at 100–600 nM each, Taq polymerase at 0.05 U/μl, 1 μl of cDNA, 0.5 μl of SYBR green (at a 1:1000 dilution of original stock) and fluorescein at 10 nM. Relative quantitation of MyoD, Myf5, MRF4, Myogenin and Pax3 expression was carried out by determining the amounts of targets and of an endogenous reference (GAPDH) from the standard curve. Target values were normalized to the endogenous reference: SQtarget/SQGAPDH (SQ=starting quantity). To amplify specific mRNAs, the following primers were used: CW-MyoD IR: 5′-GGT CTG GGT TCC CTG TTC TGT GT; CW-MyoD IF: 5′-CCC CGG CGG CAG AAT GGC TAC G; CW-Myf5 IR: 5′-CGC TGG TCG CTG GAG AG; CW-Myf5 IF: 5′-GAG GGA ACA GGT GGA GAA CTA TTA; CW-MRF4 OR: 5′-ATG GAA GAA AGG CGC TGA AGA CTG; CW-MRF4 OF: 5′-CTG CGC GAA AGG AGG AGA CTA AAG; CW-myogenin OR: 5′-AGG AGG CGC TGT GGG AGT T; CW-myogenin OF: 5′-GGG CCC CTG GAA GAA AAG; Pax3 OR: 5′-GAT CCG CCT CCT CCT CTT CTC CTT; Pax3 OF: 5′-GCC AGG GCC GAG TCA ACC AG.
Supplementary Material
Supplementary Figure I
Supplementary Figure II
Supplementary Figure III
Supplementary Figure III
Acknowledgments
We are indebted to Drs Ahmed Mansouri and Peter Gruss for supplying Pax7 and Pax7-lacZ mutant mice (MPI f Biophysikalische Chemie, Göttingen, Germany) and to Dr Charles Ordahl (UCSF, USA) for providing Pax3 antibodies. We also thank Tomek Loch for help with the quantitative PCR and Ahmed Mansouri for helpful discussions. The Deutsche Forschungsgemeinschaft, the ‘Fonds der Chemischen Industrie' and the Wilhelm-Roux-Program for Research of the Martin-Luther-University supported this work. We declare that we do not have any conflicting commercial interests related to this work.
References
- Bancroft JD, Stevens A (1990) Theory and Practice of Histological Techniques. Edinburgh, New York: Churchill Livingstone [Google Scholar]
- Barr FG (1999) The role of chimeric paired box transcription factors in the pathogenesis of pediatric rhabdomysarcoma. Cancer Res 59: 1711s–1715s [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151: 1221–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R (1994) The satellite cell and muscle regeneration. In Myogenesis, Engel AG, Franszini-Armstrong C (eds) pp 97–118. New York: McGraw-Hill [Google Scholar]
- Braun T, Rudnicki MA, Arnold HH, Jaenisch R (1992) Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71: 369–382 [DOI] [PubMed] [Google Scholar]
- Camargo FD, Green R, Capetenaki Y, Jackson KA, Goodell MA (2003) Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med 9: 1520–1527 [DOI] [PubMed] [Google Scholar]
- Chalepakis G, Gruss P (1995) Identification of DNA recognition sequences for the Pax3 paired domain. Gene 162: 267–270 [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ (2000) MyoD(−/−) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol 224: 122–137 [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ (1997) Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 191: 270–283 [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F (1998) Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279: 1528–1530 [DOI] [PubMed] [Google Scholar]
- Floss T, Arnold HH, Braun T (1997) A role for FGF-6 in skeletal muscle regeneration. Genes Dev 11: 2040–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry DJ, Meeson A, Elterman J, Zhao Y, Yang P, Bassel-Duby R, Williams RS (2000) Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc Natl Acad Sci USA 97: 5416–5421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC (1999) Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401: 390–394 [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534–551 [DOI] [PubMed] [Google Scholar]
- Jackson KA, Mi T, Goodell MA (1999) Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 96: 14482–14486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostes B, Walther C, Gruss P (1990) The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech Dev 33: 27–37 [DOI] [PubMed] [Google Scholar]
- Kim J, Lo L, Dormand E, Anderson DJ (2003) SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38: 17–31 [DOI] [PubMed] [Google Scholar]
- LaBarge MA, Blau HM (2002) Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111: 589–601 [DOI] [PubMed] [Google Scholar]
- Mansouri A (1998) The role of Pax3 and Pax7 in development and cancer. Crit Rev Oncogenesis 9: 141–149 [DOI] [PubMed] [Google Scholar]
- Mansouri A, Gruss P (1998) Pax3 and Pax7 are expressed in commissural neurons and restrict ventral neuronal identity in the spinal cord. Mech Dev 78: 171–178 [DOI] [PubMed] [Google Scholar]
- Mansouri A, Stoykova A, Torres M, Gruss P (1996) Dysgenesis of cephalic neural crest derivatives in Pax7−/− mutant mice. Development 122: 831–838 [DOI] [PubMed] [Google Scholar]
- Maulbecker CC, Gruss P (1993) The oncogenic potential of Pax genes. EMBO J 12: 2361–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerich D, Braun T (2001) Activation of myogenesis by the homeobox gene Lbx1 requires cell proliferation. EMBO J 20: 7174–7183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JE, Partridge TA (2003) Muscle satellite cells. Int J Biochem Cell Biol 35: 1151–1156 [DOI] [PubMed] [Google Scholar]
- Neuhaus P, Oustanina S, Loch T, Kruger M, Bober E, Dono R, Zeller R, Braun T (2003) Reduced mobility of fibroblast growth factor (FGF)-deficient myoblasts might contribute to dystrophic changes in the musculature of FGF2/FGF6/mdx triple-mutant mice. Mol Cell Biol 23: 6037–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA (2003) Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113: 841–852 [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R (1993) MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75: 1351–1359 [DOI] [PubMed] [Google Scholar]
- Seale P, Rudnicki MA (2000) A new look at the origin, function, and ‘stem-cell' status of muscle satellite cells. Dev Biol 218: 115–124 [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786 [DOI] [PubMed] [Google Scholar]
- Tsai FY, Orkin SH (1997) Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89: 3636–3643 [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL (2002) Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297: 2256–2259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure I
Supplementary Figure II
Supplementary Figure III
Supplementary Figure III


