Abstract
Although stinging nettle (Urtica dioica) has been shown to reduce HM (heavy metal) content in soil, its wider phytoremediation potential has been neglected. Urtica dioica was cultivated in soils contaminated with HMs or polychlorinated biphenyls (PCBs). After four months, up to 33% of the less chlorinated biphenyls and 8% of HMs (Zn, Pb, Cd) had been removed. Bacteria were isolated from the plant tissue, with the endophytic bacteria Bacillus shackletonii and Streptomyces badius shown to have the most significant effect. These bacteria demonstrated not only benefits for plant growth, but also extreme tolerance to As, Zn and Pb. Despite these results, the native phytoremediation potential of nettles could be improved by biotechnologies. Transient expression was used to investigate the functionality of the most common constitutive promoter, CaMV 35S in Urtica dioica. This showed the expression of the CUP and bphC transgenes. Collectively, our findings suggest that remediation by stinging nettle could have a much wider range of applications than previously thought.
Introduction
The long-term contamination of agronomically important soil remains a problem. Some contaminants, especially heavy metals, occur naturally through processes such as the weathering of rocks. However, most are the result of human activities, such as the mining, processing and smelting of ore, and the nuclear and automotive industries. The release of contaminants disrupts the normal biogeochemical balance by their concentration in the environment. Because current remediation technologies, based on physical-chemical processes, have several limitations [1], new methods have to be found. One such remediation technology is phytoremediation.
Phytoremediation, a technology that uses plants for the removal of pollutants from the environment, is an effective, low-cost tool for the degradation of organic compounds or accumulation of heavy metals [2]. Various plant species have mechanisms for the detoxification of xenobiotic compounds, with some being tolerant to high concentrations of toxic compounds and able to hyperaccumulate up to 1% of their weight. Despite its advantages, phytoremediation has numerous drawbacks [3], including low biomass production, short plant roots and difficulties in controlling the growth of hyperaccumulators. These limitations, as well as broad plant substrate specificity, can only be overcome by the use of microorganisms.
The microbial population provides a large reservoir of detoxification genes [4], because microorganisms are evolutionarily adapted to use diverse catabolic pathways to utilize various compounds as energy sources. The transfer of microbial degradation genes to plant species has been shown to be a promising tool. Genetically modified plants bearing microbial genes have been successfully applied in the remediation of soil contaminated by polychlorinated biphenyls [5, 6], explosives [7], pesticides [8] and heavy metals [9–11], amongst other contaminants. Genetic manipulation is not the only way to benefit from the degradation capacity of microorganisms, another way is based on using bacteria with plant growth promoting abilities for the colonization of plant tissues.
Such endophytic bacteria are usually resistant to high concentrations of pollutants and promote plant growth and remediation [12]. Moreover, the cultivation of plants with increased tolerance to pollutants improves the colonization and diversity of surrounding contaminated areas, which are otherwise sparsely populated by both plants and microbes. And finally, higher diversity usually leads to higher remediation.
Phytoremediation has been tested using various plant species and their effect on both inorganic and organic pollutants. To date, around 450 heavy metal hyperaccumulating species belonging to 45 families have been identified [13]. One such reported hyperaccumulating plant is stinging nettle (Urtica dioica) [14], which is spread worldwide in mild climate regions, and its growth is associated with human activities. However thus far, no study has investigated the use of HM hyperaccumulator stinging nettle for the phytoremediation of organic compounds. In this study, we cultivate common nettles in real soils that were long-term contaminated with polychlorinated biphenyls (PCBs) and with heavy metals. Both soils originated from dumpsites. Therefore we are focused on determining the natural remediation capabilities of stinging nettle in both soils and the benefits of endophytic bacteria. We report the way how to stimulate native phytoremediation by utilizing the common procedures for the preparation of transgenic plants. Our results show that stinging nettle does not have satisfying phytoremediation abilities, however, they can be improved by agrobacterial infiltration and the expression of genes previously reported to improve phytoremediation.
Materials and Methods
Cultivation of nettles in contaminated soils
Plants of U. dioica were cultivated in pots with two types of contaminated soil. The first soil was collected from the dumpsite of a long-term PCB-contaminated soil in Lhenice, Czechia [15] (49.0°N, 14.2°E). The second soil was obtained from mining ore at Pribram (Czechia) with excessive levels of As, Cd, Pb and Zn (49.7°N, 14.0°E). No specific permissions were required for the access and sampling of the location used, nor did the field study involve endangered or protected species. 20 seeds of U. dioica were sown into each pot containing approximately 1 l of the genuine contaminated soil. In total six pots were planted for each type of soil, meaning six biological replicas. Nettles were cultivated for four months in a cultivation chamber (Adaptis, Schoeller Instruments) with the following default program: light/dark 8/16 h, 22/20°C and a relative humidity of 30%. Pots were watered three times per week with 40 ml of water. After four months of cultivation, the soils were air-dried at room temperature and passed through a 2-mm plastic sieve.
Determination of PCB decrease in contaminated soil
The soil from Lhenice was homogenized and 1 g from each replica was extracted into 5 ml of diethyl ether for 6 h while being continuously shaken. The extract was analyzed using a gas chromatograph with a micro-electron capture detector (GC/μECD, Agilent, USA) and evaluated according to Mackova et al. (2009). The residual amount of PCBs in the soil was determined using Agilent ChemStation software (Agilent, USA). The PCB content was determined in three technical replicas. Outlying values were excluded based on the Dixon test (α = 0.05). The results are presented with standard deviations. The data were analyzed using Fisher's Least Significant Difference (LSD) test at 5% probability level.
Determination of metals decrease in contaminated soil and their amounts in plants
The soil from Pribram was homogenized and 3 g from each replica was shaken overnight in 30 ml of 2M HNO3. The extract was then analyzed by flame atomic absorption spectroscopy (Spectr AA880, Varian).
The plant material from each replica was kept separate and split into parts (roots, stems, leaves), frozen with liquid nitrogen, grinded with a pestle and mortar, and lyophilized. Approximately 0.1 g of dried powder was dry ashed over the following temperature gradient for 6 h (160–220–280–350–450–500°C, each temperature was maintained for 1 h). Subsequently, the samples were wet digested by incubating each sample for 1 h at 120°C in 1 ml of concentrated HNO3. The digested samples were dry ashed at 500°C for 1 h. The samples were dissolved in 20 ml of 1.5% HNO3 for analysis by flame atomic absorption spectroscopy (Spectr AA880, Varian).
Isolation and characterization of endophytic bacteria
Approximately 0.2 g of plant tissue samples were surface-sterilized for 10 min in 0.1% sodium hypochlorite containing 0.5 ml.l-1Tween 20. The sterilized plant parts were washed three times with sterile water. From the last washing, 100 μl of water was transferred onto an agar plate with LB (Luria-Bertani) medium, which served as the control of the sterilization process after 4 weeks of cultivation. The sterile plant parts were macerated in sterile 10 mM MgSO4 and crushed with sterile plastic pestles. 100 μl of each sample was inoculated on the agar plate with ½ LB medium as well as their dilutions in the ratio 100× and 10,000×. The plates were cultivated in a 28°C chamber for two weeks. The unique molecular fingerprints of single colonies were measured by Autoflex Speed MALDI-TOF mass spectrometer using direct transfer protocol recommended by manufacturer (Bruker Daltonics). The identification was performed by MALDI Biotyper 3.1 (Bruker Daltonics) equipped with database version 4.0.0.1 containing fingerprints of 5 627 microorganisms. The genus or at least species affiliation was identified. Bacteria non-identified by MS were characterized by sequencing their 16S rRNA using the common primers 8f and 926r, and the method described by [16].
The endophytic bacteria originating from plants cultivated in soil contaminated with PCBs were inoculated into the basal mineral salt solution saturated with biphenyl as the sole source of carbon [16]. The bacteria were cultivated in a 28°C chamber for six weeks. The turbidity, corresponding to bacterial growth, was measured spectrophotometrically each week.
The endophytic bacteria originating from plants cultivated in soil contaminated with metals were inoculated on CAS agar [17] to detect the siderophore production. The plates were cultivated in a 28°C chamber for two weeks, and then the formation of zones was detected. The production of other plant growth promoting factors was tested according to published methodologies: ACC deaminase activity [18], IAA production [19], phosphate solubilization [20] and nitrogenase activity [21].
To determine the 50% inhibitory concentration (IC50), the endophytic bacteria was inoculated to the metal-containing medium to a turbidity of 0.4 McFarland units. Culture growth was monitored with the automated growth curve analysis system Bioscreen C (Growth Curves USA) and the data were adapted to the formula Tc = T0/(1 + exp(c − IC50)/b)), where Tc is the culture turbidity at given metal concentration c, T0 is the turbidity of the culture with no added metal and b is the slope of the sigmoidal dose-dependent curve [22].
Transient expression of genes under the control of CaMV 35S promoter in nettles
The nettle seeds were planted in the soil. When the seedlings were 10 cm high (after 6 weeks), the Agrobacterium was infiltrated into the bottom part of their leaves using a plastic syringe. The bacteria inoculum was prepared according to [23]. The preparation of the strains of Agrobacterium used for transient expression has been previously published. Briefly, the Agrobacterium strain bearing the transgene bphC under the control of the constitutive promoter of Cauliflower mosaic virus incorporated into the vector pPCV provides bacteria and plants with ampicillin and hygromycin resistance [24]. The second strain of Agrobacterium, with the transgene CUP incorporated into the vector pGreen, carried the kanamycin resistance gene in its transferred DNA [25], and provides bacterial cells with both kanamycin and tetracycline resistance. The transgene bphC encodes a 2,3-dihydroxybiphenyl 1,2-dioxygenase, which is responsible for the cleavage of an aromatic ring of PCB, and the CUP gene encodes metallothionein with a high affinity for heavy metals.
Both transgenes (bphC and CUP) were under the control of the same typical plant promoter. The concentrations of antibiotics used were 5 mg.l-1 for tetracycline, 100 mg.l-1 for kanamycin and ampicillin, and finally 15 mg.l-1 for hygromycin.
48 hours after agrobacterial infiltration, the leaves were cut and frozen until the RNA was isolated (RNeasy Plant Kit, Qiagen), purified (DNase, NEB; Murine RNase inhibitor, NEB) and finally trancripted to the complementary DNA (ProtoScript AMV First Strand cDNA Synthesis Kit, NEB). The presence of mRNA responding to the transgenes was confirmed by PCR using cDNA as the template and specific primers for the gene bphC (F: ATGAGCATCAAGAGCTTGGGTTAT, R: TCACGAATTCCTTCGCACCGACTT) and CUP (F: CATCATGGTATGGCTAGCATGACTGG, R: TCATTTCCCAGAGCAGCATGACTTC). KOD Hot Start DNA polymerase (Novagen) was used for the amplification of both transgenes.
Results and Discussion
To date, nettles have not been randomly used for the remediation of contaminated soils, even if the literature describes their properties to be suitable for these techniques [26]. The main benefit of this plant species is its simplicity in terms of nutrition requirements, moreover, it is a weed species that spreads wildly around roads, canals and human habitations.
The source of the results described below is a cultivation experiment of nettles in two different types of soil contaminated by human activities. The first was polluted with polychlorinated biphenyls, the second with heavy metals. The phytoremediation potential of Urtica dioica was determined for both organic and inorganic types of pollutants.
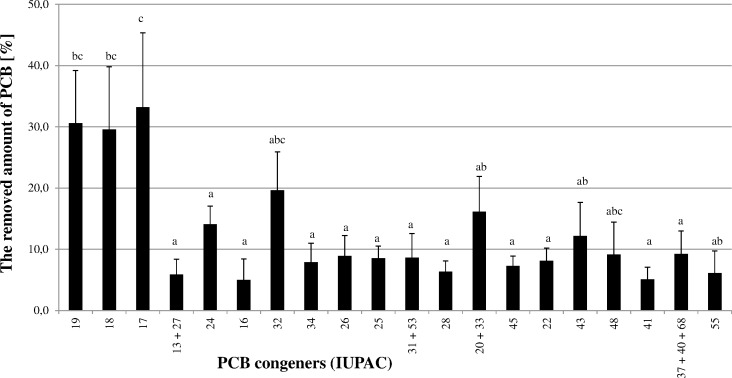
The potential of nettle phytoremediation for removing organic compounds has never been tested. This is the first report focused on the remediation of PCB by nettles (Fig 1). The metabolism of different congeners is strongly dependent on plant species, because the molecular configuration plays an important role in PCB metabolism [27]. According to our results, nettles are only able to remediate less chlorinated biphenyls. This is not very surprising, because it was previously shown that the lower chlorination grade is associated with higher metabolism rates [28]. A decrease of up to 33% was determined for trichlorinated biphenyls (congeners 13–39). Up to 12% of tetrachlorinated biphenyls (congeners 40–81) were removed. Other chlorinated biphenyls were hardly removed at all. For pentachlorinated biphenyls, up to 2.4 ± 0.8% (congener 84, 87 and 101) 1.3 ± 0.8% (congener 95, 99 and 110) were removed. Congeners 136, 147 and 134 were the only hexachlorinated biphenyls to be removed (up to 2.7 ± 1.7%). No hepta-, octo or nonachlorinated biphenyls were removed from the planted soil compared to the non-planted soil. Surprisingly, each of the above-mentioned congeners is chlorinated at both the 2 and 2´ positions. The most removed hexachlorinated conger (136) is substituted in each ortho position, i.e. positions 2, 2´, 6 and 6´. Similarly, congener 19 (2,2´,6) is one of the best removed congeners, however other congeners substituted in this way (congeners 54, 96 and 104) were not present in the soil. These highly ortho-substituted PCB congeners evidently play a role in the toxicities elicited by the PCB mixture by decreasing the dopamine content in the brain [29]. Therefore, even though their overall remediation is not very high, the remediation that they do perform is highly important.
Fig 1. Decrease in PCB congeners caused by planting nettles in the contaminated soil from Lhenice.
Standard errors are expressed as a standard deviation depending on the square root of the number of replicas [56]. The decrease in individual congeners was determined as the variance between the congener amount in the planted soil and non-planted soil (expressed in %, relative to 1 g of nettles). The data were analyzed using Fisher's Least Significant Difference (LSD) test at 5% probability level.
Even if the decrease of PCBs amount in soil is evident, the impact of nettle for removal is still unclear. Usually, the phytoremediation of organic pollutants could be conducted i) in the rhizosphere by soil bacteria whose degradative genes are induced by plant secondary metabolites secreted from plant roots or ii) after the translocation of pollutant into the plant tissue by plant enzymes [30]. To the best of our knowledge, this is to the first report focused on the removal of an organic pollutant in soil by nettle and so far no study focused on rhizodeposition of nettle’s secondary metabolites. Therefore, there is no study available for comparison of stimulation of rhizoremediation by nettle’s released secondary metabolites with many publications focused on the rhizoremediation facilitated by secondary metabolites from numerous plants (e.g. review of [31]). On the other hand, the presence of typical enzymes responsible for detoxification in plant tissue has been several times published in nettles. For example, the transformation of the initial substrates includes enzymatically catalyzed reactions involving enzymes such as P450 monooxygenases, peroxidases, reductases, dehydrogenases and esterases. The presence of peroxidase, polyphenol oxidase and catalase in nettles and their effect on catechol transformation has been previously described [32]. Another research group [33] was focused on glutathione and its related enzymes as a glutathione reductase, glutathione transferase and glutathione peroxidase clarifying the effect of these enzymes on conjugation and detoxification of xenobiotics in nettles. Although the exact mechanism of PCBs detoxification by nettles is still unknown, it is evident, that many general mechanisms for PCBs removal from soil by plants are valid for nettles as well.
The phytoremediation potential of nettles was evaluated according to translocation factor (TF, ratio of heavy metals concentration in shoot and root) [34], biological concentration factor (BCF, ratio of heavy metals concentration in root and soil) [35] and finally, according to biological accumulation factor (BAF, ratio of heavy metals concentration in shoot and soil) [35]. Plant species with a value higher than 1 are regarded as efficient in phytoextraction, phytostabilization and/or phytoaccumution. Based on our results, nettles belong in the group of zinc hyperaccumulating plant species. Zinc is an essential element in many plant proteins and enzymes. Plants have evolved the ability to accumulate and preserve considerable amounts of zinc inside cell vacuoles. Currently, 14 taxa of zinc hyperaccumulating plant species are known, including the best studied Arabidopsis halleri [36]. On the other hand, a completely different situation was described for lead, which was mostly uptaken into the root tissues and was hardly translocated to the green plant parts at all. While the TF of zinc was 1.1 ± 0.1, the TF of lead was 0.4 ± 0.1 (Table 1). A similar trend was observed by other research groups [37, 38], who explain it as an antagonistic effect of zinc on the uptake of lead. [38] also reported the same antagonistic effect of zinc on the uptake of cadmium, whose concentration in plants was practically undetectable in our experiment, in contrast to the high concentration of zinc.
Table 1. Amount of acid-extractable metals in nettles cultivated for four months in contaminated soil.
| Heavy metals concentration in nettle (mg/kg dw) | Phytoremediation potential | |||||
|---|---|---|---|---|---|---|
| Root | Shoot | Leaf | TF | BCF | BAF | |
| Pb | 108.9 ±6.5 | 43.3 ± 4.0 | 5.3 ± 0.6 | 0.4 ±0.1 | 0.3 ± 0.0 | 0.1 ± 0.0 |
| Zn | 85.1 ± 5.3 | 95.0 ± 2.6 | 46.5 ± 0.7 | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 |
The phytoremediation potential is presented as a translocation factor (TF), biological concentration factor (BCF) and biological accumulation factor (BAF). The results are presented with standard errors.
The decrease in heavy metals in soil was 4.9±0.2, 5.3±0.4 and 19.4±0.8% for lead, cadmium and zinc, respectively (Table 2). To date, phytoremediation by nettles was reported for several metals: As, Cd, Cu, Pb, Zn, Hg, Cu, Cr [26, 39–41] and bioindicating capacity for F, Zn, Fe, Mn [42, 43]. In comparison to our study, Boshoff et al. (2014) found a higher metal content in nettle (Pb = 130 mg.kg-1, Zn = 475 mg.kg-1). However, the plant accumulation of metals highly depends on the concentration of metals in the soil, and the above-mentioned publication used soil contaminated up to 4 times or 60 times (Pb and Zn, respectively) as much as our soil. Grubor et al. (2008) found a 36% decrease in lead in soil after 3 weeks of nettle cultivation, which is almost incomparable with our 5% decrease after four months. However, their soil was 5 times as contaminated with lead as our soil (Table 2). Arsenic was undetectable in nettles in our study with soil containing almost 10 mg.kg-1 of As. Boshoff et al. (2014) found 11 mg.kg-1 of As in nettles, however their soil was 14 times more contaminated with As than our soil. Therefore, our lower accumulation corresponds to the soil used, which originated from silver and uranium mining.
Table 2. The results of soil analysis in terms of heavy metals before and after four-month nettle cultivation.
| (mg/kg dw) | Pb | Cd | Zn | As |
|---|---|---|---|---|
| original soil | 354.1 ± 10.6 | 1.9 ± 0.1 | 96.7 ± 2.9 | 9.9 ± 0.3 |
| vegetated soil | 336.8 ± 0.6 | 1.8 ± 0.0 | 77.9 ± 1.0 | 10.4 ± 0.1 |
The amount of arsenic was determined to be about 11% higher after the cultivation period. The reason for this arsenic content increase could lie in the fact that only acid-extractable metals are determined. To improve our understanding we have to note that a certain amount of metals is always adsorbed to particulates, and is less likely to enter the solution for analysis. Because the growing plant produces a range of secondary metabolites at its roots, soil properties are usually changed as well as the amount of extractable and bioavailable metals.
The potential of endophytic bacteria to enhance phytoremediation has been previously reported [44]. Therefore, our efforts to support phytoremediation began with studying endophytic bacteria and their contribution to plant health and tolerance to toxic compounds. The same group of bacterial genera was isolated from both types of nettles cultivated in different contaminated soil: Arthrobacter sp., Bacillus sp. and B. pumilus. Both bacterial genera were previously identified as endophytes in other plant species, e.g. Alyssum bertolonii [45], cotton and corn [46]. Moreover, both types of nettles contained some specific bacteria (Fig 2). Both groups of endophytes were cultivated under selective conditions to find bacteria degrading PCB or producing siderophores for HM accumulation. None of the isolated endophytic bacteria were able to grow in the presence of biphenyl as the sole source of carbon; on the other hand five endophytes were able to create large zones in CAS agar. These endophytes were studied for the production of plant growth promoting (PGP) factors, and B. shackletonii and Streptomyces badius were determined to be the most beneficial for plant growth. S. badius was found previously in Asteraceae plants [47], however, its PGP have been never tested. The only bacterium to have been previously reported to be an endophyte producing PGP factors is B. pumilus. Its production of siderophores and inability to dissolve phosphates has been confirmed by several authors [48, 49]. B. shackletonii was found to be the most resistant bacteria to the presence of heavy metals in the growth medium. This rather novel species was isolated in 2004 from a volcanic soil [50] which is usually rich in heavy metals, like our soil from the mine. Our measured values for IC50 (Table 3) correspond to those determined for other HM-resistant bacterial strains [22, 51]. The As-resistance genes were characterized in Bacillus species [52] and other mechanisms of resistance were summarized in a comprehensive review [53].
Fig 2. Endophytic bacteria isolated from nettles.
Nettles growed in soil contaminated with heavy metals (left) or with polychlorinated biphenyls (right).
Table 3. The plant growth promoting factors (PGP factors) of isolated endophytes producing a high amount of siderophores.
| Name | Identification score | PGP factors | IC50 (μM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | As | Cd | Zn | Pb | ||
| Rhizobium mesosinicum | 0.981f | Y | N | Y | N | Y | 628 ±125 | 178±7 | 5 463±1 352 | 115±7 |
| Bacillus shackletonii | 2.189g | Y | Y | Y | Y | Y | 6 851±367 | 176±150 | ˃6000 | >200 |
| Streptomyces badius | 1.801g | Y | Y | Y | Y | Y | 1 556±11 | 89 ±27 | ˃6000 | >200 |
| Arthrobacter rusicus | 0.994f | Y | N | Y | N | Y | 3 977±148 | 35±12 | ˃6000 | >200 |
| Bacillus pumilus | 2.085g | Y | N | Y | N | N | >6000 | 13±1 | 3 946±996 | >200 |
The inhibition concentrations of metals, killing 50% of the population, are presented with standard deviation.
a Siderophores production
b IAA production
c ACC-deaminase activity
d Phosphate solubilisation
e Nitrogenase activity
f MS identification score. Bacteria was identified using MALDI-TOF mass spectrometer.
g 16S rRNA similarity. Bacteria was identified using 8F and 1026R primers for sequencing of 16S rRNA.
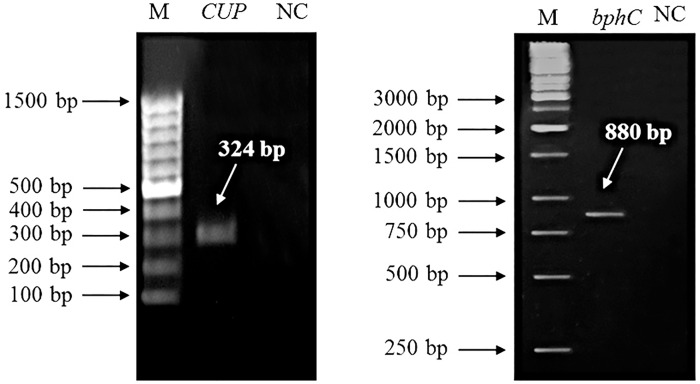
Therefore in summary, endophytic bacteria should improve the resistance of plants to heavy metals by their detoxification mechanisms. Moreover, they play an important role in plant growth promotion, because the endophytic bacteria produce significant small molecules or enzymes, e.g. plant hormones or enzymes providing plant micro- and macronutrients. Therefore, the benefit of PGP by endophytic bacteria should manifest itself in a higher biodiversity of contaminated areas. On the other hand, genetic engineering methods have demonstrated their benefits in phytoremediation [2]. Therefore, this aspect of improving the native phytoremediation potential of nettles could not be omitted. Transgenic nettles have never been prepared before, and a lot of experiments will need to be done before a permanent transgenic nettle can be obtained. To the best of our knowledge, our report is the first to focus on recombinant expression in nettle tissues; therefore we designed our experiment as a transient expression of transgenes in the leaves of this species. The constructs for agrobacterial infiltration to the plant were prepared previously. Both transgenes (bphC and CUP), playing a significant role in bacterial remediation, were cloned under the control of the strong constitutive promoter of cauliflower mosaic virus (CaMV 35S). Even though the promoter is frequently used for the preparation of transgenic plants, its functionality has to be first verified in each new species, because the CaMV promoter in transgenic DNA differs significantly from the plant´s own promoters and integrated viruses [54]. We performed the transient expression of both remediation genes. The bacterial inoculum was infiltrated into the bottom part of leaf with a syringe, as is commonly done with the plant model species tobacco [55]. The expression of both transgenes was verified at the mRNA level after the isolation and purification of RNA and reverse transcription to DNA by PCR using non-infiltrated nettle plants as a negative control (Fig 3). As was shown, Agrobacterium is able to attack the plant cells of nettles and incorporate part of its DNA to the plant genome, and plant polymerases are able to recognize CaMV 35S promoter and transcribe the transgene into the mRNA. Therefore, a lot of previously published vectors could be universally used for the agrobacterial transformation of nettles, as well as our constructs bearing the bphC or CUP gene, to improve their phytoremediation.
Fig 3. Detection of transgene expression at mRNA level in transiently transformed nettles.
The transgenes used, CUP (left) and bphC (right), originate from microorganisms, where they play an important role in remediation and tolerance to pollutants.
Conclusions
This paper is the first to take an interest in using nettles for the phytoremediation of not only heavy metals, but also organic compounds. The potential of nettles to remove heavy metals from soil was previously published and confirmed by our research. However, it was demonstrated here that nettles are able to remove up to more than 30 percent of mono- di- and tri-chlorinated biphenyls. Moreover, their endophytic bacteria and their benefits to plant growth and resistance are presented here, as well as the potential of genetic engineering for improving the native phytoremediation of nettles.
Acknowledgments
This work was supported by Technology Agency of the Czech Republic of project No. TA03011184 and by Grant Agency of the Czech Republic of project No. 15-22276S. The authors thank Ben Watson-Jones, MEng., for providing language corrections.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Technology Agency of the Czech Republic of project No. TA03011184 and by Grant Agency of the Czech Republic of project No. 15-22276S.
References
- 1.Robinson T, McMullan G, Marchant R, Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technology. 2001;77(3):247–55. [DOI] [PubMed] [Google Scholar]
- 2.Macek T, Kotrba P, Svatos A, Novakova M, Demnerova K, Mackova M. Novel roles for genetically modified plants in environmental protection. Trends in Biotechnology. 2008;26(3):146–52. 10.1016/j.tibtech.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 3.Sheoran V, Sheoran AS, Poonia P. Phytomining: A review. Minerals Engineering. 2009;22(12):1007–19. [Google Scholar]
- 4.Uhlik O, Wald J, Strejcek M, Musilova L, Ridl J, Hroudova M, et al. Identification of Bacteria Utilizing Biphenyl, Benzoate, and Naphthalene in Long-Term Contaminated Soil. Plos One. 2012;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aken BV, Correa PA, Schnoor JL. Phytoremediation of polychlorinated biphenyls: new trends and promises. Environmental science & technology. 2010;44(8):2767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viktorova J, Novakova M, Trbolova L, Vrchotova B, Lovecka P, Mackova M, et al. Characterization of transgenic tobacco plants containing bacterial bphc gene and study of their phytoremediation ability. International Journal of Phytoremediation. 2014;16(9):937–46. [DOI] [PubMed] [Google Scholar]
- 7.Panz K, Miksch K. Phytoremediation of explosives (TNT, RDX, HMX) by wild-type and transgenic plants. Journal of Environmental Management. 2012;113:85–92. 10.1016/j.jenvman.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 8.Hussain S, Siddique T, Arshad M, Saleem M. Bioremediation and Phytoremediation of Pesticides: Recent Advances. Critical Reviews in Environmental Science and Technology. 2009;39(10):843–907. [Google Scholar]
- 9.Chaney RL, Malik M, Li YM, Brown SL, Brewer EP, Angle JS, et al. Phytoremediation of soil metals. Current Opinion in Biotechnology. 1997;8(3):279–84. [DOI] [PubMed] [Google Scholar]
- 10.Macek T, Macková M, Pavlíková D, Száková J, Truksa M, Singh Cundy A, et al. Accumulation of Cadmium by Transgenic Tobacco. Acta Biotechnologica. 2002;22(1–2):101–6. [Google Scholar]
- 11.Pavlikova D, Macek T, Mackova M, Sura M, Szakova J, Tlustos P. The evaluation of cadmium, zinc and nickel accumulation ability of transgenic tobacco bearing different transgenes. Plant Soil and Environment. 2004;50(12):513–7. [Google Scholar]
- 12.Chebotar VK, Malfanova NV, Shcherbakov AV, Ahtemova GA, Borisov AY, Lugtenberg B, et al. Endophytic Bacteria in Microbial Preparations that Improve Plant Development (Review). Applied Biochemistry and Microbiology. 2015;51(3):271–7. [DOI] [PubMed] [Google Scholar]
- 13.Verbruggen N, Hermans C, Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist. 2009;181(4):759–76. 10.1111/j.1469-8137.2008.02748.x [DOI] [PubMed] [Google Scholar]
- 14.Hartley L. Characterization of a Heavy Metal Contaminated Soil in Ohio for a Phytoremediation Project: University of Toledo; 2004. [Google Scholar]
- 15.Pavlikova D, Macek T, Mackova M, Pavlik M. Technical note monitoring native vegetation on a dumpsite of PCB-contaminated soil. Int J Phytoremediation. 2007;9(1):71–8. Epub 2008/02/06. 10.1080/15226510601139433 [DOI] [PubMed] [Google Scholar]
- 16.Uhlik O, Jecna K, Mackova M, Vlcek C, Hroudova M, Demnerova K, et al. Biphenyl-Metabolizing Bacteria in the Rhizosphere of Horseradish and Bulk Soil Contaminated by Polychlorinated Biphenyls as Revealed by Stable Isotope Probing. Applied and Environmental Microbiology. 2009;75(20):6471–7. 10.1128/AEM.00466-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin SH, Lim Y, Lee SE, Yang NW, Rhee JH. CAS agar diffusion assay for the measurement of siderophores in biological fluids. Journal of microbiological methods. 2001;44(1):89–95. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 18.Dworkin M, Foster JW. Experiments with some microorganisms which utilize ethane and hydrogen. Journal of Bacteriology. 1958;75(5):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inga Miliūtė OB. IAA production and other plant growth promoting traits of endophytic bacteria from apple tree. Biologija. 2011;57(2):98–102. [Google Scholar]
- 20.Jasim B, John Jimtha C, Jyothis M, Radhakrishnan EK. Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regulation. 2013;71(1):1–11. [Google Scholar]
- 21.Dadarwal BSK K. R., Tauro P.. In vitro andin vivo nitrogenase activity ofRhizobium mutants and their symbiotic effectivity. Journal of Biosciences. 1981;3(2):117–23. [Google Scholar]
- 22.Hložková K, Šuman J, Strnad H, Ruml T, Paces V, Kotrba P. Characterization of pbt genes conferring increased Pb2+ and Cd2+ tolerance upon Achromobacter xylosoxidans A8. Research in Microbiology. 2013;164(10):1009–18. 10.1016/j.resmic.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 23.Kapila J, DeRycke R, VanMontagu M, Angenon G. An Agrobacterium-mediated transient gene expression system for intact leaves (vol 122, pg 101, 1997). Plant Science. 1997;124(2):227–. [Google Scholar]
- 24.Novakova M, Mackova M, Chrastilova Z, Viktorova J, Szekeres M, Demnerova K, et al. Cloning the Bacterial bphC Gene Into Nicotiana tabacum to Improve the Efficiency of PCB-Phytoremediation. Biotechnology and Bioengineering. 2009;102(1):29–37. 10.1002/bit.22038 [DOI] [PubMed] [Google Scholar]
- 25.Fiser J, Vrbova M, Novakova M, Mackova M, Macek T. Construction of the plant vectors with hiscup gene and their cloning into flax for higher heavy metal accumulation. 5th International Symposium on Biosorption and Bioremediation. 2012:14–7.
- 26.Shams KM, Tichy G, Fischer A, Sager M, Peer T, Bashar A, et al. Aspects of phytoremediation for chromium contaminated sites using common plants Urtica dioica, Brassica napus and Zea mays. Plant and Soil. 2010;328(1–2):175–89. [Google Scholar]
- 27.Holm FW. Effluents from Alternative Demilitarization Technologies: Springer; Netherlands; 2012. [Google Scholar]
- 28.Wilken A, Bock C, Bokern M, Harms H. Metabolism of different PCB congeners in plant cell cultures. Environmental Toxicology and Chemistry. 1995;14(12):2017–22. [Google Scholar]
- 29.Safe S. Toxicology, structure-function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environmental Health Perspectives. 1993;100:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibanez S, Talano M, Ontanon O, Suman J, Medina MI, Macek T, et al. Transgenic plants and hairy roots: exploiting the potential of plant species to remediate contaminants. New biotechnology. 2016;33(5 Pt B):625–35. Epub 2015/12/26. [DOI] [PubMed] [Google Scholar]
- 31.Musilova L, Ridl J, Polivkova M, Macek T, Uhlik O. Effects of Secondary Plant Metabolites on Microbial Populations: Changes in Community Structure and Metabolic Activity in Contaminated Environments. International Journal of Molecular Sciences. 2016;17(8):1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gullcin I, Kufrevioglu OI, Oktay M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibitory effects of some chemicals on enzyme activity. Journal of enzyme inhibition and medicinal chemistry. 2005;20(3):297–302. Epub 2005/08/27. 10.1080/1475636032000141890 [DOI] [PubMed] [Google Scholar]
- 33.Tarhan L, Kavakcioglu B. Glutathione metabolism in Urtica dioica in response to cadmium based oxidative stress. Biologia Plantarum. 2016;60(1):163–72. [Google Scholar]
- 34.Radulescu C, Stihi C, Popescu IV, Dulama ID, Chelarescu ED, Chilian A. Heavy metal accumulation and translocation in different parts of Brassica oleracea l. Romanian Journal of Physics. 2013;58(9–10):1337–54. [Google Scholar]
- 35.Obasi NA AE, Kalu KM, Ugbogu OC. Speciation of Heavy Metals and Phyto-accumulation Potentials of Selected Plants on Major Dumpsites in Umuahia, Abia State, Nigeria. International Journal of Current Biochemistry Research. 2013;1(4):16–28. [Google Scholar]
- 36.Sinclair SA, Krämer U. The zinc homeostasis network of land plants. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. 2012;1823(9):1553–67. [DOI] [PubMed] [Google Scholar]
- 37.Pachura P, Ociepa-Kubicka A, Skowron-Grabowska B. Assessment of the availability of heavy metals to plants based on the translocation index and the bioaccumulation factor. Desalination and Water Treatment. 2016;57(3):1469–77. [Google Scholar]
- 38.Gounden D, Kisten K, Moodley R, Shaik S, Jonnalagadda SB. Impact of spiked concentrations of Cd, Pb, As and Zn in growth medium on elemental uptake of Nasturtium officinale (Watercress). Journal of Environmental Science and Health Part B-Pesticides Food Contaminants and Agricultural Wastes. 2016;51(1):1–7. [DOI] [PubMed] [Google Scholar]
- 39.Boshoff M, De Jonge M, Scheifler R, Bervoets L. Predicting As, Cd, Cu, Pb and Zn levels in grasses (Agrostis sp and Poa sp.) and stinging nettle (Urtica dioica) applying soil-plant transfer models. Science of the Total Environment. 2014;493:862–71. 10.1016/j.scitotenv.2014.06.076 [DOI] [PubMed] [Google Scholar]
- 40.Grubor M. Lead uptake, tolerance, and accumulation exhibited by the plants Urtica dioica and Sedum spectabile in contaminated soil without additives. Archives of Biological Sciences. 2008;60(2):239–44. [Google Scholar]
- 41.Edwards HG, Hunt DE, Sibley MG. FT-Raman spectroscopic study of keratotic materials: horn, hoof and tortoiseshell. Spectrochimica acta Part A, Molecular and biomolecular spectroscopy. 1998;54a(5):745–57. Epub 1998/07/29. [DOI] [PubMed] [Google Scholar]
- 42.Ernst WHO, Leloup S. Perennial herbs as monitor for moderate levels of metal fallout. Chemosphere. 1987;16(1):233–8. [Google Scholar]
- 43.Koblar A, Tavcar G, Ponikvar-Svet M. Stress syndrome response of nettle (Urtica dioica L.) grown in fluoride contaminated substrate to fluoride and fluorine accumulation pattern. Journal of Fluorine Chemistry. 2015;172:7–12. [Google Scholar]
- 44.Rajkumar M, Ae N, Freitas H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere. 2009;77(2):153–60. 10.1016/j.chemosphere.2009.06.047 [DOI] [PubMed] [Google Scholar]
- 45.Barzanti R, Ozino F, Bazzicalupo M, Gabbrielli R, Galardi F, Gonnelli C, et al. Isolation and characterization of endophytic bacteria from the nickel hyperaccumulator plant Alyssum bertolonii. Microbial Ecology. 2007;53(2):306–16. 10.1007/s00248-006-9164-3 [DOI] [PubMed] [Google Scholar]
- 46.McInroy JA, Kloepper JW. Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant and Soil. 1995;173(2):337–42. [Google Scholar]
- 47.Tanvir R, Sajid I, Hasnain S. Larvicidal potential of Asteraceae family endophytic actinomycetes against Culex quinquefasciatus mosquito larvae. Natural Product Research. 2014;28(22):2048–52. 10.1080/14786419.2014.919579 [DOI] [PubMed] [Google Scholar]
- 48.Amaresan N, Jayakumar V, Kumar K, Thajuddin N. Isolation and characterization of plant growth promoting endophytic bacteria and their effect on tomato (Lycopersicon esculentum) and chilli (Capsicum annuum) seedling growth. Annals of Microbiology. 2012;62(2):805–10. [Google Scholar]
- 49.Khan AL, Waqas M, Kang SM, Al-Harrasi A, Hussain J, Al-Rawahi A, et al. Bacterial Endophyte Sphingomonas sp LK11 Produces Gibberellins and IAA and Promotes Tomato Plant Growth. Journal of Microbiology. 2014;52(8):689–95. [DOI] [PubMed] [Google Scholar]
- 50.Logan NA, Lebbe L, Verhelst A, Goris J, Forsyth G, Rodriguez-Diaz M, et al. Bacillus shackletonii sp nov., from volcanic soil on Candlemas Island, South Sandwich archipelago. International Journal of Systematic and Evolutionary Microbiology. 2004;54:373–6. 10.1099/ijs.0.02661-0 [DOI] [PubMed] [Google Scholar]
- 51.Scherer J, Nies DH. CzcP is a novel efflux system contributing to transition metal resistance in Cupriavidus metallidurans CH34. Molecular Microbiology. 2009;73(4):601–21. 10.1111/j.1365-2958.2009.06792.x [DOI] [PubMed] [Google Scholar]
- 52.Silver S, Phung LT. Genes and Enzymes Involved in Bacterial Oxidation and Reduction of Inorganic Arsenic. Applied and Environmental Microbiology. 2005;71(2):599–608. 10.1128/AEM.71.2.599-608.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silver S, Phung LT. A bacterial view of the periodic table: genes and proteins for toxic inorganic ions. Journal of Industrial Microbiology & Biotechnology. 2005;32(11–12):587–605. [DOI] [PubMed] [Google Scholar]
- 54.Ho M-W, Ryan A, Cummins J. Cauliflower Mosaic Viral Promoter—A Recipe for Disaster? Microbial Ecology in Health and Disease. 2011;11(4). Epub 2011-07-21. [Google Scholar]
- 55.Ohkama N, Hayashi H, Fujiwara T. A rapid and easy method of detecting levels of gene expression in intact tobacco plants using an Agrobacterium-mediated transient assay: Toward development of nutritional diagnosis based on gene expression. Soil Science and Plant Nutrition. 1999;45(3):767–74. [Google Scholar]
- 56.Cumming G, Fidler F, Vaux DL. Error bars in experimental biology. The Journal of Cell Biology. 2007;177(1):7–11. 10.1083/jcb.200611141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.