Abstract
Purpose of review
To evaluate relevant clinical outcomes following a transzonular intravitreal injection of a compounded triamcinolone–moxifloxacin–vancomycin (TMV) formulation for postoperative prophylaxis after cataract surgery in a retrospective review of medical records from a private practice, single-specialty ambulatory center in New Jersey, USA.
Recent findings
The analysis included 1541 cases from 922 patients who underwent cataract surgery with an intravitreal injection of TMV from November 2013 to December 2014. Cataract surgery was performed by a standard clear corneal phacoemulsification technique. Transzonular injection was used to deliver TMV directly into the anterior vitreous after implantation of an intraocular lens.
Summary
There were no major intraoperative complications associated with the transzonular injection technique. There were no cases of postoperative endophthalmitis. Nearly 92% of cases (n = 1413/1541) did not require supplemental medication after surgery. The rate of breakthrough inflammation at Days 14–21 was 9.2% (n = 132/1429). The rate of visually significant postoperative cystoid macular edema was 2.0% (n = 28/1429). The rate of clinically significant postoperative intraocular pressure increase was low: 0.9% (n = 13/1425) of cases had an at least 10 mmHg increase at Days 14–21 or 90. Four of these cases had intraocular pressure at least 30 mmHg. The rates of infection and inflammation reported in this retrospective review of a transzonular injection of TMV for prophylaxis after cataract surgery appear similar to reported rates with alternative prophylactic therapies such as topical drops. The transzonular injection of TMV may have advantages in terms of patient compliance.
Keywords: cataract postoperative care, cystoid macular edema, intravitreal injection, moxifloxacin hydrochloride, transzonular injection, triamcinolone acetonide
INTRODUCTION
All cataract surgeries are subject to the risk of postoperative complications. Persistent inflammation and cystoid macular edema (CME) can cause postoperative vision loss [1,2,3▪]. Infectious endophthalmitis, whereas rare, is another serious vision-threatening complication [4,5,6▪▪]. Prophylactic therapies are used to reduce the risk of postoperative endophthalmitis, inflammation, and CME, and their development is driven by the need to optimize surgical outcomes without unduly burdening the patient.
Topical ophthalmic medications are the standard of care for the prophylaxis of postoperative endophthalmitis. A 2014 survey of members of the American Society of Cataract and Refractive Surgery found that 97% of respondents prescribe postoperative antibiotic drops to reduce the risk of postoperative endophthalmitis [7▪]. Despite their almost unanimous use, the efficacy of topical ophthalmic antibiotics for endophthalmitis prophylaxis has never been demonstrated in a prospective randomized clinical trial.
Topical ophthalmic corticosteroids and NSAIDs are also available with varying indications for the treatment or prevention of postoperative inflammation and CME. The efficacy of these options has been demonstrated across multiple clinical studies, but safety concerns remain, including the potential for intraocular pressure (IOP) increases with the use of corticosteroid drops.
Despite being the current standard of postoperative cataract care, eye drop regimens are often associated with patient nonadherence. Prescription eye drops can be expensive, and treatment regimens are often complex, sometimes including up to three medications, each requiring different numbers of drops at various intervals throughout the day for up to several weeks [1,8]. A compliance monitoring study found that cataract surgery patients only applied half of the prescribed number of postoperative drops [9]. A separate video-based analysis found that 93% of eye-drop naïve patients used improper technique when applying postoperative drops [10]. Examples of improper techniques include the drop missing the eye, instilling an incorrect number of drops in one dose, and contaminating the bottle tip [10]. The same study also reported that less than half of the patients waited 5 min between instilling different eye-drop medications [10].
Results above suggest the need for a simpler prophylactic therapy, one that reduces or eliminates the patient burden of postoperative eye drops while remaining as well tolerated and effective. Single-use injections that are administered directly into the eye during surgery have been developed to meet this need. An intracameral injection of antibiotics, including cefuroxime, vancomycin, and moxifloxacin, has been shown to be well tolerated and effective at reducing the rate of postoperative endophthalmitis [11–13,14▪▪,15▪▪,16▪,17▪]. In the case of vancomycin, a retrospective analysis of more than 16 000 cases showed that an intracameral injection of 1-mg vancomycin reduced the rate of postoperative endophthalmitis more than 37-fold [12]. Studies have also shown that an intracameral injection of vancomycin or moxifloxacin is well tolerated for the cornea and retina [18,19▪,20▪].
Intraocular injections have also been developed to reduce the risk of postoperative inflammation, and clinical studies have shown that an intracameral injection of corticosteroids such as dexamethasone [21] or triamcinolone [22,23] can safely prevent acute or persistent postoperative inflammation without significantly increased IOP.
One concern with intracameral delivery is the ability of a therapeutic agent to access the posterior segment. Studies in rabbit eyes have shown that agents administered by intravitreal injection achieve higher intraocular concentrations, persist longer (up to 3 months) [24], and infiltrate most ocular tissues, including the retina and choroid, when compared with agents that are administered by intracameral injection [25,26].
Clinical studies support the use of intracameral corticosteroids for the prophylaxis of inflammation and CME after cataract surgery [27,28]. Control of inflammation was similar to topical medications, whereas clinically significant IOP increases were rare.
A regulated, compounded, preservative-free formulation of triamcinolone acetonide 15 mg/ml, moxifloxacin hydrochloride 1 mg/ml, and vancomycin 10 mg/ml [triamcinolone–moxifloxacin–vancomycin (TMV), Imprimis Pharmaceuticals, Inc., San Diego, California, USA] is available for intravitreal administration during cataract surgery for the prophylaxis of postoperative endophthalmitis and inflammation. The design of TMV as a single-use intraoperative prophylactic is thought to decrease both patient and surgeon burden by alleviating the need for complicated postoperative eye-drop regimens. The use of TMV for the prophylaxis of postoperative infection, inflammation, and CME has been evaluated in recently presented clinical experience reports [29,30▪▪].
The retrospective chart review reported here was conducted to evaluate the use of intravitreal TMV as an alternative to topical ophthalmic therapies for the prophylaxis of postoperative infection, inflammation, and CME in a large patient cohort.
Box 1.
no caption available
METHODS
Study and patients
The study was a retrospective analysis of the medical records of 922 patients (1541 eyes) who had cataract surgery with an intravitreal injection of TMV from November 2013 to December 2014. The analysis included surgical cases performed at a single-specialty ambulatory center (SurgiCenter of Vineland, Vineland, New Jersey, USA). Informed consent was obtained from all participants. Data extraction and analysis was HIPAA compliant. The study protocol was approved by the Wills Eye Hospital Institutional Review Board (Philadelphia, Pennsylvania, USA).
Prospective cases for inclusion in the review had cataract surgery with implantation of an intraocular lens (IOL) and at least one intravitreal injection of TMV. The following criteria were used to exclude cases from the analysis: use of topical ophthalmic antibiotics, corticosteroids, or NSAIDs up to 1-week before the scheduled day of surgery; known allergy or hypersensitivity to any of the components of the compounded TMV formulation; history of corticosteroid-responsive glaucoma; history of uncontrolled IOP; and concomitant advanced glaucoma with risk of optic nerve damage in the case of elevated IOP.
Preoperative evaluation, surgical procedure, and postoperative evaluations
All cases were evaluated preoperatively, on the day of surgery, and at three postoperative follow-up visits (Days 1, 14–21, and 90) based on anticipated timing of potential postoperative complications, including inflammation and CME. Two surgeons used identical protocols to perform preoperative and postoperative evaluations. The preoperative evaluation included slit lamp ophthalmoscopy, dilated fundoscopy, uncorrected visual acuity (UCVA), and best corrected visual acuity (BCVA) measured on a standard Snellen chart at 20 ft and IOP measured with a Goldmann applanation tonometer (AT 900, Haag-Streit Diagnostics, Koeniz, Switzerland). All patients were advised of the possible presence of floaters after TMV injection.
Standard surgical protocols were used in all cases. A slurry of 2% lidocaine gel, phenylephrine hydrochloride 2.5% (Paragon BioTeck, Inc., Portland, Oregon, USA), and Cyclogyl 2% (cyclopentolate hydrochloride, Alcon Laboratories, Inc., Fort Worth, Texas, USA) was applied to the inferior cul-de-sac of the operative eye 10 min before surgery. A 10% povidone–iodine solution was applied to the skin around the operative site, and a 5% solution was applied to the conjunctival sac 5 min before surgery. Cataract surgery was performed by a standard clear corneal phacoemulsification technique using the INFINITI Vision System (Alcon Laboratories, Inc.).
A transzonular injection of 0.2 ml TMV (3.0 mg triamcinolone acetonide, 0.2 mg moxifloxacin, and 2.0 mg vancomycin) was administered into the anterior vitreous after IOL implantation but before viscoelastic removal. Viscoelastic was used to expand the ciliary sulcus prior to injection. The TMV formulation was slowly injected through the zonules via the ciliary sulcus inferonasally with a 30-ga Rycroft cannula. Intravitreal TMV placement was visually confirmed by the appearance of a white plume in the vitreous (Fig. 1). A second or third TMV injection was permitted if the surgeon deemed the first (or second) injection to be inadequate. Viscoelastic was removed immediately after administration and confirmation of the TMV injection.
FIGURE 1.
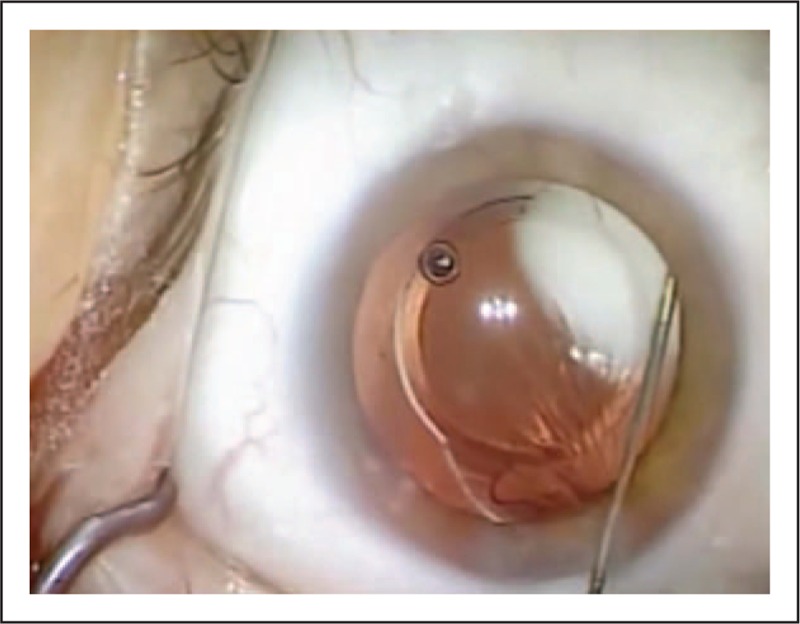
Surgical view of a transzonular intravitreal injection of a compounded triamcinolone–moxifloxacin–vancomycin formulation. A transzonular intravitreal injection of 0.2 ml triamcinolone–moxifloxacin–vancomycin was administered into the anterior vitreous after implantation of a multifocal toric intraocular lens. The triamcinolone–moxifloxacin–vancomycin formulation was injected through the zonules via the ciliary sulcus inferonasally with a 30-ga Rycroft cannula. Intravitreal triamcinolone–moxifloxacin–vancomycin placement was visually confirmed by the appearance of a white plume in the vitreous.
One drop each of PRED FORTE (prednisolone acetate) 1% (Allergan, Inc., Irvine, California, USA), a fluoroquinolone, and a topical ophthalmic antihypertensive were instilled into the operative eye immediately after surgery.
Postoperative evaluations included the following measurements and/or assessments: UCVA, BCVA, and IOP at all follow-up visits; presence of endophthalmitis at any follow-up visit; breakthrough inflammation at Days 14–21 or 90, defined as ocular pain, photophobia, ciliary flush, or other signs and symptoms that required supplemental anti-inflammatory medication; and visually significant CME at Days 14–21 and 90, defined as decreased BCVA with optical coherence tomography (OCT)-confirmed retinal thickening.
Assessments and statistical analyses
Analysis of the data records included a summary of patient demographics and a review of the surgical results. Postoperative analyses included the changes in BCVA and UCVA over time; UCVA and BCVA were converted into log MAR for aggregate analysis, with aggregate data converted back to Snellen acuity for reporting purposes. The rates of CME, breakthrough inflammation and endophthalmitis were also analyzed, as well as IOP pressure changes over time and any supplemental postoperative medication use.
A two-tailed t-test was used to determine statistically significant differences between mean values of parametric variables, with a repeated measures analysis of variance (ANOVA) used to compare parametric data over time. The chi-squared test of independence was used to determine statistically significant differences between proportions. Statistical significance was defined as a P-value less than 0.05. Statistical analyses were performed using the STATISTICA data analysis software system, version 13 (StatSoft, Inc., Tulsa, Oklahoma, USA).
RESULTS
Patient characteristics
The chart review included 1541 eyes of 922 patients; case demographics and preoperative ocular characteristics are reported in Table 1. Nearly one-third of cases (n = 504/1541) had a concomitant medical or ocular condition, the most common being diabetes (29.1%, n = 448/1541) followed by glaucoma (4.4%, n = 68/1541).
Table 1.
Case demographics and preoperative ocular characteristics
| Characteristic | |
| Number of eyes (cases) (n) | 1541 |
| Sex [number of patients (%)] | |
| Female | 554 (60.1) |
| Male | 368 (39.9) |
| Age (years) | |
| Mean (SD) | 70.4 (9.8) |
| Median (range) | 71 (18–98) |
| Ethnicity, number of patients (%)a | |
| Hispanic, Latino, or Spanish | 114 (12.4) |
| Not Hispanic, Latino, or Spanish | 807 (87.6) |
| Race [number of patients (%)]b | |
| Asian | 16 (1.7) |
| Black or African-American | 105 (11.4) |
| White | 686 (74.5) |
| Concomitant conditions [number of cases (%)] | |
| Diabetes | 448 (29.1) |
| Glaucoma | 68 (4.4) |
| Epiretinal membrane | 11 (0.7) |
| Diabetes + glaucoma | 22 (1.4) |
| Diabetes + epiretinal membrane | 1 (0.1) |
| IOP (mmHg) | |
| Number of cases (n) | 1517 |
| Mean (SD) | 15.4 (3.5) |
| Range | 5.7–30 |
| Cataract density [number of cases (%)] | |
| Number of cases (n) | 1534 |
| 1 and 1+ | 158 (10.3) |
| 2 and 2+ | 653 (42.6) |
| 3 and 3+ | 670 (43.7) |
| 4 and 4+ | 53 (3.4) |
IOP, intraocular pressure.
aEthnicity was not available for one patient (n = 921).
bRace was only recorded for patients who were not Hispanic, Latino, or Spanish (n = 807).
Surgical experience
Most cases (96.3%, n = 1484/1541) received a single injection of TMV during surgery. Fifty-six cases (3.6%, n = 56/1541) required a second TMV injection, and one case (0.1%, n = 1/1541) required a third injection. No case required more than three injections.
There were no major intraoperative complications. The unplanned vitrectomy rate was 0.45% (n = 7/1541). All vitrectomy cases received an injection of TMV without complication; in fact, the TMV served to stain the vitreous and greatly assisted in vitreous cleanup. There were no cases of zonular disruption, hyphema, or vitreous hemorrhage. There were no cases of iatrogenic retinal tear or detachment. There were isolated cases of iris prolapse in chambers that had been overfilled with viscoelastic. There were four cases of ciliary body hemorrhage. All cases were self-limiting or readily stanched with compounded lidocaine hydrochloride 1%/phenylephrine hydrochloride 1.5% (JCB Laboratories, Wichita, Kansas, USA), which was used for intracameral dilation.
Postoperative results
Visual acuity data are summarized in Table 2, with the mean log MAR UCVA and BCVA at each visit, along with the Snellen equivalent of that mean value. There was a statistically significant improvement in the postoperative BCVA and UCVA, as expected. A repeated measures ANOVA showed that the BCVA was statistically significantly different between postoperative visits (n = 158, P = 0.19), but the difference was clinically insignificant (less than half a log MAR line). A repeated measures ANOVA also showed that UCVA was statistically significantly different between visits (n = 871, P < 0.01), improving by about 1 log MAR line from Days 1 to 14–21 and another log MAR line between Days 14–21 and 90.
Table 2.
Mean visual acuity at all time points
| Mean visual acuity | |||
| Scheduled visit | Cases (n) | Snellen | Log MAR (SD) |
| UCVA | |||
| Before surgery | 919 | 20/100–1 | 0.72 (0.68) |
| Day 1 after surgery | 1511 | 20/50–2 | 0.44 (0.46) |
| Days 14–21 after surgery | 1325 | 20/40–2 | 0.33 (0.34) |
| Day 90 after surgery | 993 | 20/40+1 | 0.28 (0.34) |
| BCVA | |||
| Before surgery | 878 | 20/60+2 | 0.46 (0.46) |
| Day 1 after surgery | 381 | 20/25–2 | 0.14 (0.19) |
| Days 14–21 after surgery | 758 | 20/25–3 | 0.16 (0.25) |
| Day 90 after surgery | 720 | 20/25–2 | 0.15 (0.22) |
Note: Visual acuity data that were entered by surgeon as count fingers, hand motion, or light perception were quantified as log MAR 2.3 (Snellen 1/200), 2.6 (0.5/200), or 3.9 (0.025/200) for the purpose of analysis. BCVA, best-corrected visual acuity; UCVA, uncorrected visual acuity.
Table 3 summarizes findings related to breakthrough inflammation at Days 14–21. At Day 90, there were 26 cases of inflammation: 14 new cases and 12 cases that were persistent from Days 14 to 21. All cases with breakthrough inflammation at Days 14–21 received supplemental medication. The rate of breakthrough inflammation at Days 14–21 was significantly higher among surgeries performed during the first 7 months of the analysis period (11.2%, n = 74/661) compared with those performed in the second 7 months (7.8%, n = 58/768, P = 0.02). The rate of inflammation in cases with concomitant diabetes was not significantly different from cases without (chi-squared test, P = 0.97). However, there were significantly more cases of breakthrough inflammation in patients diagnosed with glaucoma (chi-squared test, P = 0.02). Using ethnicity and race as a general surrogate for iris color, the rate of breakthrough inflammation was compared between white and nonwhite patients; there was no statistically significant difference observed (chi-squared test, P = 0.15).
Table 3.
Summary of breakthrough inflammation after surgery with an intravitreal injection of triamcinolone–moxifloxacin–vancomycin
| Breakthrough inflammation (n = 1429a) | |
| Cases with breakthrough inflammation at Days 14–21 [n (%)] | 132 (9.2) |
| Concomitant conditions (cases with inflammation/total eyes) | |
| Diabetes | 41/448 |
| Glaucoma | 11/57b |
| Ethnicity and race (cases with inflammation/total eyes) | |
| Nonwhite | 40/347 |
| Medications prescribed | |
| Total | 147c |
| Corticosteroid | 72 |
| Nonsteroidal anti-inflammatory drug | 75 |
aA total of 112 cases did not return for a follow-up at Days 14–21.
bStatistically significantly different from the proportion of cases without the corresponding concomitant condition in the overall case set (n = 1429), chi-squared test.
cSome cases received more than one medication, so the number of medications differs from the number of cases that received medication.
Table 4 summarizes the findings related to visually significant postoperative CME at Days 14–21 or 90 was 2.0% (n = 28/1429). Most cases (79%, n = 22/28) received supplemental medication (Table 3). The rate of CME cases with concomitant diabetes was not statistically significantly different from the rate in patients without diabetes (chi-squared test, P = 0.14), though the rate in eyes with a preoperative epiretinal membrane was statistically significantly higher (chi-squared test, P < 0.01). One-third (10/28) of the cases of CME also had breakthrough inflammation, a significantly higher rate than for eyes without CME (chi-squared, P < 0.01).
Table 4.
Rates of visually significant cystoid macular edema after surgery with an intravitreal injection of triamcinolone–moxifloxacin–vancomycin
| Visually significant postoperative CMEd (n = 1429a) | |
| Cases with visually significant CME at Days 14–21 or 90 [n (%)] | 28 (2.0) |
| Cases that received medication for CME at Days 14–21 or 90 | 22/28 (78.6) |
| Concomitant conditions (cases with CME/total eyes) | |
| Diabetes | 12/448 |
| Epiretinal membrane | 2/11b |
| Medications prescribed | |
| Total | 32c |
| Corticosteroid | 12 |
| Nonsteroidal anti-inflammatory | 20 |
CME, cystoid macular edema.
aA total of 112 cases did not return for follow-up at Days 14–21.
bStatistically significantly different from the proportion of cases without the corresponding concomitant condition in the overall case set (n = 1429), chi-squared test.
cSome cases received more than one medication, so the number of medications differs from the number of cases that received medication.
dOnly includes cases of visually significant CME that emerged after cataract surgery. Excludes cases of CME that were present before surgery or at preoperative evaluation.
Mean IOP was lower than preoperative at all postoperative visits (Table 5). A repeated measures ANOVA showed the differences were statistically significantly different, but the mean differences were not clinically significant; all means were within 2 mmHg of each other. The rate of clinically significant postoperative IOP increase was low: 0.9% (n = 13/1425) of cases had an at least 10 mmHg increase at Days 14–21 or 90. Four of these cases had IOP at least 30 mmHg. One case received IOP-lowering medication. The proportion of cases with increased IOP was significantly higher in the eyes with concomitant glaucoma compared with those without glaucoma (4/68 vs 9/1361, chi-squared test, P < 0.01). Among cases with clinically significant IOP increase at Days 14–21, most were resolved at Day 90 (90.9%, n = 10/11, with IOP ≤ 21 mmHg). None of the resolved cases received IOP-lowering medications. The unresolved case (IOP 31 mmHg at Day 90) had optic neuropathy prior to surgery. No cases required surgical intervention to resolve increased IOP.
Table 5.
Mean intraocular pressure after surgery with an intravitreal injection of triamcinolone–moxifloxacin–vancomycin
| Mean IOP | ||
| Scheduled visit | Cases (n) | mmHg (SD) |
| Before surgery | 1517 | 15.4 (3.6) |
| Day 1 after surgery | 1514 | 14.3 (4.3) |
| Days 14–21 after surgery | 1301 | 14.2 (3.7) |
| Day 90 after surgery | 1067 | 13.7 (3.3) |
IOP, intraocular pressure.
aP < 0.0001 when compared with mean preoperative IOP. Analysis of statistical significance performed using two-tailed t test.
There were no cases of postoperative endophthalmitis. There were no other postoperative complications, including toxic anterior segment syndrome (TASS) or hemorrhagic occlusive retinal vasculitis (HORV). There were no cases of IOL instability, decentration, or migration. Though superiorly located (due to the inferior injection location) peripheral floaters and blurred vision were perceived among many cases in the immediate postoperative period; this did not appear to affect postoperative visual outcomes including visual acuity at Day 1. There were no reports of persistent floaters among cases throughout the remaining postoperative period. There were no cases of postoperative retinal tear or detachment through a year of follow-up.
DISCUSSION
The current retrospective chart review of 1541 cataract surgery cases evaluated the use of a transzonular intravitreal injection for the prophylaxis of postoperative infection, inflammation, and CME. The absence of postoperative endophthalmitis in this case series analysis supports the use of intravitreal TMV as a prophylactic antibiotic, but the size of the data set limits any definitive conclusion in this regard. Modern prophylactic methods have achieved postoperative endophthalmitis rates below 0.01% according to some studies [31], which means more than 10 000 eyes often need to be evaluated before an endophthalmitis-positive case is detected.
The rate of postoperative breakthrough inflammation after TMV (9.2% at Days 14–21) was similar to what has been reported for topical ophthalmic corticosteroids, such as difluprednate 0.05% (10.1% at Day 28) [32] and dexamethasone 1 mg/ml (given as TobraDex, Alcon Laboratories, Inc., 13% breakthrough inflammation at Day 21) [33]. The previous work has identified factors that are associated with increased rates of postoperative inflammation, including dark iris color and history of inflammatory conditions such as uveitis [34,35]. We did not observe a difference in breakthrough inflammation between white and nonwhite patients, though categorization by ethnicity and race is an admittedly imprecise surrogate for iris color.
The rate of visually significant postoperative CME after TMV was similar to rates previously reported with topical ophthalmic corticosteroids, including prednisolone 1% four times a day (QID) (2.1% with at least 1-month follow-up) [36]. Previous studies have suggested a higher rate of CME in diabetic patients or patients with diabetic retinopathy [37]; our results did not show this.
The rate of clinically significant IOP increase after TMV in this data set was low, and similar to what has been reported with topical ophthalmic corticosteroids, such as difluprednate 0.05% (2.8% with IOP ≥ 21 mmHg and IOP change ≥10 mmHg after) [38] and prednisolone 1% (2.4% with ≥10 mmHg change) [39]. Most cases resolved by Day 90, and those that resolved did so without IOP-lowering medication. Importantly, no cases required surgical intervention to achieve resolution of postoperative increased IOP. These findings suggest that intravitreal placement of the triamcinolone in TMV does not have a significant impact on postoperative IOP in most cases. This is supported by a previous study that demonstrated the IOP safety of low-dose (up to 3 mg) triamcinolone intravitreal injections [27].
There were no other postoperative complications, including TASS or HORV, which is a rare but serious complication that was recently identified in a small number of patients after uneventful cataract surgery that included a prophylactic intracameral injection of vancomycin [40▪].
There are questions about how the white triamcinolone plume in the vitreous affects patient vision in the early postoperative period after cataract surgery with TMV. As part of managing expectations after surgery, all patients were educated about the possibility of floaters and visual obscuration in the early postoperative period. Floaters were reported by many patients, but they did not persist.
We believe this is the first large-scale case series to evaluate the use of an intravitreal TMV injection for the prophylaxis of postoperative infection, inflammation, and CME associated with cataract surgery. The results of this retrospective chart review show that intravitreal TMV eliminated the need for supplemental postoperative medication, either topical ophthalmic medications or intraocular injections, in more than 91% of cases. There were no major intraoperative or postoperative complications observed, the rate of clinically significant IOP was 0.9%, and no cases required surgical intervention to resolve increased IOP. This suggests that TMV is a viable alternative to topical medications for postcataract prophylaxis. The transzonular intravitreal injection of TMV during cataract surgery has significant potential to ease the burden of postoperative care for patients and surgical practices.
CONCLUSION
There are many theoretical benefits of a single-use intraoperative prophylactic such as TMV: cost savings for the patient, who no longer needs to purchase postoperative eye drops; cost savings for the surgical practice, which no longer needs to spend time and human resources on explaining complicated eye drop regimens; fewer complications associated with reduced ability to self-administer postoperative eye drops, especially in cases with elderly patients; and potential improvements in surgical outcomes that are no longer dependent on patient adherence to a postoperative treatment regimen.
Acknowledgements
We would like to thank Namitha L. Gubbi for her assistance with this review.
Financial support and sponsorship
None.
Conflicts of interest
Dr Tyson is a consultant for Imprimis Pharmaceuticals. Dr Haller is currently receiving a grant from ThromboGenics, is a consultant for Merck Co. and Janssen Pharmaceutica, is a board member for Celgene, and has stock with Celgene. For the remaining authors none were declared.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.American Academy of Ophthalmology Cataract and Anterior Segment Panel Preferred practice Pattern® guidelines. Cataract in the adult eye. San Francisco, CA:American Academy of Ophthalmology; 2011. [Google Scholar]
- 2.Lobo C. Pseudophakic cystoid macular edema. Ophthalmologica 2012; 227:61–67. [DOI] [PubMed] [Google Scholar]
- 3▪.Sigler EJ, Randolph JC, Kiernan DF. Longitudinal analysis of the structural pattern of pseudophakic cystoid macular edema using multimodal imaging. Graefes Arch Clin Exp Ophthalmol 2016; 254:43–51. [DOI] [PubMed] [Google Scholar]; This article describes through spectral-domain OCT and fluorescein angiography the retinal structural changes that occur with pseudophakic cystoid macular edema (CME) and how these changes are reversed by treatment with periocular glucocorticoids.
- 4.Miller JJ, Scott IU, Flynn HW, Jr, et al. Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol 2005; 139:983–987. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Chang DF, Dewey SH, et al. ASCRS Cataract Clinical Committee Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg 2011; 37:1699–1714. [DOI] [PubMed] [Google Scholar]
- 6▪▪.Vaziri K, Schwartz SG, Kishor K, Flynn HW., Jr Endophthalmitis: state of the art. Clin Ophthalmol 2015; 9:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review article on the classification, diagnosis, and treatment of endophthalmitis.
- 7▪.Chang DF, Braga-Mele R, Henderson BA, et al. ASCRS Cataract Clinical Committee Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2014 ASCRS member survey. J Cataract Refract Surg 2015; 41:1300–1305. [DOI] [PubMed] [Google Scholar]; This ASCRS member survey shows the high usage of postoperative drops after cataract surgery and the increasing adoption of intracameral antibiotics for prophylaxis by its membership.
- 8.Shoss BL, Tsai LM. Postoperative care in cataract surgery. Curr Opin Ophthalmol 2013; 24:66–73. [DOI] [PubMed] [Google Scholar]
- 9.Hermann MM, Üstündag C, Diestelhorst M. Electronic compliance monitoring of topical treatment after ophthalmic surgery. Int Ophthalmol 2010; 30:385–390. [DOI] [PubMed] [Google Scholar]
- 10.An JA, Kasner O, Samek DA, Levesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg 2014; 40:1857–1861. [DOI] [PubMed] [Google Scholar]
- 11.ESCRS Endophthalmitis Study Group Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 2007; 33:978–988. [DOI] [PubMed] [Google Scholar]
- 12.Anijeet DR, Palimar P, Peckar CO. Intracameral vancomycin following cataract surgery: an eleven-year study. Clin Ophthalmol 2010; 4:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg 2013; 39:8–14. [DOI] [PubMed] [Google Scholar]
- 14▪▪.Javitt JC. Intracameral antibiotics reduce the risk of endophthalmitis after cataract surgery: does the preponderance of the evidence mandate a global change in practice? Ophthalmology 2016; 123:226–231. [DOI] [PubMed] [Google Scholar]; An editorial that summarizes the substantial evidence supporting the use of intracameral antibiotics for endophthalmitis prophylaxis, the barriers to adoption, and a recommended path to more widespread adoption.
- 15▪▪.Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology 2016; 123:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]; This longitudinal cohort study showed that intracameral cefuroxime or moxifloxacin was more effective for preventing postcataract extraction endophthalmitis than topical antibiotics alone.
- 16▪.Haripriya A, Chang DF, Namburar S, et al. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology 2016; 123:302–308. [DOI] [PubMed] [Google Scholar]; This is a retrospective study of over 116 000 patients which showed a four-fold reduction in postoperative endophthalmitis with the use of intracameral moxifloxacin in cataract surgery.
- 17▪.Jabbarvand M, Hashemian H, Khodaparast M, et al. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology 2016; 123:295–301. [DOI] [PubMed] [Google Scholar]; A large retrospective study revealing the effectiveness of intracameral cefuroxime over topical and subconjunctival approaches to endophthalmitis prophylaxis.
- 18.Arbisser LB. Safety of intracameral moxifloxacin for prophylaxis of endophthalmitis after cataract surgery. J Cataract Refract Surg 2008; 34:1114–1120. [DOI] [PubMed] [Google Scholar]
- 19▪.Pérez-Canales JL, Pérez-Santonja JJ, Campos-Mollo E. Evaluation of macular thickness changes after intracameral vancomycin in cataract surgery. Int Ophthalmol 2015; 35:49–57. [DOI] [PubMed] [Google Scholar]; This prospective study showed by spectral domain OCT that intracameral injection of vancomycin at the end of cataract surgery showed comparable effects to cefuroxime in terms of macular thickness changes and visual acuity and was determined to be well tolerated for intraocular use.
- 20▪.Pérez-Canales JL, Pérez-Santonja JJ, Campos-Mollo E. Corneal endothelial changes after intracameral vancomycin injection in cataract surgery. J Cataract Refract Surg 2015; 41:126–134. [DOI] [PubMed] [Google Scholar]; This prospective study showed that intracameral injection of vancomycin at the end of cataract surgery showed comparable effects to cefuroxime in terms of corneal endothelial changes and visual acuity and was determined to be well tolerated for intraocular use.
- 21.Chang DT, Herceg MC, Bilonick RA, et al. Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. Clin Ophthalmol 2009; 3:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karalezli A, Borazan M, Akova YA. Intracameral triamcinolone acetonide to control postoperative inflammation following cataract surgery with phacoemulsification. Acta Ophthalmol 2008; 86:183–187. [DOI] [PubMed] [Google Scholar]
- 23.Karalezli A, Borazan M, Kucukerdonmez C, et al. Effect of intracameral triamcinolone acetonide on postoperative intraocular pressure after cataract surgery. Eye 2010; 24:619–623. [DOI] [PubMed] [Google Scholar]
- 24.Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 2003; 110:681–686. [DOI] [PubMed] [Google Scholar]
- 25.Kadam RS, Williams J, Tyagi P, et al. Suprachoroidal delivery in a rabbit ex vivo eye model: influence of drug properties, regional differences in delivery, and comparison with intravitreal and intracameral routes. Mol Vis 2013; 19:1198–1210. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Wang M, Liu W, Lu Q, et al. Pharmacokinetic comparison of ketorolac after intracameral, intravitreal, and suprachoroidal administration in rabbits. Retina 2012; 32:2158–2164. [DOI] [PubMed] [Google Scholar]
- 27.Gills JP, Gills P. Effect of intracameral triamcinolone to control inflammation following cataract surgery. J Cataract Refract Surg 2005; 31:1670–1671. [DOI] [PubMed] [Google Scholar]
- 28.Koch PS. Intracameral injection studied to replace postop eye drops. Washington, DC, USA, April 2005:Presented at the ASCRS•ASOA Symposium & Congress; 2005. [Google Scholar]
- 29.Galloway MS. Intravitreal placement of antibiotic/steroid as substitute for postoperative drops after cataract surgery. Boston, Massachusetts, USA, April 2014:Presented at the ASCRS•ASOA Symposium & Congress; 2014. [Google Scholar]
- 30▪▪.Liegner JT, Galloway MS. Cystoid macular edema rates with intravitreal transzonular antibiotic/steroid prophylaxis. 2015; San Diego, California, USA, April 2015:Presented at the ASCRS•ASOA Symposium & Congress, [Google Scholar]; This prospective clinical study of 975 patients showed that the overall CME rate in those receiving transzonular injection of combined triamcinolone–moxifloxacin with and without vancomycin after cataract surgery was 1.2%.
- 31.Arshinoff SA, Bastianelli PA. Incidence of postoperative endophthalmitis after immediate sequential bilateral cataract surgery. J Cataract Refract Surg 2011; 37:2105–2114. [DOI] [PubMed] [Google Scholar]
- 32.Smith S, Lorenz D, Peace J, et al. Difluprednate ophthalmic emulsion 0.05% (Durezol) administered two times daily for managing ocular inflammation and pain following cataract surgery. Clin Ophthalmol 2010; 4:983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Notivol R, Amin D, Whitling A, et al. International TobraDex Study Group Prophylactic effectiveness of tobramycin-dexamethasone eye drops compared with tobramycin/vehicle eye drops in controlling post-surgical inflammation in cataract patients: prospective, randomised, double-masked, two-arm, parallel-group, placebo-controlled, multicentre study. Clin Drug Investig 2004; 24:523–533. [DOI] [PubMed] [Google Scholar]
- 34.Onodera T, Gimbel HV, DeBroff BM. Effects of cycloplegia and iris pigmentation on postoperative intraocular inflammation. Ophthalmic Surg 1993; 24:746–752. [PubMed] [Google Scholar]
- 35.Drolsum L, Davanger M, Haaskjold E. Risk factors for an inflammatory response after extracapsular cataract extraction and posterior chamber IOL. Acta Ophthalmol (Copenh) 1994; 72:21–26. [DOI] [PubMed] [Google Scholar]
- 36.Wolf EJ, Braunstein A, Shih C, Braunstein RE. Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. J Cataract Refract Surg 2007; 33:1546–1549. [DOI] [PubMed] [Google Scholar]
- 37.Schmier JK, Halpern MT, Covert DW, Matthews GP. Evaluation of costs for cystoid macular edema among patients after cataract surgery. Retina 2007; 27:621–628. [DOI] [PubMed] [Google Scholar]
- 38.Korenfeld MS, Silverstein SM, Cooke DL, et al. Difluprednate Ophthalmic Emulsion 0.05% (Durezol) Study Group Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg 2009; 35:26–34. [DOI] [PubMed] [Google Scholar]
- 39.Lane SS, Holland EJ. Loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgery. J Cataract Refract Surg 2013; 39:168–173. [DOI] [PubMed] [Google Scholar]
- 40▪.Witkin AJ, Shah AR, Engstrom RE, et al. Postoperative hemorrhagic occlusive retinal vasculitis: expanding the clinical spectrum and possible association with vancomycin. Ophthalmology 2015; 122:1438–1451. [DOI] [PubMed] [Google Scholar]; This small retrospective case series describes a rare syndrome of hemorrhagic occlusive retinal vasculitis, which the authors feel may be strongly associated with intracameral vancomycin use during cataract surgery.


