Abstract
Objective
The aim of this study was to assess the potential risk of gadobutrol-enhanced magnetic resonance imaging (MRI) in patients with moderate to severe renal impairment for the development of nephrogenic systemic fibrosis (NSF).
Materials and Methods
We performed a prospective, international, multicenter, open-label study in 55 centers. Patients with moderate to severe renal impairment scheduled for any gadobutrol-enhanced MRI were included. All patients received a single intravenous bolus injection of gadobutrol at a dose of 0.1 mmol/kg body weight. The primary target variable was the number of patients who develop NSF within a 2-year follow-up period.
Results
A total of 908 patients were enrolled, including 586 with moderate and 284 with severe renal impairment who are at highest risk for developing NSF. The mean time since renal disease diagnosis was 1.83 and 5.49 years in the moderate and severe renal impairment cohort, respectively. Overall, 184 patients (20.3%) underwent further contrast-enhanced MRI with other gadolinium-based contrast agents within the 2-year follow-up. No patient developed symptoms conclusive of NSF.
Conclusions
No safety concerns with gadobutrol in patients with moderate to severe renal impairment were identified. There were no NSF cases.
Key Words: gadobutrol, renal impairment, NSF
Clinical features of nephrogenic systemic fibrosis (NSF) were already described in the literature in 2000 with case reports dating back to 1997.1 Nephrogenic systemic fibrosis is a rare disease, but it can be severe. Indicative clinical symptoms are a thickening and hardening of the skin due to proliferation of connective tissue, which can ultimately lead to contractures and joint immobility. Other organs, for example, heart, lungs, liver, and muscles, may be affected in later stages. Disease development can last from a couple of weeks to several months or even years. Most cases of NSF have been seen in patients with severe renal impairment (estimated glomerular filtration rate [eGFR], <30 mL/min per 1.73 m2) and in patients with acute renal injury. At present, the etiology of NSF is not completely elucidated. A potential link between NSF and the application of gadolinium-based contrast agents (GBCAs) was first described by Grobner et al in 2006.2 In addition, other triggers such as metabolic acidosis,2 vascular surgery,2 treatment with erythropoietin,3 or systemic inflammation have been suggested to cause development of NSF. To establish a definitive diagnosis, a histopathological confirmation of a skin biopsy specimen is mandatory.4
The risk of a GBCA to trigger NSF seems to be related to the stability of the agent. Thus, nonionic linear GBCAs are more likely to trigger NSF than ionic linear agents both of which are distinctly more likely to trigger the disease than the macrocyclic agents in patients with reduced renal function.5
Gadobutrol is a gadolinium (Gd)-based contrast agent for magnetic resonance imaging (MRI). It is approved for a wide range of clinical indications covering the age range from full-term newborns to adults. However, approved indications and age ranges are country-specific. In Europe, gadobutrol is approved for the entire spectrum of indications in all age groups.6
Gadobutrol (Gadovist, Gadavist; Bayer Pharma AG, D-51368 Leverkusen, Germany) is a second-generation nonionic, multipurpose, extracellular, macrocyclic GBCA7,8 provided in a 1 molar concentration. In addition to its unique double concentration, gadobutrol features the highest relaxivity of all macrocyclic GBCAs.7,9,10 As a macrocyclic contrast agent, gadobutrol provides high chelate stability with substantially less—if any—in vivo release of Gd ions as opposed to linear GBCAs.11 The release of Gd ions has been linked to an increased risk of NSF in patients with impaired renal function.12,13 Because of these characteristics, gadobutrol was categorized as a low-risk GBCA for development of NSF by several medical organisations4,13 and authorities.5,14,15
The recommended standard dose of gadobutrol for intravenous injection is 0.1 mmol/kg body weight (bw), with doses up to 0.3 mmol/kg bw approved for specific indications in adults. The efficacy and safety of gadobutrol have been demonstrated in numerous clinical studies in adults and children, including full-term newborns.7,16–21
Following an FDA postmarketing requirement to all manufacturers of GBCAs in the United States in December 2009,22 we voluntarily initiated this study on gadobutrol adhering to the FDA's stipulations. The study title was “Prospective non-randomized cohort study (open-label, multicenter) to assess the magnitude of potential risk with the administration of Gadovist in patients with moderate to severe renal impairment for the development of NSF based on diagnostically specific clinical and histopathologic information” (EudraCT no. 2008-004496-22, NCT00828737). The initiation of this “GRIP” study (Gadobutrol in Renally Impaired Patients) was a preemptive measure. In 2009, gadobutrol was not yet approved in the United States, but Bayer planned its market introduction in the near future and therefore decided to voluntarily perform this study with gadobutrol following the specific FDA stipulations.
MATERIALS AND METHODS
Study Design
GRIP was a prospective, nonrandomized, open-label phase IV study performed in 55 centers in 9 countries (Germany [18 centers], Italy [10], Spain [3], Austria [6], Switzerland [1], Canada [5], Australia [2], South Korea [8], and Thailand [2]). The study period lasted from December 2008 to January 2015. The primary objective was to assess the magnitude of potential risk of developing NSF after gadobutrol administration in patients with moderate to severe renal impairment.
Study Population
The study population consisted of patients with moderate to severe renal impairment scheduled for gadobutrol-enhanced MRI within the approved indications and dose. The study was conducted in accordance with all international and local guidelines and laws stated by the involved institutional review boards. Oral and written informed consent was obtained before each examination.
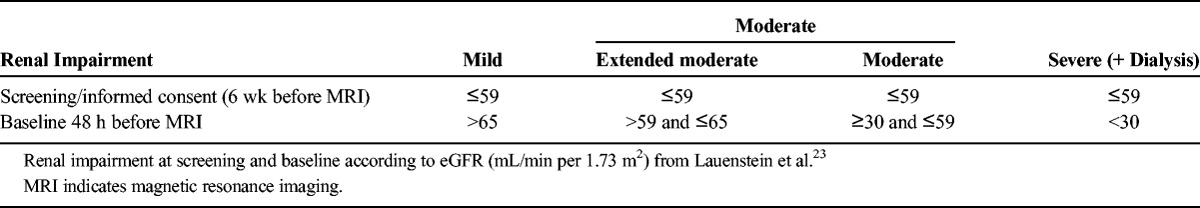
The classification of cohorts based on the degree of renal impairment was already described by Lauenstein et al23 in a study on gadoxetate disodium. The identical classification was used in the present study. The definitions in brief are as follows: “severe” renal impairment was defined by an eGFR of <30 mL/min per 1.73 m2 or dialysis. “Moderate” renal impairment was defined as an eGFR between ≥30 and ≤59 mL/min per 1.73 m2. However, renal status was tested twice—first during screening within 6 weeks before the gadobutrol injection at the local laboratory and second at baseline (ie, 48 hours before gadobutrol administration) at a central laboratory. As a result, 2 additional cohorts were defined post hoc: (1) an “extended moderate” renal impairment cohort (presenting an eGFR ≤59 mL/min per 1.73 m2 at screening, but >59 and ≤65 mL/min per 1.73 m2 at baseline) and (2) a “mild” renal impairment cohort (presenting an eGFR ≤59 mL/min per 1.73 m2 at screening, but an eGFR >65 mL/min per 1.73 m2 at baseline) (Table 1).
TABLE 1.
Classification of Study Cohorts
Treatment
All patients received a single intravenous bolus injection of 0.1 mmol/kg (0.1 mL/kg) bw gadobutrol followed by a 20 mL saline flush in the framework of the clinical routine diagnostic workup. Gadobutrol is marketed in all participating countries and was provided by the local hospital pharmacies.
Target Variables
The primary target variable was defined as the number of patients with moderate to severe renal impairment who developed NSF during the 2-year follow-up period.23 Nephrogenic systemic fibrosis was defined as described by Girardi et al.4 Secondary target variables included (1) number of patients without biopsy but who later developed NSF-like symptoms based solely on the clinical score by Girardi et al4; and (2) number and characteristics of adverse events reported in association with the administration of gadobutrol.
Study Procedures
The study procedures were identical to those described by Lauenstein et al.23 Our focus was to establish a standardized diagnostic workup and data collection for potential NSF cases in the daily clinical routine.
Blood sampling to assess eGFR was performed twice—first during screening within 6 weeks before the MRI examination at the local laboratory and second at baseline (ie, 48 hours before gadobutrol administration) at a central laboratory.
After gadobutrol administration, patients were followed for clinical examination and review of source documents after 12 and 24 months. In addition, telephone interviews were performed 1, 3, 6, and 18 months after gadobutrol administration by health care professionals who were trained on the study protocol and specifically for identification of clinical signs of NSF.
Any skin finding with the faintest suspicion of NSF was clinically assessed. In cases where a biopsy was taken, a histopathological assessment had to be performed. Here, the Girardi criteria had to be applied.4 This strategy ensured that the risk to miss a potential case of NSF was minimized.
Statistics and Sample Size
Descriptive statistics including sample size, mean, standard deviation, minimum, and maximum were calculated for quantitative variables. Frequency counts and percentages by category were generated for qualitative data. Summaries are presented by renal status cohort (mild, extended moderate, moderate, and severe renal impairment) and overall study population.23 Nephrogenic systemic fibrosis outcome had to be reported individually.
The Joint Meeting of the Cardiovascular and Renal Drugs and Drug Safety and Risk Management Advisory Committee of the FDA (December 8, 200922) stipulated a 2-year observational study for all GBCAs approved in the United States to assess the likelihood of NSF development. The FDA proposed a sample size of 1000 patients, consisting of 600 patients with moderate and 400 patients with severe renal impairment.
The FDA's sample size suggestion was based on a retrospective study of 370 patients with severe renal insufficiency who received gadodiamide. The estimated risk for development of NSF was approximately 4%.24 On June 2, 2011, the FDA released the pharmaceutical companies manufacturing GBCAs from completing study enrollment but the full 2-year follow-up was required for all patients already enrolled.25
We continued the GRIP study in accordance with the study protocol and concluded enrollment by December 31, 2012, with 927 of the 1000 originally planned patients enrolled. All patients were included in the 2-year safety follow-up.
RESULTS
The study enrolled 927 patients. Nine hundred eight patients received gadobutrol and completed the MRI examination and were included in the analysis: 284 with severe, 540 with moderate, 46 with extended moderate, and 38 with mild renal impairment (Table 2). A total of 581 patients (64.0%) completed the 24 months' follow-up.
TABLE 2.
Demographic and Baseline Characteristics by Degree of Renal Impairment (FAS)
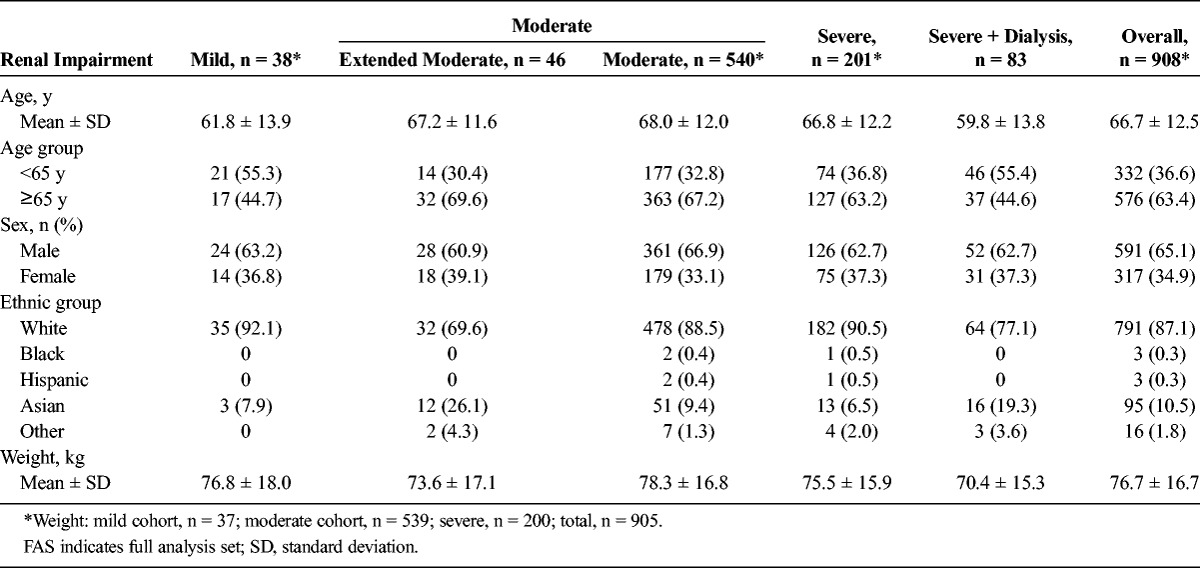
The mean age was 66.7 years (range, 19–94 years) with 63.4% of patients being 65 years or older. Almost two thirds of the patients were male, and 87.1% were white. There were no apparent differences in the degree of renal impairment among the various demographic subgroups (Table 2).
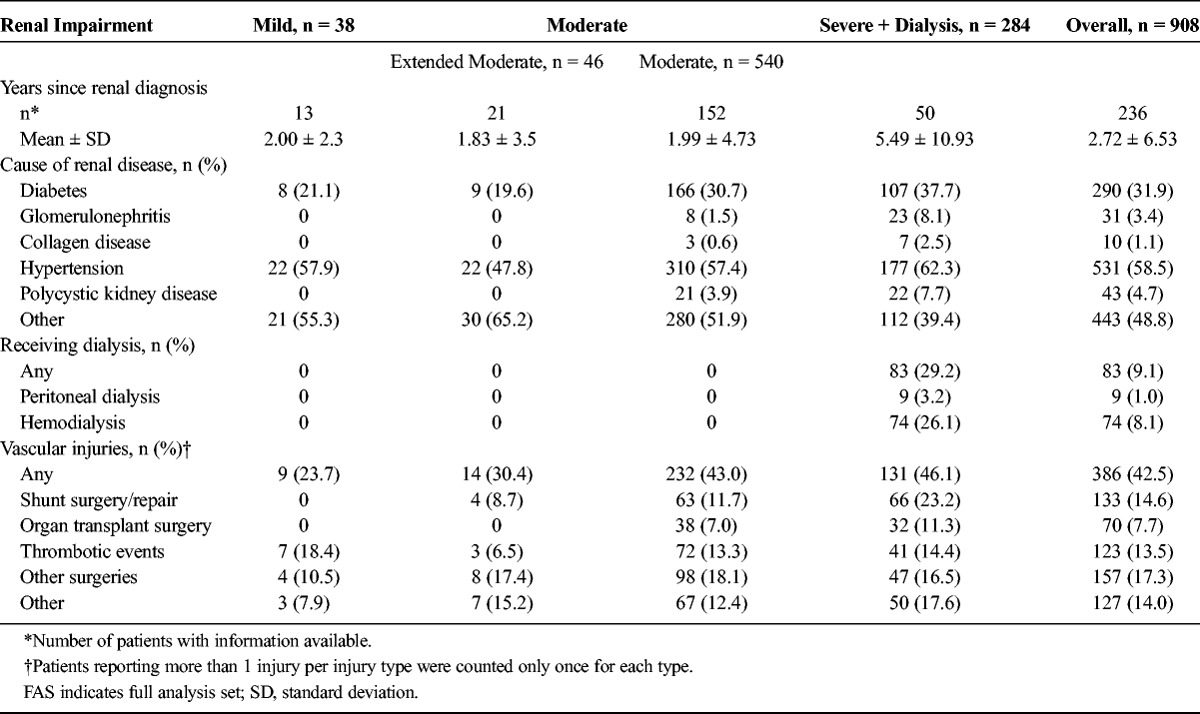
The period from initial diagnosis of renal disease increased with the severity of renal impairment and ranged from 1.83 years in the extended moderate renal impairment cohort to 5.49 years in the severe renal impairment cohort (overall range less than 0.1 to 60.4 years). Hypertension and diabetes were the most frequently reported causes of renal disease, with incidence rates of 58.5% and 31.9%, respectively. Eighty-three patients (9.1%) were dependent on dialysis and thus assigned to the severe renal impairment cohort. Vascular injuries were reported by 386 patients (42.5%), with the majority in the cohort with moderate (43.0%) and severe (46.1%) renal impairment. Seventy patients (7.7%) had a history of organ transplant surgery, all in the moderate and severe renal impairment cohort (Table 3).
TABLE 3.
History of Renal Disease by Degree of Renal Impairment (FAS)
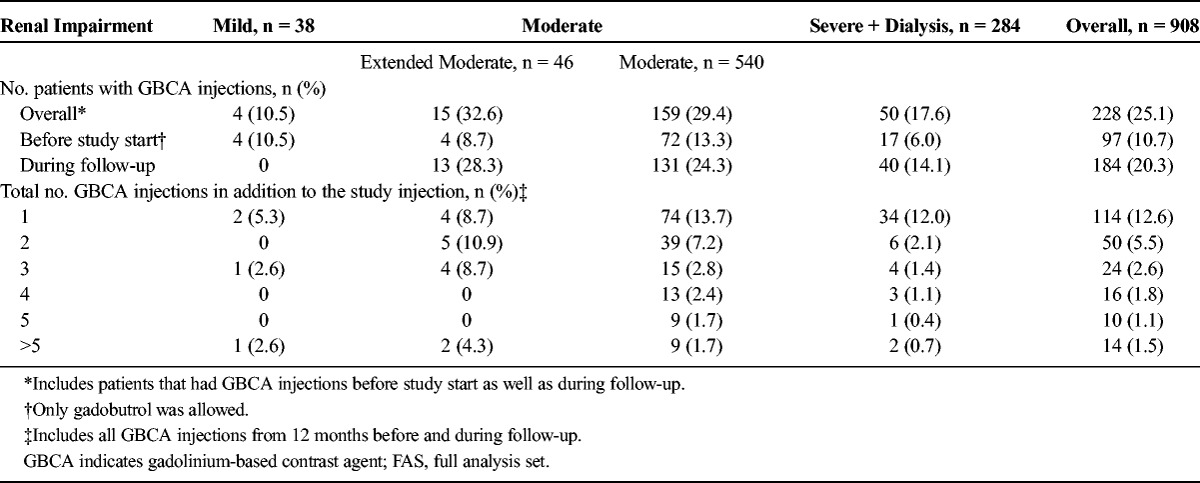
Overall, 228 patients (25.1%) underwent contrast-enhanced MRI with another GBCA within 12 months before the inclusion in the study start or in the follow-up period. Ninety-seven patients (10.7%) of the 908 were exposed to gadobutrol before the inclusion in the study, and 184 patients (20.3%) received additional different GBCAs during follow-up, that is, after gadobutrol administration at baseline. The number of GBCA administrations ranged from 1 (114 patients, 12.6%) to >5 (14 patients, 1.5%) (Table 4). Two of the patients with more than 5 additional injections of GBCAs had received these injections at baseline, and 6 patients received more than 5 additional injections during follow-up.
TABLE 4.
Patients With and Number of GBCA Injections From 12 Months Before Study Start (Gadobutrol Administration) and During Follow-up (FAS)
During the period immediately after gadobutrol administration and before leaving the MRI facility, 3 patients (0.3%) experienced drug-related adverse events (AEs): urticaria, retching, and rash. None of these AEs were considered serious or life threatening.
During the 24-month follow-up, AE reporting focused on skin-related findings and other findings suggestive of NSF. Patients with mild renal impairment were not included in the follow-up. During this follow-up, a total of 135 patients (14.9%) reported skin-related findings. The frequency was similar within the 3 cohorts of renal impairment. Four patients (0.4%) (3 in the moderate, 1 in the severe renal impairment cohort) suffered a rash, but no patient developed symptoms indicative of NSF.
No serious adverse events occurred between the date of informed consent and the date the patient left the MRI facility on the day of gadobutrol injection or during the follow-up. However, 166 patients died during the follow-up period. The frequency of deaths was related to the severity of renal impairment, with 10.9%, 18.1%, and 22.2% of patients in the cohorts with extended moderate, moderate, and severe renal impairment, respectively. Progression of renal disease was the most frequent cause of death. None of the patients with skin-related findings died.
DISCUSSION
Following the FDA's postmarketing requirement for all US-approved GBCAs,22 we voluntarily initiated this study on gadobutrol adhering to the FDA's proposed study design. This was a preemptive measure as we expected gadobutrol to be approved in the United States shortly. No case of NSF was detected in our study.
To the best of our knowledge, this is the first publication of prospective data on the incidence of NSF after gadobutrol administration. As of May 2016, several similar studies on other GBCAs have been reported. One study by Lauenstein et al,23 investigated gadoxetate disodium in 357 patients. No case of NSF was recorded. Another recent study by Amet et al26 investigated the risk of gadoteric acid in 255 patients on dialysis with no findings of NSF. In addition, Soulez et al27 reported 2 prospective 2-year studies in 534 patients with either stage 3 chronic kidney disease (CKD) or stage 4 to 5 CKD. No signs or symptoms of NSF were reported after administration of gadobenate dimeglumine or gadoteridol. Smorodinsky et al28 retrospectively evaluated 1167 patients with chronic liver disease where 72% also had some degree of renal insufficiency. They did not report any case of NSF. The GBCAs applied in that study were gadobenate dimeglumine, gadoversetamide, gadopentetate dimeglumine, gadodiamide, and gadoteridol.
Our study population consisted of 908 patients. A total of 284 patients had severe, and 586 had moderate renal impairment, consistent with the target population requested by the FDA. In a number of patients, the 2 eGFR determinations (one at screening and one immediately before the MRI examination at baseline) resulted in different patient classifications based on the cutoff values for mild, moderate, or severe renal impairment. Some patients who originally fulfilled the criterion for moderate renal impairment at the time of screening actually showed improved renal function at the time of contrast injection.23 To reflect a worst-case scenario, we subsumed those patients still in the category of moderate renal impairment and performed a 2-year follow-up. However, to be fully transparent, we reported these patients in a subgroup as “extended moderate” (Table 1). For the 38 patients in the mild renal disease group an elevated NSF risk has not been established,29 so follow-up was waived according to protocol.
Interestingly, many patients did not only receive gadobutrol during the study period. A total of 228 patients (25.1%) received other, additional GBCA administrations 12 months before study start and/or during the 2-year follow-up. Being fully aware that this might confound/harm our primary objective, we still decided to include all these patients in our analysis. Thus, we chose the most conservative approach and report the full-analysis data set that includes all patients who have received at least 1 dose of gadobutrol. Therefore, we feel confident that our study population reflects the clinical reality of patients with renal impairment. In addition, as the increasing number and dose of GBCA administrations is postulated to increase the likelihood of triggering NSF development, it appears reassuring that even in patients with exposure to multiple GBCAs no case of NSF was observed.
In our study cohort, most patients had renal disease caused by hypertension (58.5%) or diabetes (31.9%), both being very common in the modern Western world.30,31
No new safety concerns came up during the study period. No serious adverse events occurred between the date of informed consent and the date the patient left the MRI facility or on the day of gadobutrol injection or during the follow-up period. Progression of renal disease was the most frequent cause of death. Our results are in accordance with other reports on gadobutrol use in patients with renal impairment. Even in patients with chronic renal impairment, including hemodialysis, gadobutrol can safely be applied at doses up to 0.3 mmol/kg,32 providing evidence that gadobutrol is safe in patients with renal impairment.33
Today, gadobutrol is approved in more than 100 countries worldwide, including the European Union, Switzerland, Australia, United States, Canada, Japan, and China. Since its introduction to the market in February 1998 until December 2015, the cumulative patient exposure is estimated to be more than 29 million patients.21 As of May 2016, the pharmacovigilance department of Bayer Pharma AG has received 3 single-agent reports, so-called unconfounded reports, for gadobutrol consistent with the clinicohistopathological definition of NSF. This classification was rigorously performed after a most stringent and conservative approach according to the criteria by Girardi et al.4 In case clinicopathological criteria or laboratory parameters reached a score of ≥1, Bayer always assumed a worst-case scenario.21 Bayer continues to follow a policy of total transparency regarding NSF, with expedited case reporting to health authorities all over the world.21
Finally, it is important to note that the FDA34 and the European Medicines Agency35 have defined risk categories for GBCAs. Gadobutrol, as well as the 2 other macrocyclic GBCAs, gadoterate meglumine and gadoteridol, belong to the class with the lowest risk for NSF development. Also, the European Society of Urogenital Radiology (ESUR) followed those categories in their recommendations.5 The ESUR classified macrocyclic agents as “low risk” but still recommends that they should be used with caution in patients with CKD 4 and 5 (GFR <30 mL/min per 1.73 m2), and there should be at least 7 days between 2 injections. Furthermore, pregnant women should only be imaged with contrast-enhanced MRI if the expected diagnostic information is essential. However, laboratory testing of renal function (eGFR) is not mandatory, and the ESUR Contrast Medium Safety Committee guidelines state that a questionnaire on renal function should be sufficient.5 On May 23, 2007, the FDA mandated a boxed warning on the product labeling of all GBCAs.36
There are several limitations to our study that need to be taken into account when interpreting the results. Although we followed the FDA's stipulations,22 the sample size must be considered as a major limitation. In a letter dated June 2, 2011, the FDA stated that “the estimate of the incidence of NSF in patients with renal insufficiency, based on postmarketing surveillance reports, is lower than the original literature-based estimate.” Therefore, the trial's sample size became inadequate to assess the magnitude of NSF risk of GBCAs in patients with renal insufficiency.25 We stopped enrollment on December 31, 2012, but still conducted the 2-year follow-up.
The second limitation is related to the concomitant application of other GBCAs during the course of the study. However, we consider it reassuring that none of these patients developed signs of NSF.
Because the FDA asked all manufacturers of GBCAs marketed in the United States to run similar studies, further reports on other GBCAs are expected to be published soon. Once all results are available, we suggest that a final assessment of the impact of GBCA administration on NSF development should be performed.
CONCLUSIONS
Gadobutrol in patients with moderate to severe renal impairment did not raise any clinically significant safety signals. No NSF cases were observed.
ACKNOWLEDGMENTS
The authors thank all participating investigators and patients for their time dedicated to this study.
Footnotes
Conflict of interest and source of funding: M.A., H.D., G.B., M.G., R.W., W.K., and F.D.C. have no conflicts of interest to disclose. H.M., B.E.-W., and R.H. received honoraria and/or research grants from Bayer. M.R., T.B., and J.E. are Bayer employees. The study was funded by Bayer Pharma AG.
REFERENCES
- 1.Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000–1001. [DOI] [PubMed] [Google Scholar]
- 2.Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. [DOI] [PubMed] [Google Scholar]
- 3.Goveia M, Chan BP, Patel PR. Evaluating the role of recombinant erythropoietin in nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;57:725–727. [DOI] [PubMed] [Google Scholar]
- 4.Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65:1095.e7–106.e7. [DOI] [PubMed] [Google Scholar]
- 5.Thomsen HS, Morcos SK, Almén T, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23:307–318. [DOI] [PubMed] [Google Scholar]
- 6.EMC. The electronic Medicines Compendium (eMC). Available at: http://www.medicines.org.uk/emc/medicine/27209. Accessed December 12, 2015.
- 7.Scott LJ. Gadobutrol: a review of its use for contrast-enhanced magnetic resonance imaging in adults and children. Clin Drug Investig. 2013;33:303–314. [DOI] [PubMed] [Google Scholar]
- 8.Saake M, Langner S, Schwenke C, et al. MRI in multiple sclerosis: an intra-individual, randomized and multicentric comparison of gadobutrol with gadoterate meglumine at 3 T. Eur Radiol. 2016;26:820–828. [DOI] [PubMed] [Google Scholar]
- 9.Rohrer M, Bauer H, Mintorovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. [DOI] [PubMed] [Google Scholar]
- 10.Shen Y, Goerner FL, Snyder C, et al. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol. 2015;50:330–338. [DOI] [PubMed] [Google Scholar]
- 11.Frenzel T, Lengsfeld P, Schirmer H, et al. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008;43:817–828. [DOI] [PubMed] [Google Scholar]
- 12.Sieber MA, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008;18:2164–2173. [DOI] [PubMed] [Google Scholar]
- 13.Perazella MA. Gadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosis. Curr Drug Saf. 2008;3:67–75. [DOI] [PubMed] [Google Scholar]
- 14.ACR. American College of Radiology (2015) ACR Manual on Contrast Media Version 10.1. Available at: http://www.acr.org/Quality-Safety/Resources/Contrast-Manual. Accessed December 12, 2015.
- 15.EMA. /425304/2010 23 July 2010 Rev.1 Patient Health Protection. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2010/07/WC500094268.pdf. Accessed December 12, 2015.
- 16.Forsting M, Palkowitsch P. Prevalence of acute adverse reactions to gadobutrol—a highly concentrated macrocyclic gadolinium chelate: review of 14,299 patients from observational trials. Eur J Radiol. 2010;74:e186–e192. [DOI] [PubMed] [Google Scholar]
- 17.Voth M, Rosenberg M, Breuer J. Safety of gadobutrol, a new generation of contrast agents: experience from clinical trials and postmarketing surveillance. Invest Radiol. 2011;46:663–671. [DOI] [PubMed] [Google Scholar]
- 18.Hahn G, Sorge I, Gruhn B, et al. Pharmacokinetics and safety of gadobutrol-enhanced magnetic resonance imaging in pediatric patients. Invest Radiol. 2009;44:776–783. [DOI] [PubMed] [Google Scholar]
- 19.Bhargava R, Noga M. Safety and efficacy of gadobutrol-enhanced MRI in patients aged under 2 years—a single-center, observational study. Magn Reson Insights. 2013;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunze C, Mentzel HJ, Krishnamurthy R, et al. Pharmacokinetics and safety of macrocyclic gadobutrol in children aged younger than 2 years including term newborns in comparison to older populations. Invest Radiol. 2016;51:50–57. [DOI] [PubMed] [Google Scholar]
- 21.Endrikat J, Vogtlaender K, Dohanish S, et al. Safety of gadobutrol: results from 42 clinical phase II to IV studies and postmarketing surveillance after 29 million applications. Invest Radiol. 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM190850.pdf. Accessed February 19, 2016.
- 23.Lauenstein T, Ramirez-Garrido F, Kim YH, et al. Nephrogenic systemic fibrosis risk after liver magnetic resonance imaging with gadoxetate disodium in patients with moderate to severe renal impairment: results of a prospective, open-label, multicenter study. Invest Radiol. 2015;50:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–2362. [DOI] [PubMed] [Google Scholar]
- 25.FDA letter 06/02/2011. Release from postmarketing requirement. NDA 22–090. Reference ID:2954948 np.
- 26.Amet S, Launay-Vacher V, Clement O, et al. Incidence of nephrogenic systemic fibrosis in patients undergoing dialysis after contrast-enhanced magnetic resonance imaging with gadolinium-based contrast agents: the Prospective Fibrose Nephrogenique Systemique study. Invest Radiol. 2014;49:109–115. [DOI] [PubMed] [Google Scholar]
- 27.Soulez G, Bloomgarden DC, Rofsky NM, et al. Prospective cohort study of nephrogenic systemic fibrosis in patients with stage 3-5 chronic kidney disease undergoing MRI with injected gadobenate dimeglumine or gadoteridol. AJR Am J Roentgenol. 2015;205:469–478. [DOI] [PubMed] [Google Scholar]
- 28.Smorodinsky E, Ansdell DS, Foster ZW, et al. Risk of nephrogenic systemic fibrosis is low in patients with chronic liver disease exposed to gadolinium-based contrast agents. J Magn Reson Imaging. 2015;41:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanna RF, Finkelstone LA, Chow DS, et al. Nephrogenic systemic fibrosis risk and liver disease. Int J Nephrol. 2014;2014:679605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. [DOI] [PubMed] [Google Scholar]
- 31.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tombach B, Heindel W. Value of 1.0-M gadolinium chelates: review of preclinical and clinical data on gadobutrol. Eur Radiol. 2002;12:1550–1556. [DOI] [PubMed] [Google Scholar]
- 33.Tombach B, Bremer C, Reimer P, et al. Renal tolerance of a neutral gadolinium chelate (gadobutrol) in patients with chronic renal failure: results of a randomized study. Radiology. 2001;218:651–657. [DOI] [PubMed] [Google Scholar]
- 34.FDA Drug Safety Communication: New warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm. Accessed April 11, 2016.
- 35. 23 July 2010 EMA/425304/2010 Rev.1 Patient Health Protection. [Google Scholar]
- 36.FDA Requests Boxed Warning for Contrast Agents Used to Improve MRI Images. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108919.htm. Accessed April 11, 2016.


