Abstract
BACKGROUND
Infants with acute lymphoblastic leukemia (ALL) present with aggressive disease and a poor prognosis. Early relapse within 6–9 months of diagnosis is common. Approximately 75% of infants have MLL-rearranged (MLL-R) ALL with event free survival (EFS) ranging from 20–30%. Children’s Oncology Group (COG) P9407 used shortened (46 weeks), intensified therapy to address early relapse and poor EFS.
PROCEDURE
P9407 therapy was modified three times for induction toxicity resulting in three cohorts of therapy. One hundred forty-seven infants were enrolled in the third cohort.
RESULTS
We report an overall 5-year EFS and OS of 42.3 ± 6% and 52.9 ± 6.5% respectively. Poor prognostic factors included age ≤ 90 days at diagnosis, MLL-R ALL and white cell count ≥50,000/ul. For infants ≤90 days of age, the 5-year EFS was 15.5±10.1% and 48.5±6.7% for those > 90 days (p<0.0001). Among infants >90 days of age, 5-year EFS rates were 43.8±8% for MLL-R vs. 69.1±13.6% for MLL-germline ALL (p<0.0001).
CONCLUSIONS
Age ≤ 90 days at diagnosis was the most important prognostic factor. Despite shortened therapy with early intensification, EFS remained less than 50% overall in MLL-R ALL.
Keywords: Intensified therapy without SCT, infant ALL
INTRODUCTION
Acute Lymphoblastic Leukemia (ALL) in infancy (≤ 365 days of age) differs significantly from ALL in older children whose event free survival (EFS) exceeds 85% (1–6). While 90–95% of infants with ALL achieve remission, early relapse within 6–9 months of diagnosis is common. In the 1980’s, EFS was only 20–30% (7–9) but improved somewhat subsequently with the development of intensified, infant specific therapy (10–18). In the Interfant-99 trial, the largest infant ALL trial conducted to date and which used an intensive 24 month regimen, the 4-year EFS was 47%. Eight percent of patients underwent hematopoietic stem cell transplantation (HSCT) (16).
Infant ALL is characterized by hyperleukocytosis, organomegaly and an increased risk of central nervous system (CNS) disease. Translocations that interrupt the MLL gene at 11q23 with fusion to a large number of partner genes are present in 75% of cases (11, 15,19–23). Infants with MLL rearranged (MLL-R) ALL are typically younger, present with higher white blood cell counts (WBC), have more frequent CNS involvement and a far worse prognosis compared with infants with MLL germline (MLL-G) leukemia. Age above/below 6 months at diagnosis has also been an important prognostic factor (9–12).
Based on the very aggressive nature of infant ALL and the poor response to traditional therapy, the Children’s Oncology Group (COG) P9407 trial was designed to deliver shortened (46 weeks), intensified therapy with a goal of improving EFS by preventing early treatment failure. Many chemotherapy agents, with the exception of vincristine, daunomycin and triple intrathecal chemotherapy were dosed in mg/m2 rather than using traditional infant dose reductions determined in mg/kg. The therapy given originally in P9407 (Cohorts 1 and 2) was associated with a substantial risk of early treatment related mortality occurring within 90 days of diagnosis requiring several modifications to Induction therapy including alterations of daunomycin dose/schedule and substitution of prednisone for dexamethasone (24). Following these changes, early mortality was substantially reduced and an additional cohort, Cohort 3, of 141 patients was treated. Because there was no benefit to HSCT in Cohort 1 and 2 of P9407 and the parallel CCG 1953 trial (25), HSCT was not included in P9407 Cohort 3. We report outcome for these patients with infant ALL treated uniformly with chemotherapy without HSCT, which forms the basis for the regimen used currently to treat almost all infants with ALL in North America.
METHODS
Patients
One hundred forty seven infants ≥36 weeks estimated gestational age and less than 366 days of age with newly diagnosed ALL were enrolled on COG P9407 Cohort 3 between 2001–2006. The diagnosis of ALL was based on morphology, cytochemistry and immunophenotyping at local institutions. Confirmatory immunophenotyping and cytogenetic analyses were performed at COG reference laboratories or COG approved local institutional laboratories. Molecular and/or fluorescence in situ hybridization (FISH) analysis for detection of the MLL gene rearrangement was performed in COG reference labs at St. Jude Children’s Research Hospital and at the University of New Mexico. In some cases, final assignment of MLL-R vs. MLL-G status was resolved retrospectively by panhandle PCR or other PCR approaches (26). The protocol was approved by the National Cancer Institute and the Institutional Review Boards at COG member institutions. Informed consent was obtained from parents/guardians according to federal guidelines.
Treatment
COG P9407 Cohort 3 included 46 weeks of intensified therapy and is compared to therapy provided in Cohorts 1+2 in Table I. Triple intrathecal therapy (ITT) was used for CNS prophylaxis with no modification made for CNS leukemia at diagnosis. Cohort 3 protocol therapy did not include an option for HSCT. Remission was assessed at Week 8 prior to beginning Re-Induction. No bone marrow aspirate was required prior to week 8 and minimal residual disease (MRD) was not assessed routinely. Complete remission (CR) was defined as 0–5% blasts in the marrow and no evidence of extramedullary leukemia following completion of Induction and Induction Intensification. Marrow relapse was defined as >25% blasts at any point after CR was attained. CNS relapse was defined as WBC ≥ 5/microliter with positive cytology on a single lumbar puncture or 0–4 WBC/microliter with definite blasts on two consecutive lumbar punctures.
Table I.
| Induction | PDN | 40mg/m2/day PO TID days 1–21, no taper |
| DXM+ | 10 mg/m2/day PO TID days 1–21 no taper (Cohort 1+2) | |
| (Week 1–3) | VCR | 0.05 mg/kg days 0, 14, 0.03 mg/kg day 7 |
| DNM | 2mg/kg/day IV push Day 1+2 age < 6 months | |
| 2.5mg/kg/day IV push Day 1+2 age ≥6–<9 months | ||
| 3mg/kg/day IV push Day 1+2 age ≥9 months | ||
| DNM+ | 45 mg/m2/IV over 48 hours Days 1–2 (Cohort 1) | |
| 4 mg/kg IV over 48 hours (<6 months) (Cohort 2) | ||
| 5 mg/kg IV over 48 hours (≥6–<9 months) (Cohort 2) | ||
| 6 mg/kg IV over 48 hours (≥9 months) (Cohort 2) | ||
| CPM | 250 mg/m2 q 12 h × 4 doses, days 3 and 4 | |
| L-ASP | 6000 IU/m2 × 8 doses 3 × week IM | |
| ITT | Days 1, 8, 15 | |
| G-CSF | 5 ug/kg/day beginning Day 5 through count recovery | |
| Induction | MTX | 4 gm/m2 × 2, Days 22/29 |
| Intensification | CF | 10 mg/m2 PO or IV q 6 h × ≥ 5 doses, start 18 h after MTX |
| (Week 4–7) | VP-16 | 100 mg/m2 × 5 days, Days 36–40 |
| CPM | 300 mg/m2 × 5 days, Days 36–40 | |
| G-CSF | 5 ug/kg/day beginning Day 41 until count recovery | |
| ITT | Days 22, 28 | |
| Re-Induction | PDN | 40mg/m2/day PO TID days 1–21, no taper |
| (Week 8–10) | VCR | 0.05 mg/kg days 0, 14, 0.03 mg/kg day 7 |
| DNM | 2mg/kg/day IV push Day 1+2 age < 6 months | |
| 2.5mg/kg/day IV push Day 1+2 age ≥6–<9 months | ||
| 3mg/kg/day IV push Day 1+2 age ≥9 months | ||
| DNM+ | 45 mg/m2/IV over 48 hours Days 1–2 (Cohort 1) | |
| 4 mg/kg IV over 48 hours (, 6 months) (Cohort 2) | ||
| 5 mg/kg IV over 48 hours (≥6–<9 months) (Cohort 2) | ||
| 6 mg/kg IV over 48 hours (≥9 months) (Cohort 2) | ||
| CPM | 250 mg/m2 q 12 h × 4 doses, days 3 and 4 | |
| L-ASP | 6000 IU/m2 × 8 doses 3 × week IM | |
| G-CSF | 5 ug/kg/day beginning Day 5 through count recovery | |
| ITT | Days 1, 15 | |
| Consolidation | MTX | 4 gm/m2 × 2, Days 1, 8 |
| (Week 11–17) | VP-16 | 100 mg/m2 × 5 days, Days 15–19 |
| CPM | 300 mg/m2 × 5 days, Days 15–19 | |
| G-CSF | 5 ug/kg/day beginning Day 20 until count recovery | |
| AraC | 3 gm/m2 dose q 12 hours × 4 doses Day 29, 30 | |
| L-asparaginase 6000 units/m2 IM Days 31 | ||
| G-CSF | 5 ug/kg/day beginning Day 232 until count recovery | |
| ITT | Day 1 | |
| Maintenance | VCR | 0.05 mg/kg IV days Week 18, 22, 28, 32, 38, 42 |
| (Week 18–46) | DXM+ | 10 mg/m2 divided PO TID × 5 days with VCR (Cohort 1+2) |
| PDN | 40mg/m2 divided PO TID × 5 days with VCR (Cohort 3) | |
| VP-16 | 100 mg/m2/day × 5 days Week 25, 35 | |
| CPM | 300 mg/m2/day IV × 5 days Week 25, 35 | |
| G-CSF | 5 ug/kg/day beginning at completion VP-16/CPM until recovery | |
| 6MP | 75 mg/m2 PO daily and MTX 20 mg/m2 IM weekly | |
| Weeks 19–21; 23–24; 29–31; 33–34; 39–41; 43–46 | ||
| ITT Weeks 18, 22, 28, 32, 38, 42 | ||
ALL DOSES IDENTICAL REGARDLESS OF AGE UNLESS OTHERWISE SPECIFIED (DNM; ITT);
Specific to Cohort 1 & 2 and in italics; PDN, prednisone; VCR, vincristine; DNM, daunomycin; L-ASP, L-asparaginase; 6-MP, mercaptopurine; CPM, cyclophosphamide; ARA-C, cytarabine; DXM, dexamethasone; MTX, methotrexate; CF, citrovorum factor (leucovorin); IT, intrathecal; ITT, intrathecal triple therapy (MTX-methotrexate; HC-hydrocortisone; ARA-C-cytosine arabinoside); VP-16, etoposide; IV ARA-C, cytosine arabinoside; G-CSF, colony stimulating factor. ITT Cohort 3: Age < 1 year: MTX – 7.5mg; HC 7.5 mg; AraC – 15 mg, Age≥ 1 year: MTX – 8mg; HC – 8 mg; AraC – 16 mg; ITT Cohort 1+2: All ages: MTX – 7.5mg; HC 7.5mg; AraC 15 mg
Based on the high incidence of early induction deaths observed in Cohort 1+2, Cohort 3 included strict guidelines to reduce the risk of death from infection: mandatory contact with the study PI with all enrollments and with grade 3–4 toxicities; hospitalization for a minimum of 14 days but preferably 21 days post beginning induction; intravenous immunoglobulin supplementation when IgG levels dropped below 400mg/dl; broad spectrum antibiotic coverage to begin immediately with fever; antifungal therapy should fever persist more than 4 days; Respiratory syncytial virus (RSV) prophylaxis during RSV season. Infants greater than two months of age received Bactrim prophylaxis though no other antibiotic prophylaxis was recommended.
Statistical methods
Study data for COG P9407 were frozen as of February 1, 2010. Event-free survival (EFS) was defined as the time from study entry to first event (induction failure, relapse, second malignant neoplasm (SMN), or death) or date of last contact. Overall survival (OS) was defined as the time from study entry to time of death or date of last contact. Time to relapse was defined as the time from date of diagnosis to date of relapse. Complete continuous response (CCR) was defined as the time from end of Induction Intensification to first event (relapse, SMN,, or death) or date of last contact among those who achieved a CR by the end of Induction Intensification. Estimates for EFS and OS were calculated using the Kaplan-Meier method, and standard errors of the estimates were obtained by the method of Peto and Peto (27). The log-rank test was used to compare survival curves between groups. Cumulative incidence rates were estimated using the method of Gray (28). Cox proportional hazards regression and a proportional sub-distribution hazards model proposed by Fine and Gray (29) were used to identify significant prognostic factors for EFS and cumulative incidence function of competing risks data, respectively. The Chi-square test and Fisher’s exact test were used to compare proportions. Statistical significance was defined as a p-value less than 0.05. All analyses were performed using SAS® software. All graphics were generated using R (http://www.R-project.org, version 2.13.1).
RESULTS
Patient Population
COG P9407 Cohort 3 enrolled 147 infants with ALL. Six patients were ineligible: 4 9 (misdiagnosed); 2 (treatment started prior to enrollment). Characteristics of eligible patients are summarized in Table II. Median age was 208 days (range 1–365 days); 19.2% were ≤ 90 days old at diagnosis. Median WBC was 141,000/microliter (0.6–1,500,000/microliter), with 71.6% having a WBC >50,000/microliter. Approximately thirty-two percent (45/141) were CNS2 (< 5 WBC/microliter with blasts) and 16.3% (25/141) were CNS3 (≥ 5 WBC/microliter with blasts) at diagnosis. Of those 23 patients considered to have CNS3 disease, 7 had bloody taps. Of 135 infants with informative MLL results, 70.9% had an MLL gene rearrangement. Within the group with informative MLL results, 67/100 had MLL partner genes associated with a poor prognosis particularly in the younger infants: AF4 (47) and ENL (22). A separate report describes preliminary analyses of outcome as related to the different MLL partner genes (30).
Table II.
Patient Characteristics for Cohort by Age at Diagnosis (≤ 90 days, > 90 days)
| Overall Total n (%) |
Cohort 3 (N=141)
|
|||
|---|---|---|---|---|
| ≤ 90 days of age n (%) |
> 90 days of age n (%) |
p-value (age comparison for Cohort 3) | ||
|
Race | ||||
| Black | 9 (6.4) | 3 (11.1) | 6 (5.3) | |
| White | 115 (81.6) | 21 (77.8) | 94 (82.5) | 0.58 |
| Other/Unknown | 17 (12.1) | 3 (11.1) | 14 (12.3) | |
|
Gender | ||||
| Male | 80 (56.7) | 18 (66.7) | 62 (54.4) | 0.29 |
| Female | 61 (43.3) | 9 (33.3) | 52 (45.6) | |
|
WBC | ||||
| < 50K | 40 (28.4) | 1 (3.7) | 39 (34.2) | |
| 50K – 299K | 58 (41.1) | 10 (37.0) | 48 (42.1) | 0.0002 |
| ≥300K | 43 (30.5) | 16 (59.3) | 27 (23.7) | |
|
CNS status at diagnosis | ||||
| CNS 1 | 73 (51.8) | 4 (14.8) | 69 (60.5) | |
| CNS 2 | 45 (31.9) | 12 (44.4) | 33 (29) | <0.0001 |
| CNS 3 | 23 (16.3) | 11 (40.7) | 12 (10.5) | |
|
CD 10 status | ||||
| CALLA + | 21 (14.9) | 2 (7.4) | 19 (16.7) | 0.64 |
| CALLA − | 17 (12.1) | 3 (11.1) | 14 (12.3) | |
|
MLL status | ||||
| MLL-R | 100 (70.9) | 22 (81.5) | 78 (68.4) | 0.22 |
| MLL-G | 35 (24.8) | 4 (14.8) | 31 (27.2) | |
|
Death (first event) | ||||
| Induction death | 8 (5.7) | 4 (14.8) | 4 (3.5) | 0.35 |
| Remission death | 13 (9.2) | 3 (11.1) | 10 (8.8) | |
Note: 110 patients with missing CD10 status; 5 patients with unknown/missing MLL status
Treatment outcomes
Response Rate
Complete remission was determined at the completion of Induction Intensification prior to beginning Re-induction at Week 8 of therapy. Six patients were not evaluable for response at the end of Induction Intensification: wrong induction steroid (1); unable to perform marrow due to clinical status (4); unknown (1). The overall CR rate was 91.8%% (123/134), 3 patients had refractory leukemia, and 8 had an early treatment related death (within 90 days of enrollment) while receiving Induction/Induction Intensification (24).
Event-Free and Overall Survival
The 5-year EFS was 42.3 ± 6% and 5-year OS was 52.9 ± 6.5% for infants treated in Cohort 3(Figure 1). The 5-year EFS rate was 15.5 ± 10.1% for infants ≤ 90 days and 48.5 ± 6.7% for those > 90 days (p<0.0001; Figure 2). The median EFS was 552 days and median overall survival 1102 days. EFS in three month age increments was 15.5 ± 10.1% (≤ 90 days), 31.5 ± 9.9% (>3 months to 6 months), 43.4 ± 12.3% (>6 months to 9 months) and 67.5 ± 10.7% (>9 months to 12 months. Cumulative incidence rates at five years were 39.0 ± 4.3% for relapse, 15.1 ± 3.0% for death, 2.13 ± 1.2% for progressive disease and 1.45 ± 1.0% for second malignancy (Supplemental Figure 1).
Figure 1. Overall survival (OS) and event free survival (EFS) for Cohort 3.
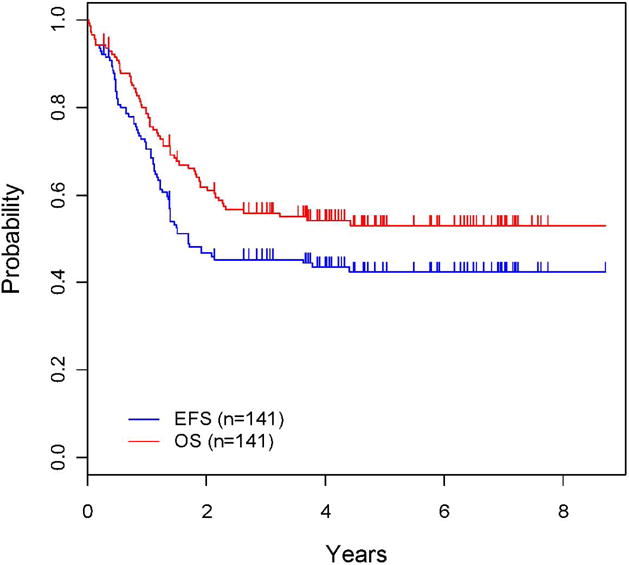
The OS and EFS are presented.
Figure 2. EFS curves for Cohort 3: ≤90 days vs. > 90 days at diagnosis.
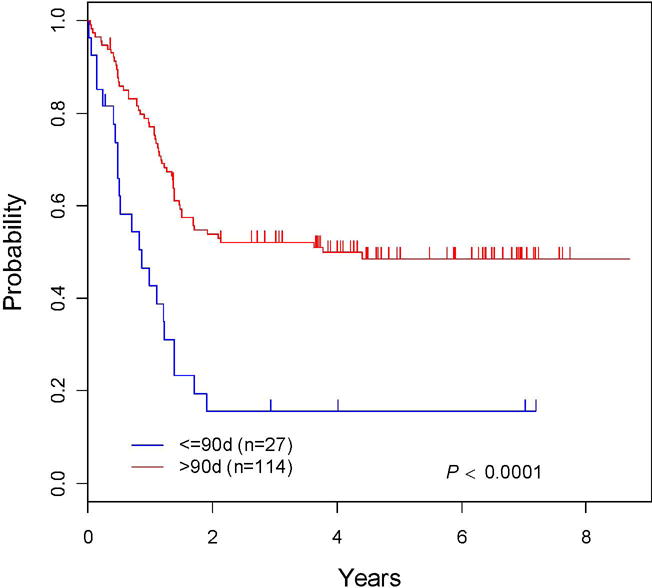
The EFS is compared by age at diagnosis: ≤90 days vs. > 90 days.
Causes and Timing of Treatment Failure
The median time to relapse in Cohort 3 was 402 days. There were 53 disease related events among 134 patients: 27 marrow, 8 testes, 7 blood (> 25% blasts in peripheral exam +/− marrow confirmation), 4 isolated CNS, 3 marrow + CNS, 1 subcutaneous tissue, 1 marrow + CNS + other, 1 CNS + other, and 1 other (cerebrospinal fluid, meninges and spaces, dural sinus, ventricular system and choroid plexus). Twenty-three (43.4%) relapses occurred within one year of diagnosis, with 9 (17%) occurring < 6 months post diagnosis and 16 (30%) < 9 months post diagnosis. Twelve were isolated marrow relapses. An additional 25 relapses (47.2%) occurred between 1 and 2 years following diagnosis. There were only three late relapses occurring >2.5 years post diagnosis. Those relapses occurred at 1,326 days (MLL-R marrow), 1,381 days (MLL-G subcutaneous tissue), and 1,611 days (MLL-R testes).
Early toxic deaths within 90 days of diagnosis occurred in 8/141 patients. Five deaths occurred within the first 30 days following diagnosis: infection (3), tumor lysis syndrome (1), pulmonary hemorrhage (1). Infectious deaths in the first 90 days included: Pseudomonas spp (2); Adenovirus (1); Candida spp (1); Staphylococcus spp (1); sepsis nos. In comparison, 17/68 patients in Cohort 1+2 experienced early toxic deaths of which 14/17 were infection related: RSV (3); Aspergillus spp. (2); Enterobacter spp. (2); Enterococcus spp. (1); Staphylococcus spp. (1); Pneumocystis jiroveci (1); Pseudomonas spp. (1); sepsis nos (3) described in detail by Salzer et al (24). Treatment related deaths in Cohort 3 that occurred greater than 90 days following diagnosis were due to infection/septic shock (4), fungal pneumonia (1). Stevens-Johnson syndrome (1), pulmonary edema (1), accidental death (1) and unknown (1).
Non-fatal Toxicities
The most common grade 3–4 toxicities that occurred in Cohort 3 included hematologic toxicity, bacterial sepsis/infection NOS, liver enzyme (ALT/AST) elevation, diarrhea and stomatitis (Supplemental Table I). The worst grade for each toxicity was reported comparing Induction/Induction Intensification (I/II) to post Induction Intensification (post I/II) therapy. Though not reported by specific phases post I/II (Re-Induction, Consolidation, Continuation), it is reasonable to assume the peak in infection rates and hematologic toxicity were a result of toxicity experienced during Consolidation, identical to II with an additional course of high dose cytarabine. Liver enzyme elevation was observed during both phases temporarily related to high dose methotrexate with 14% in I/II and 19.8% post I/II experiencing grade 3–4 toxicity yet returned to less that grade 3 quickly. Central nervous system events (seizures) were more common in infants < 90 days of age at diagnosis and occurred in 5/27 infants: febrile with high dose cytarabine (1); as a result of hypocalcemia (2); post IT methotrexate (1); associated with CNS hemorrhage at diagnosis (1). There were 8 CNS events in 115 infants > 90 days of age at diagnosis of which four events were seizures: etiology unclear (1); hemorrhage (1); hyponatremia with vincristine (1); iatrogenic hyperglycemia (1).
Prognostic factors
Important prognostic factors included age, initial WBC and MLL status (Table III). Age ≤ 90 days at diagnosis was a highly significant negative risk factor for EFS (Figure 2). The 5-year EFS rates were 69.7 ± 12.8% for the patients with MLL-G vs. 35.5 ± 6.4% for the infants with MLL-R (p<0.0008; Supplemental Figure 2). There was a statistically significant difference (p<0.0001) in EFS for age (≤ 90 days/> 90 days) by MLL status (MLL-R/MLL-G). Among infants ≤ 90 days, the 5 year EFS was 75 ±37.5% for MLL-G but only 4.8 ± 4.7% for MLL-R (p=<0.0001). The MLL-G group was extremely small including only 4 infants. The 5-year EFS rates were 69.1 ± 13.6% and 43.8 ± 7.5% for infants >90 days who were MLL-G and MLL-R, respectively (p<0.0207; Figure 3). Among infants 3 – 6 months of age, the 5-year EFS was 50 ± 20.4% and 32.1 ± 7.98% for MLL-G and MLL-R, respectively (p=0.36).
Table III.
5-year EFS (±SE) for Cohort 3
| Variable | Cohort 3
|
||
|---|---|---|---|
| n | 5 yr EFS ± s.e. (%) |
p-value | |
|
| |||
| MLL Status | |||
| MLL-R | 100 | 35.5± 6.4 | 0.0008 |
| MLL-G | 35 | 69.7 ± 12.8 | |
|
| |||
| WBC Count | |||
| WBC < 50k | 40 | 73.8 ± 9.4 | <0.0001 |
| WBC ≥ 50k | 101 | 30.2 ± 7 | |
|
| |||
| WBC Count | |||
| WBC < 300k | 98 | 50.1 ± 7.1 | 0.002 |
| WBC ≥ 300k | 43 | 25.1 ± 10.9 | |
|
| |||
| CNS Status | 73 | ||
| CNS 1 | 45 | 47.3 ± 8.1 | 0.17 |
| CNS 2 | 23 | 37.8 ± 10.5 | |
| CNS 3 | 35.2 ± 16.4 | ||
|
| |||
| Age at diagnosis | |||
| Age ≤ 90 days | 27 | 15.5 ± 10.1 | <0.0001 |
| Age > 90 days | 114 | 48.5 ± 6.7 | |
|
| |||
| WBC/Age at diagnosis | |||
| <50k, ≤90 days | 1 | — | <0.0001 |
| <50k, >90 days | 39 | 73.7 ± 9.5 | |
| ≥50k, ≤90 days | 26 | 15.4 ± 10 | |
| ≥50k, >90 days | 75 | 35.4 ± 8.6 | |
|
| |||
| WBC/Age at diagnosis | |||
| <300k, ≤90 days | 11 | ---- | <0.0001 |
| <300k, >90 days | 87 | 53.7 ± 7.3 | |
| ≥300k, ≤90 days | 16 | 12.5 ± 8.3 | |
| ≥300k, >90 days | 27 | 32.4 ± 18.8 | |
|
| |||
| Age/MLL | |||
| ≤90 days/G | 4 | 75 ± 37.5 | <0.0001 |
| ≤90 days/R | 22 | 4.8 ± 4.7 | |
| >90 days/G | 31 | 69.1 ± 13.6 | |
| >90 days/R | 78 | 43.8 ± 7.5 | |
|
| |||
| Age/MLL | |||
| 0 to <=3 months | 27 | 15.5 ± 10.1 | <0.0001 |
| >3 to 6 months | 34 | 31.5 ± 9.9 | |
| >6 to 9 months | 36 | 43.4 ± 12.3 | |
| >9 to 12 months | 44 | 67.5 ± 10.7 | |
Figure 3. EFS curves for Cohort 3: MLL vs. age at diagnosis.
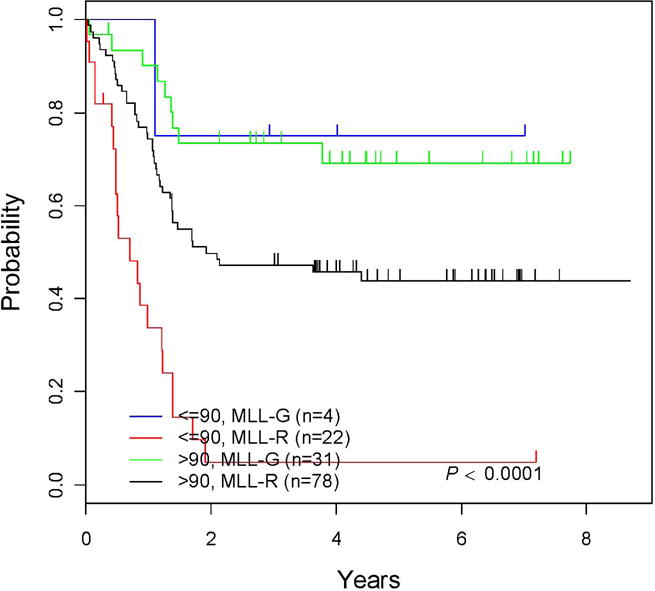
The EFS using MLL status vs. age at diagnosis.
The WBC at diagnosis was a significant prognostic factor, with a 5-year EFS of 73.8 ± 9.4% for those with a presenting WBC < 50,000 × 109/l versus 30.2 ± 4.7% for those with WBC ≥50,000 × 109/l (p<0.0001; Supplemental Figure 3). A presenting WBC above or below 300,000 × 109/l has been an important prognostic factor in some infant ALL studies (14, 16) and our data also demonstrated a statistically significant difference in EFS between these groups (p=0.0020) (Table III). Age at diagnosis was also a significant factor in determining the impact of WBC on EFS (Supplemental Figure 4 and Table III).
Central nervous system status (CNS1, 2 or 3) did not have a statistically significant impact on EFS, although there was a non-significant trend toward better 5-year EFS for patients that were CNS1 (47.3 ± 8.1%) versus those who were CNS 2 (37.8 ± 10.5% or CNS3 (35.2 ± 16.4%) (Supplemental Figure 5). There was an association between WBC and age (p=0.0002) and CNS status and age (p<0.0001).
Age at diagnosis, initial WBC, MLL status, and CNS status were included in the Cox proportional hazards regression model (Supplemental Table II). Adverse prognostic factors included age ≤ 90 days (HR=2.29, p=0.0058), MLL-R. (HR=2.95, p=0.0021) and WBC ≥ 50,000 × 109/l (HR=3.4, p=0.0017). These 3 factors were also significant in the multivariate analyses. Supplemental Table III describes events that occurred in the MLL-G and MLL-R subgroups. Significant prognostic factors for relapse were identified using a subdistribution hazard model: MLL-R (subdistribution hazard ratio [sHR] = 3.16 and WBC ≥ 50,000 × 10 9/ul (sHR =4, p=0.064). Age at diagnosis (≤ 90 days/>90 days) and CNS status (CNS 1/2) were not significant prognostic factors in this hazard ratio (Supplemental Table 4).
DISCUSSION
Infant ALL is far more difficult to treat and cure than ALL occurring in children older than one year. Risk factors associated with a poorer prognosis include: MLL gene rearrangement (10–11, 15,19–23), absence of CD10 expression (7,9) co-expression of myeloid antigens (31), higher WBC counts (9–11,32–34), organomegaly (8) and younger age (less than 3–6 months) (9–12). Gender is not prognostic in infant ALL (9–10,12,20,31). Until recently, most studies used a traditional approach to ALL therapy including prolonged maintenance therapy (typically lasting 2 years) and dose reductions based on age and weight.
Outcome prior to the late 1990s was uniformly poor with 4-year EFS of less than 40% for combined populations of both MLL-R and MLL-G infant ALL and 20–30% for those with MLL-R ALL when evaluated independently (10–11,14–15, 32). Recently, EFS has improved somewhat. Japanese investigators reported a 3 year EFS of 43% in MLL-R infants > 6 months of age who underwent HSCT and a 95% EFS for a relatively small subgroup with MLL-G ALL (18, 35). The results for patients with MLL-G reported by the Japanese exceed any previous report, have not been replicated and are perplexing since more than 90% were male (33). The Dana Farber Cancer Institute Consortium reported results for infants with ALL that are somewhat better than those in this study, but the population appears different as fewer than half of the infants reported in those trials were either CD10 negative or MLL-R (32). The largest infant ALL trial reported to date is the European Interfant-99 study in which 482 infants were stratified by prednisone prophase response and randomized to +/– late intensification and 8% of patients underwent HSCT (16, 36). The 4-year EFS was 47% for all patients, 37% for MLL-R and 74% for patients with MLL-G (16). In the CCG 1953 trial, a parallel pilot to COG P9407 Cohorts 1 and 2 for the first nine weeks of treatment, overall EFS was 41.7% (17).
COG P9407 delivered shortened, highly intensified therapy with the elimination of age and weight related dose reductions for most chemotherapy agents. However, vincristine was dosed by mg/kg and ITT dosed by age. Daunomycin, originally dosed in mg/m2 continuous infusion over 48 hours was not tolerated in Cohort 1 and dosing changed to mg/kg/age continuous infusion over 48 hours in Cohort 2. Ultimately, daunomycin was changed to mg/kg/day IV over 30 minutes in infants ≤ 90 days in Cohort 2 though remained continuous infusion in older infants. These changes had little impact on toxicity in Cohort 2. In Cohort 3, daunomycin was given as mg/kg/age daily × two days IV push. Following a switch in induction steroid from dexamethasone in Cohorts 1+2 to less immunosuppressive prednisone in Cohort 3, acceptable rates of treatment related mortality were observed in the latter cohort although higher than that seen in older children with ALL.
Parallel pilots, POG 9407 (Cohort 1+2) and CCG 1953, had identical therapy until the time at which eligible MLL-R infants could receive HSCT. An analysis of the role of HSCT in 53 infants compared with 47 who received chemotherapy alone demonstrated no advantage for HSCT over chemotherapy with 5-year EFS of 48.8% vs. 48.7%, respectively (25). Therefore, unlike other recent studies, HSCT was not included as part of the therapy for Cohort 3. Thus, this study is the largest cohort of infants with ALL that received uniform treatment without HSCT. Several important conclusions can be drawn from this study, particularly in conjunction with results of Interfant-99 (16). Overall, the results obtained in these two studies were quite similar: P9407 5-year EFS 42% vs. Interfant 4 year EFS 47%; MLL-R -P9407 5-year EFS 36% vs. Interfant 4 year EFS 37%; MLL-G - P9407 5-year EFS 70% vs. Interfant 4 year EFS 74%. While the comparability of the results of P9407 Cohort 3 (46 weeks of treatment/no HSCT) and Interfant-99 (24 months of treatment with +/− HSCT) raise questions about how HSCT or prolonged therapy affect long term survival, more than half of infants with ALL die including almost two-thirds of those with MLL-R.
Intensified therapy given in Cohort 3 did not appear to affect early relapse, the major cause of treatment failure on this study. Twenty three of 53 (43.4%) total relapses occurred on therapy within one year of diagnosis and another 25 (47.2%) relapses occurred between 1 and 2 years following diagnosis. Thus further intensification alone with conventional agents is unlikely to offer meaningful improvements in outcome for infant ALL. Late relapses that occurred > 2.5 years after diagnosis were rare. The very high relapse rate observed within 12 months of cessation of therapy using an early intensification platform prompted the COG to extend maintenance therapy until two years following diagnosis in the successor COG AALL0631 trial.
The early toxic death rate was much improved in Cohort 3 (8/141= 5.6%) compared with Cohort 1+2 (18/70 = 25.7%) due primarily to the substitution of prednisone for dexamethasone during induction though infants < 90 days of age at diagnosis remained at highest risk (24). Overall, treatment related deaths (induction or in CCR within one month of completion of therapy) on P9407 Cohort 3 were 13% (19/141). Treatment related deaths on Interfant-99 were 9.5% (46/482) but the death rate did not differ by age at diagnosis (16). Grade 3–4 toxicities, in particular bacterial sepsis/infection NOS, stomatitis and neurologic toxicity in Cohort 3 were not significantly different than observed in Interfant-99.
The poor outcomes for infants with ALL in these very large clinical trials despite very intensive therapy and use of HSCT in some studies, suggest that novel approaches are needed to improve cure rates for this challenging disease. Efforts to exploit the underlying biology of MLL-R ALL may offer promise in developing novel approaches to therapy. Gene expression analyses have demonstrated very high levels of the FMS-like tyrosine kinase 3 (FLT3) gene in infants and children with MLL-R ALL (37–39). Although clinical responses using lestaurtinib in adults with relapsed/refractory AML have been mixed, encouraging preclinical data have been reported using the FLT3 inhibitor, lestaurtinib, in ALL cell lines and marrow samples from infants and children with various subtypes of ALL (38, 40, 41). The COG is currently conducting a clinical trial (AALL0631; #NCT00557193) with a chemotherapy backbone based on COG P9407 that includes intensification and prolonged maintenance therapy +/− lestaurtinib for infants with MLL-R ALL. New understanding of the of MLL partner genes such as AF4 and ENL may contribute to a better understanding and the development of better treatment options for such infants. Interfant group continues to explore other modes of treatment intensification in Interfant-06 trial. Due to the small population of infants with ALL (<5% of all childhood ALL), efforts are underway to develop a transatlantic collaboration for future trials. This next study proposes to study epigenetic based therapy in addition to chemotherapy given the widespread epigenetic alterations in MLL-R infant ALL.
Supplementary Material
Acknowledgments
Sincere thanks to Patricia A. Dinndorf, MD for her work in the development of and oversight as the Principal Investigator for Children’s Cancer Group (CCG) 1953 – the parallel pilot study to COG P9407.
Grant support: This work was supported by grants from the NCI to the Children’s Oncology Group including U10 CA98543 (COG Chair’s grant), U10 CA98413 (COG Statistics & Data Center), and U24 CA114766 (COG Specimen Banking), R01CA80175 (CAF), and Leukemia & Lymphoma Society SCOR 7372-07 (CAF). SPH is the Ergen Family Chair in Pediatric Cancer. CAF is the Joshua Kahan Endowed Chair in Pediatric Leukemia.
Footnotes
Prior presentation:
These data were presented in part at the American Society of Pediatric Hematology-Oncology 2007 (abstract-oral presentation) and the 39th Congress of the International Society of Pediatric Oncology in Mumbai, India 2007.
Conflict of Interest
The following authors have nothing to disclose: ZoAnn E. Dreyer, Joanne M. Hilden, Meenakshi Devidas, Cheryl L. Willman, I-Ming Chen, Brent L. Wood, Andrew J. Carroll, Nyla A. Heerema, Blaine Robinson, Stephen P. Hunger, Fred G. Behm, William L. Carroll, Bruce M. Camitta, Jeanette Pullen, Tamekia L. Jones, Gregory H. Reaman, Wanda L. Salzer, Naomi J. Winick, Richard C. Harvey. Carolyn A. Felix has the following disclosure: Carolyn A. Felix owns the following patent: Methods and Kits for Analysis of Chromosomal Rearrangements Associated with Leukemia – US Patent #6,368,791 (issued April 9, 2002).
Author Contribution/Justification:
I: Designed research, performed research, collected data, analyzed and interpreted data, wrote manuscript: ZoAnn E. Dreyer MD, Joanne M. Hilden MD, Gregory H. Reaman MD, Stephen P. Hunger MD, William L. Carroll, Bruce M. Camitta MD. II. Performed research, collected data, analyzed and interpreted data, wrote manuscript: Wanda L. Salzer MD, Jeanette Pullen MD, Naomi J. Winick MD III: Designed research, collected data, analyzed and interpreted data, performed statistical analyses, wrote manuscript: Tamekia Jones PhD, Meenakshi Devidas PhD. IV: Contributed analytical tools, collected data, analyzed and interpreted data, wrote manuscript: Cheryl L. Willman MD, Richard C. Harvey PhD, I-Ming Chen PhD, Fred G. Behm MD, Brent L. Wood MD PhD, Andrew J. Carroll PhD, Nyla A. Heerema PhD, Carolyn A. Felix MD, Blaine Robinson PhD
References
- 1.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the Children’s Oncology Group studies for childhood acute lymphoblastic leukemia 1983–2002: A Children’s Oncology Group report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. Epub 2009 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moricke A, Zimmerman M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM group 1981 to 2000. Leukemia. 2010;24(2):265–84. doi: 10.1038/leu.2009.257. Epub 2009 Dec 10. [DOI] [PubMed] [Google Scholar]
- 3.Salzer WL, Devidas M, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: a report from the Children’s Oncology Group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman LB, Stevenson KE, O’Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24:320–34. doi: 10.1038/leu.2009.253. Epub 2009 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–82. doi: 10.1038/leu.2009.252. Epub 2009 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia from 1990–2005: A report from the Children’s Oncology Group. Journal of Clinical Oncology. 30:1663–69. doi: 10.1200/JCO.2011.37.8018. Epub 2012 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reaman GH, Zeltzer P, Bleyer WA, et al. Acute lymphoblastic leukemia in infants less than one year of age; a cumulative experience of the Children’s Cancer Study Group. J Clin Oncol. 1985;3:1513–21. doi: 10.1200/JCO.1985.3.11.1513. [DOI] [PubMed] [Google Scholar]
- 8.Lauer SJ, Camitta BM, Leventhal BG, et al. Intensive alternating drug pairs after remission induction for treatment of infants with acute lymphoblastic leukemia; a Pediatric Oncology Group pilot study. J Pediatr Hematol Oncol. 1998;20:229–33. doi: 10.1097/00043426-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Chessells JM, Eden OB, Bailey CC, et al. Acute lymphoblastic leukemia in infancy; experience in MRC UKALL trials. Report from the Medical Research Council Working Party on Childhood Leukemia. Leukemia. 1994;8:1275–9. [PubMed] [Google Scholar]
- 10.Dordelmann M, Reiter A, Borkhardt A, et al. Prednisone response is the strongest predictor of treatment outcomein infant acute lymphoblastic leukemia. Blood. 1999;94:1209–17. [PubMed] [Google Scholar]
- 11.Reaman GH, Sposto R, Sensel MG, et al. Treatment outcome and prognostic factors for infants with acute lymphoblastic leukemia treated on two consecutive trails of the Children’s Cancer Group (see comments) J Clin Oncol. 1999;17:445–55. doi: 10.1200/JCO.1999.17.2.445. [DOI] [PubMed] [Google Scholar]
- 12.Frankel LS, Ochs J, Shuster JJ, et al. Therapeutic trial for Infant acute lymphoblastic leukemia: the Pediatric Oncology Group experience (POG 8493) J Pediatr Hematol Oncol. 1997;19:35–7. doi: 10.1097/00043426-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ferster A, Bertrand Y, Benoit Y, et al. Improved survival for acute lymphoblastic leukaemia in infancy: Experience of EORTC-Childhood Leukaemia Cooperative Group. Br J Haematol. 1994;86:284–90. doi: 10.1111/j.1365-2141.1994.tb04727.x. [DOI] [PubMed] [Google Scholar]
- 14.Chessells JM, Harrison CJ, Kempski H, et al. Clinical Features, cytogenetics and outcome in acute lymphoblastic and myeloid leukaemia of infancy: report from the MRC Childhood Leukaemia working party. Leukemia. 2002;16:776–84. doi: 10.1038/sj.leu.2402468. [DOI] [PubMed] [Google Scholar]
- 15.Isoyama K, Eguchi M, Hibi S, et al. Risk-directed treatment of infant acute lymphoblastic leukaemia based on early assessment of MLL gene status: results of the Japan infant Leukaemia Study (MLL96) Br J Hoematol. 2002;118:999–1010. doi: 10.1046/j.1365-2141.2002.03754.x. [DOI] [PubMed] [Google Scholar]
- 16.Pieters R, Schrappe M, De Lorenzo P, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukemia (Interfant-99): an observational study and multicentre randomized trial. Lancet. 2007;370:240–50. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 17.Hilden JM, Dinndorf PA, Meerbaum SO, et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children’s Oncology Group. Blood. 2006;108:441–51. doi: 10.1182/blood-2005-07-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosata Y, Koh K, Kinukawa N, et al. Infant acute lymphoblastic leukemia with MLL gene rearrangements: outcome following intensive chemotherapy and hematopoietic stem cell transplantation. Blood. 2004;104:3527–34. doi: 10.1182/blood-2004-04-1390. [DOI] [PubMed] [Google Scholar]
- 19.Hilden JM, Frestedt JL, Moore RO, et al. Molecular analysis of infant acute lymphoblastic leukemia: MLL gene rearrangement and reverse transcriptase-polymerase chain reaction for t(4;11)(q21:q23) Blood. 1995;86:3876–82. [PubMed] [Google Scholar]
- 20.Cimino G, Rapanotti MC, Rivolta A, et al. Prognostic relevance of ALL-1 gene rearrangement in infant acute leukemias. Leukemia. 1995;9:391–5. [PubMed] [Google Scholar]
- 21.Chen CS, Sorensen PH, Domer PH, et al. Molecular rearrangements on chromosome 11q23 predominate in infant acute lymphoblastic leukemia and are associated with specific biologic variables and poor outcome. Blood. 1993;81:2386–93. [PubMed] [Google Scholar]
- 22.Rubnitz JE, Link MP, Shuster JJ, et al. Frequency and prognostic significance of HRX rearrangements in infant acute lymphoblastic leukemia: A Pediatric oncology group study. Blood. 1994;84:570–3. [PubMed] [Google Scholar]
- 23.Heerema NA, Arthur DC, Sather H, et al. Cytogenetic features of infants less than 12 months of age at diagnosis of acute lymphoblastic leukemia: impact of the 11q23 breakpoint on outcome: a report of the Children’s Cancer Group. Blood. 1994;83:2274–84. [PubMed] [Google Scholar]
- 24.Salzer W, Jones T, Devidas M, et al. Modification of induction therapy decreased risk of early death in infants with acute lymphoblastic leukemia treated on Children’s oncology Group study P9407. Pediatric Blood and Cancer. 2012;59:834–839. doi: 10.1002/pbc.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreyer Z, Dinndorf P, Camitta B, et al. Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: A report from the Children’s Oncology Group. J Clin Oncol. 2011;29(2):214–22. doi: 10.1200/JCO.2009.26.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson BW, Felix CA. Panhandle PCR approaches to cloning MLL genomic breakpoint junctions and fusion scripts. Methods Mol Biol. 538:85–114. 209. doi: 10.1007/978-1-59745-418-6_6. [DOI] [PubMed] [Google Scholar]
- 27.Peto R, Peto J. Asymptotically efficient rank invariant test procedure. J Royal Stat. 1972;135:185–98. [Google Scholar]
- 28.Gray R. A class of K-sampling tests for comparing the cumulative incidence of a competing risk. Annal Stat. 1988;16:1141–1154. [Google Scholar]
- 29.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Jrnl of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 30.Robinson B, Devidas M, Carroll WL, et al. Specific MLL partner genes in infant acute lymphoblastic leukemia (ALL) associated with outcome are linked to age and white blood cell count (WBC) at diagnosis: A report of the Children’s Oncology group (COG) P9407. Blood (American Society of Hematology Annual Meeting Abstracts) 2009 Nov;114:907. [Google Scholar]
- 31.Basso G, Putti MC, Cantu-Bajnoldi A. The immunophenotype In infant acute lymphoblastic leukemia; correlation with clinical outcome. An Italian multicentre study (AIEOP) Br J Haematol. 1992;81:184–91. doi: 10.1111/j.1365-2141.1992.tb08205.x. [DOI] [PubMed] [Google Scholar]
- 32.Silverman LB, McLean TW, Gelber RD, et al. Intensified therapy for infants with acute lymphoblastic leukemia –Results from the Dana Farber Cancer Institute Consortium. Cancer. 1997;80:2285–95. [PubMed] [Google Scholar]
- 33.Ishii E, Okamura J, Tsuchida M, et al. Infant leukemia in Japan: clinical and biological analysis of 48 cases. Med Pediatr Oncol. 1991;19:28–32. doi: 10.1002/mpo.2950190106. [DOI] [PubMed] [Google Scholar]
- 34.Pui CH. Acute leukemia in children. Curr Opin Hematol. 1996;3:249–58. doi: 10.1097/00062752-199603040-00002. [DOI] [PubMed] [Google Scholar]
- 35.Nagayama J, Tomizawa D, Koh K, et al. Infants with acute lymphoblastic leukemia and germline MLL gene are highly curable with use of chemotherapy alone: results from the Japan Infant Leukemia Study Group. Blood. 2006;107:4663–5. doi: 10.1182/blood-2005-11-4728. [DOI] [PubMed] [Google Scholar]
- 36.Mann G, Attarbaschi A, Schrappe P, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukaemia: Results from the Interfant-99 study. Blood. 2010;116:2644–50. doi: 10.1182/blood-2010-03-273532. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong SA, Staunton JE, Silverman LB, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong SA, Mabon ME, Silverman LB, et al. FLT3 mutations in childhood acute lymphoblastic leukemia. Blood. 2004;103:3544–3546. doi: 10.1182/blood-2003-07-2441. [DOI] [PubMed] [Google Scholar]
- 39.Brown P, Levis M, Shurtleff S, et al. FLT3 inhibition selectively skills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood. 2005;105:812–820. doi: 10.1182/blood-2004-06-2498. [DOI] [PubMed] [Google Scholar]
- 40.Smith BD, Levis M, Beran M, et al. Single agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 41.Levis M, Smith BD, Beran M, et al. A randomized, open-label study of lestaurtinib (CEP-701), an oral FLT3 inhibitor, administered in sequence with chemotherapy in patients with relapsed AML harboring FLT3 activating mutations: clinical response correlates with successful FLT3 inhibition. Blood. 2005;106:403a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


