Abstract
BACKGROUND
Preterm birth (PTB) (<37 completed weeks gestation) is a pathological outcome of pregnancy and a major global health problem. Babies born preterm have an elevated risk for long term adverse medical and neurodevelopmental sequelae. Substantial evidence implicates intrauterine infection and/or inflammation in PTB. However, these are often relatively late findings in the process, when PTB is inevitable. Identification of earlier markers of PTB may make successful intervention possible. Although select proteins, notably, those related to the inflammatory pathways, have been associated with PTB, there has been a lack of research into the role of other protein pathways in the development of PTB. The purpose of this study was to investigate, using a previously described biomarker discovery approach, a subset of circulating proteins and their association with PTB focusing on samples from early pregnancy.
OBJECTIVES
1) To perform a large-scale biomarker discovery, utilizing an innovative platform to identify proteins associated with preterm birth in plasma taken between 10 and 15 weeks’ gestation and, 2) To determine which protein pathways are most strongly associated with preterm birth. To address these aims we measured 1129 proteins in a plasma sample from early pregnancy using a multiplexed aptamer–based proteomic technology developed in Colorado by SomaLogic.
STUDY DESIGN
Using a nested case-control approach, we measured proteins at a single time point in early pregnancy in 41 women who subsequently delivered preterm and 88 women who had term uncomplicated deliveries. We measured 1129 proteins using a multiplexed aptamer–based proteomic technology developed by SomaLogic. Logistic regressions and random forests were used to compare protein levels.
RESULTS
The complement factors B and H and the coagulation factors IX and IX ab were the highest ranking proteins distinguishing cases of preterm birth from term controls. The top 3 pathways associated with preterm birth were the complement cascade, the immune system and the clotting cascade.
CONCLUSIONS
Using a discovery approach, these data provide further confirmation that there is an association of immune and coagulation–related events in early pregnancy with preterm birth. Thus, plasma protein profiles at 10–15 weeks of gestation are related to the development of preterm birth later in pregnancy.
Keywords: Complement system, coagulation cascade, early pregnancy, preterm birth
Introduction
Preterm birth (PTB) (<37 completed weeks gestation) is a pathological1 outcome of pregnancy and a major global health problem.2 Babies born preterm have an elevated risk for long term adverse medical and neurodevelopmental sequelae.3 The pathological mechanisms leading to PTB are complex with multiple pathways involved.3,4 Substantial evidence implicates intrauterine infection and/or inflammation in PTB.3 However, these are often relatively late findings in the process, when PTB is inevitable. Identification of earlier markers of PTB may make successful intervention possible. Although select proteins, notably, those related to the inflammatory pathways, have been associated with PTB, there has been a lack of research into the role of other protein pathways in the development of PTB.3
The purpose of this study was to investigate, using a previously described biomarker discovery approach,5,6 a subset of circulating proteins and their association with PTB focusing on samples from early pregnancy. The goals of this study, using a broad-based proteomic screening platform were to: 1) identify top ranked proteins associated with PTB in early (10 to 15 weeks’ gestation) pregnancy and, 2) determine the top ranked pathways associated with PTB. To address these aims we measured 1129 proteins in a plasma sample from early pregnancy using a multiplexed aptamer–based proteomic technology developed in Colorado by SomaLogic.5–7
MATERIAL AND METHODS
This is an analysis of data and samples collected as part of the Denver Complement Study.8–10 This prospective cohort study was approved by the Colorado Multiple Institutional Review Board. In brief, 1287 women were recruited in the first half of pregnancy from the University of Colorado Hospital prenatal clinics and two affiliated sites. Informed consent was obtained, and an additional EDTA-plasma tube was obtained with the routine prenatal labs. Data were gathered on the maternal medical and obstetrical history. After delivery, outcome data were collected and the gestational age at blood draw was assigned based on the best overall obstetrical estimate, incorporating assessment at the first visit and in the great majority, on early ultrasound examination.
We conducted a nested-case control study within this longitudinal cohort. We made an a priori decision to concentrate the analysis on women who had a blood sample taken between 10 and 15 weeks gestation (n = 856) to reduce the potential confounding effect of gestational age at blood draw and to consider early predictors of PTB. Only the first delivery of women who had greater than one delivery during the study period was included (6 records deleted) in the analysis. The following records were also removed: multiple births (n = 34), congenital or chromosomal anomalies or deliveries less than 20 weeks’ gestation (n = 24), lost to follow-up (n = 32), chronic medical disease (cardiac disease, chronic hypertension, coagulation disorders, uterine anomalies, type 1 diabetes, and autoimmune disease, n = 111), consent not provided to research outside of the primary study (n = 22), and, deviation in study protocol (missing or inadequate sample, n = 24). Following these exclusions 603 records remained in the analytic dataset (41 cases of PTB and 562 term controls). As we were interested in the pathogenic mechanisms of PTB, we excluded (n = 474) from the term deliveries records of patients with preeclampsia, gestational hypertension, disorders of placentation, intrauterine growth restriction, induction of labor or cesarean delivery. Therefore, all term controls had an uncomplicated spontaneous vaginal delivery. The exclusion of these comorbidities allowed a more systematic comparison with PTB thereby increasing power to find differences attributed to PTB without being obscured by additional complications. Ultimately, 41 cases of PTB and 88 term controls were included in the analytic dataset.
The primary outcome was PTB (between 20 and less than 37 completed weeks gestation) resulting from spontaneous PTB [spontaneous preterm labor (SPTL) or preterm premature rupture of the membranes (PPROM), n = 22] or from a medical indication (n = 19). Ten of the medically indicated PTB resulted from hypertensive diseases of pregnancy, 5 from placental problems and 4 from intrauterine growth restriction.
Sample preparation
Each sample was centrifuged within an average time of 9 minutes from phlebotomy (median = 6 minutes, range = 1 to 38 minutes).. The supernatant was removed, aliquotted, placed on dry ice and placed in a freezer at −80°C. All samples had one freeze thaw prior to proteomic analysis in this study.10
SomaLogic Technology:5
The SOMAscan proteomic assay is supported by a new generation of protein-capture Slow Off-rate Modified Aptamer (SOMAmer™) reagents. SOMAmers bind to pre-selected targets including proteins and peptides with high affinity and specificity. The SOMAscan multiplex assay consists of 1129 individual affinity molecules SOMAmer reagents (described in detail elsewhere5,6). In brief, a biological sample in each well of a 96 well plate is incubated with a mixture of the 1129 SOMAmer reagents. Two sequential bead-based immobilization and washing steps eliminate unbound or non-specifically bound proteins and the unbound SOMAmer reagents, leaving only protein target-bound SOMAmer reagents. These remaining SOMAmer reagents are isolated, and each reagent is quantified simultaneously on a custom Agilent hybridization array. The amount of each SOMAmer measured is quantitatively proportional to the protein concentration in the original sample.
Statistical Analysis
Descriptive statistics were calculated and compared across groups using t-tests or chi-squared tests. Concentrations for each of 1129 proteins were log (base 2) transformed and compared between PTB and term groups using a logistic regression. P-values were adjusted for multiple comparisons using the False Discovery Rate.11 Proteins that were significant in this analysis were evaluated across the three subgroups (term, medically indicated and spontaneous PTB) using an Analysis of Variance. Random forests consisting of 5,000 classification trees were used to multivariately evaluate proteins, the mean decrease in the Gini index was used to rank proteins and the out-of-bag error rate was used to describe classification error.12 Proteins were classified into pathways using Reactome13 and were ranked using Fisher’s combined probability test which assesses the association for the pathway by combining test statistics from the individual proteins contained within that pathway.
RESULTS
Baseline statistics on select variables (maternal age, race, parity, and gestational age at the first prenatal visit) from the 41 cases of preterm birth (22 spontaneous and 19 medically indicated) and 88 uncomplicated term deliveries from the nested case control study show comparable demographics between groups, with the exception of race (Table 1).
Table 1.
Characteristics of Women with Preterm Birth and Term Deliveries
| Term Deliveries n = 88 |
All PTB n = 41 |
P Value | ||
|---|---|---|---|---|
| Mean maternal age ±SD (years) | 34.2 (4.0) | 33.7 (7.2) | 0.60 | |
| Mean Gestational age at blood draw ±SD (weeks) | 12.4 (1.0) | 12.3 (1.1) | 0.62 | |
| Race/ethnicity | ||||
| Non-Hispanic White | 78 (89%) | 24 (59%) | ||
| Hispanic | 7 (8%) | 12 (29%) | ||
| African American | 1 (1%) | 3 (7%) | ||
| Asian and other race/ethnicities | 2 (2%) | 2 (5%) | < 0.01 | |
| Nulliparous | 43 (49%) | 16 (39%) | 0.30 | |
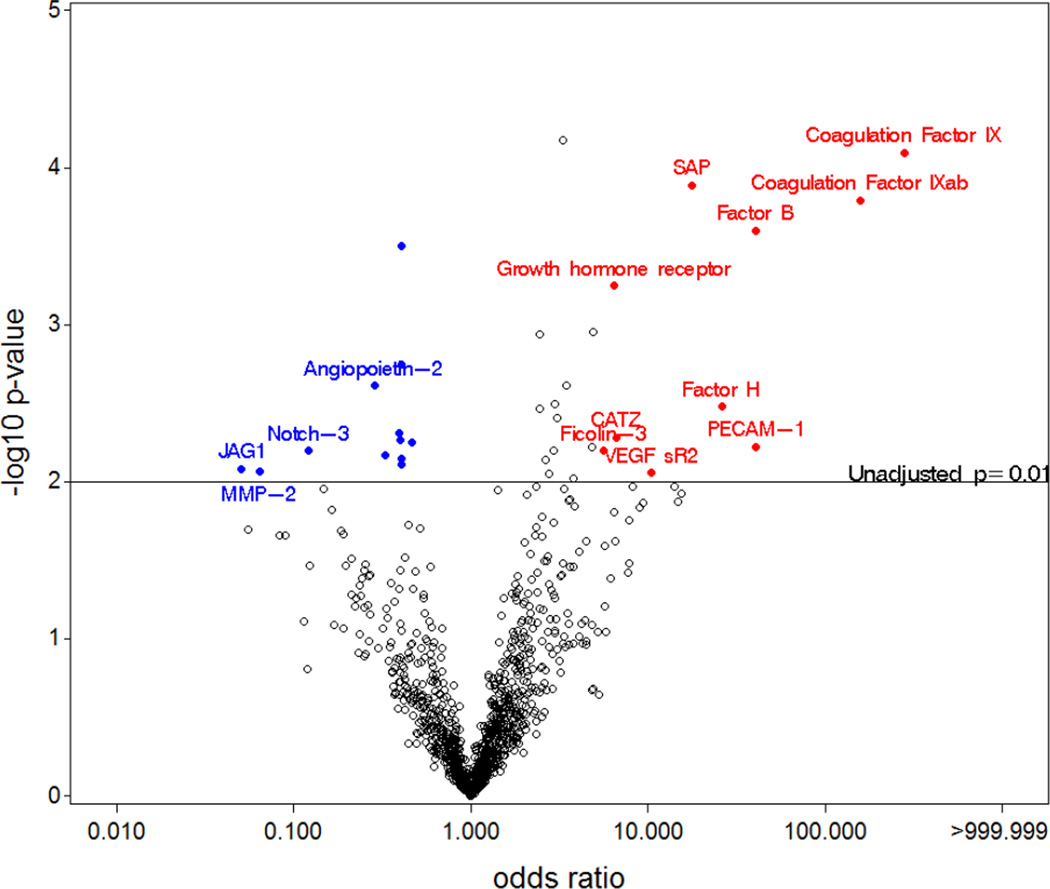
The SOMAmer proteins were compared in women with PTB and term delivery. The significance (−log10 p-value) and the magnitude (OR) for all the SOMAmer proteins are displayed graphically (Figure 1). Proteins with the largest magnitude of difference between groups and smallest p-value are named in the figure and appear in the upper left and upper right hand corners of the plot. Several proteins related to the clotting (coagulation factors IX and IX ab) and complement pathways (factor B, factor H and ficolin-3) were higher in PTB cases compared with controls. In addition, we found links between platelet endothelial cell adhesion molecule (PECAM-1), serum amyloid P-component (SAP), vascular endothelial growth factor receptor-2 (VEGF SR2), cathepsin Z (CATZ), growth hormone receptor (GHR) with PTB. Other proteins such as protein jagged-1 (JAG1), matrix metalloproteinase-2 (MMP-2), neurogenic locus notch homolog protein 3 (Notch 3), and angiopoietin-2 (ANGPT2), were lower in cases compared with controls. We demonstrate in Table 2 the magnitude of the association [(odds ratio (OR)] with 95% confidence intervals (CI) of the relationship of the top ranked proteins (n=34) with PTB along with the unadjusted p-value and the p-value adjusted for multiple comparisons. The coagulation factors IX, IX ab and SAP remained significantly related to PTB following adjustment for multiple comparisons, as did leptin. Factor B, GHR and insulin-like growth factor-binding protein-1 (IGFBP1) were of borderline significance after multiple comparisons adjustment.
Figure 1. Significance and magnitude for association between PTB and all proteins.
Volcano plot displaying the -log p-values (statistical significance) versus the odds ratio (magnitude of difference) for each individual protein. Those proteins which were significant (unadjusted p < 0.01 indicated by the horizontal line) and had an OR > 5 (red) or < 0.5 (blue) are distinguished by color.
Table 2.
| UniProt3 | Target | Odds Ratio |
95% CI | Unadjusted p-value |
FDR4 p-value |
|
|---|---|---|---|---|---|---|
| P00740 | Coagulation Factor IX | 280.7 | 17.04 | 4623 | 0.00 | 0.05 |
| P00740 | Coagulation Factor IX ab | 158.4 | 11.41 | 2201 | 0.00 | 0.05 |
| P00751 | Factor B | 40.66 | 5.59 | 295.8 | 0.00 | 0.06 |
| P16284 | PECAM-1 | 40.41 | 2.89 | 564.7 | 0.01 | 0.29 |
| P08603 | Factor H | 26.16 | 2.96 | 231.1 | 0.00 | 0.26 |
| P02743 | SAP | 17.81 | 4.08 | 77.78 | 0.00 | 0.05 |
| P35968 | VEGF sR2 | 10.51 | 1.81 | 61.01 | 0.01 | 0.31 |
| Q9UBR2 | CATZ | 6.61 | 1.76 | 24.84 | 0.01 | 0.29 |
| P10912 | Growth hormone receptor | 6.42 | 2.23 | 18.47 | 0.00 | 0.09 |
| O75636 | Ficolin-3 | 5.64 | 1.63 | 19.52 | 0.01 | 0.29 |
| Q8TE58 | ATS15 | 4.93 | 1.89 | 12.86 | 0.00 | 0.15 |
| P22223 | P-Cadherin | 4.80 | 1.57 | 14.73 | 0.01 | 0.29 |
| P36507 | MP2K2 | 3.82 | 1.39 | 10.52 | 0.01 | 0.32 |
| P10619 | Cathepsin A | 3.47 | 1.55 | 7.77 | 0.00 | 0.23 |
| P41159 | Leptin | 3.30 | 1.83 | 5.93 | 0.00 | 0.05 |
| P01031 | C5a | 3.06 | 1.43 | 6.53 | 0.00 | 0.28 |
| P01833 | PIGR | 2.98 | 1.44 | 6.17 | 0.00 | 0.26 |
| P63098 | Calcineurin B a | 2.94 | 1.36 | 6.37 | 0.01 | 0.29 |
| O00408 | cGMP-stimulated PDE | 2.77 | 1.29 | 5.95 | 0.01 | 0.31 |
| O60603 | TLR2 | 2.65 | 1.30 | 5.41 | 0.01 | 0.29 |
| Q9UK85 | Soggy-1 | 2.46 | 1.35 | 4.50 | 0.00 | 0.26 |
| P07949 | RET | 2.44 | 1.43 | 4.19 | 0.00 | 0.15 |
| P78504 | JAG1 | 0.05 | 0.01 | 0.46 | 0.01 | 0.31 |
| P08253 | MMP-2 | 0.06 | 0.01 | 0.50 | 0.01 | 0.31 |
| Q9UM47 | Notch-3 | 0.12 | 0.03 | 0.55 | 0.01 | 0.29 |
| O15123 | Angiopoietin-2 | 0.29 | 0.13 | 0.64 | 0.00 | 0.23 |
| Q16644 | MAPKAPK3 | 0.33 | 0.14 | 0.73 | 0.01 | 0.29 |
| Q13219 | PAPP-A | 0.39 | 0.21 | 0.75 | 0.00 | 0.29 |
| P02751 | FN1.3 | 0.40 | 0.21 | 0.76 | 0.01 | 0.29 |
| P18065 | IGFBP-2 | 0.40 | 0.21 | 0.78 | 0.01 | 0.29 |
| P08833 | IGFBP-1 | 0.41 | 0.25 | 0.66 | 0.00 | 0.06 |
| P02751 | Fibronectin | 0.41 | 0.23 | 0.72 | 0.00 | 0.20 |
| P45985 | MP2K4 | 0.41 | 0.21 | 0.79 | 0.01 | 0.30 |
| P02790 | Hemopexin | 0.47 | 0.27 | 0.80 | 0.01 | 0.29 |
This table only includes the proteins significantly related to PTB from the univariate analysis
Adjusted for multiple comparisons
UniProt = Universal protein resource (UniProt). Protein specific ID from a well-curated, centralized and freely accessible database:
FDR= false discovery rate
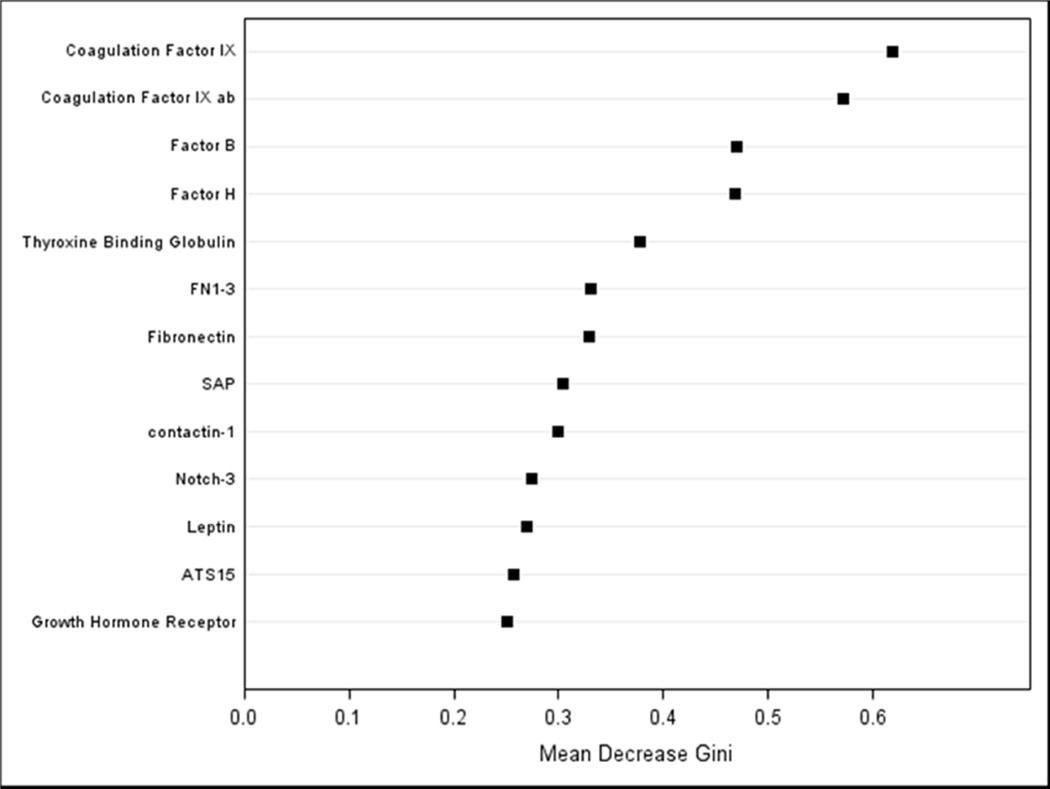
The results from the random forest reiterate those from the univariate logistic regressions; the coagulation Factors IX and IXab, Factor B and H were the highest ranking factors distinguishing cases of PTB from term controls (Figure 2). The random forest resulted in a classification error rate (ability of the random forest to accurately classify women into PTB/full term based only on their protein profiles) of 31%, which was reduced to 26.4% (17% for full term and 46% for PTB) after dimension reduction.
Figure 2. Variable importance plot from random forest.
A variable importance plot is useful for visualizing the rank importance of variables. Mean Decrease Gini index identifies features that are ranked as more important for distinguishing women who had a PTB from women who had a term delivery. Features with a larger value indicate variables that contribute more information compared to random noise, the separation in the indices between predictor variables degrades as the features become less useful for distinguishing between groups.
We show in Table 3 the pathways that are potentially involved in PTB. Proteins can be classified into multiple pathways and pathways are hierarchical, resulting in pathways that are not mutually exclusive. Smaller more specific pathways are nested into larger more general pathways (please see legend for Table 3). In this interactive context, the complement system ranked highest, followed by the immune system and the clotting cascade.
Table 3.
Ranking of Protein Pathways1
| Rank | Pathway | Number of proteins in pathway |
|---|---|---|
| 1 | Complement cascade | 24 |
| 2 | Immune System | 222 |
| 3 | Formation of Fibrin Clot (Clotting Cascade) | 21 |
| 4 | Metabolism of proteins | 33 |
| 5 | Signal Transduction | 236 |
| 6 | Post-translational protein modification | 15 |
| 7 | PTM: gamma carboxylation, hypusine formation and arylsulfatase activation | 12 |
| 8 | Regulation of Insulin-like Growth Factor (IGF) Activity by Insulin-like Growth Factor Binding Proteins (IGFBPs) | 17 |
| 9 | Innate Immune System | 120 |
| 10 | Diabetes pathways | 25 |
| 11 | Integrin cell surface interactions | 25 |
| 12 | Hemostasis | 119 |
| 13 | Transcriptional Regulation of White Adipocyte Differentiation | 5 |
| 14 | Signaling by NOTCH | 16 |
| 15 | Signaling by NOTCH3 | 5 |
| 16 | Integrin alpha IIb beta3 signaling | 12 |
| 17 | Activation of Genes by ATF4 | 3 |
| 18 | Disease | 137 |
| 19 | Platelet Aggregation (Plug Formation) | 16 |
| 20 | Prolactin receptor signaling | 6 |
| 21 | Signaling by NOTCH1 | 14 |
| 22 | Cytokine Signaling in Immune system | 64 |
| 23 | Growth hormone receptor signaling | 10 |
| 24 | Cell surface interactions at the vascular wall | 38 |
| 25 | Amyloids | 13 |
Top 25 ranked pathways. The proteins were classified into known pathways using Reactome, these pathways can overlap with each other and are hierarchical (i.e., one pathway could be a subset of another pathway). The ranking of each pathway was determined by combining the individual p-values from the logistic regression for the proteins in the pathway.
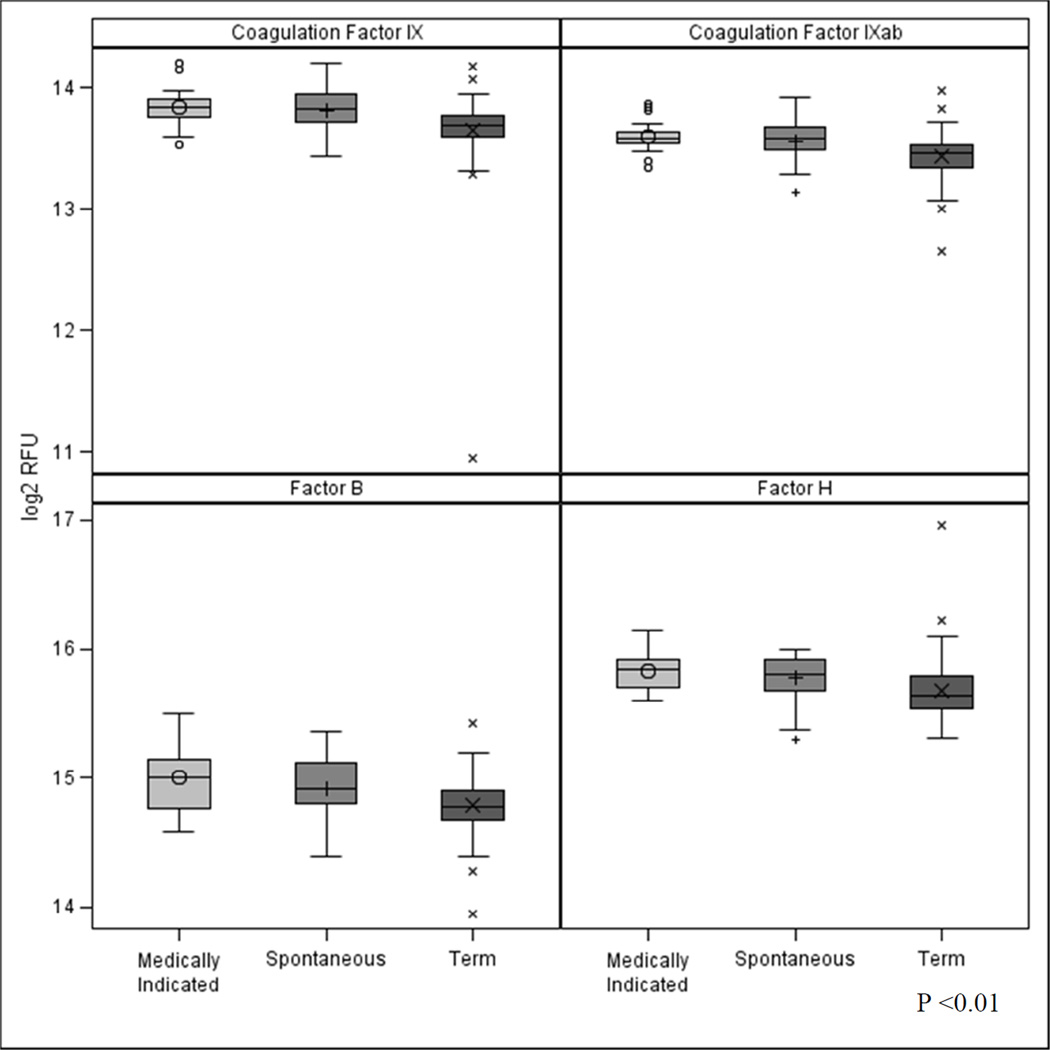
As a follow-up analysis, we explored the differences in the top ranking complement and coagulation proteins across the 3 categories of PTB. Of note, we found a significant difference in factor B, factor H, coagulation factors IX and IX ab in the medically indicated (n=19) and spontaneous groups (n=22) compared with the term group (Figure 3). In addition to evaluating the important term/PTB dichotomous outcome, we also considered gestational age as a continuous outcome. This analysis revealed similar top ranked proteins from the random forest including factor B, FN1–3, Coagulation Factor IX and Fibronectin but also indicated PECAM-1 and EG-VEGF increased in importance.
Figure 3.
Distribution of coagulation factors IX and IX ab and complement factor B and factor H across medically indicated PTB (n = 19), spontaneous PTB (n = 22) and term deliveries (n = 88) (p<0.01 for all four proteins). The box indicates the interquartile range (IQR: 25th to 75th percentile), the median and mean are indicated by the lines and points, respectively. The whiskers indicate data within 1.5 times the IQR and points are data outside 1.5 times the IQR. RFU = relative fluorescence units.
COMMENT
In this nested case-control study we used the innovative SOMAscan proteomic assay technology5 to measure proteins in a plasma sample taken at a single time point in early (10 to 15 weeks’ gestation) pregnancy. The coagulation factors IX and IX ab and the complement factors B and H were the highest ranking proteins distinguishing cases of PTB from term controls. The top 3 pathways associated with PTB were the complement cascade, the immune system and the clotting cascade. These data suggest that there is a contribution of immune-and coagulation–related events in early pregnancy to PTB. Of particular note, these potentially associated plasma protein profiles, evident very early in pregnancy, at 10–15 weeks of gestation, are related to the development of PTB several months later highlighting the possibility of developing early interventions to prevent these later gestational complications
We propose that activation of the complement cascade (described elsewhere14) at this time in early pregnancy is triggered by events linked with inflammation that could be infectious or non-infectious in origin. Subclinical microbial invasion of the amniotic cavity in early pregnancy3,15 is an event that has been implicated in PTBs occurring specifically at earlier gestational ages.16 We suggest that placental-derived injured or apoptotic cells17,18 may be trigger for a non-microbial activation of the complement cascade. This finding would be in keeping with recognition by the complement pathway of danger-associated molecular patterns (DAMPS) of not only pathogens but also of injured or apoptotic cells.18 Factors B and H were the leading complement factors related to PTB. Complement factor B is a member of the alternative complement pathway. In our previous research we demonstrated that elevated levels of the factor B-derived Bb activation fragment in early pregnancy were related to PTB at less than 34 weeks’ gestation.8 In the current study using a broad-based screening approach we have again demonstrated a role for enhanced involvement of the proteins related to the alternative complement pathway in PTB. We also observed upregulation of the regulatory complement protein, Factor H in early pregnancy in women who subsequently had a PTB. The role of this complement regulator19 is to prevent uncontrolled activation of the complement system, as dysregulation of control can lead to a severe inflammatory response with collateral damage to host tissue as seen in other human diseases.20 We suggest that Factor H is elevated in an effort to self-regulate as a response to inflammatory events originating in the placenta in early pregnancy. It is noteworthy that Ingram et al. recently suggested that elevated complement factor H is a biomarker of multiple sclerosis disease state.21
We demonstrated a role for the clotting cascade (Table 3) and, specifically thrombosis-related coagulation factor IX (Christmas factor)22 in PTB. This finding is aligned with the results from other authors (reviewed in3) who described a role for defective decidual hemostasis/vaginal bleeding in PTB. Indeed, Mackenzie et al. demonstrated that thrombin resulting from placental abruption promotes PTB by mediating fetal membrane extracellular matrix degradation via enhanced decidual cell matrix mettaloproteinase-3 expression.23 This finding is in agreement from other investigators who have observed crosstalk between the coagulation and the complement system.24,25
We also found other proteins that were higher in PTB cases compared with term controls. These proteins include: PECAM-1, a cell adhesion and signaling receptor that is expressed on hematopoietic and endothelial cells that has a role in the inflammatory process26; SAP, a protein found in amyloid deposits that binds to apoptotic cells and the nuclear component of necrotic cells27 and has links with the complement system;28 VEGF sR2, a soluble receptor involved in vascular endothelial cell development29; cathepsin Z which has links with protein turnover in cells and tumor progression30; the GHR, that mediates the actions of growth hormone which promotes cell division, regeneration and growth31; ficolin-3, a protein that is related to the complement system32 and mediates the clearance of apoptotic cells,33 and leptin, an adipocyte-derived hormone linked with obesity and preeclampsia.34 In contrast, other proteins were lower in cases of PTB compared with term controls and included: JAG1, a protein linked with the notch signaling pathway,35 MMP-2, a metalloproteinase involved in the breakdown of extracellular matrix that has links with tumor progression36 notch-3, shown to have a role in fetal-maternal communication during implantation and placentation,37 and, angiopoietin-2, which has a role in the regulation of endothelial cell survival and vascular maturation.38 We suggest that alterations in levels of these proteins in early pregnancy are potential markers of subsequent PTB.
Regarding the pathway analysis, we found that in addition to the complement, clotting and immune system pathways, top ranked pathways included metabolism of proteins (synthesis and catabolism of proteins), signal transduction (activation of cell-specific receptors by an extracellular signal), and post translational protein modification (a component of protein biosynthesis) in PTB. These findings could reflect the high turnover/remodeling of cells in the developing placenta at this time of pregnancy and the alterations due to the underlying pathogenic process promoting future PTB.17 A role for regulation of IGF activity by Insulin-like Growth Factor (IGF) Binding Proteins was also demonstrated. This finding in early gestation may be important as this family of proteins has significant connections with intra-uterine growth restriction.39 Finally, we found the innate immunity and diabetes pathways to be among the top ranked pathways related to PTB.
Notwithstanding the important results from this study, there are some limitations. Validation5 of the findings and this technology is required in a much larger PTB cohort. Of relevance to this suggestion are the results from a recent study where this biomarker discovery platform was used to investigate protein profiles in two independent cohorts of patients with Duchenne muscular dystrophy and age-matched control. Forty-two proteins showed significant differences that were consistent in both cohorts suggesting the utility of this approach to biomarker discovery.40 An additional limitation was the heterogeneous group of women studied and the small sample size. Careful stratification of the cases by the major antecedent of PTB is required. We view as important to also stratify by gestational age at delivery in the analysis of future pregnancy cohorts. The protein profiles may be different in early pregnancy in women who have babies born at the lower extremities of PTB, as different pathways may be involved.3 Despite these limitations, our cohort was very well characterized, with measurement of a tremendous range of protein markers very early in the PTB causal pathway.
In summary, we have used an innovative technology to uncover new proteins along several coalescing pathways related to the development of PTB. Moreover, using this approach, we have validated our previous findings of a role for the complement system in PTB8 and prior studies of the coagulation pathway.23 Moving forward, investigators should focus attention on the role of the complement and clotting cascades in PTB in the early pathogenesis of PTB. Ultimately, identification of protein(s) that can be targeted for therapeutic intervention, particularly early in the course of pregnancy, as described here, could provide an improved prognosis for the many women and their babies who suffer the consequences of PTB.
Acknowledgments
Funding: Funding: 5 K23 HD4984-04 and R21 HD68961-01A1 (AML) from the Eunice Kennedy Shriver National Institute of Child Health and Development, Newborn Hope Colorado and, SomaLogic Inc. Boulder, CO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study Performed: Aurora, Colorado
Conflict of Interest/Disclosure statement: Dr. Deterding has a Children's Hospital Colorado innovations grant to develop Kids Personalized Protein Signatures (KIPPS) panels for children using the SomaLogic technology. In addition she serves on the Joint Steering Committee for KIPPS. The other co-authors report no conflict of interest.
Paper Presentation: Presented at the Frontiers in Pregnancy Research Symposium, University of Colorado School of Medicine, Aurora, Colorado. May 15, 2015.
References
- 1.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 2.Howson CP, Kinney MV, McDougall L, Lawn JE. Born too soon: preterm birth matters. Reprod Health. 2013;10(Suppl 1):S1. doi: 10.1186/1742-4755-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014 Aug 15;345(6198):760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001 Jul;15(Suppl 2):78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 5.SomaLogic. SOMAscan technical white paper. [Accessed 6/17/2015];2013 http://www.somalogic.com/somalogic/media/Assets/PDFs/White-paper-11-12-13final.pdf. 2015. [Google Scholar]
- 6.Rohloff JC, Gelinas AD, Jarvis TC, et al. Nucleic Acid Ligands With Protein-like Side Chains: Modified Aptamers and Their Use as Diagnostic and Therapeutic Agents. Mol Ther Nucleic Acids. 2014;3:e201. doi: 10.1038/mtna.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehan MR, Ostroff R, Wilcox SK, et al. Highly multiplexed proteomic platform for biomarker discovery, diagnostics, and therapeutics. Adv Exp Med Biol. 2013;735:283–300. doi: 10.1007/978-1-4614-4118-2_20. [DOI] [PubMed] [Google Scholar]
- 8.Lynch AM, Gibbs RS, Murphy JR, et al. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2008 Oct;199(4):354, e351–e358. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch AM, Gibbs RS, Murphy JR, Giclas PC, Salmon JE, Holers VM. Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet Gynecol. 2011 Jan;117(1):75–83. doi: 10.1097/AOG.0b013e3181fc3afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch AM, Murphy JR, Byers T, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008 Apr;198(4):385, e381–e389. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001 Nov 1;125(1–2):279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 12.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 13.Reactome. Reactome project. 2012 http://www.reactome.org. [Google Scholar]
- 14.Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol. 2014 Mar 21;32:433–459. doi: 10.1146/annurev-immunol-032713-120154. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166(5):1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 16.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992 Mar;79(3):351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002 Jun;87(6):2954–2959. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- 18.Kohl J. The role of complement in danger sensing and transmission. Immunol Res. 2006;34(2):157–176. doi: 10.1385/IR:34:2:157. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira VP, Pangburn MK, Cortes C. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol. 2010 Aug;47(13):2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol. 2014 Oct;61(2):118–125. doi: 10.1016/j.molimm.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingram G, Hakobyan S, Loveless S, Robertson N, Morgan BP. Complement regulator factor H in multiple sclerosis. J Cell Biochem. 2011 Oct;112(10):2653–2654. doi: 10.1002/jcb.23204. [DOI] [PubMed] [Google Scholar]
- 22.Heikal NM, Murphy KK, Crist RA, Wilson AR, Rodgers GM, Smock KJ. Elevated factor IX activity is associated with an increased odds ratio for both arterial and venous thrombotic events. Am J Clin Pathol. 2013 Nov;140(5):680–685. doi: 10.1309/AJCPAGOR4Q2IIKUG. [DOI] [PubMed] [Google Scholar]
- 23.Mackenzie AP, Schatz F, Krikun G, Funai EF, Kadner S, Lockwood CJ. Mechanisms of abruption-induced premature rupture of the fetal membranes: Thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol. 2004 Dec;191(6):1996–2001. doi: 10.1016/j.ajog.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007 Apr;28(4):184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Amara U, Flierl MA, Rittirsch D, et al. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010 Nov 1;185(9):5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010 Jul 17;87(3–4):69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bickerstaff MC, Botto M, Hutchinson WL, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999 Jun;5(6):694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 28.Du Clos TW, Mold C. Pentraxins (CRP, SAP) in the process of complement activation and clearance of apoptotic bodies through Fcgamma receptors. Curr Opin Organ Transplant. 2011 Feb;16(1):15–20. doi: 10.1097/MOT.0b013e32834253c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007 Oct;19(10):2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Santamaria I, Velasco G, Pendas AM, Fueyo A, Lopez-Otin C, Cathepsin Z. a novel human cysteine proteinase with a short propeptide domain and a unique chromosomal location. J Biol Chem. 1998 Jul 3;273(27):16816–16823. doi: 10.1074/jbc.273.27.16816. [DOI] [PubMed] [Google Scholar]
- 31.Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol. 2010 Sep;6(9):515–525. doi: 10.1038/nrendo.2010.123. [DOI] [PubMed] [Google Scholar]
- 32.Michalski M, Swierzko AS, Pagowska-Klimek I, et al. Primary Ficolin-3 deficiency - Is it associated with increased susceptibility to infections? Immunobiology. 2015 Jun;220(6):711–713. doi: 10.1016/j.imbio.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Honore C, Hummelshoj T, Hansen BE, Madsen HO, Eggleton P, Garred P. The innate immune component ficolin 3 (Hakata antigen) mediates the clearance of late apoptotic cells. Arthritis Rheum. 2007 May;56(5):1598–1607. doi: 10.1002/art.22564. [DOI] [PubMed] [Google Scholar]
- 34.Taylor BD, Ness RB, Olsen J, et al. Serum leptin measured in early pregnancy is higher in women with preeclampsia compared with normotensive pregnant women. Hypertension. 2015 Mar;65(3):594–599. doi: 10.1161/HYPERTENSIONAHA.114.03979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penton AL, Leonard LD, Spinner NB. Notch signaling in human development and disease. Semin Cell Dev Biol. 2012 Jun;23(4):450–457. doi: 10.1016/j.semcdb.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005 May 25;1755(1):37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Cuman C, Menkhorst E, Winship A, et al. Fetal-maternal communication: the role of Notch signalling in embryo implantation. Reproduction. 2014 Mar;147(3):R75–R86. doi: 10.1530/REP-13-0474. [DOI] [PubMed] [Google Scholar]
- 38.Kappou D, Sifakis S, Konstantinidou A, Papantoniou N, Spandidos DA. Role of the angiopoietin/Tie system in pregnancy (Review) Exp Ther Med. 2015 Apr;9(4):1091–1096. doi: 10.3892/etm.2015.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003 Aug;13(4):113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 40.Hathout Y, Brody E, Clemens PR, et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):7153–7158. doi: 10.1073/pnas.1507719112. [DOI] [PMC free article] [PubMed] [Google Scholar]