Abstract
Background
Investigations into the distribution of sinus irrigations have been limited by labor-intensive methodologies that do not capture the full dynamics of irrigation flow. The purpose of this study was to validate the accuracy of a computational fluid dynamics (CFD) model for sinonasal irrigations through a cadaveric experiment.
Methods
Endoscopic sinus surgery was performed on two fresh cadavers to open all eight sinuses, including a Draf III procedure for cadaver 1, and Draf IIb frontal sinusotomies for cadaver 2. Computed tomography maxillofacial scans were obtained preoperatively and postoperatively, from which CFD models were created. Blue-dyed saline in a 240 mL squeeze bottle was used to irrigate cadaver sinuses at 60 mL/s (120 mL per side, over 2 seconds). These parameters were replicated in CFD simulations. Endoscopes were placed through trephinations drilled through the anterior walls of the maxillary and frontal sinuses, and sphenoid roofs. Irrigation flow into the maxillary, frontal, and sphenoid sinuses was graded both ipsilateral and contralateral to the side of nasal irrigation, and then compared with the CFD simulations.
Results
In both cadavers, preoperative and postoperative irrigation flow into maxillary, frontal, and sphenoid sinuses matched extremely well when comparing the CFD models and cadaver endoscopic videos. For cadaver 1, there was 100% concordance between the CFD model and cadaver videos, and 83% concordance for cadaver 2.
Conclusions
This cadaveric experiment provided potential validation of the CFD model for simulating saline irrigation flow into the maxillary, frontal, and sphenoid sinuses before and after sinus surgery.
Keywords: sinus irrigation, computational fluid dynamics, endoscopic sinus surgery, Draf III, frontal sinus, sphenoid sinus
Introduction
Evidence continues to accumulate with regard to the mechanisms and efficacy of different topical therapies utilized in patients with chronic rhinosinusitis, both before and after sinus surgery. Optimizing topical therapies is an active area of rhinologic research, with various microscopic and macroscopic factors of topical delivery being studied.1 Among macroscopic factors, optimizing the delivery and distribution of sinus irrigations has been one of the more heavily studied areas.2-7
Various types of studies on topical irrigation delivery to the sinuses have been conducted. Live patient and cadaver studies have been performed using blue-dyed irrigations with direct sinus visualization by endoscopy,2,3 or irrigations with iodinated contrast followed by computed tomography (CT) scans to determine which sinuses collect contrast material.4,5 Technetium 99m sulfur colloid6 and fluorescein7 labelled irrigations have also been used in attempts to quantify distribution of irrigations to the sinonasal cavities. These methods are generally labor intensive, and do not capture the full dynamics of irrigation flow. Therefore a more efficient and potentially more accurate method was pursued to model sinonasal irrigation delivery. The purpose of this study was to validate a recently published computational fluid dynamics (CFD) model8 for sinonasal irrigations in both operated and nonoperated sinuses, through a cadaveric experiment.
Methods
Two fresh cadaver heads were obtained from the University of Pennsylvania morgue, and the study was approved by the University of Pennsylvania Institutional Review Board. The following step-by-step protocol was carried out for both of the cadaver heads: 1) preoperative CT scan, 2) preoperative sinonasal irrigation and endoscopic video recording of the sinuses, 3) endoscopic sinus surgery, 4) postoperative CT scan, 5) postoperative sinonasal irrigation and recording, 6) preoperative and postoperative CFD models created to simulate the sinonasal irrigations, 7) irrigation gradings compared between the CFD models and cadaver endoscopic videos. Of note, protocols 1-5 were performed by two of the experimenters (JRC, JNP), while protocol 6 was performed by two different experimenters (KZ, ND). While performing the cadaver irrigations and endoscopic video recordings in protocols 2 and 5, experimenters were blinded to the results of the CFD models created in protocol 6. Likewise, the experimenters creating the CFD models in protocol 6 were blinded to the endoscopic video results from protocols 2 and 5. For protocol 7, irrigation flow patterns seen on cadaver endoscopic videos and CFD models were graded by three of the experimenters independently. Specifically, the experimenters assigned grades to the preoperative and postoperative cadaver endoscopic videos prior to grading the CFD model simulations. Irrigation penetration of the maxillary, frontal, and sphenoid sinuses was graded ipsilateral and contralateral to the side of irrigation, both before and after surgery. The following grading scale was used to classify and compare the degrees of sinus irrigant penetration seen in both the CFD model and endoscopic cadaver videos: 0- no penetration, 1- minimal or slow filling, 2- complete or rapid filling.
To begin the study, preoperative 1 mm slice CT maxillofacial scans were obtained on both of the cadaver heads. Next the cadaver noses were irrigated and endoscopically recorded before any sinus surgery was performed. To allow endoscopic visualization of the irrigations penetrating the maxillary, frontal, and sphenoid sinuses, 4 mm trephinations were drilled through the anterior walls of the maxillary and frontal sinuses, and the roofs of the sphenoid sinuses. This was achieved by first performing craniotomies and removing all brain and meningeal tissues. Holes were then drilled with a 4 mm diamond burr through the sphenoid roofs, and the anterior walls of the frontal and maxillary sinuses bilaterally. To evaluate irrigation flow into the sinuses, a 70° endoscope was placed through the frontal sinus trephination, and a 30° endoscope was placed through the trephinations of the maxillary and sphenoid sinuses. These scopes allowed optimal views of the respective sinus ostia and inflow of irrigations.
Prior to irrigations being performed, each cadaver head was secured in a position with the face oriented parallel to the ground, and nasal cavity perpendicular to the ground. The oropharynx was packed tightly with a ball of gauze wrapped in Vaseline gauze, so as to elevate the soft palate and create an impermeable barrier between the nasopharynx and oropharynx. This allowed irrigations to flow between nasal cavities as they would in live patients with functional velopharyngeal closure, preventing premature exit of the irrigations down the oropharynx. Also of note, to ensure watertight seals of all the sinuses during the irrigations, epoxy putty was used to plug all of the holes drilled in the walls of the sinuses. For the sinus being evaluated endoscopically during irrigation, putty was also placed around the shaft of the scope at the level of the trephination to prevent any irrigant egress.
Irrigations were then performed on each cadaver, with one experimenter performing the irrigation, and the other holding the endoscope in the sinus being studied. A NeilMed SinusRinse® squeeze bottle was filled with 240 mL of saline dyed with blue food coloring. The nozzle tip of the squeeze bottle was seated in the nasal vestibule, and the bottle was angled at approximately 20° to the nasal floor. Irrigations were then performed to one of the nasal cavities in each cadaver at a volume flow rate of 60 mL/s (120 mL per side over two seconds). The flow rate was determined by having one of the authors irrigate his own nasal cavity multiple times, and averaging the time taken to irrigate 120 mL. Efforts were made to standardize the flow rate during the cadaveric experiment by having the same author perform the irrigations, only after multiple trials yielded the desired flow rate consistently. For cadaver 1, the left nasal cavity was irrigated. For cadaver 2, the right nasal cavity was irrigated. The maxillary, frontal, and sphenoid sinuses ipsilateral and contralateral to the side of irrigation were recorded endoscopically during the nasal irrigations, and irrigation penetration was graded 0-2 for each sinus as described previously. Figure 1 depicts the cadaveric experiment setup.
Figure 1.

Cadaveric experiment setup. (A) Components of experiment labeled, and cadaver head secured in the face down position, with nasal cavity perpendicular to the floor. (B) Diagram depicting the orientation of the head and irrigation bottle during irrigations, and how angled scopes were placed through sinus wall trephinations to visualize the respective sinuses during irrigations.
Once the preoperative cadaveric irrigations were completed, endoscopic sinus surgery was performed on both cadavers to open each of the eight paranasal sinuses. Wide sphenoidotomies were performed by removing the entire anterior walls of the sphenoid sinuses. For the frontal sinuses, a Draf III procedure was performed on cadaver 1, and bilateral Draf IIb frontal sinusotomies were performed on cadaver 2. CT scans were then obtained after surgery on each head. Of note, prior to obtaining the CT scans, the sinus wall trephinations were all plugged with epoxy putty to recreate sinus wall continuity and avoid any alteration to the flow characteristics in the CFD model. Irrigations were then performed on the cadavers in the same manner as described in the preoperative state, and endoscopic recordings of each sinus were graded 0-2 as described.
Next the CFD models were created. Based on previously published methods,8-10 preoperative and postoperative CFD models were created based on the preoperative and postoperative CT scans obtained on the cadavers. The technique used to create the CFD models for sinus irrigations is complex, and the complete details of the method can be found in a previous work by the authors.11 In brief, AMIRA® software (Visualization Sciences Group, Burlington, MA) was used to establish the interface between the nasal mucosa and air on the CT scans. The air space of the nasal cavity was filled with tetrahedral elements using ICEMCFD® software (Ansys, Inc., Canonsburg, PA). After refinement and correction for errors, the final models contained elements for pre- and postoperative models, respectively. The same irrigation parameters used in the cadaver experiment were used for the CFD simulations: Face parallel to the ground head position, 120 mL saline irrigated through one of the nasal cavities at a rate of 60mL/s for 2 seconds, and an irrigation angle of 20° to the nasal floor. The simulations were carried out by a commercial CFD software, CFX® (Ansys, Inc., Canonsburg, PA), using a multiphase free surface method. The multiphase model served to define the interaction between different fluid phases: air and saline solution in this case. Video files of the CFD models were created, but only still images are presented in the paper.
Lastly, the irrigation grades given for the cadaver endoscopic videos were compared to the irrigation grades given for the CFD model. By evaluating the three pairs of sinuses ipsilateral and contralateral to the side of irrigation, both pre- and postoperatively, a total 12 comparisons were made for each cadaver.
Results
There was 100% inter-observer agreement between the irrigation grades given by the three grading experimenters for the cadaver endoscopic videos and CFD models. In both cadavers, preoperative and postoperative irrigations to the maxillary, frontal, and sphenoid sinuses ipsilateral and contralateral to the sides of irrigation were found to match extremely well when comparing the CFD simulation models with the endoscopic videos (Tables 1-2). For cadaver 1, there was 100% concordance (12/12) between the grades given for irrigation penetration of each of the sinuses studied, in both the CFD model and cadaver endoscopic videos. For cadaver 2, there was 83% concordance (10/12) between the grades given for sinus irrigation in the CFD model and cadaver videos. The discrepant grades were as follows. Preoperative irrigation of the frontal sinus, contralateral to the side of irrigation was graded as a 0 in the cadaver endoscopic video, while the CFD model was graded as a 1. Preoperative irrigation of the sphenoid sinus, ipsilateral to the side of irrigation was graded as a 1 in the cadaver endoscopic video, while the CFD model was graded as a 0.
Table 1.
Grading (0-2) and comparison of irrigation penetration into respective sinuses before and after surgery in cadaver experiment and computational fluid dynamics model (Cadaver 1). Note the left nasal cavity was irrigated in this cadaver.
| Cadaver 1 | Cadaver | CFD |
|---|---|---|
| Maxillary, Preoperative | Maxillary, Preoperative | |
| Left, ipsilateral | 2 | 2 |
| Right, contralateral | 1 | 1 |
| Maxillary, Postoperative | Maxillary, Postoperative | |
| Left, ipsilateral | 2 | 2 |
| Right, contralateral | 1 | 1 |
| Frontal, Preoperative | Frontal, Preoperative | |
| Left, ipsilateral | 1 | 1 |
| Right, contralateral | 0 | 0 |
| Frontal, Postoperative | Frontal, Postoperative | |
| Left, ipsilateral | 2 | 2 |
| Right, contralateral | 2 | 2 |
| Sphenoid, Preoperative | Sphenoid, Preoperative | |
| Left, ipsilateral | 0 | 0 |
| Right, contralateral | 0 | 0 |
| Sphenoid, Postoperative | Sphenoid, Postoperative | |
| Left, ipsilateral | 0 | 0 |
| Right, contralateral | 0 | 0 |
Table 2.
Grading (0-2) and comparison of irrigation penetration into respective sinuses before and after surgery in cadaver experiment and computational fluid dynamics model (Cadaver 2). Note the right nasal cavity was irrigated in this cadaver.
| Cadaver 2 | Cadaver Video | CFD Model |
|---|---|---|
| Maxillary, Preoperative | Maxillary, Preoperative | |
| Right, ipsilateral | 2 | 2 |
| Left, contralateral | 1 | 1 |
| Maxillary, Postoperative | Maxillary, Postoperative | |
| Right, ipsilateral | 1 | 1 |
| Left, contralateral | 2 | 2 |
| Frontal, Preoperative | Frontal, Preoperative | |
| Right, ipsilateral | 1 | 1 |
| Left, contralateral | 0 | 1 |
| Frontal, Postoperative | Frontal, Postoperative | |
| Right, ipsilateral | 2 | 2 |
| Left, contralateral | 2 | 2 |
| Sphenoid, Preoperative | Sphenoid, Preoperative | |
| Right, ipsilateral | 1 | 0 |
| Left, contralateral | 0 | 0 |
| Sphenoid, Postoperative | Sphenoid, Postoperative | |
| Right, ipsilateral | 0 | 0 |
| Left, contralateral | 0 | 0 |
Additionally, while the grading scale could not be applied to the dynamic moment-to-moment flow of the irrigations, the flow patterns seen in the CFD model appeared strikingly similar to the flow seen endoscopically within the sinus cavities. Representative still images of the preoperative and postoperative CFD models are shown in Figures 2-3. Video recordings of the cadaveric videos and CFD irrigation models could not be presented in the paper.
Figure 2.
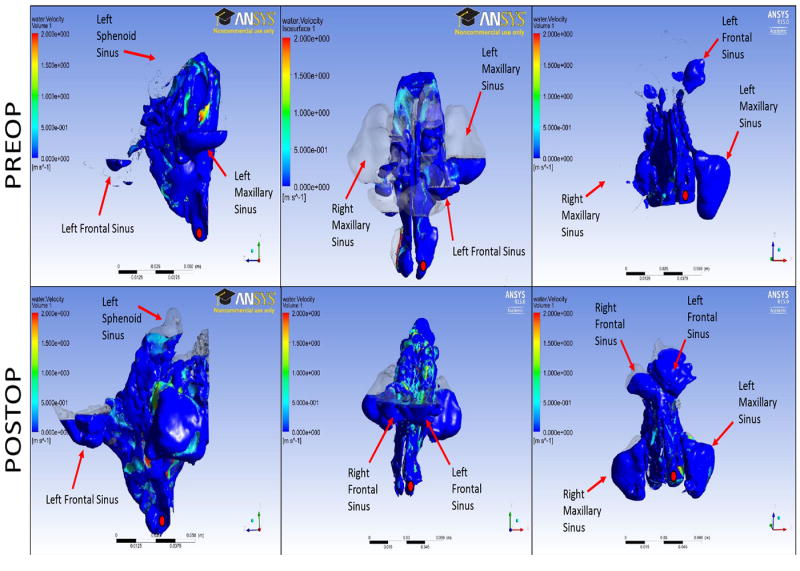
Still images of the preoperative and postoperative computational fluid dynamics models of sinus irrigations for cadaver 1. A Draf III procedure was performed for the frontal sinus. The red dot indicates the side of irrigation (left side for this cadaver). Cadaveric endoscopic videos confirmed the CFD findings in 100% of comparisons.
Figure 3.

Still images of the preoperative and postoperative computational fluid dynamics models of sinus irrigations for cadaver 2. Bilateral Draf IIb procedures were performed for the frontal sinus. The red dot indicates the side of irrigation (right side for this cadaver). Cadaveric endoscopic videos confirmed these findings in 83% of comparisons.
Discussion
Studying the distribution of topical irrigations to the sinus cavities is an exciting but challenging area to research. To understand how and where topical irrigations travel within the sinonasal cavities, the complete flow path of the irrigations should be evaluated. Studies to date, however, have generally focused on the final destination of the irrigations, demonstrating that irrigations either do or don’t reach certain regions within the sinonasal cavities before or after sinus surgery.2-7 However, no studies have evaluated the moment-to-moment dynamics of irrigation flow through the nasal and sinus cavities.
When studying the distribution of sinonasal irrigations, one significant advantage of the CFD model over previously described methods is that it can simulate the moment-to-moment flow dynamics of irrigations as they traverse the sinonasal cavities. This gives the potential of studying the complete path of irrigations, how the paths are affected by the removal of different structures in the nasal cavity, and whether altering the path of flow through the nose improves irrigant penetration of the sinuses. Another advantage over other techniques is that it is less labor intensive with regard to man hours, requiring only a CT scan and the computer software. Additionally, the software allows multiple important variables to be manipulated, such as irrigation delivery angle, irrigation velocity, and head position. This can save a significant amount of time when studying the distribution of sinus irrigations compared with using cadavers or live patients. The main drawback to the CFD model is the significant computing power required to create and run the simulations. Each preoperative and postoperative CFD model took seven days to complete, with the four models created for this study requiring a month of dedicated computing time. However, with supercomputers, this time could be cut down significantly.
Some potential criticisms of this study also deserve mention. One important point to highlight with the study is that the results only reflect the potential validation of the CFD model. Given the low number of cadavers used in the study, there was insufficient power to validate the CFD model statistically. However, given the 100% inter-observer agreement and high concordance rates between irrigation grades assigned to the CFD models and cadaver endoscopic videos, an impressive trend toward validation was demonstrated. Another point to discuss from the study was the discrepancy seen in two sets of grades compared between the CFD model and endoscopic videos for cadaver 2. While it is impossible to determine whether the discrepancies were the result of errors with the CFD model or the cadaver experiment, it seems more likely that the discrepancies were due to the cadaver irrigation portion of the experiment rather than flaws in the CFD model. A number of confounding variables were present during the experimenters’ attempts at standardizing the irrigation and recording of the cadaveric nasal cavities. Variables such as angle of irrigation, squeezing force, and irrigation flow rate were difficult to reproduce perfectly by the experimenter, and so this introduced confounding when compared to the CFD model where these variables were easily controlled.
Despite the aforementioned concerns with the study, the overwhelming agreement between the CFD model and cadaver videos was very encouraging for establishing the CFD model as a novel method for studying sinonasal irrigations. Multiple potential clinical benefits remain with CFD modeling of sinonasal irrigations. It may give surgeons the ability to predict topical delivery throughout the entire sinonasal cavity and tailor surgical intervention to the degree of topical delivery desired to the sinonasal mucosa. It may also help surgeons educate patients as to the most optimal delivery devices and parameters to utilize when performing their irrigations. Because of these potential benefits, CFD modeling of sinus irrigations definitely warrants further investigation.
Conclusions
CFD modeling of sinonasal irrigations is an intriguing technique for evaluating the dynamic flow of sinonasal irrigations through the entire sinonasal cavity. This cadaveric experiment provided potential validation of the CFD model for simulating saline irrigation flow into the maxillary, frontal, and sphenoid sinuses before and after sinus surgery.
Acknowledgments
The authors would like to thank the University of Pennsylvania morgue, for their assistance in storing and handling the cadaveric specimens for the dissection portion of the study.
Kai Zhao receives funding from NIH NIDCD R01 DC013626
Footnotes
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Craig, Zhao, and Palmer. Acquisition of data: Craig, Zhao, Doan and Palmer. Analysis and interpretation of data: Craig, Zhao, and Palmer. Drafting of the manuscript: Craig. Critical revision of the manuscript for important intellectual content: Craig, Zhao, Doan, Khalili, Lee, Adappa, and Palmer. Administrative, technical, and material support: Palmer. Study supervision: Palmer.
Oral Presentation: ARS (September 26, 2015, Dallas, TX)
There are no financial disclosures or other conflicts of interest to report for this study.
Contributor Information
John R Craig, Dept. of Otolaryngology, Henry Ford Health System, Detroit, MI.
Kai Zhao, Dept. of Otolaryngology, Ohio State University, Columbus, OH.
Ngoc Doan, Dept. of Engineering, Drexel University, Philadelphia, PA.
Sammy Khalili, Dept. of Otolaryngology, University of Pennsylvania, Philadelphia, PA.
John YK Lee, Dept. of Otolaryngology, University of Pennsylvania, Philadelphia, PA.
Nithin D Adappa, Dept. of Otolaryngology, University of Pennsylvania, Philadelphia, PA.
James N Palmer, Dept. of Otolaryngology, University of Pennsylvania, Philadelphia, PA.
References
- 1.Harvey RJ, Schlosser RJ. Local drug delivery. Otolaryngol Clin North Am. 2009;42:829–845. doi: 10.1016/j.otc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Grobler A, Weitzel EK, Buele A, et al. Pre- and postoperative sinus penetration of nasal irrigation. Laryngoscope. 2008;118:2078–2081. doi: 10.1097/MLG.0b013e31818208c1. [DOI] [PubMed] [Google Scholar]
- 3.Singhal D, Weitzel EK, Lin E, et al. Effect of head position and surgical dissection on sinus irrigant penetration in cadavers. Laryngoscope. 2010;120:2528–2531. doi: 10.1002/lary.21092. [DOI] [PubMed] [Google Scholar]
- 4.Harvey RJ, Goddard JC, Wise SK, et al. Effects of endoscopic sinus surgery and delivery device on cadaver sinus irrigation. Otolaryngol Head Neck Surg. 2008;139:137–142. doi: 10.1016/j.otohns.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Snidvongs K, Chaowanapanja P, Aeumjaturapat S, et al. Does nasal irrigation enter paranasal sinuses in chronic rhinosinusitis? Am J Rhinol Allergy. 2008;22:483–486. doi: 10.2500/ajr.2008.22.3221. [DOI] [PubMed] [Google Scholar]
- 6.Wormald PJ, Cain T, Oates L, et al. A comparative study of three methods of nasal irrigation. Laryngoscope. 2004;114:2224–2227. doi: 10.1097/01.mlg.0000149463.95950.c5. [DOI] [PubMed] [Google Scholar]
- 7.Bleier BS, Preena D, Schlosser RJ, et al. Dose quantification of topical drug delivery to the paranasal sinuses by fluorescein luminosity calculation. Int Forum Allergy Rhinol. 2012;2:316–320. doi: 10.1002/alr.21034. [DOI] [PubMed] [Google Scholar]
- 8.Zhao K, Craig JR, Cohen NA, et al. Sinus irrigations before and after surgery – visualization through computational fluid dynamics simulations. Laryngoscope. doi: 10.1002/lary.25666. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao K, Scherer PW, Hajiloo SA, et al. Effect of anatomy on human nasal air flow and odorant transport patterns: implications for olfaction. Chem Senses. 2004;29:365–379. doi: 10.1093/chemse/bjh033. [DOI] [PubMed] [Google Scholar]
- 10.Zhao K, Pribitkin EA, Cowart BJ, et al. Numerical modeling of nasal obstruction and endoscopic surgical intervention: outcome to airflow and olfaction. Am J Rhinol. 2006;20:308–316. doi: 10.2500/ajr.2006.20.2848. [DOI] [PubMed] [Google Scholar]
- 11.Zhao K, Craig JR, Cohen NA, Adappa ND, Khalili S, Palmer JN. Sinus irrigations before and after surgery – visualization through computational fluid dynamics simulations. Laryngoscope. doi: 10.1002/lary.25666. print pending. [DOI] [PMC free article] [PubMed] [Google Scholar]


