Abstract
For 40 years ebolaviruses have been responsible for sporadic outbreaks of severe and often fatal hemorrhagic fever in humans and nonhuman primates. In December 2013 an unprecedented Zaire ebolavirus epidemic began in West Africa. Although “patient zero” has finally been reached after 2 years, the virus is again causing disease in the region. Currently there are no licensed vaccines or therapeutic countermeasures against ebolaviruses; however, the epidemic in West Africa has focused attention on the potential vaccine platforms developed over the past 15 years. There has been remarkable progress using a variety of platforms including DNA, subunit, and several viral vector approaches, replicating and non-replicating, which have shown varying degrees of protective efficacy in the “gold-standard” nonhuman primate models for Ebolavirus infections. A number of these vaccine platforms have moved into clinical trials over the past year with the hope of finding an efficacious vaccine to prevent future outbreaks/epidemics of Ebola hemorrhagic fever on the scale of the West African epidemic.
Keywords: Ebolavirus, filovirus, prophylaxis, vaccine, animal model
1. INTRODUCTION
Ebolaviruses and marburgviruses, the causative agents of Ebola and Marburg hemorrhagic fever (HF), are members of the family Filoviridae [1]. Filoviruses are filamentous, enveloped, non-segmented, negative sense RNA viruses with genomes approximately 19 kb in length. Seven gene products are encoded within the genomes of these viruses from 3’ to 5’: the nucleoprotein (NP), virion protein (VP)35, VP40, the glycoprotein (GP), VP30, VP24, and the polymerase (L). In addition, the Ebolavirus species express two additional nonstructural proteins from the GP gene: soluble GP (sGP) [2] and small soluble GP (ssGP) [3]. The wild-type 7U genotype expresses ~80% sGP and ~20% GP, whereas tissue culture-adapted viruses with an 8U genotype express only GP [1]. The GP, with potential contribution from the NP and VP40, have been the key immunogenic antigens in vaccine development and protection.
Ebolavirus [species Zaire ebolavirus (ZEBOV), Sudan ebolavirus (SEBOV), Taï Forest ebolavirus (TFEBOV) and Bundibugyo ebolavirus (BEBOV)] and Marburgvirus (species Marburg marburgvirus, MARV) cause a severe form of viral HF with human case fatality rates up to 90% [1]. In 1967, MARV was discovered in Marburg, Germany, however, the origin of the virus was found to be in Africa. The discovery of ZEBOV occurred nine years later in 1976 in Central Africa with two simultaneous outbreaks in Sudan and Zaire (now Democratic Republic of the Congo, DRC) [1]. Before the 2013–2016 epidemic in West Africa, Ebola hemorrhagic fever (EHF) outbreaks caused exclusively by ZEBOV were frequently reported from Central Africa with initial outbreaks in the Democratic Republic in the Congo (DRC) followed in the first decade of the new millennium by multiple outbreaks in the border region between Gabon and the Republic of Congo (ZEBOV). ZEBOV continues to emerge/re-emerge in this region including as recent as 2014 when 66 individuals were infected in DRC and 49 died [4]. Subsequently, EHF outbreaks have been more commonly reported from East Africa or the eastern region of Central Africa, in all cases with BEBOV and SEBOV as the causative pathogens. Over the past 30 years, ZEBOV pathogenesis has been well characterized in animal disease models such as nonhuman primates (NHPs); however, the data from human cases has been limited. The most recent epidemic in West Africa has shed light on disease progression in humans, albeit with limitations as the affected area is amongst the poorest in the world.
To date, experimental vaccine platforms have shown promise in the macaque models though safety and manufacturing concerns for the platforms still exist as such there is still no licensed vaccine or treatment available. Traditionally, vaccines and treatments against filovirus HF are initially screened in guinea pigs, mice, and hamsters [5–11]. Unfortunately, filovirus isolates from humans or NHPs do not cause severe disease in rodents upon first exposure to the virus, typically this requires serial adaptation to produce a uniformly lethal infection. During the adaptation process genomic changes occur that increase the pathogenesis in rodent models; however, disease in rodent models does not often mimic the human disease progression, which is observed in NHPs [6, 9]. As an example, the coagulation disorders that are hallmark features of disease in filovirus-infected humans and NHPs are lacking in filovirus-infected mice or inbred strain 13 guinea pigs [6, 9], though a recent ZEBOV outbred Hartley guinea pig model has shown some coagulation disorders [12]. Another example includes the observed bystander apoptosis of large numbers of uninfected lymphocytes in filovirus-infected humans [13], which can be found in NHP but not necessarily in mouse models [14, 15]. Recently, a described hamster model of ZEBOV infection revealed more similarity with primate disease than mice or guinea pigs [8]; however, further studies need to be conducted to fully assess the utility of this model. These differences raise the concern that efficacy data generated in rodents may not be very predictive of efficacy in NHP models and humans. It is uncertain whether studies performed in rodents would be suitable for supporting applications for licensure of filovirus vaccines and treatments; therefore, this review focuses on vaccine studies performed in NHPs. Additionally, the NHP model for EHF seems pivotal for testing the efficacy of therapeutics, antivirals and vaccines as these cannot routinely be tested in humans. Therefore, the US Food and Drug Administration (FDA) has implemented the animal rule for diseases like filovirus HF that would allow licensing of filovirus countermeasures based on efficacy data in animal models that recapitulate human disease in combination with safety and immunogenicity studies in humans [16].
An objective comparison of vaccine platforms tested in NHPs is impeded by the conditions under which the animal studies are conducted. Challenge virus stocks vary between the different BSL4 facilities and so do the endpoint criteria for animal studies using NHPs. In regard to the challenge virus stocks, the various facilities have successfully used different isolates of ZEBOV (Kikwit, Mayinga, Makona) for NHP challenge underlining that there is indeed cross-protection within the ZEBOV species. However, some of the isolates were an 8U genotype and others a 7U genotype. While both genotypes cause disease in NHPs, virus replication and disease progression are delayed in the 8U genotype variant [17, 18]. Importantly, rAd5-based ZEBOV vaccines, which had previously shown 100% efficacy against virus stocks with a large proportion of 8U genomes were not 100% efficacious against high percentage 7U ZEBOV genome stocks in NHPs [19]. A recent additional complication has resulted from lower challenge doses as in the past the standard challenge dose for ZEBOV was always 1,000 plaque-forming-units (pfu), in more recent studies the bar has been lowered to 100 pfu making it difficult to judge if the immunization scheme is as effective as with previous vaccine approaches subjected to a 1,000 pfu challenge, a dose chosen to represent a lab or healthcare worker accident.
The development of vaccines and treatments for EHF has been an international focus of research laboratories and a number of pharmaceutical companies, which has resulted in a variety of platforms. The vaccine platforms range from DNA and subunit vaccines to non-replicating and replication-competent viral vectors with most targeting the ZEBOV species. These include virus-like particles (VLPs), Venezuelan equine encephalitis virus (VEEV) replicons, adenoviruses, human parainfluenza virus type 3 (HPIV3), cytomegalovirus (CMV), and the rhabdoviruses: rabies virus (RABV) and vesicular stomatitis virus (VSV). While ZEBOV vaccines that have shown efficacy in NHPs are covered in Table 1, every vaccine cannot be covered in detail in this review. Therefore, the most developed vaccine platforms will be presented in detail with Table 1 as a reference point to compare other vaccine platforms that have been 100% efficacious in NHPs.
Table 1.
Efficacy of vaccine platforms in NHPs
| Vaccine candidate | Number of vaccine doses |
Time to challenge |
Survival rate |
References |
|---|---|---|---|---|
| Inactivated virus | 3 | 78 d | 25% | 9 |
| Virus-like particles (VLPs) | 2–3 | 126-70 d | 100 % | 22 & 23 |
| Rec. ZEBOVΔVP30 | 1 | 28 d | 100 % | 46 |
| Alphavirus replicon | 1 | 28 d | 100 % | 30 |
| DNA | 3 | 112 d | 83% | 31 |
| DNA + rec. Adenovirus | 4 | 224 d | 100% | 33 |
| Rec. Adenovirus | 1 | 35-28 d | 100% | 34 & 44 |
| Rec. Adenovirus + MVA | 2 | 273 d | 100% | 44 |
| Rec. Cytomegalovirus | 2 | 112 d | 75% | 89 |
| Rec. Vaccinia virus | 3 | 98 d | 0 % | 9 |
| Rec. Paramyxovirus | 1 | 28 d | 100% | 50 |
| Rec. Vesicular stomatitis virus | 1 | 28-7 d | 100% | 66 & 75 |
| Rec. Rabiesvirus | 1 | 75 d | 100% | 52 |
2. Virus-Like Particles (VLPs)
A non-replicating, protein subunit-based vaccine platform using VLPs has shown utility against filovirus challenge in NHPs. VLPs are produced from cells transfected with plasmids encoding filovirus VP40 matrix protein and GP which release filamentous particles containing these two proteins into the cell culture supernatant [20, 21]. Co-expression of additional filovirus proteins such as NP and VP24 can create VLPs composed of four filovirus proteins; NP, VP24, VP40, and GP [21]. VLPs containing ZEBOV NP, VP40, and GP proteins were created in insect cells using a baculovirus expression system and used in NHPs as a part of a 3-dose vaccine regimen plus RIBI adjuvant at 42 day intervals with subsequent homologous lethal challenge [22]. All five cynomolgus macaques challenged in this study were protected from challenge with evidence of circulating anti-ZEBOV GP antibodies that correlated with complement-mediated and antibody-dependent cell-mediated cytoxicity [22]. Recently, 2 doses of VLPs with QS-21 adjuvant were shown to protect a small number of NHPs against ZEBOV challenge (1,000 pfu; 7A variant) [23]. Interestingly, this platform also showed complete protection with 2 doses of SEBOV-like particles plus QS-21 adjuvant against uniformly lethal SEBOV challenge; furthermore 2-doses of the ZEBOV-like particles with adjuvant conferred cross-protective immunity to TFEBOV [23]. The VLP platform is considered a safe vaccine approach in comparison to some of the replication-competent platforms, which will be discussed in the following sections. Furthermore, VLPs are highly immunogenic and vaccination has been shown to induce innate, humoral and cellular immune responses in rodents, macaques and chimpanzees [24, 25]. However, the mechanism of protection for the VLP platform has not yet been defined, but most certainly humoral immune responses are significant as shown for other protein-based vaccines like the human papilloma vaccine [26]. The incorporation of NP into the VLP preparation may add further targets for T cell mediated immunity, but these immune responses have not yet been investigated.
3. DNA vaccines
Over the last twenty years, DNA vaccination has been developed for a number of viruses including ZEBOV. These vaccines can be rapidly adapted as pathogens evolve, are non-infectious, are able to be used in multiple boost regimens, and can be produced in large quantities; all attractive qualities for vaccines against emerging and re-emerging pathogens. DNA vaccines have been shown to induce cellular as well as humoral immune responses, but regularly require administration of several doses to achieve the desired immunity [27]. While DNA vaccines have shown some efficacy in ZEBOV rodent models [28, 29], the efficacy of ZEBOV GP in NHPs has yet to reach 100% with a recent report describing codon optimized DNA vaccines providing 83% protection against ZEBOV challenge [30].
4. NON-REPLICATING VACCINE VECTORS
4.1 Venezuelan Equine Encephalitis Virus (VEEV) Replicons
Alphaviruses have a broad host range, replicate in multiple vertebrate and invertebrate cells, and can be employed as vaccine vectors, referred to as “replicons”, by cloning the antigen of interest in place of the alphavirus structural genes. These replicons have the ability to transcribe messenger RNA and replicate the genome but do not make virus particles in the absence of the alphaviral structural proteins. VEEV replicons expressing ZEBOV GP, NP, or both GP and NP have been assessed in NHPs with inconsistent results. Vaccination of NHPs with multiple injections of VEEV replicons expressing ZEBOV GP, NP, or both GP and NP at doses in the 107 pfu range, failed to protect any animals from a lethal intramuscular (i.m.) ZEBOV challenge [9]. However, a single injection of NHPs with a blend of 1010 VEEV replicons expressing the ZEBOV GP and SEBOV GP was able to protect animals from i.m. challenge with ZEBOV or SEBOV [31]. Unfortunately, when this vaccine regimen was tested against aerosol challenge with SEBOV, complete protection was not afforded even when the challenge dose was reduced 10-fold. However, increasing the vaccination regimen to two injections was able to provide protection of macaques against lethal outcome but not clinical signs of disease [31].
4.2 Recombinant Adenovirus-based Vectors
Replication-defective adenoviruses, such as the recombinant adenovirus serotype 5 (rAd5) are commonly used as vaccine platforms [32]. The first successful protection of NHPs from EHF was a result of using a prime-boost strategy [33]; cynomolgus macaques were vaccinated three times with DNA expressing the GPs of ZEBOV, SEBOV, and TFEBOV, and the NP of ZEBOV. After 3 months a booster vaccination of a rAd5 vector expressing the ZEBOV GP was administered. The vaccinated animals survived challenge after exposure to a low dose (6 pfu) of ZEBOV. The data for this study suggested that humoral immunity and T memory helper cells were strongly associated with protection; while cell-mediated immunity was important, it was not an absolute requirement for protection [33]. The need for the DNA plasmid component of this regimen is not clear, as a single injection of 1010 infectious units (IFU) of rAd5 expressing the ZEBOV GP resulted in complete protection from death and illness of cynomolgus macaques with a high dose (1,000 pfu) i.m. challenge of homologous ZEBOV 28 days later [34]. Recently, a dose of 1010 IFU of a codon optimized ZEBOV GP vaccine was tested in NHPs in combination with 109 IFU rAd5 expressing interferon α (rAd5/IFNα) resulting in 100% survival of the immunized animals [35]. Additionally, a DNA prime-rAd5 boost strategy was also employed to demonstrate cross-protective vaccine efficacy against the most recently discovered species of Ebolavirus, BEBOV [36]. Briefly, NHPs were vaccinated with DNA expressing ZEBOV GP and SEBOV GP and then boosted (DNA) at weeks 4, 8, and 14. One year later (week 53) animals were boosted with a rAd5-base vaccine expressing the ZEBOV GP. Seven weeks after the rAd5 boost the animals were challenged with BEBOV. All vaccinated macaques survived challenge, with only one animal showing evidence of clinical illness from the BEBOV exposure. Although the vaccine doses are high in titer, the rAd5 platform is non-replicating and therefore considered safe. However, the production of this high-titered vaccine could be problematic.
A significant concern for the use of this vaccine platform is the pre-existing immunity to Ad5 as humans have between 60% and 90% in certain populations [37]. In experimental infections of rodents and NHPs it was demonstrated that pre-existing immunity significantly lowered protective efficacy of rAd5-based vaccines [35, 38–42]; however, improvements to the immunization route or the vaccine vector itself have been used to overcome this problem. For instance, delivery of the vaccine via the oral, nasal or intratracheal route can circumvent pre-existing immunity without having an effect on the protective efficacy against lethal challenge; in addition, the stimulation of T cell responses was significantly improved [35, 39–41]. These efforts have been pushed further by replacing the rAd5 vector with a different serotype-based backbone with less or no pre-existing immunity in humans like Ad26 and Ad35 [42] or to primate-specific adenoviruses such as chimpanzee (Ch)Ad and simian Ad21 [38, 43]. Recently, rAd26 and rAd35 have been employed to develop a pan-filovirus vaccine approach as vaccination of NHPs with rAd35 expressing the ZEBOV GP failed to completely protect animals against a lethal ZEBOV challenge. Similarly, vaccination of NHPs with either rAd26 or a modified adenovirus in which only the seven short hexon hypervariable regions of Ad5 were exchanged from human adenovirus serotype 48 (each expressing the ZEBOV GP) failed to protect animals against a lethal ZEBOV challenge. However, a strategy to prime with rAd26 vectors expressing ZEBOV GP and boost with rAd35 vectors expressing ZEBOV GP was able to protect NHPs against a lethal ZEBOV challenge [42]. Recently, the rChAd3 vaccine vector expressing ZEBOV GP provided 100% protection at a single dose of 1011 and 1010 IFU five weeks after vaccination [44] and was able to provide 50% protection 10 months post-vaccination with a single dose of 1011 IFU and 100% protection using a prime-boost regimen in combination with modified vaccinia Ankara (MVA) expressing ZEBOV and SEBOV GP, MVA-BN-Filo. The data presented suggest long term protection requiring strong memory CD8+ T cells to reestablish effector and memory CD8+ T cell responses after challenge with ZEBOV.
4.3 Recombinant ZEBOVΔVP30
Reverse genetics systems for RNA viruses, including ZEBOV, have enabled the establishment of a recombinant biologically contained ZEBOV [45]. This recombinant (r)ZEBOV has the essential VP30 (a viral-specific transcription activator) deleted and the resulting rZEBOVΔVP30 no longer has the ability to replicate. Providing VP30 in trans allows for propagation of rZEBOVΔVP30 whereas no progeny virus is produced in the absence of VP30 [45]. This vaccine vector was recently shown to confer 100% protection of NHPs against ZEBOV challenge using a single dose, two doses, or H2O2 inactivated two doses of vaccine regimen [46]. rZEBOVΔVP30 is an interesting vaccine approach as the vector resembles wild-type (wt) ZEBOV very closely and contains all but one protein to elicit ZEBOV-specific immune responses. The H2O2 inactivated prime-boost vaccine strategy minimizes safety concerns compared to other platforms.
5. REPLICATION-COMPETENT VACCINE VECTORS
5.1 Recombinant Human parainfluenza virus type 3
Human parainfluenza virus type 3 (HPIV3), a member of the family Paramyxoviridae, is a common pediatric respiratory pathogen. Live-attenuated vectors based on HPIV3 are actively being investigated as vaccines for HIPV3 and other pediatric pathogens [47, 48]. Recently, a HPIV3 vector expressing the ZEBOV GP and a vector expressing two antigens, the ZEBOV NP and GP, have been developed [49]. Combining an intranasal and intratracheal vaccination in NHPs with the ZEBOV GP vector afforded the best protection against a high dose (1,000 pfu) intraperitoneal challenge of homologous ZEBOV 28 days post vaccination [49]. In this study, 85% of HPIV3 ZEBOV GP-vaccinated animals survived challenge and 57% of animals were protected against clinical illness. When the vaccine regimen using the HPIV3 ZEBOV GP vaccine was expanded to two doses over a 67-day period the efficacy was improved, with 100% of NHPs protected against clinical illness and death. This vector was further assessed by aerosol vaccination route and was able to protect 100% of NHPs challenged with 1,000 pfu i.m. with ZEBOV from severe disease however, some clinical signs were noted in survivors [50].
The HPIV3 system is replication competent; therefore, safety concerns require further analyses such as the tolerance in immunocompromised individuals and neurovirulence testing. Additionally, a majority of all adult humans have pre-existing immunity to this common childhood pathogen, which presents a potential challenge using this vaccine vector analogous to rAd5. However, this concern was addressed as it was demonstrated that infection of HPIV3-positive NHPs with HPIV3 expressing ZEBOV GP is possible and results in an immune response to ZEBOV GP. These results indicate that vaccination might be feasible despite preexisting immunity [51], however, this study did not provide data on protective efficacy as production of antibodies to ZEBOV GP does not always correlate with protection against ZEBOV infection [52].
5.2 Recombinant Rabies Virus-based Vectors
A Rabies virus-based vector used as a dual vaccine against Rabies virus (RABV) and ZEBOV for potential use in both at risk populations in Africa, humans and nonhuman primates, has been developed recently. The vector BNPSP333 is based on the reverse genetics system for the RABV vaccine strain SAD B19, which is currently used for wildlife immunizations. A mutation at amino acid 333 in the RABV glycoprotein (RABV-G) attenuates this vector as demonstrated by reduced neurovirulence in adult mice [53]. Development of this platform led to three different versions of the vaccine vector; a replicating vector expressing both RABV-G and ZEBOV-GP (rRABV/ZEBOV-GP), a vector deleted of RABV-G but expressing ZEBOV-GP (rRABVΔG/ZEBOV-GP) and an inactivated form of the replicating vector (inacRABV/ZEBOV-GP) [54]. These novel vaccine vectors for ZEBOV have recently been evaluated in NHPs demonstrating a single dose of the replicating vector rRABV/ZEBOV-GP was 100% protective [52]. In contrast, one dose of rRABVΔG/ZEBOV-GP and a prime/boost regimen with the inacRABV/ZEBOV-GP resulted in 50% protection. Humoral and adaptive immune responses were carefully investigated concluding that survival was largely dependent on the quality of antibody responses to ZEBOV-GP [52]. Interestingly, ZEBOV-GP-specific antibodies with an IgG1-bias seemed to be critical for protection against ZEBOV challenge. Recently, the inactivated RABV vaccine vector was improved using a codon-optimized antigen and showed promising results in a prime/boost regimen with adjuvant in NHPs against challenge with 100 pfu i.m. ZEBOV [55]. These data are very encouraging toward the development of a dual vaccine for RABV and ZEBOV, although the challenge virus dose was 10-fold lower compared to most other studies. Regardless, GMP-production of this vaccine for human clinical trials has been initiated [55].
5.3 Recombinant Vesicular Stomatitis Virus-based Vectors
Over the last few decades, Rose and colleagues have pioneered the use of recombinant vesicular stomatitis virus (rVSV), the prototypic member of the Rhabdoviridae family, as an expression and vaccine vector [56–58]. The main vector used for the Ebolavirus vaccines lacks the VSV glycoprotein (G), which contributes to VSV neurotropism and pathogenicity, and resulting in an attenuated version of VSV serotype Indiana [59, 60]. Furthermore, pre-existing immunity is rare, and directed towards VSV-G, a protein no longer present in this vector [58, 61]. The rVSV Ebolavirus vectors encode the GP as the immunogen(s) in place of VSV-G (rVSVΔG); while this vaccine virus is attenuated it is easily propagated and is highly immunogenic with immunized individuals showing transient viremia [59, 62–64]. An alternative rVSV vaccine vector still expressing VSV-G (rVSV-N4CT1-ZEBOV-GP) has recently shown utility as a ZEBOV vaccine in NHPs [65] although the rVSVΔG vectors described below have been characterized for filoviruses over a longer period of time.
To date, the Ebolavirus vaccine platform based on rVSVΔG (here referred to as rVSV) has proven to be successful. A single i.m. vaccination of NHPs with 1×107 pfu of a rVSV vector expressing only ZEBOV GP (rVSV-ZEBOV) elicited complete protection against a high dose (1,000 pfu) i.m. challenge of homologous ZEBOV given 28 days later [66]. However, cross-protection against another species of Ebolavirus, SEBOV, was not achieved as re-challenge of the survivors with SEBOV resulted in fatal disease [66]. A single i.m. vaccination of NHPs with rVSV-ZEBOV was also able to completely protect animals against a homologous aerosol challenge of ZEBOV given 28 days later [67]. It should be noted that protection against ZEBOV challenge can be conferred by i.m. and mucosal [68]. The mechanism of rVSV-ZEBOV protection from lethal challenge with ZEBOV has been evaluated and results suggested that antibodies were necessary and correlated with protection of NHPs [69]. This platform has been used to generate vaccines for all known Ebolavirus species [70].
The ideal filovirus vaccine should be a single dose vaccine that can protect against the various species and strains of Ebolavirus and MARV as the filoviruses overlap in their endemic areas in addition to the unknown nature of a filovirus attack through bioterrorism. To investigate this possibility, this vector platform was assessed in NHPs as a multivalent vaccine consisting of equal parts of the rVSV-filovirus-GP vaccine vector for MARV, ZEBOV, and SEBOV [71]. After 28 days the NHPs were challenged with ZEBOV, SEBOV, TFEBOV, or MARV. None of the vaccinated NHPs succumbed to any filovirus challenge, showing the utility this platform could have as a single dose multivalent vaccine. Additionally, the utility of combining rVSV-SEBOV and rVSV-ZEBOV vectors using either a single dose blended vaccination approach or a prime-boost regimen against heterologous BEBOV challenge in NHPs was evaluated and resulted in 100% protection [72]. In addition, the rVSV-ZEBOV vaccine caused no adverse events in SHIV-infected NHPs [73]. Despite the immunocompromised status of these animals protection against lethal ZEBOV challenge was achieved and correlated with the level of the humoral immune response supporting the importance of antibodies elicited by this vaccine for protection [73]. In order to address a different safety aspect, pigs representing a livestock species were infected with rVSV-ZEBOV; the animals showed no disease and rVSV-ZEBOV shedding was minimal further underlining the safety of this vaccine [74]. Recently, it was shown that in NHPs the rVSV-ZEBOV vector conferred 100% protection when administered within 7 days of a lethal against ZEBOV challenge [75]. These data are intriguing as they suggest the vaccine can be efficacious and therefore being used for ring vaccination strategies.
6. HUMAN CLINICAL TRIALS
Several of the above described ZEBOV vaccine platforms have been preclinically tested over a decade ago, but until 2014 very few phase I human clinical trials were approved and had been conducted (www.ClinicalTrials.gov; www.pactr.org). Due to the severity of the West African ZEBOV epidemic, the case importations into the European and American continent, and the global public health impact, phase I clinical trials were accelerated for the most promising vaccine platforms providing 100% protection in NHPs (table 1), mainly for the adenovirus/MVA platform and rVSV-ZEBOV (Fig. 1). There are completed and ongoing phase I and II clinical trials worldwide (Fig. 2) and initial phase III clinical trials in West Africa with encouraging preliminary results.
Fig. 1. Overview of vaccine approaches successfully tested in EBOV animal disease models (rodents and macaques) and currently in human clinical trials.
Fig. 2. Distribution of ongoing phase I–III clinical trials in the world.
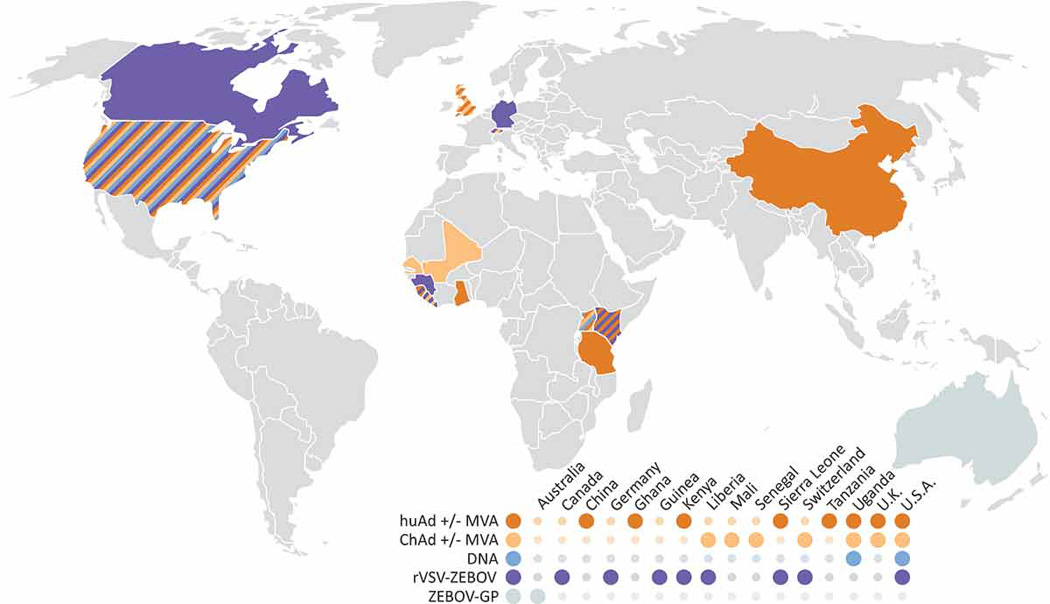
Immunization with Human Adenovirus (HuAd) or Chimpanzee Adenovirus (ChAd) alone or in combination with Modified Vaccinia Ankara (MAV); DNA vaccination; recombinant Vesicular stomatitis virus (rVSV-ZEBOV); recombinant ZEBOV glycoprotein (ZEBOV-GP). Sources: www.ClinicalTrials.gov; www.pactr.org
The first phase I clinical trials for a ZEBOV vaccine using a 3-dose DNA vaccination approach started in November 2003 in the USA [76]. While the vaccine was safe and well tolerated in all volunteers, suboptimal immunogenicity of this particular vaccine formulation was revealed thus triggering efforts to modify the vaccine. An improved 4-dose DNA vaccine formulation was tested and again was safe and well tolerated in all volunteers resulting in enhanced immunogenicity [77]. Similarly, in a phase I trial in Uganda a 3-dose DNA vaccination scheme against ZEBOV and MARV was safe and well tolerated in the trial participants and resulted in good immunogenicity [78]. While the DNA vaccine is a very safe approach, it needs several boosts and therefore requires a longer time to achieve protective immunity making it less desirable in an outbreak but potentially suitable for a population-based immunization.
The second ZEBOV vaccine platform ever tested in humans was the rAd5-based vaccine, which is 100% protective in NHPs [33]. The immunization was safe and well tolerated in the study participants, but pre-existing immunity to the Ad5 vector compromised immunogenicity and particularly humoral immune responses to the ZEBOV antigen [79]. Regardless, in 2015 an updated rAD5 vaccine encoding the antigen from the West African ZEBOV isolate was generated and tested in humans in China. This trial resulted in improved humoral and adaptive immune responses compared to the previous study likely due to the administration of a higher vaccine dose [80]. The pre-existing immunity to the Ad5 vector still remains a concern and could only be overcome in the high-dose vaccination group. The participants developed mild adverse effects (pain at injection site); the vaccine otherwise appeared to be safe.
In order to address the problem of pre-existing immunity to the Ad5 vector, a chimpanzee Ad3 (ChAd3)-based ZEBOV vaccine was developed and demonstrated protective efficacy in NHPs [44]. As this vaccine is a non-replicating vector, it was accelerated for phase I human trials in the USA and Switzerland. The vaccine was safe and well tolerated by all participants for every dose tested, although mild to moderate systemic effects like pain at the injection site, fever, headache and fatigue/malaise were reported [81, 82]. After 4 weeks of immunization with a single ChAd3-ZEBOV vaccine dose of ≥2×1010 or 2×1011 of the ChAd3-ZEBOV/SEBOV combination vaccine, the ZEBOV GP-specific antibody titers reached similar levels as observed in NHPs protected from disease [81, 82]. These results are encouraging for short-term protection but durability of the immune response is questionable as the antigen-specific titers dropped in all participants after 6 months [82]. This concern was addressed in a separate trial when participants in Mali received the ChAd3-ZEBOV vaccine as a prime followed by MVA-BN-Filo as a booster immunization [83], a strategy previously shown to enhance the durability of protection in NHPs [44]. Again, mild to moderate side effects to the vaccination were reported but otherwise both vaccines were safe and well tolerated in all participants. Already 7 days after the MVA-BN-Filo boost the antibody titers to ZEBOV GP were drastically enhanced, but no data on the durability of this response are available yet [83]. Unfortunately, no efficacy data in humans (phase III) are available for the ChAd3/MVA vaccine approach.
The third ZEBOV vaccine in phase I, II and III clinical trials worldwide is the rVSV-ZEBOV (Fig. 2). Similar to the ChAd3 vaccine, several vaccine doses ranging from 3× 105 to 5× 107 pfu were analyzed in the phase I trial participants resulting in dose-dependent variation of antibody responses and mild to moderate side effects (pain at the injection site, fever, chills, headache, fatigue, myalgia), with some severe side-effects in the highest dose group tested (mild arthralgia, arthritis, skin lesions) [62, 63, 84]. Overall, the vaccine dose chosen for future human use (2× 107 pfu) was well tolerated, safe and immunogenic and the vaccine was quickly moved into a phase III ring vaccination trial in Guinea while the 2014–15 ZEBOV outbreak was still ongoing. The preliminary report by Henao-Restrepo and colleagues included 7651 individuals who received the vaccine either immediately after being exposed to an infected person or 21 days later (one ZEBOV incubation period) [64]. Similar to observations in NHPs, this vaccine was fast-acting and the vaccinees seem to be protected from ZEBOV infection within 10 days. While the trials resulted in 16 cases of new Ebolavirus infections in the delayed vaccination group, no cases were reported in the immediately immunized group, demonstrating promising efficacy of the rVSV-ZEBOV vaccine against the circulating ZEBOV-Makona strain in Guinea [64]. This is encouraging data and underlines the importance of a fast-acting vaccine for emergency use like in outbreak situations. To date little is known about the durability of protection after a single rVSV-ZEBOV vaccination with only a limited dataset in Guinea pigs being published [85], but with the ongoing clinical trials this question could be addressed.
7. EXPERT COMMENTARY
Four decades ago in 1976, ZEBOV was recognized as the causative agent of a human HF outbreak in Zaire (now the Democratic Republic of the Congo). Over the past 20 years efforts have been made towards the development of vaccines and therapeutics, but we still do not have an approved/licensed countermeasure. While the most recent EHF epidemic in West Africa has accelerated phase I–III testing of a few promising ZEBOV vaccine candidates as well as a few therapeutics and antivirals (www.ClinicalTrials.gov; www.pactr.org), the major impediments for licensure of countermeasures are still funding for performing large NHP studies following the Animal rule and phase I–II clinical trials as well as opportunities to perform phase III trials. In the past, EHF outbreaks occurred sporadically in Central Africa affecting only small numbers of people in often rural areas. The most recent and first epidemic in West Africa with almost 29,000 cases and over 11,000 fatalities highlighted the potential of this virus to spread quickly in more populated areas of Africa with the potential to cause epidemics and the fear of a pandemic. Fortunately, now there seems to be at least temporary interest of large pharmaceutical companies in ZEBOV vaccine development. With the unexpected and unprecedented response of the global community to the West African epidemic, military personnel, aid workers, charity or medical non-profit organizations and military personnel are vulnerable and would certainly benefit from a vaccine. These groups could be prepared by prophylactic vaccination ahead of upcoming missions but could also benefit from a fast-acting, single-shot vaccine in case of emergency deployments. However, vaccines should primarily be produced and stock-piled for at-risk populations in the endemic areas in Africa, in particular for healthcare workers as they are the first to encounter an infected individual. For this group a single-dose approach with long-lasting immunity is desirable but we are not there yet as there are limited durability data available for the different vaccine platforms currently in clinical trials. For now the ring vaccination in Guinea has been shown to be effective and could easily be repeated in future outbreaks/epidemics. For that it would be advantageous if emergency clinical trial protocols could be put in place and used immediately after confirmation of clinical cases. Researchers working in containment facilities are another group that would benefit from prophylactic vaccination or from treatment following an exposure. Taking this a step further, a similar scenario could be imagined for non-ZEBOV ebolaviruses, MARV and even Lassa virus for which vaccine candidates have gone successful through preclinical trials [86]. If clinical trials for these vaccines could be conducted soon the world could put an emergency response plan in place for a few emerging infectious diseases to counteract before the situation gets out of control.
Ebolaviruses and related pathogens are emerging/re-emerging zoonoses. Thus, special focus should be given to the identification of the reservoir, which is still unknown for ebolaviruses in general albeit fruit bats might be one reservoir species [87]. This could allow for more effective intervention strategies at early stages in transmission and prevent introduction into the human population and other end-host species such as the great apes in case of ZEBOV. Wildlife vaccination needs to be more seriously considered, in particular for great apes in case of ZEBOV. A first step has been made with the RABV-based ZEBOV vaccine [52], which could be delivered mucosally through oral baits similar to the licensed RABV vaccine used to vaccinate foxes and raccoons in Europe and the United States, respectively [88]. Very recently, a replication-competent recombinant cytomegalovirus vaccine expressing the ZEBOV GP has been described and first protective efficacy has been demonstrated in the ZEBOV rhesus macaque model [89]. This vaccine has the potential to be developed into a disseminating vaccine approach as cytomegaloviruses are known for their strict species-specificity and spread through the target population without affecting other mammalian species. Immunization strategies like this are of great value to both wildlife preservation as well as human public health.
8. FIVE-YEAR VIEW
The goal over the next five years should be that at least a few of the ZEBOV vaccine approaches successfully tested in clinical trials will be moved through licensing for stockpiling in case of public health emergencies such as other outbreaks, case importations or intentional release. These vaccines should be first considered for at risk groups like healthcare workers in the endemic areas in Africa, aid workers that might be deployed for public health missions and researchers in high-containment facilities. In outbreak situations ring vaccination should be considered. African countries affected by ebolaviruses should have mechanisms for immediate vaccination of larger parts of their population in place in case of emergencies.
The mechanism of protection for a few ZEBOV vaccine platforms has been studied but so far there has been no common correlate identified. Further studies into defining the mechanisms of protection mediated by ZEBOV vaccines need to follow. Notably, the level of total IgG antibodies seems to be a common correlate of protection in response to different ZEBOV vaccine approaches. This important read-out for effective vaccination needs to be verified and further defined together with studying the durability of the protective immune responses elicited by each vaccine.
Lastly, it is important to identify the reservoir(s) - and perhaps potential interim/amplifying hosts should they exist - for the different Ebolavirus species in order to educate people effectively in preventing virus transmissions into the human population. From a wildlife conservation and public health standpoint it is reasonable to consider wildlife vaccination in the ZEBOV-affected areas in Africa, most importantly the great ape populations. The strategy of bi- or even multivalent vaccine approaches, simultaneously targeting pathogens with higher animal and/or public health impact (i.e. rabies virus, malaria), could help increase the interest of industry partners for financing options and compliance in the population.
KEY ISSUES.
Ebolaviruses cause sporadic outbreaks of HF in Africa with increasing frequency and high case fatality rates.
There are no approved vaccines or treatment options available, making efforts towards effective prophylaxis and therapy urgent.
The reservoir species for ebolaviruses remains to be identified; evidence pointing towards bats needs to be confirmed.
EHF outbreaks in the human population are often linked to exposure to wildlife. More effort needs to be made for wildlife vaccination approaches.
In response to the recent West African ZEBOV epidemic, experimental vaccine approaches protective in nonhuman primate models of EHF have been accelerated into phase I–III clinical trials with promising results.
While antibodies have been shown to be important for protection for the rhabdovirus-based vaccine vectors, protective efficacy with the rAd5 platform seems to be more dependent on CD8+ T cells.
The development of various vaccine platforms is encouraged as they have their advantages/disadvantages for distinct application approaches, i.e. emergency fast-acting vaccination versus population-based durable immunization.
Acknowledgments
Filovirus research is supported in part by the Division of Intramural Research, NIAID, NIH and UC7AI094660 for BSL-4 operations support of the Galveston National Laboratory. H Feldmann and TW Geisbert claim intellectual property regarding the vesicular stomatitis virus-based filovirus vaccine.
The authors would like to thank Austin Athman and Ryan Kissinger (NIAID) for assistance with figure production.
Footnotes
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
Reference annotations
* Of interest
** Of considerable interest
- 1.Feldmann H, Sanchez A, Geisbert TW. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 923–956. [Google Scholar]
- 2.Volchkova VA, Feldmann H, Klenk HD, Volchkov VE. The nonstructural small glycoprotein sGP of Ebola virus is secreted as an antiparallel-orientated homodimer. Virology. 1998;250:408–414. doi: 10.1006/viro.1998.9389. [DOI] [PubMed] [Google Scholar]
- 3.Mehedi M, Falzarano D, Seebach J, Hu X, Carpenter MS, Schnittler HJ, et al. A new Ebola virus nonstructural glycoprotein expressed through RNA editing. Journal of virology. 2011;85:5406–5414. doi: 10.1128/JVI.02190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Ebola virus disease - Democratic Republic of Congo. Disease outbreak news. 2014
- 5.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. The Journal of infectious diseases. 1998;178:651–661. doi: 10.1086/515386. [DOI] [PubMed] [Google Scholar]
- 6.Bray M. The role of the Type I interferon response in the resistance of mice to filovirus infection. The Journal of general virology. 2001;82:1365–1373. doi: 10.1099/0022-1317-82-6-1365. [DOI] [PubMed] [Google Scholar]
- 7.Connolly BM, Steele KE, Davis KJ, Geisbert TW, Kell WM, Jaax NK, et al. Pathogenesis of experimental Ebola virus infection in guinea pigs. The Journal of infectious diseases. 1999;179(Suppl 1):S203–S217. doi: 10.1086/514305. [DOI] [PubMed] [Google Scholar]
- 8.Ebihara H, Zivcec M, Gardner D, Falzarano D, LaCasse R, Rosenke R, et al. A Syrian golden hamster model recapitulating ebola hemorrhagic fever. The Journal of infectious diseases. 2013;207:306–318. doi: 10.1093/infdis/jis626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisbert TW, Pushko P, Anderson K, Smith J, Davis KJ, Jahrling PB. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerging infectious diseases. 2002;8:503–507. doi: 10.3201/eid0805.010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryabchikova E, Kolesnikova L, Smolina M, Tkachev V, Pereboeva L, Baranova S, et al. Ebola virus infection in guinea pigs: presumable role of granulomatous inflammation in pathogenesis. Archives of virology. 1996;141:909–921. doi: 10.1007/BF01718165. [DOI] [PubMed] [Google Scholar]
- 11.Warfield KL, Bradfute SB, Wells J, Lofts L, Cooper MT, Alves DA, et al. Development and characterization of a mouse model for Marburg hemorrhagic fever. Journal of virology. 2009;83:6404–6415. doi: 10.1128/JVI.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross RW, Fenton KA, Geisbert JB, Mire CE, Geisbert TW. Modeling the Disease Course of Zaire ebolavirus Infection in the Outbred Guinea Pig. The Journal of infectious diseases. 2015;212(Suppl 2):S305–S315. doi: 10.1093/infdis/jiv237. [DOI] [PubMed] [Google Scholar]
- 13.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nature medicine. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 14.Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Invest. 2000;80:171–186. doi: 10.1038/labinvest.3780021. [DOI] [PubMed] [Google Scholar]
- 15.Bradfute SB, Braun DR, Shamblin JD, Geisbert JB, Paragas J, Garrison A, et al. Lymphocyte death in a mouse model of Ebola virus infection. The Journal of infectious diseases. 2007;196(Suppl 2):S296–S304. doi: 10.1086/520602. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009;7:393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisbert TW, Strong JE, Feldmann H. Considerations in the Use of Nonhuman Primate Models of Ebola Virus and Marburg Virus Infection. The Journal of infectious diseases. 2015;212(Suppl 2):S91–S97. doi: 10.1093/infdis/jiv284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trefry JC, Wollen SE, Nasar F, Shamblin JD, Kern SJ, Bearss JJ, et al. Ebola Virus Infections in Nonhuman Primates Are Temporally Influenced by Glycoprotein Poly-U Editing Site Populations in the Exposure Material. Viruses. 2015;7:6739–6754. doi: 10.3390/v7122969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirschberg R, Ward LA, Kilgore N, Kurnat R, Schiltz H, Albrecht MT, et al. Challenges, progress, and opportunities: proceedings of the filovirus medical countermeasures workshop. Viruses. 2014;6:2673–2697. doi: 10.3390/v6072673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasenosky LD, Neumann G, Lukashevich I, Kawaoka Y. Ebola virus VP40-induced particle formation and association with the lipid bilayer. Journal of virology. 2001;75:5205–5214. doi: 10.1128/JVI.75.11.5205-5214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Licata JM, Johnson RF, Han Z, Harty RN. Contribution of ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. Journal of virology. 2004;78:7344–7351. doi: 10.1128/JVI.78.14.7344-7351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. The Journal of infectious diseases. 2007;196(Suppl 2):S430–S437. doi: 10.1086/520583. *First study demonstrating efficacy of the VLP vaccine in NHPs.
- 23.Warfield KL, Dye JM, Wells JB, Unfer RC, Holtsberg FW, Shulenin S, et al. Homologous and heterologous protection of nonhuman primates by Ebola and Sudan virus-like particles. PloS one. 2015;10:e0118881. doi: 10.1371/journal.pone.0118881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warfield KL, Aman MJ. Advances in virus-like particle vaccines for filoviruses. The Journal of infectious diseases. 2011;204(Suppl 3):S1053–S1059. doi: 10.1093/infdis/jir346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warfield KL, Goetzmann JE, Biggins JE, Kasda MB, Unfer RC, Vu H, et al. Vaccinating captive chimpanzees to save wild chimpanzees. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8873–8876. doi: 10.1073/pnas.1316902111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines--immune responses. Vaccine. 2012;30(Suppl 5):F83–F87. doi: 10.1016/j.vaccine.2012.04.106. [DOI] [PubMed] [Google Scholar]
- 27.Lu S, Wang S, Grimes-Serrano JM. Current progress of DNA vaccine studies in humans. Expert review of vaccines. 2008;7:175–191. doi: 10.1586/14760584.7.2.175. [DOI] [PubMed] [Google Scholar]
- 28.Vanderzanden L, Bray M, Fuller D, Roberts T, Custer D, Spik K, et al. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology. 1998;246:134–144. doi: 10.1006/viro.1998.9176. [DOI] [PubMed] [Google Scholar]
- 29.Riemenschneider J, Garrison A, Geisbert J, Jahrling P, Hevey M, Negley D, et al. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine. 2003;21:4071–4080. doi: 10.1016/s0264-410x(03)00362-1. [DOI] [PubMed] [Google Scholar]
- 30.Grant-Klein RJ, Altamura LA, Badger CV, Bounds CE, Van Deusen NM, Kwilas SA, et al. Codon-optimized filovirus DNA vaccines delivered by intramuscular electroporation protect cynomolgus macaques from lethal Ebola and Marburg virus challenges. Human vaccines & immunotherapeutics. 2015;11:1991–2004. doi: 10.1080/21645515.2015.1039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbert AS, Kuehne AI, Barth JF, Ortiz RA, Nichols DK, Zak SE, et al. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. Journal of virology. 2013;87:4952–4964. doi: 10.1128/JVI.03361-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hitt MM, Graham FL. Adenovirus vectors for human gene therapy. Adv Virus Res. 2000;55:479–505. doi: 10.1016/s0065-3527(00)55014-3. [DOI] [PubMed] [Google Scholar]
- 33. Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. *Introduces the first vaccine protective against ZEBOV in NHPs.
- 34.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson JS, Pillet S, Bello AJ, Kobinger GP. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. Journal of virology. 2013 doi: 10.1128/JVI.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hensley LE, Mulangu S, Asiedu C, Johnson J, Honko AN, Stanley D, et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus Species. PLoS pathogens. 2010;6:e1000904. doi: 10.1371/journal.ppat.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 38.Kobinger GP, Feldmann H, Zhi Y, Schumer G, Gao G, Feldmann F, et al. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology. 2006;346:394–401. doi: 10.1016/j.virol.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 39.Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, et al. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PloS one. 2008;3:e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson JS, Abou MC, Tran KN, Kumar A, Sahai BM, Kobinger GP. Impact of systemic or mucosal immunity to adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs. The Journal of infectious diseases. 2011;204(Suppl 3):S1032–S1042. doi: 10.1093/infdis/jir332. [DOI] [PubMed] [Google Scholar]
- 41.Choi JH, Schafer SC, Zhang L, Kobinger GP, Juelich T, Freiberg AN, et al. A single sublingual dose of an adenovirus-based vaccine protects against lethal Ebola challenge in mice and guinea pigs. Molecular pharmaceutics. 2012;9:156–167. doi: 10.1021/mp200392g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geisbert TW, Bailey M, Hensley L, Asiedu C, Geisbert J, Stanley D, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. Journal of virology. 2011;85:4222–4233. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy S, Zhi Y, Kobinger GP, Figueredo J, Calcedo R, Miller JR, et al. Generation of an adenoviral vaccine vector based on simian adenovirus 21. The Journal of general virology. 2006;87:2477–2485. doi: 10.1099/vir.0.81989-0. [DOI] [PubMed] [Google Scholar]
- 44. Stanley DA, Honko AN, Asiedu C, Trefry JC, Lau-Kilby AW, Johnson JC, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nature medicine. 2014;20:1126–1129. doi: 10.1038/nm.3702. *First study demonstrating efficacy of the ChAd3 vaccine in NHPs.
- 45.Halfmann P, Kim JH, Ebihara H, Noda T, Neumann G, Feldmann H, et al. Generation of biologically contained Ebola viruses. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1129–1133. doi: 10.1073/pnas.0708057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marzi A, Halfmann P, Hill-Batorski L, Feldmann F, Shupert WL, Neumann G, et al. Vaccines. An Ebola whole-virus vaccine is protective in nonhuman primates. Science. 2015;348:439–442. doi: 10.1126/science.aaa4919. *First study showing efficacy of the ZEBOVΔVP30 vaccine in NHPs.
- 47.Durbin AP, Skiadopoulos MH, McAuliffe JM, Riggs JM, Surman SR, Collins PL, et al. Human parainfluenza virus type 3 (PIV3) expressing the hemagglutinin protein of measles virus provides a potential method for immunization against measles virus and PIV3 in early infancy. Journal of virology. 2000;74:6821–6831. doi: 10.1128/jvi.74.15.6821-6831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karron RA, Belshe RB, Wright PF, Thumar B, Burns B, Newman F, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr Infect Dis J. 2003;22:394–405. doi: 10.1097/01.inf.0000066244.31769.83. [DOI] [PubMed] [Google Scholar]
- 49.Bukreyev A, Rollin PE, Tate MK, Yang L, Zaki SR, Shieh WJ, et al. Successful topical respiratory tract immunization of primates against Ebola virus. Journal of virology. 2007;81:6379–6388. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meyer M, Garron T, Lubaki NM, Mire CE, Fenton KA, Klages C, et al. Aerosolized Ebola vaccine protects primates and elicits lung-resident T cell responses. J Clin Invest. 2015;125:3241–3255. doi: 10.1172/JCI81532. *First study demonstrating efficacy of the HPIV3 vaccine in NHPs.
- 51.Bukreyev AA, Dinapoli JM, Yang L, Murphy BR, Collins PL. Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology. 2010;399:290–298. doi: 10.1016/j.virol.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Blaney JE, Marzi A, Willet M, Papaneri AB, Wirblich C, Feldmann F, et al. Antibody quality and protection from lethal ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS pathogens. 2013;9:e1003389. doi: 10.1371/journal.ppat.1003389. *First study showing efficacy of the RABV vaccine in NHPs.
- 53.McGettigan JP, Pomerantz RJ, Siler CA, McKenna PM, Foley HD, Dietzschold B, et al. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic. Journal of virology. 2003;77:237–244. doi: 10.1128/JVI.77.1.237-244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, Juelich TL, et al. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. Journal of virology. 2011;85:10605–10616. doi: 10.1128/JVI.00558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willet M, Kurup D, Papaneri A, Wirblich C, Hooper JW, Kwilas SA, et al. Preclinical Development of Inactivated Rabies Virus-Based Polyvalent Vaccine Against Rabies and Filoviruses. The Journal of infectious diseases. 2015;212(Suppl 2):S414–S424. doi: 10.1093/infdis/jiv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts A, Buonocore L, Price R, Forman J, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. Journal of virology. 1999;73:3723–3732. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose NF, Roberts A, Buonocore L, Rose JK. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. Journal of virology. 2000;74:10903–10910. doi: 10.1128/jvi.74.23.10903-10910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106:539–549. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 59.Marzi A, Feldmann H, Geisbert TW, Falzarano D. Vesicular Stomatitis Virus-Based Vaccines for Prophylaxis and Treatment of Filovirus Infections. Journal of bioterrorism & biodefense. 2011:S1. doi: 10.4172/2157-2526.S1-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mire CE, Miller AD, Carville A, Westmoreland SV, Geisbert JB, Mansfield KG, et al. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS neglected tropical diseases. 2012;6:e1567. doi: 10.1371/journal.pntd.0001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. Journal of virology. 2004;78:5458–5465. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Munoz P, et al. A Recombinant Vesicular Stomatitis Virus Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, et al. Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe - Preliminary Report. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–866. doi: 10.1016/S0140-6736(15)61117-5. *First study demonstrating efficacy of the rVSV-ZEBOV vaccine in humans.
- 65.Mire CE, Matassov D, Geisbert JB, Latham TE, Agans KN, Xu R, et al. Single-dose attenuated Vesiculovax vaccines protect primates against Ebola Makona virus. Nature. 2015;520:688–691. doi: 10.1038/nature14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nature medicine. 2005;11:786–790. doi: 10.1038/nm1258. *First study showing efficacy of the rVSV-ZEBOV vaccine in NHPs.
- 67.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, Reed DS, Feldmann F, Grolla A, et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26:6894–6900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, et al. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PloS one. 2009;4:e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marzi A, Engelmann F, Feldmann F, Haberthur K, Shupert WL, Brining D, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1893–1898. doi: 10.1073/pnas.1209591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marzi A, Ebihara H, Callison J, Groseth A, Williams KJ, Geisbert TW, et al. Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. The Journal of infectious diseases. 2011;204(Suppl 3):S1066–S1074. doi: 10.1093/infdis/jir348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. Journal of virology. 2009;83:7296–7304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mire CE, Geisbert JB, Marzi A, Agans KN, Feldmann H, Geisbert TW. Vesicular stomatitis virus-based vaccines protect nonhuman primates against Bundibugyo ebolavirus. PLoS neglected tropical diseases. 2013;7:e2600. doi: 10.1371/journal.pntd.0002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, Geisbert JB, Grolla A, Leung A, et al. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS pathogens. 2008;4:e1000225. doi: 10.1371/journal.ppat.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Wit E, Marzi A, Bushmaker T, Brining D, Scott D, Richt JA, et al. Safety of recombinant VSV-Ebola virus vaccine vector in pigs. Emerging infectious diseases. 2015;21:702–704. doi: 10.3201/eid2104.142012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, Scott DP, et al. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science. 2015;349:739–742. doi: 10.1126/science.aab3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, Koup RA, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clinical and vaccine immunology : CVI. 2006;13:1267–1277. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarwar UN, Costner P, Enama ME, Berkowitz N, Hu Z, Hendel CS, et al. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a phase I clinical trial. The Journal of infectious diseases. 2015;211:549–557. doi: 10.1093/infdis/jiu511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kibuuka H, Berkowitz NM, Millard M, Enama ME, Tindikahwa A, Sekiziyivu AB, et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet. 2015;385:1545–1554. doi: 10.1016/S0140-6736(14)62385-0. [DOI] [PubMed] [Google Scholar]
- 79.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304–313. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 80.Zhu F-C, Hou L-H, Li J-X, Wu S-P, Liu P, Zhang G-R, et al. Safety and immunogenicity of a nocel recombinant adenovirus type 5 vector based Ebola vaccine in healthy adults in China: preliminary report of a phase 1, randomized, double-blind, placebo-controlled clinical trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)60553-0. [DOI] [PubMed] [Google Scholar]
- 81.Ledgerwood JE, DeZure AD, Stanley DA, Novik L, Enama ME, Berkowitz NM, et al. Chimpanzee Adenovirus Vector Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 82.De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis. 2015 doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 83.Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2016;16:31–42. doi: 10.1016/S1473-3099(15)00362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2015;15:1156–1166. doi: 10.1016/S1473-3099(15)00154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong G, Audet J, Fernando L, Fausther-Bovendo H, Alimonti JB, Kobinger GP, et al. Immunization with vesicular stomatitis virus vaccine expressing the Ebola glycoprotein provides sustained long-term protection in rodents. Vaccine. 2014;32:5722–5729. doi: 10.1016/j.vaccine.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Falzarano D, Feldmann H. Vaccines for viral hemorrhagic fevers--progress and shortcomings. Current opinion in virology. 2013;3:343–351. doi: 10.1016/j.coviro.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones ME, Schuh AJ, Amman BR, Sealy TK, Zaki SR, Nichol ST, et al. Experimental Inoculation of Egyptian Rousette Bats (Rousettus aegyptiacus) with Viruses of the Ebolavirus and Marburgvirus Genera. Viruses. 2015;7:3420–3442. doi: 10.3390/v7072779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vos A, Pommerening E, Neubert L, Kachel S, Neubert A. Safety studies of the oral rabies vaccine SAD B19 in striped skunk (Mephitis mephitis) J Wildl Dis. 2002;38:428–431. doi: 10.7589/0090-3558-38.2.428. [DOI] [PubMed] [Google Scholar]
- 89. Marzi A, Murphy AA, Feldmann F, Parkins CJ, Haddock E, Hanley PW, et al. Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Sci Rep. 2016;6:21674. doi: 10.1038/srep21674. *First study demonstrating efficacy of the CMV-based vaccine in NHPs.


