Abstract
Staphylococcus aureus is a Gram-positive coccus that interacts with human hosts on a spectrum from quiet commensal to deadly pathogen. S. aureus is capable of infecting nearly every tissue in the body resulting in cellulitis, pneumonia, osteomyelitis, endocarditis, brain abscesses, bacteremia and more. S. aureus has a wide range factors that promote infection and each site of infection triggers a different response in the human host. In particular, the different patterns of inflammasome activation mediate tissue-specific pathogenesis in S. aureus infection. Although still a nascent field, understanding the unique host-pathogen interactions in each infection and the role of inflammasomes in mediating pathogenesis may lead to novel strategies for treating S. aureus infections. Reviews addressing S. aureus virulence and pathogenesis (Thammavongsa et al. 2015), as well as epidemiology and pathophysiology (Tong et al. 2015), have recently been published. This review will focus on S. aureus factors that activate inflammasomes and their impact on innate immune signaling and bacterial survival.
Keywords: S. aureus, pathogenesis, virulence, hemolysin, leukotoxin, neutrophil, macrophage, monocyte, innate immune, host-pathogen interaction
1. Introduction
S. aureus has numerous mechanisms to evade and subvert the immune system allowing it to produce infection broadly in immune-competent hosts. Many virulence factors, such as pore-forming toxins (PFTs) and phenol-soluble modulins (PSMs), activate inflammasomes and will be discussed here (Fig. 1). Most S. aureus PFTs (i.e., alpha-hemolysin [Hla], gamma-hemolysin [HlgAB, HlgCB], leukocidin AB [LukAB, also known as LukGH], leukocidin ED [LukED], and Panton-Valentine leukocidin [PVL, also known as LukFS]) are two-component toxins that initiate attack when one component binds a toxin-specific cell surface receptor and inserts itself into the host cell membrane. This is followed by recruitment of the second component then additional pairs to complete a hexameric beta-barrel pore (Badarau et al. 2015; Yamashita et al. 2011; Pedelacq et al. 1999; Guillet et al. 2004). The notable exception to this pattern is Hla which forms a pore by binding to its receptor then forming a heptameric beta-barrel pore made up of only a single component (Sugawara et al. 2015; Gouaux et al. 1997).
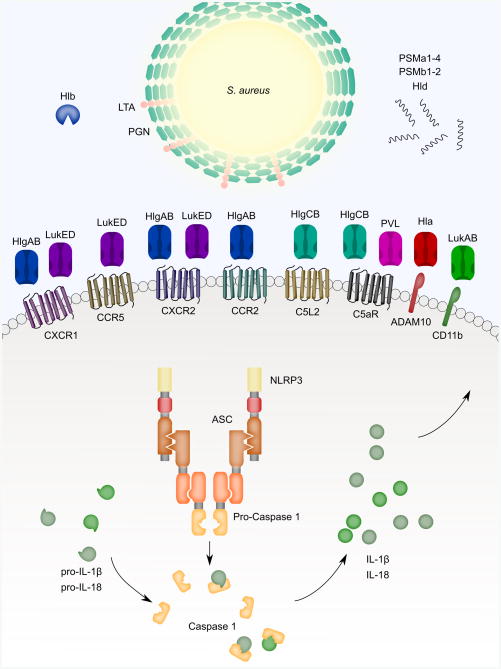
Fig. 1.
S. aureus PAMPs and virulence factors activate inflammasome signaling. A wide variety of S. aureus bacterial molecular patterns and virulence factors, including pore-forming toxins (PFTs), peptidoglycan (PGN), phenol-soluble modulins (PSMs and Hld), activate inflammasome signaling. The NLRP3 inflammasome is depicted as the primary example of inflammasome signaling in this figure. The PFTs - Hla, LukAB, LukED, PVL, HlgAB, and HlgCB – bind cellular receptors, as seen in the center of the figure, to promote pore formation. Pore formation causes potassium efflux and leads to NLRP3 inflammasome activation. When activated, NLRP3 binds ASC through PYD-PYD interactions and ASC binds pro-Caspase 1 through CARD-CARD interactions. Formation of the NLRP3 inflammasome complex causes activation of Caspase 1 that can process pro-IL-1B and pro-IL-18 for activation and secretion. Beta hemolysin (Hlb), a sphingomyelinase, and the PSMs also activate inflammasome-like signaling. Purified Hlb and PSMs can trigger IL-1B and IL-18 secretion but direct activation of the NLRP3 inflammasome by these virulence factors has not yet been demonstrated.
Each of these toxins is cytotoxic to a range of cell types based on expression of the toxins' cognate receptors: Hla and A Disintegrin And Metalloprotease 10 (ADAM10) (Wilke and Bubeck Wardenburg 2010); HlgAB and CXCR1, CXCR2 and CCR2; HlgCB and C5aR and C5L2 (Spaan et al. 2014); LukAB and CD11b (DuMont et al. 2013a), LukED and CCR5 (Alonzo et al. 2013), CXCR1 and CXCR2 (Reyes-Robles et al. 2013); and PVL and C5aR (Spaan et al. 2013). Many of these toxin-receptor pairs lend a level of species specificity as well. In primary human monocytes, the rank order potency of the PFTs is LukAB and PVL (tied), Hla and HlgCB (tied), HlgAB, then LukED (Melehani et al. 2015). The lukAB operon promoter is preferentially activated when USA300 strain S. aureus is exposed to human polymorphonuclear leukocytes (PMNs) (DuMont et al. 2013b). Despite these hints at a prominent role for LukAB in S. aureus pathogenesis, the role of LukAB in infections has largely been overlooked because of its lack of effect in mice. LukAB binds to human CD11b with 1000-fold higher affinity than murine CD11b, rendering mouse leukocytes resistant to LukAB (DuMont et al. 2013a). Murine neutrophils are also completely resistant to HlgCB and are also partially resistant to HlgAB because CCR2 is the only compatible HlgAB murine receptor (Spaan et al. 2014). Both rabbit and human neutrophils are susceptible to PVL while murine and java monkey neutrophils are resistant (Loffler et al. 2010). As predicted by this susceptibility pattern, HEK cells exogenously expressing rabbit and human C5aR both bind the LukS component of PVL while cells expressing mouse or macaque C5aR do not bind LukS (Spaan et al. 2013). Thus, S. aureus PFTs have the potential to play varying roles depending on the site of S. aureus infection and species of the host.
Like alpha-hemolysin (Hla), beta hemolysin (Hlb) and delta hemolysin (Hld) are named for their ability to lyse red blood cells in vitro. However, they are functionally distinct toxins that do not form a beta-barrel pore like Hla. Hlb is a hemolytic and cytotoxic sphingomyelinase (Walev et al. 1996; Huseby et al. 2007). Delta-hemolysin is a 26-amino acid membrane-damaging peptide that is classified as a phenol-soluble modulin (Fitton et al. 1980).
Phenol-soluble modulins (PSMs – reviewed (Peschel and Otto 2013)) are cytotoxic and pro-inflammatory peptides produced by S. aureus (Wang et al. 2007). S. aureus produces four PSMα peptides (PSMα1-PSMα4), two PSMβ peptides (PSMβ1 and PSMβ2), and delta-hemolysin (Hld). All seven PSMs are sensed by formyl peptide receptor 2 (FPR2) in human neutrophils. Activation of FPR2 by PSMs promotes chemotaxis of neutrophils into a site of infection in response to S. aureus (Kretschmer et al. 2010). PSMs also have demonstrated roles in cytolysis, biofilm development, and immunomodulation and are thought to be partially responsible for the increased virulence of community-acquired S. aureus over hospital-acquired strains (reviewed (Otto 2010)).
Lipoteichoic acid (LTA) is a component of the bacterial wall in Gram-positive bacteria that is essential for bacterial growth, cell division and survival (Grundling and Schneewind 2007). The innate immune system has evolved multiple strategies for detecting and responding to LTA, including Toll-like receptor 2 (TLR2), Scavenger Receptor A (Dunne et al. 1994; Greenberg et al. 1996), CD36 (Hoebe et al. 2005), CD14 and soluble CD14 (Hermann et al. 2002; Han et al. 2003), Surfactant Protein D (van de Wetering et al. 2001), Paired Ig-like Receptor B (Nakayama et al. 2012), and L-ficolin (Lynch et al. 2004) (reviewed (Weidenmaier and Peschel 2008)). Having multiple methods for sensing LTA is probably important to fine tune the immune response to infections. For example, LTA binding to TLR2 upregulates cytokine production through NF-κB signaling, while LTA binding to Paired Ig-like receptor B suppresses IL-6 and IL-1β secretion (Nakayama et al. 2012). LTA is not unique to S. aureus and is also found in other Firmicutes, such as Bacillus subtilis and Listeria monocytogenes (Percy and Grundling 2014). Because these bacteria last shared a common ancestor approximately 1.5 billion years ago (Hedges et al. 2006; Kumar and Hedges 2011), the utilization of LTA likely evolved well before the establishment of multicellular life. This suggests that LTA sensing in humans is the result of selective pressures favoring LTA detection by the host rather than evolution of LTA to manipulate immune signaling.
Additionally, other integral bacterial components such as bacterial RNA (Sha et al. 2014; Eigenbrod et al. 2012), lipoproteins (Munoz-Planillo et al. 2009), and peptidoglycan (Muller et al. 2015; Shimada et al. 2010) are important molecular patterns detected by the immune system through inflammasomes. However, due to space limitations, these will not be discussed here.
2. Inflammasomes that are activated by S. aureus
Multiple inflammasomes are activated during S. aureus infections. The first demonstration of inflammasome activation by S. aureus was the observation that NLRP3 deficient macrophages were dramatically impaired in secretion of IL-1β after exposure to live S. aureus (Mariathasan et al. 2006). Activation of the NLRP3 inflammasome involves two steps: (1) a priming step that involves NF-κB-mediated upregulation of pro-IL-1β transcription and post-translational modifications of inflammasome components and (2) NLRP3 inflammasome oligomerization leading to Caspase 1 activation and secretion of mature IL-1β and IL-18 and pyroptosis (Figure 2).
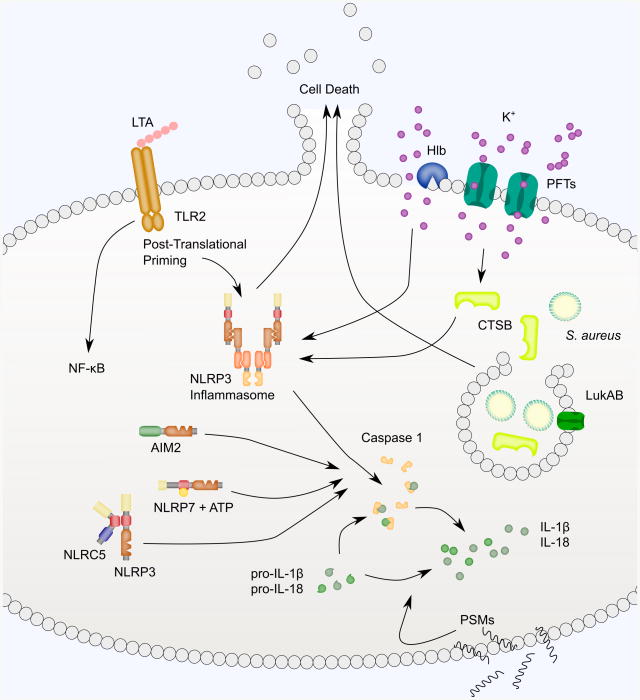
Fig. 2.
NLRP3, NLRC5, NLRP7, AIM2 and other inflammasome-like signaling is activated in response to S. aureus. Activation of inflammasome signaling typically proceeds in two steps. First, TLRs engage PAMPS to prime inflammasome signaling. S. aureus lipoteichoic acid (LTA), a major constituent of the cell wall of Gram-positive bacteria binds TLR2. TLR signaling activates NF-κB-mediated transcription of pro-IL-1β and also triggers post-translational modifications of the inflammasome signaling pathway. Activation of the NLRP3 inflammasome occurs through pore-mediated and phagocytosis-mediated processes. PFTs and Hlb damage the plasma membrane and cause potassium efflux necessary for inflammasome activation. PVL-induced NLRP3 inflammasome signaling requires CTSB activation. PVL, Hla and LukAB also promote Caspase 1-independent cell death. PFTs may also destabilize lysophagosomes during phagocytosis of S. aureus. In particular, LukAB has been shown to bind CD11b on the phagosomal membrane to promote S. aureus escape. This triggers NLRP3-dependent cytokine secretion and NLRP3-independent cell death. Lysophagosomal rupture is thought to lead to CTSB leakage into the cytoplasm, though this has not been shown directly with S. aureus. A variety of other inflammasomes have also been implicated in sensing S. aureus. NLRC5 binds to NLRP3 and enhances cytokine secretion. NLRP7 and its ATP-binding activity are required for Caspase 1 activation in response to S. aureus. AIM2 is activated in response to S. aureus central nervous system abscess formation, though it isn't clear if AIM2 is sensing S. aureus or a danger-associated molecular pattern resulting from S. aureus infection. PSMs also trigger secretion of IL-1β and IL-18, but through a Caspase 1-independent mechanism.
In the case of an S. aureus infection, TLR2 detects LTA to provide an initial priming signal (Schwandner et al. 1999; Yoshimura et al. 1999). In experimental systems, cells are often primed by the addition of purified LTA (Melehani et al. 2015) or heat-killed S. aureus (HKSA) (Craven et al. 2009), or other TLR-ligand such as LPS.
Numerous studies have since delineated the contribution of various S. aureus virulence factors to NLRP3 inflammasome activation. All six PFTs from S. aureus activate the NLRP3 inflammasome in THP1 cells and primary human monocytes, leading to canonical cytokine processing and cell death (Melehani et al. 2015; Holzinger et al. 2012; Craven et al. 2009; Munoz-Planillo et al. 2009).
Caspase 1 is critical for processing IL-1β and IL-18 for secretion and is required for a type of necrotic cell death termed pyroptosis. However, Hla-, PVL- or LukAB-triggered cell death is not blocked in human cells pre-treated with Caspase 1 inhibitors (Craven et al. 2009; Melehani et al. 2015; Holzinger et al. 2012). Additionally, Casp1/Casp11 knockout mouse macrophages are killed by treatment with Hla just as Caspase-1/Caspase 11 sufficient macrophages (Craven et al. 2009; Melehani et al. 2015; Holzinger et al. 2012). However, loss of NLRP3 protects cells against PFT-induced cell death. Therefore, S. aureus PFTs seem to trigger a host cell death pathway with an incompletely described molecular mechanism through NLRP3 inflammasome activation that abrogates a requirement for the classical Caspase 1 dependent pathway.
S. aureus activation of the NLRP3 inflammasome with PFTs, Hla (Craven et al. 2009), LukAB (Melehani et al. 2015), and PVL (Holzinger et al. 2012), is blocked by supraphysiologic concentrations of extracellular potassium suggesting a requirement for potassium efflux. PVL also causes activation of Cathepsin B (CTSB) and inhibition of CTSB protease activity by CA-074, a CTSB inhibitor, blocks PVL-induced cell death (Holzinger et al. 2012). CTSB is a lysosomal cysteine protease required for NLRP3 activation by disruption of lysophagosomes after ingestion of inflammation inducing particulate matter like silica (Hornung et al. 2008). CTSB is thought to spill into to the cytoplasm after lysophagosomal disruption, but the mechanism by which CTSB activates NLRP3 is unknown. It is also not known whether the requirement for CTSB in PVL-induced NLRP3 activation is toxin specific or common to all S. aureus PFTs. One possible explanation for how CTSB is liberated from lysophagosomes is that S. aureus PFTs can bind to their cognate receptor on phagosomal membranes. This binding promotes S. aureus escape from phagocytosis to enable survival (DuMont et al. 2013b). Interestingly, the route of exposure (i.e., extracellular versus within a phagocytic vacuole) also seems to influence signaling in response to LukAB (Melehani et al. 2015). When LukAB interacts with CD11b at the plasma membrane, NLRP3 is activated, driving IL-1β and IL-18 secretion and cell death. Alternatively, when LukAB expressed from phagocytosed S. aureus interact with CD11b on the phagocytic vacuole membrane, cell death becomes independent of NLRP3 activation. Phagosomal delivery of LukAB does not generate an alternative Caspase 1 activating pathway as NLRP3 is still required for IL-1β and IL-18 secretion. It is not clear whether this separate cell death mechanism engaged by phagosome-localized LukAB is activated in addition to NLRP3-dependent pathways or if the NLRP3-dependent pathways activated by extracellular toxin are not activated by toxin in this setting (Melehani et al. 2015).
S. aureus can also trigger necroptosis, cell death dependent on RIPK1, RIPK3, and MLKL that can be blocked by necrostatin-1 and necrosulfonamide (NSA). Pretreatment of THP1 cells with NSA prior to exposure to S. aureus or purified Hla decreased cell death. NSA shifted the potency of Hla and at higher doses of Hla cells still died suggesting that Hla is able to engage additional cell death pathways at these doses. NSA diminished S. aureus-induced IL-1β secretion suggesting this agent or the necroptotic pathway may tie in with inflammasome activation (Kitur et al. 2015).
While the cytotoxicity of PFTs has been demonstrated widely in primary human monocytes, macrophages, neutrophils and dendritic cells, most studies of PFTs and inflammasome activation have been carried out in THP1 cells, mouse macrophages, or human peripheral blood monocytes. The prevailing assumption is that the cytotoxicity of these toxins depends on host NLRP3 across all susceptible human cell types, though this has not been demonstrated directly (Dumont et al. 2011; Holzinger et al. 2012; Ventura et al. 2010).
Few other mechanistic details between PFT exposure and NLRP3 inflammasome activation have been determined. Many other host proteins have been implicated along the NLRP3 inflammasome signaling cascade with other triggers, but have yet to be confirmed for S. aureus.
Like the pore-forming Hla, purified Hlb also triggers human monocytes to secrete IL-1β (Walev et al. 1996). Though these studies preceded the discovery of the NLRP3 inflammasome, Hlb-induced IL-1β secretion was suppressed by extracellular potassium, which strongly suggests a role for the NLRP3 inflammasome. Despite purified toxin being sufficient to induce IL-1β secretion, S. aureus Hlb-deficient mutants had no observable defect in inducing IL-1β and IL-18 secretion in mouse macrophages. S. aureus lacking the combination of Hla, Hlb, and Hlg caused very little IL-1β and IL-18 secretion when compared to S. aureus lacking any pair of these hemolysins (Munoz-Planillo et al. 2009). These studies suggest that all three hemolysins in S. aureus have redundant capacity to activate the NLRP3 inflammasome. It is also possible that hemolysin production is context dependent and different hemolysins (or combinations of hemolysins) may be required in infection of separate tissues, which has not been recapitulated in in vitro cell culture of immune cells to date. Hlb also limits production of IL-8, a potent neutrophil chemoattractant, by human endothelial cells (Tajima et al. 2009) and as such, may play a dual role in establishing and promoting infection.
Other NLR proteins may interact with NLRP3 to enhance inflammasome signaling. In one instance, NLRC5 was shown to co-immunoprecipitate with NLRP3 and knockdown of NLRC5 in THP1 cells by shRNA led to dramatic reduction in IL-1β secretion in response to live S. aureus (Davis et al. 2011). The S. aureus factors that might contribute to activation of NLRC5 in this context have not been identified.
In addition to NLRP3, NLRP7, a sensor of acylated lipopeptides, leads to signaling in response to S. aureus. Knockdown of NLRP7 in THP1 cells resulted in decreased S. aureus-induced IL-1β secretion and increased intracellular bacteria following infection (Khare et al. 2012). NLRP7 function requires ATP binding and hydrolysis. Overexpression of wildtype myc-tagged NLRP7, but not myc-tagged ATPase-deficient NLRP7, by lentiviral transduction led to an increase in S. aureus induced IL-1β secretion as compared to transduction of myc alone (Radian et al. 2015). The role of NLRP7 in cell death is unclear. Depletion of NLRP7 by siRNA did not impact S. aureus-induced cell death as measured by LDH release (Khare et al. 2012) but viral transduction of THP1 cells to overexpress NLRP7 enhanced S. aureus-induced cell death as measured by PI uptake (Radian et al. 2015). More work is needed on endogenous NLRP7 to better understand its role in S. aureus induced cell death.
AIM2, a sensor responsible for inflammasome formation in response to cytoplasmic DNA, may also detect S. aureus. Mice lacking AIM2 demonstrated reduced IL-1β recovered from central nervous system abscesses resulting from intracranial injection of S. aureus. Asc-/- mice, but not Nlrp3-/- mice, exhibited the same defect in IL-1β production during intracranial abscess formation. Interestingly, mice lacking AIM2 or ASC also demonstrated dramatically decreased production of non-inflammasome dependent cytokines including: IL-6, CXCL1 and CXCL10 production in this model. This suggests either production of these cytokines is influenced by production of IL-1β or that there are non-inflammasome dependent signaling pathways activated by AIM2 and ASC. Because no studies of S. aureus-exposed AIM2 deficient cultured immune cells, including CNS microglia, have been published, it is not clear whether the defect in IL-1β production observed in infected brain tissues of AIM2 deficient mice is the result of a failure to sense a component of S. aureus or to sense a host factor produced as a result of S. aureus infection (Hanamsagar et al. 2014).
Inflammasome-like or non-canonical inflammasome responses may also initiate an immune response against S. aureus. When purified, each of the seven S. aureus PSMs are sufficient to induce IL-18 secretion from human keratinocytes. S. aureus with all seven PSMs deleted induced lower levels of IL-18 and IL-1β secretion from keratinocytes. Unexpectedly, secretion of IL-18 or IL-1β from keratinocytes in response to live S. aureus could not be blocked by a panel of Caspase inhibitors (Syed et al. 2015). These data suggest that keratinocytes may rely upon a non-Caspase protease for processing and secretion of IL-1β and IL-18 in response to PSMs.
While many studies of inflammasome activation by S. aureus have been conducted in in vitro cell culture using primary human monocytes as well as other cell types (THP1 cells, mouse macrophages, etc.), in natural infections, S. aureus and its secreted toxins interact with multiple cell types each with a unique capacity to sense and respond to the bacteria. This leads to the possibility that a variety of inflammasomes may be activated in different cell types over the course of an infection with variable effects on infection outcomes. To understand the role of inflammasome activation in the natural course of infection, we turn our review to studies of S. aureus infection in animal models, primarily in mice. In most cases, mouse studies were carried out using only the C57BL/6 strain in which inflammasome components have been genetically deleted. Mice as a model for S. aureus infections have serious limitations as mice are relatively naturally resistant to S. aureus and non-responsive to many virulence factors that act specifically on human cells. These studies would benefit from incorporating more genetic diversity.
3. Role of inflammasome activation in infection models
3.1 The NLRP3 inflammasome responds to hemolysins to control S. aureus dermal infections
S. aureus is the number one cause of skin infections. These infections range in severity from pimples and boils to abscesses and cellulitis. A mouse model for S. aureus skin infections has been developed (Miller et al. 2006) and adapted to study the role of inflammasomes in this setting (Miller et al. 2007; Cho et al. 2012; Soong et al. 2015).
Tissue biopsies were collected from mice injected subcutaneously with a variety of isogenic hemolysin knockout S. aureus strains. When hla, hlb, and hlg genes were deleted together, secretion of IL-1β and IL-18 was almost completely eliminated whereas only minor reductions were observed with any single or double hemolysin knockout as compared to the parental S. aureus strain. Subcutaneous injection of mice with individual deletions of Nlrp3, Asc, or Casp1, did not elicit IL-18 secretion, demonstrating a role for the NLRP3 inflammasome in this setting (Munoz-Planillo et al. 2009).
Mice deficient in IL-1β (Il1b-/-) develop larger lesions with higher bacterial burdens and reduced neutrophil recruitment following subcutaneous injection of S. aureus as compared to parental strain mice (Miller et al. 2007). Adoptive transfer of Il1b-/- bone marrow into irradiated parental strain mice mirrored the defect seen in wholly Il1b-/- mice, suggesting that immune cells are a critical source of IL-1β in this model skin infection. In Il1b-/- mice, recombinant active IL-1β helped to control infections and promote bacterial clearance (Miller et al. 2007). In the same subcutaneous injection model, multispectral noninvasive imaging during infection was used to localize different types of phagocytes and fluorescent staining of IL-1β revealed a stronger spatial correlation between neutrophils and IL-1β than between macrophages and IL-1β. Adoptive transfer of IL-1β-expressing neutrophils into an Il1b-/- host was sufficient to restore the impaired neutrophil abscess formation, suggesting neutrophils are the critical source of immune cell-derived IL-1β in this model. S. aureus induced IL-1β production during subcutaneous injection in mice in a Hla-dependent manner that required intact genes encoding Tlr2, Nod2, Fpr1, Asc, Casp1, and Nlrp3 (Cho et al. 2012). In humans, neutrophils are an important component of controlling cutaneous S. aureus infections (Borregaard 2010) as neutrophil functional defects, such as chronic granulomatous disease, result in susceptibility to invasive S. aureus cutaneous infections (Miller and Cho 2011). The importance of neutrophils during subcutaneous infection of mice suggests that the mouse model is appropriately recapitulating this feature of the human infection.
Although these studies of S. aureus skin infection in mice have been done with direct inoculation of bacteria through the epidermal barrier, S. aureus can cause infection by invading through a human keratinocyte barrier. In an organotypic culture of human keratinocytes grown at an air-liquid interface on a dermal substitute matrix, S. aureus can be seen by microscopy to disrupt the apical keratinocyte layer. Hla triggers Caspase 1 and Calpain (Ca(2+)-dependent intracellular protease) activation leading to IL-1β secretion and cell death. If Caspase 1 or Calpain are inhibited during this process, invasion efficiency is partially decreased (Soong et al. 2012).
Patients with atopic dermatitis and psoriasis are commonly colonized with S. aureus; however, only those with atopic dermatitis suffer from increased risk of bacterial superinfections. Expression of NLRP3 and Caspase 1 by immunohistochemistry was reduced in skin in a cohort of patients with lesional atopic dermatitis as compared to patients with psoriatic or healthy skin. Primary human keratinocytes from healthy skin and primary human monocytes have been found to downregulate NLRP3 and ASC expression in response to IL-4, IL-5 and IL-13 (cytokines abundant in atopic dermatitis). Accordingly, Hla-induced IL-1β secretion was diminished in monocytes from patients with atopic dermatitis compared to patients with psoriasis and healthy controls (Niebuhr et al. 2014). These data suggest that the Th2 dominant immunologic milieu of patients with atopic dermatitis leads to defects in inflammasome signaling promoting susceptibility to S. aureus infection. It is also possible that the Th2 dominant immunologic milieu associated with asthma and other atopic diseases opposition of NLRP3 inflammasome activation explains epidemiologic data showing patients with these conditions have increased risk of S. aureus infection (Juhn 2014).
PSMs also play a role in pathogenesis of S. aureus-induced skin inflammation. Primary human keratinocytes exposed to S. aureus with genes encoding all seven PSMs knocked out by allelic replacement or start codon disruption secreted significantly less IL-1β, IL-18, and LDH, a marker of cell death, than keratinocytes exposed to wild type S. aureus. Keratinocytes exposed to any purified PSM secreted IL-18 but only the four PSMαs and delta-toxin were cytotoxic. Interestingly, caspase inhibitors and extracellular potassium were unable to block cytokine secretion or cytotoxicity, suggesting that this combination of cell death and cytokine secretion that is normally associated with inflammasome activation of Caspase 1 is triggered by a Caspase 1-independent process in keratinocytes that remains unidentified. In mice challenged epicutaneously with S. aureus and an isogenic mutant lacking PSM, the parental S. aureus strain triggered a predominantly neutrophil infiltrate and the PSM-deficient S. aureus did not. This difference in host neutrophil recruitment to infection site persisted in mice lacking IL-18, suggesting that PSM-mediated killing of keratinocytes led to release of chemoattractive factors or that PSM themselves are chemoattractive (Syed et al. 2015). Previous research has shown that blocking formyl peptide receptor 2, the receptor for S. aureus PSMs, blocked PSM-mediated leukocyte infiltration in a mouse air pouch and mouse peritoneal infection model, suggesting that PSMs themselves are chemoattractive or trigger a chemoattractive cascade (Kretschmer et al. 2010).
Currently though, the translatability of these models to the human condition remains an open question. There is limited published evidence for the role of IL-1β or inflammasome activation in cellulitis and other skin infections in humans. In clinical trials of IL-1β antagonists, a trend towards increased risk of serious infections was observed in patients with inflammatory conditions treated with these agents (Galloway et al. 2011). There are no reports regarding the use of these agents and specific risk of S. aureus infection of the skin. Experimental models in mice offer an opportunity to study this but face major limitations in their relevance. Mouse skin differs significantly from human skin. Mouse epidermis is made up of only three cell layers and is less than 25 micrometers thick whereas human epidermis is usually 6-10 cell layers and is greater than 50 micrometers thick. The epithelial turnover in mouse skin is faster and mouse skin regenerates without significant scarring. These differences and more are reviewed in Gudjonsson et al. (2007).
Considerable interest exists in humanized mouse models of skin infections and these have been deployed to study the human specific pathogen Neisseria meningitides (Melican et al. 2013). While human skin grafting on mice may eventually be a useful model for studying S. aureus skin infections, this methodology has yet to be deployed in this arena. Humanized NSG mice with humanized immune systems have been used in S. aureus skin infection models. A S. aureus inoculum one to two log lower induced consistent skin lesions as compared with non-humanized mice. This model aided in studying the role of PVL in dermonecrosis. Blocking the PVL receptor, human C5aR, with PMX53 or an anti-C5aR antibody eliminated the enhanced cytotoxicity of PVL-positive S. aureus but also reduced recruitment of neutrophils and exacerbated the infection (Tseng et al. 2015). Caution must be taken in interpreting results, however, given changes observed in skin grafts over time (Kappes et al. 2004). Additionally, mice with human skin grafts still carry myeloid-derived cells from mice, which may be a significant cause of differences seen in S. aureus susceptibility between human and mice.
With those caveats in mind, current evidence suggests that S. aureus activates inflammasomes in keratinocytes to promote invasion and establish infection. PSMs that induce NLRP3-independent cell death in keratinocytes may provide a redundant mechanism to support invasion. Once infection is established, neutrophils are recruited and secrete IL-1β, probably through NLRP3 inflammasome activation, to promote clearance of S. aureus infections (Figure 3, top panels). If this holds true, therapeutic intervention to suppress inflammasome signaling may have a desirable effect in preventing invasion through keratinocytes but may unduly hinder clearance of already invaded S. aureus.
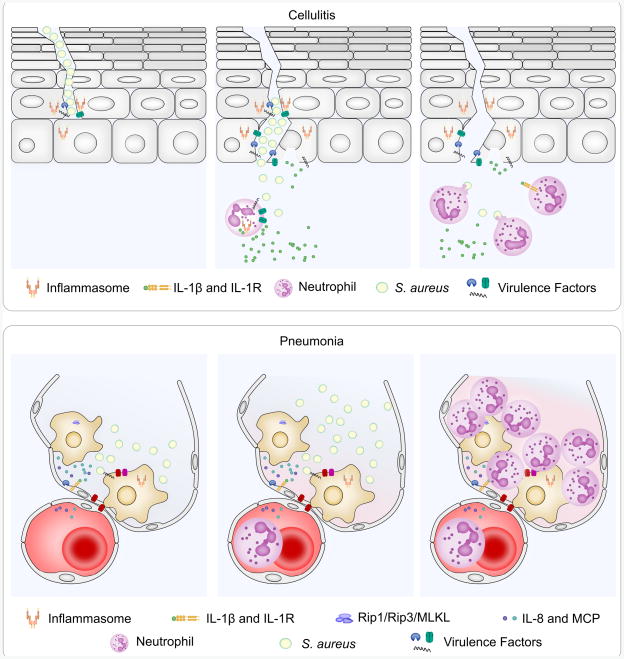
Fig. 3.
NLRP3 inflammasome signaling plays unique rolls in skin and lung infections. Skin (top row) and lung (bottom row) infections are modeled here as an example of the unique effects of inflammasome signaling during S. aureus infections. In model skin infection (top row), S. aureus is cocultured with an organotypic culture of human keratinocytes grown at an air-liquid interface on a dermal substitute matrix, S. aureus utilizes Hla to activate inflammasome signaling to enhance invasion through the keratinocyte barrier (left). Once invasion has occurred (experimentally induced by subcutaneous injection), neutrophils are recruited to the site of infection and activate the NLRP3 inflammasome to promote IL-1β secretion (center). Neutrophil-derived IL-1β is required for abscess formation and bacterial clearance (right). In the lung (bottom row), S. aureus triggers alveolar macrophages to secrete IL-1β that promotes secretion of the neutrophil attracting chemokines, IL-8 and MCP-1, from human lung epithelial cells (left). Neutrophil recruitment leads to further NLRP3 inflammasome activation by Hla (middle) that ultimately destroys the lung tissue (right), as loss of NLRP3 in mice improves pathology with no change in bacterial burden. Loss of the IL-1 receptor does not impact the infection suggesting that IL-1 signaling is not required. Hla, LukAB and PSMs also induce necroptosis, a RIPK1-, RIPK3-, MLKL-dependent cell death process that hinders clearance of S. aureus, as deletion of RIPK3 improves bacterial control. MLKL also plays a role in IL-1β secretion as loss of MLKL blocks Caspase 1 activation suggesting an intersection between the necroptosis signaling pathway and the inflammasome signaling pathway.
3.2 IL-1β signaling is critical for combating soft tissue infections
Staphylococcus aureus is a common culprit in soft tissue and medical device infections. Patients with ulcers, commonly resulting from advanced complications of diabetes, deep injuries, recent surgery, or indwelling medical devices are particularly at risk. Recent work has demonstrated a differential effect of S. aureus strains in inducing inflammasome activation and IL-1β secretion in surgical site infections, with strain PS80 leading to substantially more IL-1β than SH1000. In this study, loss of NLRP3 did not completely diminish IL-1β secretion, suggesting a coordinated role with other inflammasomes. However, deletion of Nlrp3 or IL-1 receptor (Il1r) compromised control of bacterial burden in infected surgical wounds (Maher et al. 2013). Little else has been done to characterize the role of inflammasome signaling in soft tissue infections, though it is tempting to assume these would be similar to dermal infections once S. aureus crosses the keratinocyte barrier.
3.3 S. aureus hijacks the NLRP3 inflammasome to exacerbate lung infection pathology
S. aureus pneumonia is a difficult disease to effectively treat. Even with proper antimicrobial therapy, S. aureus triggers a massive immune response that causes significant tissue destruction with substantial lethality.
Hla is an essential virulence factor in mouse models of severe pneumonia. S. aureus lacking Hla have reduced bacterial burden and causes less lung pathology and host death when delivered intranasally or intratracheally to the mouse lung when compared to S. aureus with an intact Hla gene (Bubeck Wardenburg and Schneewind 2008). Blocking Hla with an antibody also promotes bacterial clearance from the lung, indicating that expression of Hla is important in the ability of the bacteria to cause lung infection in this model (Bubeck Wardenburg and Schneewind 2008; Ragle and Bubeck Wardenburg 2009). Highly purified recombinant Hla, alone or in conjunction with HKSA, delivered intratracheally causes a clinical syndrome resembling S. aureus pulmonary infection in mice. Pathologic changes in the lung including destruction of normal alveolar architecture, tissue necrosis, hemorrhage, and inflammation are consistent with pathologic findings in human lungs with severe S. aureus pneumonia. This syndrome is largely abrogated when Hla is administered to mice lacking NLRP3 (Kebaier et al. 2012). There was no difference in bacterial burdens between mice with and without NLRP3 inoculated with intratracheal S. aureus. Thus, unlike the skin where signaling from the NLRP3 inflammasome contributes to host elimination of the bacteria, NLRP3 inflammasome signaling is not essential for clearing S. aureus from the mouse lung. There was no difference in bacterial burden or lung inflammation between wild type and NLRP3 null mice that were infected with S. aureus lacking Hla, suggesting Hla–induced activation of NLRP3 is a major cause of pneumonia pathology in this model of S. aureus infection (Figure 3, bottom panels). IL-1 receptor deficient mice exhibited severe inflammatory responses to pulmonary delivery of Hla, thus tissue destruction mediated by this toxin in the lung did not depend on IL-1β signaling (Kebaier et al. 2012). These findings suggest that cell death and/or other Caspase1-dependent cytokines downstream of NLRP3 activation were sufficient to cause severe lung pathology in the setting of S. aureus infection.
There is significant debate as to whether PVL is a critical virulence factor in pneumonia. Mice infected intranasally with PVL-expressing S. aureus experienced substantial neutrophil recruitment, inflammation in the lung parenchyma, bronchial epithelial damage, tissue necrosis and hemorrhage. These same effects were seen upon administration of both components of the purified PVL toxin but were absent in isogenic PVL-negative S. aureus or upon administration of a single component of the PVL toxin (Labandeira-Rey et al. 2007). A second report replicated these findings, demonstrating that deletion of PVL from the LAC S. aureus strain improves Balb/c mouse survival following intranasal inoculation. Additionally, immunization against PVL showed a trend towards protection in subsequent S. aureus challenge by intranasal infection (Brown et al. 2009). These results are in contrast to separate findings that deletion of PVL from the LAC S. aureus strain actually enhances virulence and decreases survival of Balb/c mice infected using the same protocol (Bubeck Wardenburg et al. 2008; Villaruz et al. 2009). Given the general resistance of mouse neutrophils to S. aureus PVL (Loffler et al. 2010), the differences in these studies may result from differences in susceptibility of non-immune cells to PVL. However, the use of Balb/c mice in both groups of studies suggests the difference is not strain-intrinsic. In general, because of the species specificity of S. aureus PFTs, humanized mice may be required to more fully appreciate the impact of PVL in S. aureus pneumonia as was seen when applied to the humanized mouse cellulitis model (Tseng et al. 2015).
In primary human monocyte-derived macrophages, HKSA followed by administration of recombinant PVL induced secretion of high levels of IL-1β. IL-1β secretion was enhanced by co-administration PVL and the PSMs Hld and PSMalpha3, the PFTs Hlg and LukED, and Hlb, greater than the sum of their parts (Perret et al. 2012). PVL also triggered IL-1β secretion in human alveolar macrophages that goes on to stimulate the secretion of neutrophil attracting chemokines IL-8 and MCP-1 by A549 human lung epithelial cells in a mixed culture model. Blocking the IL-1 receptor with IL-1R antagonist abolishes PVL-induced secretion of IL-8 and MCP-1 (Perret et al. 2012). These data suggest that blockade of IL-1β signaling may diminish pathology in S. aureus pulmonary infection. However, a subsequent study in rabbits demonstrated that Anakinra, an IL-1 receptor antagonist, could block pathology induced by treatment with recombinant PVL and HKSA but not in live S. aureus infection. Furthermore, treatment of infected rabbits with Anakinra led to increased bacterial burden in the lungs (Labrousse et al. 2014). These data demonstrate that IL-1β secretion triggered by PVL plays a protective role in S. aureus pneumonia in rabbits, where the toxin is active. While these findings seem at odds with the apparent pathologic role of NLRP3 inflammasome activation in mice with S. aureus pneumonia, the studies in IL-1 receptor deficient mice demonstrate that toxin mediated activation of NLRP3 drove pathogenic findings in the lung and clinical severity of disease independently of IL-1β production. Additionally, because mice are deficient from NLRP3 through the entire course of the model pneumonia, there is no way to separate effects of NLRP3 on the ability of S. aureus to initiate infection from the effects of NLRP3 on the response to ongoing infection.
Recently, S. aureus toxins were shown to induce necroptosis in the lung through RIPK1/RIPK3/MLKL signaling. In primary human macrophage co-culture with S. aureus, inhibitors of necroptosis, including necrostatin-1 and necrosulfonamide (NSA), or siRNA-mediated knockdown of RIPK3 or MLKL blocked S. aureus-induced cell death. NSA also decreased purified Hla-induced THP1 cell death. In murine S. aureus pneumonia, blocking necroptosis with NSA or knockout of RIPK3 improved bacterial clearance. RIPK3 deficient mice had improved lung architecture and less disruption of the pulmonary barrier, resulting in less protein in bronchoalveolar lavage fluid. Interestingly, NSA pretreatment of THP1 cells exposed to S. aureus supernatants in culture or RIPK3 knockout in S. aureus pneumonia led to decreased IL-1β secretion, suggesting a possible link between necroptosis and inflammasome activation (Kitur et al. 2015).
Currently, there is strong evidence supporting the use of systemic corticosteroids in hospitalized patients with severe community acquired pneumonia (Siemieniuk et al. 2015). Interestingly, a glucocorticosteroid-responsive negative regulatory element has been identified just upstream of the IL-1β transcription start site (Zhang et al. 1997) and, as such, suppression of IL-1β production might be contributing to improved outcomes in these patients. However, conflicting results as to whether IL-1 signaling enhances or diminishes lung pathology will be important to clarify before targeted therapeutic immunosuppression is used clinically in the setting of severe S. aureus pneumonia. Clinical trials of anakinra suggest patients with asthma or other pulmonary comorbidities might be at increased risk of infectious complications, though results were not statistically significant (Schiff et al. 2004; Fleischmann et al. 2003). Suppression of necroptosis through RIPK3 inhibition may also be a viable strategy but understanding the contributions of necroptosis and NLRP3 inflammasome-mediated cell death to pathology and bacterial clearance is still in its infancy. Finally, activation of IL-1 independent effects of NLRP3, particularly inflammatory cell death, by S. aureus virulence factors may be sufficient to drive severe lung pathology even in the setting of neutralizing IL-1. Better animal models of S. aureus pneumonia that are responsive to the major PFT and other S. aureus virulence factors are needed to propel this field forward.
3.4 Microglia activate NLRP3 in vitro but depend on AIM2 to clear S. aureus central nervous system infections
S. aureus can also cause brain abscesses usually as a complication of surgery, trauma or bacteremia. Microglia are immunologically competent cells in the brain activated early in the process of S. aureus abscess formation (Kielian 2004). Exposure of primary microglia isolated from C57BL/6 mice to live S. aureus strain USA300 induced both IL-1β and IL-18 secretion. Microglia from mice lacking NLRP3 or ASC were deficient in IL-1β secretion but responded to live S. aureus with similar levels of IL-18 as wild type mice. Deletion of Hla or Hlg, but not LukAB or LukED reduced Newman strain S. aureus-induced IL-1β secretion from mouse-derived microglia (Hanamsagar et al. 2011). However, this is not surprising given the general resistance of mouse to LukAB (DuMont et al. 2013a) and the low level of expression of LukED (DuMont et al. 2013b) under the conditions the bacteria were cultured. Caspase1 and CTSB inhibitors blocked S. aureus-induced microglial IL-1β secretion in these in vitro cell culture experiments. Consistent with a second pathway controlling IL-18 secretion in this setting, the CTSB inhibitor had no effect on IL-18 secretion (Hanamsagar et al. 2011).
In a model of brain abscess, Asc and Casp1/Casp11 knockout mice had earlier mortality and diminished detectable IL-1β recovered from abscesses as compared to the parental mouse strain. Unexpectedly, the survival of Nlrp3 knockout mice in this brain abscess model was identical to the parental strain. Upon further investigation, loss of AIM2 mimicked the sensitivity to S. aureus infection and diminished IL-1β production seen in ASC deficient mice, suggesting that AIM2 is the primary upstream sensor for the inflammasome activating ASC and Caspase 1 in this model. Besides IL-1β, other key inflammatory mediators, including IL-6, CXCL1, CXCL10, and CCL2 were significantly reduced in the CNS of Aim2 and Asc knockout mice (Hanamsagar et al. 2014). Immune cell infiltrates, including neutrophils and macrophages, and other cytokines, such as IL-10, TNF-α and IFN-γ, were not changed between Asc knockout and the parental strain mice. Also, the bacterial burden of Aim2 knockout mice and the parental strain were equivalent in the first 18 hours of infection. Most Aim2 knockout mice that died were recorded as having died at approximately 20 hours post infection, leaving us without a satisfying explanation for why these mice suddenly succumbed to disease given the similarities in bacterial burden and immune cell infiltrates (Hanamsagar et al. 2014). Previous studies of S. aureus brain abscesses have demonstrated damage to the blood-brain barrier (Kielian et al. 2001) and impaired capillary perfusion and parenchymal cell death has been noted in soft tissue infections (Harding et al. 2014), both explanation that might be worth further investigation in this setting. The discrepancy between IL-1β production being NLRP3 dependent in cultured microglia exposed to S. aureus and AIM2-dependent after intracranial inoculation of S. aureus points to the need to push through additional mechanistic studies to understand the role of inflammasome activation in brain abscess. Host derived DAMPs, like extracellular chromatin, arising from cellular injury during inoculation or cytotoxic factors from the bacteria may be responsible for activating AIM2 in the in vivo brain abscess model. Further investigation of AIM2 deficient microglia in vitro would be beneficial for characterizing the response seen in vivo. Also, study of Aim2 knockout mice in the context of other S. aureus infections would help determine if the role of AIM2 is brain/microglia-specific or is a general feature of the immune response to S. aureus.
4. How the host inflammasome can affect other inflammatory processes
The Th17 response has emerged as a major focus of studies looking downstream of inflammasome activation. The most direct hint that IL-17-driven responses are critical for defense against S. aureus is seen in patients with hyper IgE syndrome (HIES). In HIES, mutation in STAT3 causes impaired Th17 cell function and recurrent and severe S. aureus infections (Milner et al. 2008). Similar impairments have been recapitulated in mouse models of S. aureus infection.
NLRP3-mediated secretion of IL-1β by bone marrow dendritic cells promotes secretion of IL-17 by γδ-T cells, as loss of the IL-1 receptor limits this response. Importantly, the ability to control surgical site infections is enhanced by IL-1β stimulated γδ-T cells as bacterial burden is increased in δTCR deficient and IL-17R deficient mice (Maher et al. 2013). In S. aureus cutaneous infections, loss of γδ-T cells led to larger skin lesions with higher bacterial counts as a result of impaired neutrophil recruitment. IL-17R-deficient mice had a similar phenotype. Treatment of γδ-T cell-deficient mice with a single dose of recombinant IL-17 rescued the impaired immune response to S. aureus (Cho et al. 2010). A population of CD44+ CD27- memory γδ-T cells that is expanded upon peritoneal infection of C57BL/6 mice with S. aureus produces high levels of IL-17 and promotes bacterial clearance during reinfections. IL-1 signaling was not required for activation or expansion of memory γδ-T cells during reinfection (Murphy et al. 2014). γδ-T cells also play a prominent role in defense against S. aureus pneumonia. γδ-T cell-deficient mice had impaired neutrophil recruitment and an increased bacterial burden in the lung. However, the absence of γδ-T cells decreases lung pathology and improves survival (Cheng et al. 2012), similar to studies with loss of NLRP3 (Kebaier et al. 2012).
5. Integrating inflammasome studies to improve patient care
Early evidence suggests that vaccines against PFTs may be beneficial in reducing virulence and severity of infections. In hospitalized patients with S. aureus infections, the risk of sepsis was significantly lower in those patients with higher levels of IgG against Hla, Hld, PVL, staphylococcal enterotoxin C-1 and PSMα3 (Adhikari et al. 2012). Some children with culture-proven S. aureus infection developed anti-LukAB antibodies that potently neutralize cytotoxicity in vitro, suggesting that the toxin is produced in vivo and that it elicits a humoral response (Thomsen et al. 2014). Immunization of mice with a mutant form of Hla that cannot form pores, Hla H35L, generates antigen-specific immunoglobulin G responses and affords protection against staphylococcal pneumonia. Additionally, transfer of Hla-specific antibodies or anti-Hla monoclonal antibodies also protects naïve animals against S. aureus challenge and prevents injury of human lung epithelial cells during infection (Bubeck Wardenburg and Schneewind 2008; Ragle and Bubeck Wardenburg 2009). This is presumably the result of decreased NLRP3 inflammasome activation in this setting, however, that has not been measured in vivo directly.
Therapies targeting specific virulence factors may provide a useful adjuvant to antibiotic therapy. As recently declared in the Annals of Internal Medicine, many believe it is “time to change clinical practice” and recommend systemic administration of corticosteroids in patients with severe community acquired pneumonia. Theoretically, tailoring therapy more narrowly to reduce virulence and hyperactive immune responses without compromising bacterial clearance would provide further benefit over this broadly immunosuppressive therapy.
For understanding tissue-specific pathogenesis, we will need improved infection models that better recapitulate human tissues and immune cells to overcome the natural resistance of mice to S. aureus. Tissue-specific knockouts and bone marrow chimeras will also help differentiate tissue-driven immune responses from those of bone marrow origin. Ultimately, investigations into inflammasome-mediated responses to S. aureus infections may provide us with the therapeutic tools we need to push back against this growing threat.
6. Concluding Remarks
The rapid pace of discovery of new S. aureus toxins and other virulence factors has led to a dramatically improved understanding of the interactions between S. aureus and the innate immune system during infection. The NLRP3 inflammasome is now recognized as a critical driver of pro-inflammatory signaling in S. aureus infections. Additional studies provide evidence that other inflammasomes can also contribute to the pro-inflammatory milieu during S. aureus infection. However, significant shortcomings in the frequently used mouse models raise doubts about relative importance of many potential S. aureus virulence factors as well as the role of host inflammasome activation during infection. Novel approaches in studying S. aureus in clinical infections would provide much needed insight as to the relevance of pre-clinical studies of S. aureus pathogenesis. Further clarification of the protective and pathogenic roles of virulence factor-induced inflammasome signaling in S. aureus infections is needed to guide the development of novel therapeutics and vaccines to diminish the virulence of and treat S. aureus infections.
Acknowledgments
The authors' research has been supported by the National Institutes of Health: JHM received support from UNC MSTP T32GM008719 and UNC Predoctoral Training in Pharmacologic Sciences T32GM007040, JAD was supported by research grant R01AI088255. JAD is an awardee of the Burroughs Wellcome Fund Career Award for Medical Scientists.
Contributor Information
Jason H. Melehani, Email: jason_melehani@med.unc.edu, University of North Carolina at Chapel Hill, School of Medicine, Department of Pharmacology, Chapel Hill, North Carolina, USA
Joseph A. Duncan, Email: jaduncan@med.unc.edu, University of North Carolina at Chapel Hill, School of Medicine, Department of Pharmacology, Chapel Hill, North Carolina, USA, University of North Carolina at Chapel Hill, School of Medicine, Department of Medicine, Division of Infectious Diseases, Chapel Hill, North Carolina, USA, phone: 919-843-0715; University of North Carolina at Chapel Hill, School of Medicine, Lineberger Comprehensive Cancer Center, Chapel Hill, North Carolina, USA
References
- Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, Johnson JK, Nguyen C, Chen WH, Roghmann MC. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. The Journal of infectious diseases. 2012;206(6):915–923. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- Alonzo F, 3rd, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2013;493(7430):51–55. doi: 10.1038/nature11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badarau A, Rouha H, Malafa S, Logan DT, Hakansson M, Stulik L, Dolezilkova I, Teubenbacher A, Gross K, Maierhofer B, Weber S, Jagerhofer M, Hoffman D, Nagy E. Structure-function analysis of heterodimer formation, oligomerization, and receptor binding of the Staphylococcus aureus bi-component toxin LukGH. The Journal of biological chemistry. 2015;290(1):142–156. doi: 10.1074/jbc.M114.598110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch F, Bowden MG. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2009;15(2):156–164. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. The Journal of infectious diseases. 2008;198(8):1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. The Journal of experimental medicine. 2008;205(2):287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Liu T, Zhou WY, Zhuang Y, Peng LS, Zhang JY, Yin ZN, Mao XH, Guo G, Shi Y, Zou QM. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC immunology. 2012;13:38. doi: 10.1186/1471-2172-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS pathogens. 2012;8(11):e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. The Journal of clinical investigation. 2010;120(5):1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PloS one. 2009;4(10):e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BK, Roberts RA, Huang MT, Willingham SB, Conti BJ, Brickey WJ, Barker BR, Kwan M, Taxman DJ, Accavitti-Loper MA, Duncan JA, Ting JP. Cutting edge: NLRC5-dependent activation of the inflammasome. Journal of immunology. 2011;186(3):1333–1337. doi: 10.4049/jimmunol.1003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Molecular microbiology. 2011;79(3):814–825. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuMont AL, Yoong P, Day CJ, Alonzo F, 3rd, McDonald WH, Jennings MP, Torres VJ. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proceedings of the National Academy of Sciences of the United States of America. 2013a;110(26):10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuMont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, Torres VJ. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infection and immunity. 2013b;81(5):1830–1841. doi: 10.1128/IAI.00095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne DW, Resnick D, Greenberg J, Krieger M, Joiner KA. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(5):1863–1867. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrod T, Franchi L, Munoz-Planillo R, Kirschning CJ, Freudenberg MA, Nunez G, Dalpke A. Bacterial RNA mediates activation of caspase-1 and IL-1beta release independently of TLRs 3, 7, 9 and TRIF but is dependent on UNC93B. Journal of immunology. 2012;189(1):328–336. doi: 10.4049/jimmunol.1103258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton JE, Dell A, Shaw WV. The amino acid sequence of the delta haemolysin of Staphylococcus aureus. FEBS letters. 1980;115(2):209–212. doi: 10.1016/0014-5793(80)81170-7. [DOI] [PubMed] [Google Scholar]
- Fleischmann RM, Schechtman J, Bennett R, Handel ML, Burmester GR, Tesser J, Modafferi D, Poulakos J, Sun G. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trial. Arthritis and rheumatism. 2003;48(4):927–934. doi: 10.1002/art.10870. [DOI] [PubMed] [Google Scholar]
- Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Watson KD, Lunt M, Consortium BCC. Symmons DP British Society for Rheumatology Biologics R. The risk of serious infections in patients receiving anakinra for rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology. 2011;50(7):1341–1342. doi: 10.1093/rheumatology/ker146. [DOI] [PubMed] [Google Scholar]
- Gouaux E, Hobaugh M, Song L. alpha-Hemolysin, gamma-hemolysin, and leukocidin from Staphylococcus aureus: distant in sequence but similar in structure. Protein science : a publication of the Protein Society. 1997;6(12):2631–2635. doi: 10.1002/pro.5560061216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JW, Fischer W, Joiner KA. Influence of lipoteichoic acid structure on recognition by the macrophage scavenger receptor. Infection and immunity. 1996;64(8):3318–3325. doi: 10.1128/iai.64.8.3318-3325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundling A, Schneewind O. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(20):8478–8483. doi: 10.1073/pnas.0701821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. The Journal of investigative dermatology. 2007;127(6):1292–1308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- Guillet V, Roblin P, Werner S, Coraiola M, Menestrina G, Monteil H, Prevost G, Mourey L. Crystal structure of leucotoxin S component: new insight into the Staphylococcal beta-barrel pore-forming toxins. The Journal of biological chemistry. 2004;279(39):41028–41037. doi: 10.1074/jbc.M406904200. [DOI] [PubMed] [Google Scholar]
- Han SH, Kim JH, Martin M, Michalek SM, Nahm MH. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infection and immunity. 2003;71(10):5541–5548. doi: 10.1128/IAI.71.10.5541-5548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Aldrich A, Kielian T. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. Journal of neurochemistry. 2014;129(4):704–711. doi: 10.1111/jnc.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Torres V, Kielian T. Inflammasome activation and IL-1beta/IL-18 processing are influenced by distinct pathways in microglia. Journal of neurochemistry. 2011;119(4):736–748. doi: 10.1111/j.1471-4159.2011.07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding MG, Zhang K, Conly J, Kubes P. Neutrophil crawling in capillaries; a novel immune response to Staphylococcus aureus. PLoS pathogens. 2014;10(10):e1004379. doi: 10.1371/journal.ppat.1004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SB, Dudley J, Kumar S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22(23):2971–2972. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- Hermann C, Spreitzer I, Schroder NW, Morath S, Lehner MD, Fischer W, Schutt C, Schumann RR, Hartung T. Cytokine induction by purified lipoteichoic acids from various bacterial species--role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-gamma release. European journal of immunology. 2002;32(2):541–551. doi: 10.1002/1521-4141(200202)32:2<541∷AID-IMMU541>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433(7025):523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- Holzinger D, Gieldon L, Mysore V, Nippe N, Taxman DJ, Duncan JA, Broglie PM, Marketon K, Austermann J, Vogl T, Foell D, Niemann S, Peters G, Roth J, Loffler B. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. Journal of leukocyte biology. 2012;92(5):1069–1081. doi: 10.1189/jlb.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature immunology. 2008;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby M, Shi K, Brown CK, Digre J, Mengistu F, Seo KS, Bohach GA, Schlievert PM, Ohlendorf DH, Earhart CA. Structure and biological activities of beta toxin from Staphylococcus aureus. Journal of bacteriology. 2007;189(23):8719–8726. doi: 10.1128/JB.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? The Journal of allergy and clinical immunology. 2014;134(2):247–257. doi: 10.1016/j.jaci.2014.04.024. quiz 258-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappes U, Schliemann-Willers S, Bankova L, Heinemann C, Fischer TW, Ziemer M, Schubert H, Norgauer J, Fluhr JW, Elsner P. The quality of human skin xenografts on SCID mice: a noninvasive bioengineering approach. The British journal of dermatology. 2004;151(5):971–976. doi: 10.1111/j.1365-2133.2004.06191.x. [DOI] [PubMed] [Google Scholar]
- Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. The Journal of infectious diseases. 2012;205(5):807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity. 2012;36(3):464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Immunopathogenesis of brain abscess. Journal of neuroinflammation. 2004;1(1):16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. Journal of immunology. 2001;166(7):4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS pathogens. 2015;11(4):e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, van Kessel KA, van Strijp JA, Otto M, Peschel A. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell host & microbe. 2010;7(6):463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Hedges SB. TimeTree2: species divergence times on the iPhone. Bioinformatics. 2011;27(14):2023–2024. doi: 10.1093/bioinformatics/btr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, Vandenesch F, Bowden MG. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315(5815):1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- Labrousse D, Perret M, Hayez D, Da Silva S, Badiou C, Couzon F, Bes M, Chavanet P, Lina G, Vandenesch F, Croisier-Bertin D, Henry T. Kineret(R)/IL-1ra blocks the IL-1/IL-8 inflammatory cascade during recombinant Panton Valentine Leukocidin-triggered pneumonia but not during S. aureus infection. PloS one. 2014;9(6):e97546. doi: 10.1371/journal.pone.0097546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS pathogens. 2010;6(1):e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch NJ, Roscher S, Hartung T, Morath S, Matsushita M, Maennel DN, Kuraya M, Fujita T, Schwaeble WJ. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. Journal of immunology. 2004;172(2):1198–1202. doi: 10.4049/jimmunol.172.2.1198. [DOI] [PubMed] [Google Scholar]
- Maher BM, Mulcahy ME, Murphy AG, Wilk M, O'Keeffe KM, Geoghegan JA, Lavelle EC, McLoughlin RM. Nlrp-3-driven interleukin 17 production by gammadeltaT cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infection and immunity. 2013;81(12):4478–4489. doi: 10.1128/IAI.01026-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Melehani JH, James DB, DuMont AL, Torres VJ, Duncan JA. Staphylococcus aureus Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular. PLoS pathogens. 2015;11(6):e1004970. doi: 10.1371/journal.ppat.1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melican K, Michea Veloso P, Martin T, Bruneval P, Dumenil G. Adhesion of Neisseria meningitidis to dermal vessels leads to local vascular damage and purpura in a humanized mouse model. PLoS pathogens. 2013;9(1):e1003139. doi: 10.1371/journal.ppat.1003139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nature reviews Immunology. 2011;11(8):505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24(1):79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O'Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. Journal of immunology. 2007;179(10):6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Wolf AJ, Iliev ID, Berg BL, Underhill DM, Liu GY. Poorly Cross-Linked Peptidoglycan in MRSA Due to mecA Induction Activates the Inflammasome and Exacerbates Immunopathology. Cell host & microbe. 2015;18(5):604–612. doi: 10.1016/j.chom.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. Journal of immunology. 2009;183(6):3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AG, O'Keeffe KM, Lalor SJ, Maher BM, Mills KH, McLoughlin RM. Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. Journal of immunology. 2014;192(8):3697–3708. doi: 10.4049/jimmunol.1303420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Kurokawa K, Nakamura K, Lee BL, Sekimizu K, Kubagawa H, Hiramatsu K, Yagita H, Okumura K, Takai T, Underhill DM, Aderem A, Ogasawara K. Inhibitory receptor paired Ig-like receptor B is exploited by Staphylococcus aureus for virulence. Journal of immunology. 2012;189(12):5903–5911. doi: 10.4049/jimmunol.1201940. [DOI] [PubMed] [Google Scholar]
- Niebuhr M, Baumert K, Heratizadeh A, Satzger I, Werfel T. Impaired NLRP3 inflammasome expression and function in atopic dermatitis due to Th2 milieu. Allergy. 2014;69(8):1058–1067. doi: 10.1111/all.12428. [DOI] [PubMed] [Google Scholar]
- Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annual review of microbiology. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- Pedelacq JD, Maveyraud L, Prevost G, Baba-Moussa L, Gonzalez A, Courcelle E, Shepard W, Monteil H, Samama JP, Mourey L. The structure of a Staphylococcus aureus leucocidin component (LukF-PV) reveals the fold of the water-soluble species of a family of transmembrane pore-forming toxins. Structure. 1999;7(3):277–287. doi: 10.1016/s0969-2126(99)80038-0. [DOI] [PubMed] [Google Scholar]
- Percy MG, Grundling A. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annual review of microbiology. 2014;68:81–100. doi: 10.1146/annurev-micro-091213-112949. [DOI] [PubMed] [Google Scholar]
- Perret M, Badiou C, Lina G, Burbaud S, Benito Y, Bes M, Cottin V, Couzon F, Juruj C, Dauwalder O, Goutagny N, Diep BA, Vandenesch F, Henry T. Cross-talk between Staphylococcus aureus leukocidins-intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cellular microbiology. 2012;14(7):1019–1036. doi: 10.1111/j.1462-5822.2012.01772.x. [DOI] [PubMed] [Google Scholar]
- Peschel A, Otto M. Phenol-soluble modulins and staphylococcal infection. Nature reviews Microbiology. 2013;11(10):667–673. doi: 10.1038/nrmicro3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radian AD, Khare S, Chu LH, Dorfleutner A, Stehlik C. ATP binding by NLRP7 is required for inflammasome activation in response to bacterial lipopeptides. Molecular immunology. 2015;67(2 Pt B):294–302. doi: 10.1016/j.molimm.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infection and immunity. 2009;77(7):2712–2718. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Robles T, Alonzo F, 3rd, Kozhaya L, Lacy DB, Unutmaz D, Torres VJ. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell host & microbe. 2013;14(4):453–459. doi: 10.1016/j.chom.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff MH, DiVittorio G, Tesser J, Fleischmann R, Schechtman J, Hartman S, Liu T, Solinger AM. The safety of anakinra in high-risk patients with active rheumatoid arthritis: six-month observations of patients with comorbid conditions. Arthritis and rheumatism. 2004;50(6):1752–1760. doi: 10.1002/art.20277. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. The Journal of biological chemistry. 1999;274(25):17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- Sha W, Mitoma H, Hanabuchi S, Bao M, Weng L, Sugimoto N, Liu Y, Zhang Z, Zhong J, Sun B, Liu YJ. Human NLRP3 inflammasome senses multiple types of bacterial RNAs. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(45):16059–16064. doi: 10.1073/pnas.1412487111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Gotz F, Liu GY, Underhill DM. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell host & microbe. 2010;7(1):38–49. doi: 10.1016/j.chom.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemieniuk RA, Meade MO, Alonso-Coello P, Briel M, Evaniew N, Prasad M, Alexander PE, Fei Y, Vandvik PO, Loeb M, Guyatt GH. Corticosteroid Therapy for Patients Hospitalized With Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Annals of internal medicine. 2015;163(7):519–528. doi: 10.7326/M15-0715. [DOI] [PubMed] [Google Scholar]
- Soong G, Chun J, Parker D, Prince A. Staphylococcus aureus activation of caspase 1/calpain signaling mediates invasion through human keratinocytes. The Journal of infectious diseases. 2012;205(10):1571–1579. doi: 10.1093/infdis/jis244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong G, Paulino F, Wachtel S, Parker D, Wickersham M, Zhang D, Brown A, Lauren C, Dowd M, West E, Horst B, Planet P, Prince A. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. mBio. 2015;6(2) doi: 10.1128/mBio.00289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJ, van Kessel KP, Vandenesch F, Lina G, van Strijp JA. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell host & microbe. 2013;13(5):584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Spaan AN, Vrieling M, Wallet P, Badiou C, Reyes-Robles T, Ohneck EA, Benito Y, de Haas CJ, Day CJ, Jennings MP, Lina G, Vandenesch F, van Kessel KP, Torres VJ, van Strijp JA, Henry T. The staphylococcal toxins gamma-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nature communications. 2014;5:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Yamashita D, Kato K, Peng Z, Ueda J, Kaneko J, Kamio Y, Tanaka Y, Yao M. Structural basis for pore-forming mechanism of staphylococcal alpha-hemolysin. Toxicon : official journal of the International Society on Toxinology. 2015;108:226–231. doi: 10.1016/j.toxicon.2015.09.033. [DOI] [PubMed] [Google Scholar]
- Syed AK, Reed TJ, Clark KL, Boles BR, Kahlenberg JM. Staphlyococcus aureus phenol-soluble modulins stimulate the release of proinflammatory cytokines from keratinocytes and are required for induction of skin inflammation. Infection and immunity. 2015;83(9):3428–3437. doi: 10.1128/IAI.00401-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima A, Iwase T, Shinji H, Seki K, Mizunoe Y. Inhibition of endothelial interleukin-8 production and neutrophil transmigration by Staphylococcus aureus beta-hemolysin. Infection and immunity. 2009;77(1):327–334. doi: 10.1128/IAI.00748-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nature reviews Microbiology. 2015;13(9):529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen IP, Dumont AL, James DB, Yoong P, Saville BR, Soper N, Torres VJ, Creech CB. Children with invasive Staphylococcus aureus disease exhibit a potently neutralizing antibody response to the cytotoxin LukAB. Infection and immunity. 2014;82(3):1234–1242. doi: 10.1128/IAI.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical microbiology reviews. 2015;28(3):603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CW, Biancotti JC, Berg BL, Gate D, Kolar SL, Muller S, Rodriguez MD, Rezai-Zadeh K, Fan X, Beenhouwer DO, Town T, Liu GY. Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Staphylococcus aureus Skin Infection. PLoS pathogens. 2015;11(11):e1005292. doi: 10.1371/journal.ppat.1005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering JK, van Eijk M, van Golde LM, Hartung T, van Strijp JA, Batenburg JJ. Characteristics of surfactant protein A and D binding to lipoteichoic acid and peptidoglycan, 2 major cell wall components of gram-positive bacteria. The Journal of infectious diseases. 2001;184(9):1143–1151. doi: 10.1086/323746. [DOI] [PubMed] [Google Scholar]
- Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, DeLeo FR. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PloS one. 2010;5(7):e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaruz AE, Bubeck Wardenburg J, Khan BA, Whitney AR, Sturdevant DE, Gardner DJ, DeLeo FR, Otto M. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. The Journal of infectious diseases. 2009;200(5):724–734. doi: 10.1086/604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walev I, Weller U, Strauch S, Foster T, Bhakdi S. Selective killing of human monocytes and cytokine release provoked by sphingomyelinase (beta-toxin) of Staphylococcus aureus. Infection and immunity. 1996;64(8):2974–2979. doi: 10.1128/iai.64.8.2974-2979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nature medicine. 2007;13(12):1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nature reviews Microbiology. 2008;6(4):276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(30):13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Kawai Y, Tanaka Y, Hirano N, Kaneko J, Tomita N, Ohta M, Kamio Y, Yao M, Tanaka I. Crystal structure of the octameric pore of staphylococcal gamma-hemolysin reveals the beta-barrel pore formation mechanism by two components. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(42):17314–17319. doi: 10.1073/pnas.1110402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. Journal of immunology. 1999;163(1):1–5. [PubMed] [Google Scholar]
- Zhang G, Zhang L, Duff GW. A negative regulatory region containing a glucocorticosteroid response element (nGRE) in the human interleukin-1beta gene. DNA and cell biology. 1997;16(2):145–152. doi: 10.1089/dna.1997.16.145. [DOI] [PubMed] [Google Scholar]