Abstract
Experimental studies conducted on animal and human endothelium suggested that higher systolic blood pressure (SBP) variability reduces bioavailability of nitric oxide and increases vascular smooth muscle cell proliferation. These vascular wall changes could stiffen the arterial wall. Using data from the Multi-Ethnic Study of Atherosclerosis, we investigated the association between long-term SBP variability and ten-year percent change in arterial stiffness among 1122 individuals (mean age 57 years, 46% Males at baseline) who were not taking anti-hypertensive medications. Within-individual standard deviation (SD), variability independent of the mean (VIM), and coefficient of variation (CV) of SBP across 5 visits were used to capture long-term SBP variability. Carotid arterial stiffness was measured using distensibility coefficient (DC) and Young’s elastic modulus (YEM) at baseline and after a mean of 9.5 years of follow-up (visit 5). In a multivariate linear regression model, individuals in the 5th quintile as compared to those in the 1st quintile of SD, VIM, and CV of SBP had a 9.8% (95% CI: −17.0%, −2.7%), 6.4% (95% CI: −13.2%, 0.4%), and 8.7% (95% CI: −15.4%, −1.9%) higher decline in DC and a 27.5% (95% CI: 15.8%, 39.3%), 25.8% (95% CI: 14.7%, 36.9%), and 27.9% (95% CI: 16.8%, 39.1%) higher progression in YEM, respectively, after ten years of follow-up. Linear trends in the decline of DC and progression of YEM were observed across the quintiles of SBP variability indices. These findings suggest that higher long-term SBP variability may be a risk factor for arterial stiffness progression independent of mean BP.
Keywords: Blood pressure variability, arterial stiffness, distensibility coefficient, Young’s elastic modulus, race/ethnicity, cohort study
Introduction
Elevated mean blood pressure (BP) is widely recognized as a major risk factor for target organ damage and all-cause mortality.1, 2 However, other components of blood pressure, such as short- and long-term variability in BP, have been recently recognized as an important risk factors for development and progression of vascular events.3–7 Long-term (visit-to-visit) BP variability was shown to be reproducible over time,8 more common among individuals with higher cardiovascular (CV) risk,5 and higher among individuals at risk for stroke,9 indicating that long-term BP variability is a non-random phenomenon. In fact, independent of mean BP, long term variability in BP has been found to be associated with development of coronary heart disease, heart failure, cardiovascular mortality,10 stroke,10, 11 chronic kidney disease,6 and cognitive function.7 However, the mechanism through which higher BP variability causes vascular events is poorly understood.12, 13
Findings from experimental studies in animals and cultured human endothelium suggested that higher BP variability may cause arterial remodeling, such as vascular smooth muscle cell proliferation and extracellular matrix deposition, and also lead to increased oscillatory shear stress to the vascular endothelium, potentially contributing to increased expression of adhesion molecules and reduced bioavailability of nitric oxide.12–15 These structural and functional changes on the vascular wall are common antecedents of arterial stiffness.16–19 Thus, the effect of higher long-term BP variability on vascular events may partly occur through a gradual increase in arterial stiffness – which is known to be an independent risk factor for CV events.20, 21 The objective of this study was to investigate whether there is an association between long-term systolic BP variability and ten-year change in arterial stiffness independent of mean BP level.
Methods
Study design and participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal population based study of risk factors for subclinical and clinical cardiovascular diseases (CVD) among individuals who were free of clinical CVD at baseline. Participants were recruited from 6 centers across the United States (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan, New York; and St. Paul, Minnesota). The study protocol was approved by the institutional review boards at all 6 field centers and informed consent was obtained from all participants. Baseline assessment was conducted between July 2000 and August 2002 (visit 1) among 6,814 men and women aged 44 to 84 years. Four follow up examinations were conducted between September 2002 and February 2004 (visit 2), March 2004 and September 2005 (visit 3), September 2005 and May 2007 (visit 4), and April 2010 and December 2011 (visit 5). Details on design and objectives of MESA can be found in Bild et at.22 Participants were eligible for the present analysis if: (i) they had BP measured on at least 3 out of 5 visits to calculate indices of BP variability, and (ii) they were not taking antihypertensive medications at all the visits because BP medication masks pathophysiologic variations in BP.23 In addition, participants were included in the analysis if they had ultrasonography of the common carotid artery at visits 1 and 5.
Long-term BP variability
After participants rested for 5 minutes in a quiet environment, three seated brachial BP measurements were taken by a trained and certified research staff in the right arm at an interval of 1 minute using a calibrated Dinamap PRO 100 automated oscillometric device (Critikon, Tampa, FL)24 with the back and arm supported during each visit. The last two measurements were used to calculate averaged BP at each visit and these averaged values were used to calculate BP variability across the five visits (the exposure variable). Long-term systolic BP variability was quantified using three indices: (i) within-individual standard deviation (SD), (ii) coefficient of variation (CV - ratio of SD to the mean), and (iii) variability independent of mean (VIM). VIM was calculated as the SD divided by the within-individual mean to the power p and multiplied by the average value of systolic BP in the cohort to the power p.11, 25 The power p is obtained by fitting a curve of SD against within-individual mean systolic BP using the model SD=a times meanp, where p was derived by nonlinear regression analysis as implemented in the SAS PROC NLIN procedure.11, 25 VIM was shown to correlate highly with other indices of BP variability26 while its correlation with mean BP level is almost zero.11, 27 VIM allows assessing the association of BP variability with outcomes while removing the confounding effect of BP level.
Additional BP phenotypes calculated were within-individual mean systolic BP by averaging BP values across visits 1 to 5, mean arterial pressure calculated as (systolic BP + 2diastolic BP)/3 and averaged across the five visits, and cumulative exposure to systolic BP from visit 1 to visit 5, defined as the sum of averaged systolic BPs between two consecutive visits multiplied by the time between these two consecutive visits in years as shown in Yano et al.7
Carotid artery stiffness
B-mode ultrasound video image of a longitudinal section of the right common carotid artery was taken at visit 1 using a Logiq 700 ultrasound system (General Electric Medical Systems, transducer frequency 13 MHz) by trained and certified sonographers at each MESA site and images were recorded in a videotape. Video images were digitized at high resolution using a Medical Digital Recording (MDR) device (PACSGEAR, Pleasanton, CA). Similar recording was made using the same ultrasound and digitizing equipment at visit 5; however, the video images were directly digitized using the MDR settings without use of videotape. The University of Wisconsin-Madison Atherosclerosis Imaging Research Program interpreted the images. The systolic and diastolic diameters of the carotid artery were determined by the largest and smallest diameters during a cardiac cycle. Mean internal diameter at peak systole and mean internal and external diameters at end-diastole of carotid artery were calculated from measurements taken from 2–3 consecutive cardiac cycles. Intra- and inter-reader reliabilities showed excellent agreement between images read.28
Two indicators of carotid artery stiffness were calculated. Based on formulas recommended by expert consensus,29 carotid artery distensibility coefficient (DC) was calculated as (D2s−D2d)/(ΔP*D2d) and Young’s elastic modulus (YEM) as 3(1+(D2d/(D2e − D2i))]/DC. Ds and Dd are the internal diameters of the common carotid artery at peak-systole and end-diastole, ΔP is brachial pulse pressure, De and Di are the external and internal carotid artery diameters at end-diastole, DC is the distensibility coefficient. Ten-year percent change in DC and YEM (the outcomes) were calculated by subtracting visit 1 score of DC and YEM from the corresponding score at visit 5 and dividing the resulting value by the absolute value of visits 1 score and multiplying by 100, i.e. ((V5 − V1)/|V1|) * 100. Arterial stiffness corresponds to a higher score on YEM and a lower score on DC.
In addition, demographics (age, sex, and race), anthropometrics (height, weight, body mass index - BMI), behavioral factors (physical activity, alcohol intake and pack-years of cigarette smoked), and laboratory data (total cholesterol, high density lipoprotein - HDL-C, serum glucose, creatinine, and glomerular filtration rate - GFR), and diabetes mellitus status were determined as described in appendix A - online-only Data Supplement.
Statistical analysis
The association between long-term systolic BP variability indices (SD, VIM and CV) and ten-year percent change in carotid artery DC and YEM was evaluated using linear regression model while adjusting for potential confounders. To identify potential confounders, we drew a priori directed acyclic graph30 and applied Pearl’s back-door criterion31 using DAGitty.32 Potential confounders identified were age, sex, race, smoking, physical activity, alcohol intake, BMI, hypercholesterolemia, diabetes, GFR, C-reactive protein, menopausal status, and baseline arterial stiffness. In addition, mean arterial pressure and cumulative BP were adjusted when the exposure was SD of systolic BP and cumulative BP when the exposure was CV of systolic BP. SD, VIM and CV of systolic BP across visits 1 to 5 were included in separate regression models. Percent change in DC and YEM were regressed separately on continuous and quintiles of systolic BP variability indices and the lowest quintile was used as a reference group. Test of trend across the quintiles was assessed by including indicator of quintiles as a continuous ordinal variable. We assessed interaction of systolic BP variability indices with age, sex, and race using the most fully adjusted models. In all the analyses, statistical significance was set at P<0.05. Plots of the residuals against the fitted values for all the models were checked to assess assumptions of linearity and homoscedasticity and to check outlier observations in the data.33 All analyses were performed using Stata 13 (StataCorp. 2013, TX: StataCorp LP).34
Results
Participant characteristics
In the MESA, there were 2,314 participants who had complete data for systolic BP for at least three visits out of the total five visits and were not also taking antihypertensive medications at all the visits. Among those, 1,122 had ultrasonography imaging of the common carotid artery at both visits 1 and 5 and complete data on adjusted variables. There was no significant difference on most demographic and clinical characteristics between all eligible participants (N=2,314) and those who were included in the analysis (N=1,122) (Table 1, online-only Data Supplement). However, the participants included in our analysis were slightly younger (56.8 vs. 58.3 years, P<0.001), had lower systolic BP (113.5 vs.115.4 mm Hg, P<0.001), mean arterial pressure (83.0 vs.84.0 mm Hg, P=0.004), higher GFR (81.3 vs. 80.4 ml/min/1.73m2, P=0.04) and were less likely to be diabetic (5.3% vs. 13.5%, P<0.001) than all eligible participants.
Our participants’ age at baseline ranged 45–84 years (mean age: 56.8 years), 46.2% were male, 44.2% were White, 17.6% were Black, 16.0% were Chinese, and 22.1% were Hispanic (Table 1). Compared to individuals with lower SD of systolic BP (quintile 1), those who had higher SD (quintile 5) tended to be older, female, to have higher BMI, systolic and diastolic BP, cumulative systolic and diastolic BP, mean arterial pressure, total cholesterol, C-reactive protein, to have lower HDL, GFR, and had higher proportion of female who reached menopause (Table 1). Similarly, participants with higher VIM of systolic BP (quintile 5) were older, female, less likely to exercise, and had lower GFR, higher systolic BP, C-reactive protein, but lower diastolic BP, cumulative diastolic BP, and mean arterial pressure than those with lower VIM (quintile 1) (Table 2). Similar findings were found across quintiles of CV of systolic BP (Table 2, online-only Data Supplement).
Table 1.
Participant characteristics by quintiles of long-term systolic blood pressure standard deviation.
| All Participants (N=1122) | Quintiles of systolic blood pressure standard deviation (mm Hg)
|
||||||
|---|---|---|---|---|---|---|---|
| Q1 (n=226) (0.84–4.88) | Q2 (n=223) (4.90–6.67) | Q3 (n=225) (6.68–8.80) | Q4 (n=224) (8.81–12.13) | Q5 (n=224) (12.16–33.11) | |||
|
| |||||||
| Participant characteristics | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | P |
| Age (years) | 56.8 (8.6) | 53.8 (7.1) | 54.9 (8.4) | 56.2 (7.8) | 59.7 (9.0) | 59.3 (9.3) | <0.001 |
| Male (%) | 46.2 | 48.2 | 49.3 | 52.0 | 43.3 | 37.9 | 0.02 |
| Ethnicity (%) | |||||||
| White | 44.2 | 46.0 | 41.7 | 44.4 | 47.3 | 41.5 | 0.11 |
| Chinese | 16.0 | 16.4 | 19.7 | 15.1 | 16.5 | 12.5 | |
| Black | 17.6 | 12.8 | 15.7 | 17.8 | 16.5 | 25.4 | |
| Hispanic | 22.1 | 24.8 | 22.9 | 22.7 | 19.6 | 20.5 | |
| Pack years of smoking | 8.8 (17.7) | 7.1 (15.3) | 7.7 (15.6) | 9.1 (17.6) | 10.0 (18.4) | 10.0 (20.8) | 0.26 |
| Former drinker (%) | 37.8 | 37.2 | 37.7 | 39.6 | 41.5 | 33.0 | 0.71 |
| Current drinker (%) | 47.7 | 46.9 | 48.4 | 47.6 | 46.0 | 49.6 | |
| Activity (MET-hr/wk) | 99.7 (77.0) | 108.8 (89.4) | 104.8 (82.7) | 95.8 (73.6) | 95.1 (72.4) | 94.2 (64.1) | 0.15 |
| BMI (kg/m2) | 26.7 (4.6) | 26.3 (4.3) | 26.3 (4.3) | 27.2 (4.9) | 26.4 (4.2) | 27.4 (5.0) | 0.01 |
| Systolic BP (mm Hg) | 113.5 (13.3) | 106.7 (10.1) | 108.2 (9.9) | 112.0 (11.0) | 116.3 (13.5) | 124.6 (13.1) | <0.001 |
| Diastolic BP (mm Hg) | 67.7 (8.1) | 66.2 (7.7) | 66.3 (7.8) | 67.9 (7.9) | 68.5 (8.3) | 69.9 (8.3) | <0.001 |
| Cumulative Systolic BP (mm Hg | 1075.6 (145.8) | 1009.9 (118.5) | 1024.5 (113.3) | 1065.4 (110.4) | 1096.5 (155.9) | 1182.3 (156.3) | <0.001 |
| Cumulative Diastolic BP (mm Hg) | 639.6 (89.8) | 625.6 (84.0) | 627.3 (86.4) | 643.6 (81.7) | 642.4 (94) | 659.4 (98.4) | <0.001 |
| Mean arterial pressure (mm Hg) | 83.0 (8.7) | 79.7 (7.7) | 80.2 (7.6) | 82.6 (8.1) | 84.4 (8.8) | 88.1 (8.6) | <0.001 |
| Diabetes mellitus (%) | 5.3 | 4.9 | 3.1 | 5.3 | 6.3 | 7.1 | 0.40 |
| Total cholesterol (mg/dL) | 195.7 (28.8) | 193.2 (25.6) | 193.0 (28.7) | 196.3 (31.6) | 200.9 (27.1) | 195 (30.1) | 0.03 |
| HDL (mg/dL) | 54.3 (15.6) | 56.1 (15.9) | 53.6 (14.9) | 51.8 (16.0) | 55.3 (15.6) | 54.5 (15.6) | 0.04 |
| GFR (ml/min/1.73m2) | 81.3 (12.5) | 83.3 (12) | 82.8 (11.3) | 81.0 (12.6) | 79.7 (12.7) | 79.7 (13.4) | 0.003 |
| Log-CRP (mg/L) | 0.4 (1.1) | 0.1 (1.1) | 0.2 (1.1) | 0.4 (1.1) | 0.5 (1.1) | 0.6 (1.1) | <0.001 |
| Heart rate (beats/min) | 64.0 (7.7) | 64.4 (7.5) | 64 (7.5) | 64.8 (8) | 63.4 (7.3) | 63.4 (8) | 0.22 |
| Baseline menopause (% female) | 71.7 | 57.3 | 63.7 | 71.3 | 80.3 | 82.7 | <0.001 |
Abbreviations: BP, blood pressure; CRP, C-reactive protein; GFR, glomerular filtration rate; HDL, high density lipoprotein; MET, Metabolic Equivalent of Task; SD, standard deviation; Q, quintiles. Continuous variables were averaged across 5 visits. Individuals identified as diabetic at least once across 5 visits were considered as diabetic.
Table 2.
Participant characteristics by quintiles of long-term systolic blood pressure variability independent of the mean.
| All Participants (N=1122) | Quintiles of systolic blood pressure variability independent of the mean (mm Hg)
|
||||||
|---|---|---|---|---|---|---|---|
| Q1 (n=225) (1.15–5.32) | Q2 (n=224) (5.33–6.94) | Q3 (n=225) (6.95–8.93) | Q4 (n=224) (8.93–11.30) | Q5 (n=224) (11.31–37.60) | |||
|
| |||||||
| Participant characteristics | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | P |
| Age (years) | 56.8 (8.6) | 55.0 (7.7) | 56.6 (9.2) | 56.9 (8.4) | 56.9 (8.2) | 58.6 (9.4) | <0.001 |
| Male (%) | 46.2 | 54.7 | 55.4 | 46.2 | 41.5 | 33.0 | <0.001 |
| Ethnicity (%) | |||||||
| White | 44.2 | 43.1 | 42.4 | 43.6 | 50.0 | 42.0 | 0.44 |
| Chinese | 16.0 | 14.2 | 18.8 | 16.9 | 12.9 | 17.4 | |
| Black | 17.6 | 16.4 | 14.7 | 17.8 | 18.3 | 21.0 | |
| Hispanic | 22.1 | 26.2 | 24.1 | 21.8 | 18.8 | 19.6 | |
| Pack years of smoking | 8.8 (17.7) | 7.9 (16.6) | 7.1 (15.9) | 9.7 (17.0) | 10.5 (22.2) | 8.6 (15.7) | 0.24 |
| Former drinker (%) | 37.8 | 39.1 | 41.1 | 41.3 | 30.4 | 37.1 | 0.41 |
| Current drinker (%) | 47.7 | 46.7 | 46.4 | 44.9 | 53.1 | 47.3 | |
| Activity (MET-hr/wk) | 99.7 (77.0) | 116.6 (95.7) | 101.1 (79.2) | 97.2 (72.7) | 93.8 (68.8) | 89.9 (62.3) | 0.003 |
| BMI (kg/m2) | 26.7 (4.6) | 27.0 (4.0) | 27.0 (5.0) | 26.5 (4.5) | 26.6 (4.6) | 26.4 (4.7) | 0.55 |
| Systolic BP (mm Hg) | 113.5 (13.3) | 112.5 (11.6) | 114.9 (14.4) | 111.9 (12.9) | 114.8 (14.4) | 113.6 (12.7) | 0.05 |
| Diastolic BP (mm Hg) | 67.7 (8.1) | 68.7 (7.7) | 69.1 (8.2) | 67.1 (8.2) | 67.5 (8.3) | 66.3 (7.8) | 0.03 |
| Cumulative Systolic BP (mm Hg | 1075.6 (145.8) | 1063.2 (127.1) | 1080.1 (159.8) | 1069.6 (132.8) | 1091.2 (157.4) | 1074 (148.9) | 0.31 |
| Cumulative Diastolic BP (mm Hg) | 639.6 (89.8) | 648.2 (84.4) | 647.5 (91.1) | 639.5 (85.3) | 639.2 (94.7) | 623.8 (91.7) | 0.03 |
| Mean arterial pressure (mm Hg) | 83.0 (8.7) | 83.3 (8.0) | 84.3 (9.1) | 82 (8.6) | 83.3 (9.3) | 82.1 (8.3) | 0.03 |
| Diabetes mellitus (%) | 5.3 | 4.9 | 5.4 | 4.9 | 4.9 | 6.7 | 0.90 |
| Total cholesterol (mg/dL) | 195.7 (28.8) | 193.0 (26.0) | 193.5 (28.6) | 196.8 (31.7) | 198.1 (29.1) | 197 (28.2) | 0.23 |
| HDL (mg/dL) | 54.3 (15.6) | 54.5 (15.6) | 52.9 (15.9) | 53.1 (14.0) | 56.0 (17.2) | 54.8 (15.3) | 0.21 |
| GFR (ml/min/1.73m2) | 81.3 (12.5) | 82.6 (12.2) | 81.9 (13.0) | 81.5 (11.7) | 81.2 (12.5) | 79.3 (12.7) | 0.07 |
| Log-CRP (mg/L) | 0.4 (1.1) | 0.2 (1.1) | 0.3 (1.0) | 0.3 (1.2) | 0.5 (1.1) | 0.5 (1.1) | 0.01 |
| Heart rate (beats/min) | 64.0 (7.7) | 64.3 (7.5) | 64.6 (8.0) | 64.2 (7.6) | 63.8 (7.5) | 63.2 (7.8) | 0.34 |
| Baseline menopause (% female) | 71.7 | 63.7 | 70.0 | 69.4 | 76.3 | 76.0 | 0.17 |
Abbreviations: BP, blood pressure; CRP, C-reactive protein; GFR, glomerular filtration rate; HDL, high density lipoprotein; MET, Metabolic Equivalent of Task; SD, standard deviation; Q, quintiles. Continuous variables were averaged across 5 visits. Individuals identified as diabetic at least once across 5 visits were considered as diabetic.
Mean SD of systolic BP was 8.7 mm Hg (95% confidence interval - CI: 8.4, 8.9), mean VIM was 8.5 mm Hg (95% CI: 8.3, 8.7) and mean CV was 7.5% (95% CI: 7.3%, 7.7%) across the five visits. Almost all participants (97.8%) had complete data on systolic BP at all five visits, 1.9% had complete date at only 4 visits, and 0.3% at only 3 visits. Mean DC was 4.1×10−3 mmHg−1 at baseline and decreased to 3.4×10−3 mmHg−1 at visit 5. Mean YEM was 2.5×103 mmHg at baseline and increased to 2.9×103 mmHg at visit 5. On average, DC decreased by 11% and YEM increased by 25.3% over ten years of follow-up (Table 3, online-only Data Supplement). Mean (SD) of DC and YEM at visits 1 and 5 as well as the absolute and percent change across the visits by the quintiles of BP variability indices is given in tables 3, 4, and 5 in the online-only Data Supplement.
Long-term systolic BP variability and arterial stiffness progression
After adjusting for age, sex, race, study center, pack-years, alcohol intake, physical activity, BMI, heart rate, diabetes status, total cholesterol, HDL, GFR, C-reactive protein, and menopausal status, DC declined by 13.2% (95% CI: −20.7, −5.6) and YEM increased by 31.6% (95% CI: 19.6, 43.5) higher among those in the 5th quintile of SD of systolic BP as compared to those in the 1st quintile after ten years of follow-up (Table 3, Model 1). After adjusting further for cumulative systolic BP and mean arterial pressure (Table 3, Model 2), the decline in DC was higher by 8.6% (95% CI: −16.6, −0.8) and progression in YEM was higher by 26.8% (95% CI: 14.2, 39.4) among those in the 5th as compared to those in the 1st quintile of SD of systolic BP. Further adjustment for baseline arterial stiffness showed a 9.8% (95% CI: −17.0, −2.7) higher decline in DC and a 27.5% (95% CI: 15.8, 39.3) higher increment in YEM comparing individuals in the 5th against the 1st quintile of SD of systolic BP variability (Table 3, Model 3). There was a significant trend in decline of DC (linear trend P=0.049) and progression of YEM (linear trend P<0.001) across the quintiles of SD of systolic BP in the fully adjusted model.
Table 3.
Multivariate regression for the association between long-term systolic blood pressure standard deviation and ten-year change in distensibility coefficient and Young’s elastic modulus.
| N=1122 | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
|
| |||
| Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | |
| Outcome: Percent change in DC | |||
| Continuous SD-SBP (mm Hg) | −1.15 (−1.68, −0.62)‡ | −0.75 (−1.32, −0.18)† | −0.73 (−1.25, −0.22)† |
| Quintile of SD-SBP (mm Hg) | |||
| 1st quintile (0.84–4.88) (ref) | |||
| 2nd quintile (4.90–6.67) | −1.32 (−8.60, 5.95) | −1.74 (−8.79, 5.31) | −3.36 (−9.70, 2.98) |
| 3rd quintile (6.68–8.80) | −1.21 (−8.55, 6.13) | −1.38 (−8.53, 5.77) | −1.28 (−7.70, 5.15) |
| 4th quintile (8.81–12.13) | 0.46 (−7.07, 7.99) | −0.13 (−7.57, 7.32) | −0.81 (−7.51, 5.88) |
| 5th quintile (12.16–33.11) | −13.16 (−20.74, −5.59)‡ | −8.64 (−16.56, −0.77)* | −9.84 (−16.96, −2.72)† |
| Trend P-value | 0.005 | 0.11 | 0.049 |
| Outcome: Percent change in YEM | |||
| Continuous SD-SBP (mm Hg) | 3.21 (2.38, 4.04)‡ | 2.86 (1.96, 3.76)‡ | 2.86 (2.03, 3.70)‡ |
| Quintile of SD-SBP (mm Hg) | |||
| 1st quintile (0.84–4.88) (ref) | |||
| 2nd quintile (4.90–6.67) | 2.92 (−8.55, 14.39) | 3.62 (−7.59, 14.83) | 4.06 (−6.39, 14.50) |
| 3rd quintile (6.68–8.80) | 1.06 (−10.51, 12.63) | 1.74 (−9.63, 13.11) | 0.89 (−9.70, 11.48) |
| 4th quintile (8.81–12.13) | 12.88 (1.02, 24.75)* | 14.41 (2.57, 26.25)* | 14.61 (3.58, 25.64)† |
| 5th quintile (12.16–33.11) | 31.57 (19.63, 43.51)‡ | 26.81 (14.21, 39.40)‡ | 27.53 (15.79, 39.27)‡ |
| Trend P-value | <0.001 | <0.001 | <0.001 |
P-value:
<0.05,
≤0.01,
≤0.001.
Abbreviations: CI, confidence interval; Coef., regression coefficient; DC, distensibility coefficient; ref, reference group; SBP, systolic blood pressure; SD, standard deviation. Model 1 adjusted for age, sex, race/ethnicity, study center, and menopausal status at baseline, and averaged pack years, alcohol intake, physical activity, body mass index, heart rate, total cholesterol, high density lipoprotein, glomerular filtration rate, c-reactive protein across visits 1 to 5 and diabetes status across visits 1 to 5. Model 2 adjusted for model 1 variables, baseline mean arterial pressure, averaged mean arterial pressure across visits 1 to 5, and cumulative exposure to systolic BP from visit 1 to visit 5. Model 3 adjusted for model 2 variables and baseline DC or YEM.
Mean and SD of blood pressure were shown to correlate moderately.11, 27 Thus, we used VIM of systolic BP as a second index of BP variability. Figure 1 in the online-only Data Supplement shows scatter plot of mean systolic BP against SD and VIM of systolic BP. In our data, mean systolic BP correlated moderately with SD (Pearson’s correlation=0.49) while the correlation with VIM was zero (Pearson’s correlation=0.002).
There was a 9.0% (95% CI: −16.6, −1.5) higher deceleration in DC and a 27.8% (95% CI: 15.9, 39.6) higher progression in YEM after ten years of follow-up among individuals in the 5th quintile of VIM of systolic BP as compared to those in the 1st quintile after adjusting for age, sex, race, study center, pack-years, alcohol intake, physical activity, BMI, heart rate, diabetes status, total cholesterol, HDL, GFR, C-reactive protein, and menopausal status (Table 4, Model a). DC decreased 6.4% (95% CI: −13.19, 0.43) and YEM increased 25.8% (95% CI: 14.7, 36.9) higher after adjusting further for baseline arterial stiffness among individuals in the 5th as compared to those in the 1st quintile of systolic BP VIM (Table 4, Model b). There was a significant trend of higher progression in YEM (linear trend P<0.001) across the quintiles of VIM of systolic BP. When CV of systolic BP was used as the exposure, consistent findings were observed (Table 4, lower portion). Likewise, a decline in DC and an increment in YEM were observed when systolic BP variability indices were included as a continuous variable in the models.
Table 4.
Multivariate regression for the association of long-term systolic BP variability independent of the mean and coefficient of variation with ten-year change in distensibility coefficient and Young’s elastic modulus.
| N=1122 | Percent change in DC | Percent change in YEM | ||
|---|---|---|---|---|
|
| ||||
| Coef. (95% CI)a | Coef. (95% CI)b | Coef. (95% CI)a | Coef. (95% CI)b | |
| Continuous VIM-SBP (mm Hg) | −1.04 (−1.65, −0.44)‡ | −0.75 (−1.30, −0.20)‡ | 3.23 (2.29, 4.17)‡ | 3.00 (2.11, 3.88)‡ |
| Quintile of VIM-SBP (mm Hg) | ||||
| 1st quintile (1.15–5.32) (ref) | ||||
| 2nd quintile (5.33–6.94) | −0.51 (−7.81, 6.78) | −0.83 (−7.42, 5.77) | −1.16 (−12.64, 10.33) | −0.99 (−11.75, 9.78) |
| 3rd quintile (6.95–8.93) | 2.47 (−4.84, 9.79) | 1.88 (−4.73, 8.50) | 1.02 (−10.50, 12.54) | 1.82 (−8.98, 12.62) |
| 4th quintile (8.93–11.30) | 1.30 (−6.07, 8.67) | 0.36 (−6.31, 7.03) | 9.98 (−1.63, 21.59) | 10.94 (0.05, 21.82)* |
| 5th quintile (11.31–37.60) | −9.03 (−16.55, −1.51)* | −6.38 (−13.19, 0.43) | 27.76 (15.91, 39.61)‡ | 25.80 (14.70, 36.92)‡ |
| Trend P-value | 0.062 | 0.143 | <0.001 | <0.001 |
| Continuous CV-SBP (%) | −1.11 (−1.80, −0.43)‡ | −0.90 (−1.50, −0.29)† | 3.69 (2.62, 4.76)‡ | 3.51 (2.52, 4.49)‡ |
| Quintile of CV-SBP (%) | ||||
| 1st quintile (0.86–4.54) (ref) | ||||
| 2nd quintile (5.54–6.10) | −3.62 (−10.86, 3.61) | −4.22 (−10.61, 2.18) | 3.34 (−8.05, 14.73) | 2.81 (−7.70, 13.33) |
| 3rd quintile (6.10–7.63) | 2.14 (−5.18, 9.47) | 3.03 (−3.45, 9.50) | −2.55 (−14.07, 8.98) | −3.21 (−13.85, 7.43) |
| 4th quintile (7.63–10.27) | −0.03 (−7.42, 7.36) | −1.58 (−8.12, 4.95) | 9.48 (−2.16, 21.11) | 11.30 (0.55, 22.04)* |
| 5th quintile (10.29–27.98) | −10.56 (−18.21, −2.91)† | −8.66 (−15.43, −1.90)* | 29.98 (17.94, 42.02)‡ | 27.94 (16.82, 39.05)‡ |
| Trend P-value | 0.049 | 0.059 | <0.001 | <0.001 |
P-value:
<0.05,
≤0.01,
≤0.001.
Abbreviations: CI, confidence interval; Coef., regression coefficient; CV, coefficient of variation; DC, distensibility coefficient; ref, reference group; SBP, systolic blood pressure; VIM, variability independent of the mean; YEM, Young’s elastic modules.
Model adjusted for age, sex, race/ethnicity, study center, and menopausal status at baseline, and averaged pack years, alcohol intake, physical activity, body mass index, hear rate, total cholesterol, high density lipoprotein, glomerular filtration rate, c-reactive protein across visits 1 to 5, diabetes status across visits 1 to 5, and cumulative systolic BP when CV was the exposure.
Model adjusted for model “a” variables and baseline DC or YEM.
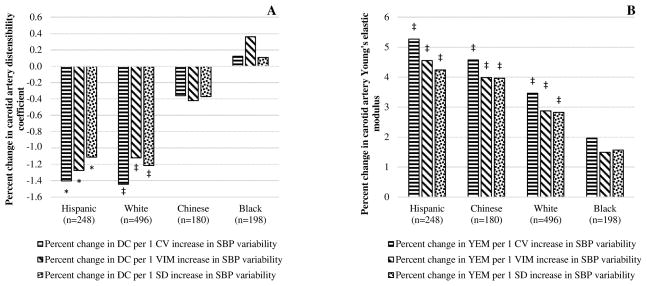
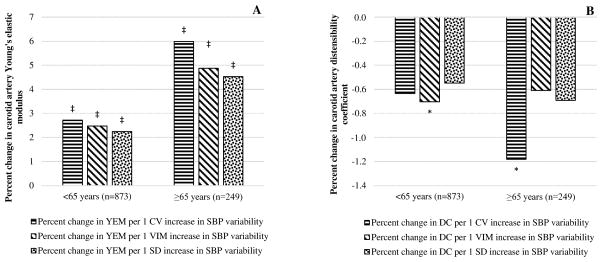
Both arterial stiffness and BP variability tend to be higher among the elderly, male and the minorities,35–37 and we assessed if age, gender, and race modify the association of BP variability with arterial stiffness. There was no interaction of gender with indices of systolic BP variability (interaction P≥0.29 in all). However, when the outcome was percent change in YEM, there was significant interaction of age with SD (interaction P=0.03), VIM (interaction P=0.03), and CV (interaction P= 0.003) of systolic BP indicting higher progression of arterial stiffness among those ≥65 years. There was also a borderline interaction of race with systolic BP SD (interaction P=0.08 for Blacks and 0.08 for Chinese), VIM (interaction P=0.09 for Blacks) and CV (interaction P=0.06 for Blacks) when the outcome was percent change in DC suggesting Blacks had slower progression of arterial stiffness than the other races. Race stratified analysis showed, there was higher decline in DC and progression in YEM among Whites, Hispanic, and Chinese but a non-significant change in DC and YEM among Blacks for a unit increase in systolic BP variability indices (Figure 1A and B). Similarly, the increase in YEM was higher by about two fold among individuals aged ≥65 years than those <65 years for a one unit increase in systolic BP variability indices (Figure 2A) while there was no appreciable difference on DC (Figure 2B).
Figure 1.
Association between systolic BP variability indices and ten-year percent change in carotid artery DC (A) and YEM (B) by race. Adjusted for age, sex, study center, mean arterial pressure when exposure was SD, DC/YEM, and menopausal status at baseline, and averaged pack years, alcohol intake, physical activity, BMI, heart rate, total cholesterol, HDL, GFR, C-reactive protein, mean arterial pressure when the exposure was SD of systolic BP, and cumulative exposure to systolic BP across visits 1 to 5 when exposures were SD and CV of systolic BP, and diabetes status across visits 1 to 5. Abbreviations: CV, coefficient variation, DC, distensibility coefficient; SBP, systolic blood pressure; SD, standard deviation; VIM, variability independent of the mean; YEM, Young’s elastic modulus. P-value: *<0.05, †<0.01, ‡<0.001.
Figure 2.
Association between systolic BP variability indices and ten-year percent change in carotid artery YEM (A) and DC (B) by age. Adjusted for sex, race/ethnicity, study center, mean arterial pressure when exposure was SD, DC/YEM, and menopausal status at baseline, and averaged pack years, alcohol intake, physical activity, BMI, heart rate, total cholesterol, HDL, GFR, C-reactive protein, mean arterial pressure when exposure was SD of systolic BP, cumulative exposure to systolic BP across visits 1 to 5 when exposures were SD and CV of systolic BP, and diabetes status across visits 1 to 5. Abbreviations: CV, coefficient variation, DC, distensibility coefficient; SBP, systolic blood pressure; SD, standard deviation; VIM, variability independent of the mean; YEM, Young’s elastic modulus. P-value: *<0.05, †<0.01, ‡<0.001.
Discussion
Using three indictors of long-term systolic BP variability and two indicators of carotid arterial stiffness, we found that higher long-term systolic BP variability was associated with greater progression in arterial stiffness over ten years among individuals who were not taking antihypertensive medications. This association persisted after adjusting for mean BP level, cumulative BP, baseline arterial stiffness, and other confounders. There was significant interaction of race and age with systolic BP variability. Higher systolic BP variability was consistently associated with a decline in DC and progression in YEM only among Whites, Hispanic, and Chines but not among Blacks. The decline in DC and progression in YEM was also higher among individuals ≥65 years than those <65 years for a similar increase in systolic BP variability.
To our knowledge, there is only one previous study that assessed the association between long-term BP variability and arterial stiffness.38 In the Asymptomatic Polyvascular Abnormalities Community (APAC) study, Wang et al.38 assessed the variability in systolic BP across four visits (each visit separated by two years) using standard deviation and examined its association with brachial-arterial pulse wave velocity (baPWV) at the end of the follow-up year among 3,994 Chinese adults. Consistent with our findings, they found that higher systolic BP variability was associated with higher ba-PWV (β=6.17 cm/sec 95% CI: 4.24, 8.12) after adjusting for multiple risk factors. In the Multi-Ethnic Study of Atherosclerosis, baseline arterial stiffness has been found to predict future long-term variability in systolic BP.39 It is therefore, difficult to decipher from Wang et al.’s38 study whether higher variability in BP caused stiffer arteries or the vice versa because baseline arterial stiffness was not known and adjusted in their analysis.
Several studies have shown an association between higher short-term systolic BP variability and stiffer arteries. In a cross-sectional study, Schillaci et al.40 found a correlation between SD of day-time (r=0.17) and night time (r=0.19) systolic BP with cfPWV among 911 untreated and nondiabetic hypertensive patients. Another study by Ichihara et al.41 showed that a one SD higher 24-h ambulatory systolic BP was associated with an increase in baPWV by 38.7 mm/sec (95% CI: 3.4, 73.9) among 203 newly diagnosed and untreated hypertensive patients after adjusting for confounders. Ozawa et al.42 also found that independent of other risk factors, baPWV was lower by 0.23 cm/sec (P=0.03) for a SD higher nighttime systolic BP among 92 hospitalized Japanese hypertensive patients.
It was suggested that higher variability in systolic BP for a prolonged time may result in stiffer artery by promoting proliferation of smooth muscle cells and accelerating the development of atherosclerosis through neuro-humoral processes by inducing the expression of several inflammatory factors, such as TNF-α and IL-1.38 Findings from experimental studies on cultured endothelium from animals and humans also suggested that higher BP variability may cause structural changes in the arterial wall, such as extracellular matrix deposition and vascular smooth muscle cells proliferation, and may also increase oscillatory shear stress in the vascular endothelium, potentially promoting expression of adhesion molecules and reducing bioavailability of nitric oxide.12–15 These structural and functional changes on the vascular wall are common findings in stiffer arteries. Histological examination of stiffened arteries consistently show inflammatory activity, increased collagen content, broken elastin molecules, hypertrophied smooth muscle layer, and increased matrix metalloproteinases.16–19
Arterial stiffness and BP variability may affect each other in a bidirectional manner. Shimbo et al.39 found systolic BP variability to be higher among individuals with lower baseline aortic distensibility after adjusting for demographic variables, cardiovascular risk factors, mean systolic BP, and antihypertensive medication. Therefore, unless high BP is controlled and maintained in a steady state, greater BP variability may accelerate the rate of arterial stiffness progression, which in turn, may lead to a vicious cycle of more elevated BP variability causing further increase in arterial stiffness. Furthermore, both higher BP variability and arterial stiffness were shown to be independent risk factors for CV diseases,10, 21 and this implies that individuals with higher BP variability and arterial stiffness could be at increased risk for CV events and mortality. Future studies should investigate the combined adverse effect of high BP variability and increased arterial stiffness on CV risk.
An interesting finding in our study is that higher systolic BP variability was associated consistently with progression in arterial stiffness only among Whites, Hispanics and Chinese but not among Blacks. On the other hand, mean systolic BP was associated with a greater progression in arterial stiffness in Blacks than in the other races (Table 6, online-only Data Supplement). This suggests that mean systolic BP instead of variability in systolic BP may be an important risk factor for the progression of arterial stiffness among Blacks while both mean and variation in systolic BP may be risk factors among other races. Vascular wall changes, such as elastin degradation and excessive deposition of collagen and calcium are common in the elderly.43, 44 Thus, the higher progression in arterial stiffness among the elderly as compared to non-elderly for a similar increase in BP variability could be due to the combined stiffening effects of increased age and BP variability on arterial wall.
Some studies found the predictive capacity of long-term BP variability for a clinical outcome diminishing and becoming non-significant when BP variability was calculated from 3 or 4 visits while BP variability calculated from ≥5 visits was a significant predictor.11, 45 We performed a sensitivity analysis generating long-term systolic BP variability only from the first 3 or 4 visits of MESA. We found a diminished predictive ability of long-term systolic BP variability for arterial stiffness progression (Table 7, online-only Data Supplement).
Our study is the first population based cohort study, to our knowledge, to show a temporal association between higher long-term systolic BP variability and accelerated progression in arterial stiffness independent of mean BP, baseline arterial stiffness and other confounders. Our study is also the first to show variation by race and age in the association between systolic BP variability and progression in arterial stiffness. An additional strength of this study is the inclusion of participants with diverse ethnic backgrounds from the general population, which would increase generalizability of our findings.
However, brachial artery BP was measured only at 5 visits over a mean of 9.5 years of follow-up, and this may not accurately reflect participants’ level of systolic BP variability during that follow-up time. In addition, in the calculation of DC and YEM, brachial artery BP was used as a surrogate for carotid arterial BP. Brachial artery pulse pressure was found to overestimate central pulse pressure,29, 46 and this may have over-estimated arterial stiffness. This error is likely to be non-differential by systolic BP variability and may have underestimated the association between systolic BP variability and arterial stiffness. Furthermore, participants who had arterial stiffness measurement were slightly younger, had slightly lower systolic BP, mean arterial pressure, higher GFR, and more likely to be diabetic than all eligible participants (Table 1, online-only Data Supplement). However, these difference were very small and unlikely to cause significant change in the association between systolic BP variability and arterial stiffness.
Perspective
Individuals who had higher variability in systolic BP over ten years showed greater ten-year progression in arterial stiffness from their baseline value independent of their mean BP, baseline arterial stiffness and other confounders among Whites, Hispanics, and Chinese but not among Blacks. Our findings suggest that mean systolic BP instead of variability in systolic BP may be an important risk factor for the progression of arterial stiffness among Blacks while mean and variation in systolic BP may be risk factors among the other races. In addition, the progression in arterial stiffness was higher by about two fold in the elderly compared to the non-elderly for a similar increase in systolic BP variability. Based on these novel findings, we suggest that higher long-term systolic BP variability merits consideration as a new risk factor for arterial stiffness progression.
Supplementary Material
Novelty and Significance.
What Is New?
This study is the first population based cohort study, to our knowledge, (i) to show a temporal association between higher long-term systolic BP variability and accelerated progression in arterial stiffness independent of mean BP, and (ii) to show variation in the association between systolic BP variability and progression in arterial stiffness by race and age.
What is Relevant
Experimental studies conducted on animal and human endothelium suggested that higher systolic blood pressure (BP) variability reduces bioavailability of nitric oxide and increases vascular smooth muscle cell proliferation. These vascular wall changes could stiffen the arterial wall.
It is difficult to interpret whether higher variability in BP caused stiffer arteries or the vice versa from the only available study on this topic because higher arterial stiffness was also shown to increase BP variability and was not taken into account.
Summary
Using three indices of BP variability and two indices of arterial stiffness, we showed that higher variability in systolic BP over ten years was associated with greater ten-year progression in arterial stiffness independent of mean BP level and other confounders among Whites, Hispanics, and Chinese but not among blacks. In addition, the progression in arterial stiffness was higher in the elderly compared to the non-elderly for a similar increase in systolic BP variability. Based on these findings, we suggest that higher long-term systolic BP variability merits consideration as a new risk factor for arterial stiffness progression.
Acknowledgments
Sources of Funding: This work was supported by contracts HC95159-HC95169 and HL07936 from the National Heart Lung and Blood Institute, grant ES015915 from the National Institute of Environmental Health Sciences, and grants RR024156 and RR025005 from the National Center for Research Resources. This publication was developed under Science to Achieve Results research assistance agreement RD831697 from the Environmental Protection Agency. It has not been formally reviewed by the Environmental Protection Agency. The views expressed in this document are solely those of the authors. The Environmental Protection Agency does not endorse any products or commercial services mentioned in this publication. Dr. Yacob Tedla was supported by a T32 HL 069771 Ruth L. Kirschstein National Research Service Award from the National Heart, Lung, and Blood Institute to the Northwestern University, Department of Preventive Medicine.
Footnotes
Disclosures: No disclosure to report.
Conflicts of interest: There are no conflicts of interest.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Schmieder RE. End organ damage in hypertension. Dtsch Arztebl Int. 2010;107:866–73. doi: 10.3238/arztebl.2010.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Parati G, Lantelme P. Blood pressure variability, target organ damage and cardiovascular events. J Hypertens. 2002;20:1725–1729. doi: 10.1097/00004872-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Darabont R, Tautu OF, Pop D, Fruntelata A, Deaconu A, Onciul S, Salaru D, Micoara A, Dorobantu M. Visit-to-visit blood pressure variability and arterial stiffness independently predict cardiovascular risk category in a general population: results from the SEPHAR II study. Hellenic J Cardiol. 2015;56:208–216. [PubMed] [Google Scholar]
- 6.Yano Y, Fujimoto S, Kramer H, et al. Long-term blood pressure variability, new-onset diabetes mellitus, and new-onset chronic kidney disease in the Japanese general population. Hypertension. 2015;66:30–36. doi: 10.1161/HYPERTENSIONAHA.115.05472. [DOI] [PubMed] [Google Scholar]
- 7.Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P, Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the coronary artery risk development in young adults (CARDIA) study. Hypertension. 2014;64:983–988. doi: 10.1161/HYPERTENSIONAHA.114.03978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard SC, Rothwell PM. Reproducibility of measures of visit-to-visit variability in blood pressure after transient ischaemic attack or minor stroke. Cerebrovasc Dis. 2009;28:331–340. doi: 10.1159/000229551. [DOI] [PubMed] [Google Scholar]
- 9.Cuffe RL, Howard SC, Algra A, Warlow CP, Rothwell PM. Medium-term variability of blood pressure and potential underdiagnosis of hypertension in patients with previous transient ischemic attack or minor stroke. Stroke. 2006;37:2776–2783. doi: 10.1161/01.STR.0000244761.62073.05. [DOI] [PubMed] [Google Scholar]
- 10.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, Levitan EB, Whelton PK, Cushman WC, Louis GT, Davis BR, Oparil S. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 12.Aoki Y, Kai H, Kajimoto H, Kudo H, Takayama N, Yasuoka S, Anegawa T, Iwamoto Y, Uchiwa H, Fukuda K, Kage M, Kato S, Fukumoto Y, Imaizumi T. Large blood pressure variability aggravates arteriolosclerosis and cortical sclerotic changes in the kidney in hypertensive rats. Circ J. 2014;78:2284–2291. doi: 10.1253/circj.cj-14-0027. [DOI] [PubMed] [Google Scholar]
- 13.Miao CY, Xie HH, Zhan LS, Su DF. Blood pressure variability is more important than blood pressure level in determination of end-organ damage in rats. J Hypertens. 2006;24:1125–1135. doi: 10.1097/01.hjh.0000226203.57818.88. [DOI] [PubMed] [Google Scholar]
- 14.Silacci P, Desgeorges A, Mazzolai L, Chambaz C, Hayoz D. Flow pulsatility is a critical determinant of oxidative stress in endothelial cells. Hypertension. 2001;38:1162–1166. doi: 10.1161/hy1101.095993. [DOI] [PubMed] [Google Scholar]
- 15.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 16.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 17.Tsioufis C, Bafakis I, Kasiakogias A, Stefanadis C. The role of matrix metalloproteinases in diabetes mellitus. Curr Top Med Chem. 2012;12:1159–1165. doi: 10.2174/1568026611208011159. [DOI] [PubMed] [Google Scholar]
- 18.Jadhav UM, Kadam NN. Non-invasive assessment of arterial stiffness by pulse-wave velocity correlates with endothelial dysfunction. Indian Heart J. 2005;57:226–232. [PubMed] [Google Scholar]
- 19.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–217. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Parati G, Bilo G. Calcium antagonist added to angiotensin receptor blocker: a recipe for reducing blood pressure variability? Evidence from day-by-day home blood pressure monitoring. Hypertension. 2012;59:1091–1093. doi: 10.1161/HYPERTENSIONAHA.112.193037. [DOI] [PubMed] [Google Scholar]
- 24.Critikon Dinamap Pro - Integrated Medical Systems. DINAMAP PRO Series 100–400 Monitor Operation Manual. Tampa, FL: [Google Scholar]
- 25.Asayama K, Wei FF, Hara A, Hansen TW, Li Y, Staessen JA. Prognosis in relation to blood pressure variability: con side of the argument. Hypertension. 2015;65:1170–1179. doi: 10.1161/HYPERTENSIONAHA.115.04808. [DOI] [PubMed] [Google Scholar]
- 26.Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Relationships between metrics of visit-to-visit variability of blood pressure. J Hum Hypertens. 2013;27:589–593. doi: 10.1038/jhh.2013.19. [DOI] [PubMed] [Google Scholar]
- 27.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, Poulter NR, Sever PS. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 28.Gepner AD, Korcarz CE, Colangelo LA, Hom EK, Tattersall MC, Astor BC, Kaufman JD, Liu K, Stein JH. Longitudinal effects of a decade of aging on carotid artery stiffness: the multiethnic study of atherosclerosis. Stroke. 2014;45:48–53. doi: 10.1161/STROKEAHA.113.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 31.Pearl J. Causal diagrams and the identification of causal effects. In: Pearl J, editor. Causality: Models, reasoning, and inference. 2. New York, NY: Cambridge University Press; 2000. [Google Scholar]
- 32.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analysing causal diagrams. Epidemiology. 2011;22:745–745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 33.Greenland S. Introduction to Regression Modeling. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3. New York, NY: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 34.StataCorp LP. Stata Statistical Software: Release 13. College Station, TX: 2013. [Google Scholar]
- 35.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Snieder H, Su S, Harshfield GA, Treiber FA, Wang X. A longitudinal study of blood pressure variability in African-American and European American youth. J Hypertens. 2010;28:715–722. doi: 10.1097/HJH.0b013e328336ed5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markert MS, Della-Morte D, Cabral D, Roberts EL, Jr, Gardener H, Dong C, Wright CB, Elkind MS, Sacco RL, Rundek T. Ethnic differences in carotid artery diameter and stiffness: the Northern Manhattan Study. Atherosclerosis. 2011;219:827–832. doi: 10.1016/j.atherosclerosis.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Yang Y, Wang A, An S, Li Z, Zhang W, Liu X, Ruan C, Liu X, Guo X, Zhao X, Wu S. Association of long-term blood pressure variability and brachial-ankle pulse wave velocity: a retrospective study from the APAC cohort. Sci Rep. 2016;6:21303. doi: 10.1038/srep21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimbo D, Shea S, McClelland RL, Viera AJ, Mann D, Newman J, Lima J, Polak JF, Psaty BM, Muntner P. Associations of aortic distensibility and arterial elasticity with long-term visit-to-visit blood pressure variability: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertens. 2013;26:896–902. doi: 10.1093/ajh/hpt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, Battista F, Settimi L, Desamericq G, Dolbeau G, Faini A, Salvi P, Mannarino E, Parati G. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension. 2012;60:369–377. doi: 10.1161/HYPERTENSIONAHA.112.197491. [DOI] [PubMed] [Google Scholar]
- 41.Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Hayashi M. Ambulatory blood pressure variability and brachial-ankle pulse wave velocity in untreated hypertensive patients. J Hum Hypertens. 2006;20:529–536. doi: 10.1038/sj.jhh.1002023. [DOI] [PubMed] [Google Scholar]
- 42.Ozawa M, Tamura K, Okano Y, Matsushita K, Ikeya Y, Masuda S, Wakui H, Dejima T, Shigenaga A, Azuma K, Ishigami T, Toya Y, Ishikawa T, Umemura S. Blood pressure variability as well as blood pressure level is important for left ventricular hypertrophy and brachial-ankle pulse wave velocity in hypertensives. Clin Exp Hypertens. 2009;31:669–679. doi: 10.3109/10641960903407033. [DOI] [PubMed] [Google Scholar]
- 43.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74:2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 44.Zimlichman R. Treatment of hypertension and metabolic syndrome: lowering blood pressure is not enough for organ protection, new approach-arterial destiffening. Current hypertension reports. 2014;16:479. doi: 10.1007/s11906-014-0479-z. [DOI] [PubMed] [Google Scholar]
- 45.Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M, Manson JE, Wassertheil-Smoller Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women’s Health Initiative. Hypertension. 2012;60:625–630. doi: 10.1161/HYPERTENSIONAHA.112.193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001;38:1461–1466. doi: 10.1161/hy1201.097723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.