Abstract
CADASIL, the most frequent genetic cause of stroke and vascular dementia, is caused by highly stereotyped mutations in the NOTCH3 receptor, which is predominantly expressed in vascular smooth muscle. The well-established TgNotch3R169C mouse model develops characteristic features of the human disease, with deposition of NOTCH3 and other proteins, including TIMP3 (tissue inhibitor of metalloproteinase 3), on brain vessels as well as reduced maximal dilation, and attenuated myogenic tone of cerebral arteries, but without elevated blood pressure. Increased TIMP3 levels were recently shown to be a major determinant of altered myogenic tone. In this study, we investigated the contribution of TIMP3 and Notch3 signaling to the impairment of maximal vasodilator capacity caused by the archetypal R169C mutation. Maximally dilated cerebral arteries in TgNotch3R169C mice exhibited a decrease in lumen diameter over a range of physiological pressures that occurred prior to myogenic tone deficits. This defect was not prevented by genetic reduction of TIMP3 in TgNotch3R169C mice and was not observed in mice overexpressing TIMP3. Knock-in mice with the R169C mutation (Notch3R170C/R170C) exhibited similar reductions in arterial lumen, and both TgNotch3R169C and Notch3R170C/R170C mice showed increased cerebral artery expression of Notch3 target genes. Reduced maximal vasodilation was prevented by conditional reduction of Notch activity in smooth muscle of TgNotch3R169C mice and mimicked by conditional activation of Notch3 in smooth muscle, an effect that was blood pressure-independent. We conclude that increased Notch3 activity mediates reduction in maximal dilator capacity of cerebral arteries in CADASIL and may contribute to reductions in cerebral blood flow.
Keywords: Cerebral small vessel disease, CADASIL, Notch3, vasodilator capacity, brain artery
Introduction
Structural changes in small arteries and arterioles leading to smaller lumen and reduced maximal dilation are commonly seen in chronic hypertension in both patients and mice.1, 2 These changes include various combinations of inward remodeling, hypertrophy and increased stiffness depending on the vessel caliber and form of hypertension. Such changes are of particular importance because, although they can protect the downstream circulation against elevated pressure, they can be maladaptive, adversely affecting local blood flow by decreasing microvascular pressure and maximal vasodilator capacity, thereby contributing to an increased risk of vascular events.3, 4,5 Mechanisms that control the development of such changes in arterial structure, particularly genetic determinants, are still poorly defined.
Cerebral small vessel diseases (cSVDs) are involved in about a fifth of all strokes and account for up to 45% of cases of elderly dementia. The majority of cSVDs are sporadic, with age and hypertension deemed the most important risk factors.6 CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy), caused by dominant mutations in the NOTCH3 receptor, is the most common inherited cSVD.7, 8 Remarkably, CADASIL shares many of the clinical and pathological features of sporadic forms of cSVDs, except for its earlier age of onset and common occurrence in normotensive individuals.7 The well-established TgNotch3R169C mouse model of CADASIL expresses an archetypal CADASIL-associated NOTCH3 mutation that does not affect Notch3 signaling in vitro.9, 10 TgNotch3R169C mice recapitulate the salient features of the disease, including deposition on brain vessels of the extracellular domain of NOTCH3 (Notch3ECD) and aggregates of other proteins in extracellular deposits called granular osmiophilic material (GOM).11 Interestingly, TgNotch3R169C mice exhibit a reduction in lumen diameter of maximally dilated cerebral arteries, despite the fact that blood pressure levels are normal, consistent with the clinical observation that CADASIL usually occurs in normotensive patients.11 Also, cerebral arteries exhibit attenuated pressure-induced constriction (myogenic tone) and cerebral blood flow (CBF) hemodynamics are compromised. 11,12
We and others recently demonstrated that mutant Notch3ECD triggers accumulation of vascular extracellular matrix proteins, including TIMP3 (tissue inhibitor of metalloproteinases-3), among others, which complex with Notch3ECD and are further deposited in GOM.13,14 Importantly, we have established that an elevated level of TIMP3 plays a key role in myogenic tone deficits and altered CBF hemodynamics in the TgNotch3R169C CADASIL model.15 However, mechanisms that control structural changes in cerebral arteries in this model remains to be elucidated. Specifically, whether this defect is related to vascular deposition of Notch3ECD or extracellular matrix proteins is not known.
Recent reports have highlighted the role of the extracellular matrix, matrix metalloproteinases, and the actin cytoskeleton of smooth muscle cells (SMCs) in structural changes of small arteries leading to a smaller lumen.2, 16, 17 TIMP3, like other members of the TIMP family, is a key regulator of extracellular matrix-degrading metalloproteinases.18 Notably, complete elimination of TIMP3 in the mouse results in pathological dilation of mesenteric arteries with increased distensibility.19 The Notch3 receptor is predominantly expressed in arterial SMCs and is a critical regulator of the developmental formation of small arteries, especially in the brain.20, 21 Importantly, both in vivo and in vitro studies have documented a role for Notch3 in the rearrangement of the SMC cytoskeleton.20 Motivated by these results, we here examined the possible role of TIMP3 and Notch3 signaling in mutant Notch3-mediated reductions in lumen diameter.
Methods
Experimental animals
Genetically modified mice (Table 1) were bred and housed in pathogen-free animal facilities and fed a standard diet ad libitum with free access to water. All experiments were conducted in full accordance with French guidelines for the Care and Use of Laboratory Animals and were approved by the “Lariboisière-Villemin” Institutional Animal Care and Use Committee (C2EA 09), with every effort made to minimize the number of animals used. All mice were male, and all experiments were carried out in accordance with ARRIVE guidelines.
Table 1. Mouse strains used in the study.
| Nomenclature | Description | Purpose |
|---|---|---|
| TgNotch3R169C | Mice overexpressing a rat Notch3 protein with the R169C mutation (line 88), 4 fold over the endogenous Notch3 (11) | Characterize mutant Notch3-induced structural changes (Figures 1 and S1) and assess Notch3 activity in cerebral arteries (Figure 2) |
| TgNotch3WT | Mice overexpressing a rat Notch3 protein with the wild type sequence (line 129), about 4 fold over the endogenous Notch3 (11) | |
| non-Tg | Non transgenic littermates | |
|
| ||
| TgNotch3R169C;Timp3+/+ | TgNotch3R169C mice with normal expression of TIMP3 (15) | Analyze the involvement of excess TIMP3 in structural changes caused by the R169C mutation (Figure S2) |
| TgNotch3R169C;Timp3+/- | TgNotch3R169C mice with reduced expression of TIMP3 (15) | |
| Non-Tg;Timp3+/+ | Non transgenic mice with normal expression of TIMP3 (15) | |
| Non-Tg;Timp3+/- | Non transgenic mice with reduced expression of TIMP3 (15) | |
| TgBAC-TIMP3 | Mice overexpressing TIMP3 (15) | |
|
| ||
| Notch3R170C/R170C | Mice with targeted insertion of the R169C mutation into the endogenous Notch3 locus 10 | Assess the consequence of the presence of the R169C mutation in the endogenous Notch3 locus on the cerebral arteries (Figure 3) |
| Notch3WT/WT | Littermate mice with the wildtype Notch3 sequence 10 | |
|
| ||
| TgN3R169C;Rbpjdel-SMC | Tamoxifen-treated TgNotch3R169C; SMMHC-CreERT2;Rbpjflox/flox mice to generate TgNotch3R169C mice with SMC-specific deletion of Rbpj | Examine the contribution of elevated Notch3 activity to structural changes caused by the R169C mutation (Figure 4) |
| TgN3R169C;RbpjWT | Tamoxifen-treated TgNotch3R169C; SMMHC-CreERT2;Rbpj+/+ mice to generate TgNotch3R169C mice with wild-type expression of RBPJ | |
| non-Tg;Rbpjdel-SMC | Tamoxifen-treated non-Tg;SMMHC-CreERT2;Rbpjflox/flox mice to generate non transgenic mice with SMC-specific deletion of Rbpj | |
|
| ||
| non-Tg;RbpjWT | Tamoxifen-treated non-Tg;SMMHC-CreERT2;Rbpj+/+ mice to generate non transgenic mice with wild-type expression of RBPJ | |
|
| ||
| TgNotch3ΔE(B)Act-SMC | Tamoxifen-treated SMMHC-CreERT2; TgNotch3ΔE from line B to generate mice expressing a constitutively activated Notch3 receptor in SMC | Investigate the consequence of elevated Notch3 activity on cerebral arteries (Figure 5, Figures S3-6) |
| TgNotch3ΔE(C)Act-SMC | Tamoxifen-treated SMMHC-CreERT2; TgNotch3ΔE from line C to generate mice expressing a constitutively activated Notch3 receptor in SMC | |
| WT | Tamoxifen-treated SMMHC-CreERT2; non transgenic littermates from lines B and C were used as controls | |
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). Myogenic tone, passive diameter, cross-sectional area of the vascular media and incremental distensibility were analyzed by two-way repeated-measure analysis of variance (ANOVA) followed by a Bonferroni post hoc test. Gene expression levels and Notch3ECD deposits were analyzed by Student's t-test (2 groups) and one-way ANOVA followed by Bonferroni or Tukey post hoc tests (> 2 groups). All statistics were performed using Graph Pad Prism software. Differences with P-values < 0.05 were considered statistically significant.
A detailed description of experimental procedures is available in the online-only material.
Results
Cerebral arteries from TgNotch3R169C mice are less distensible with reductions in maximal dilation
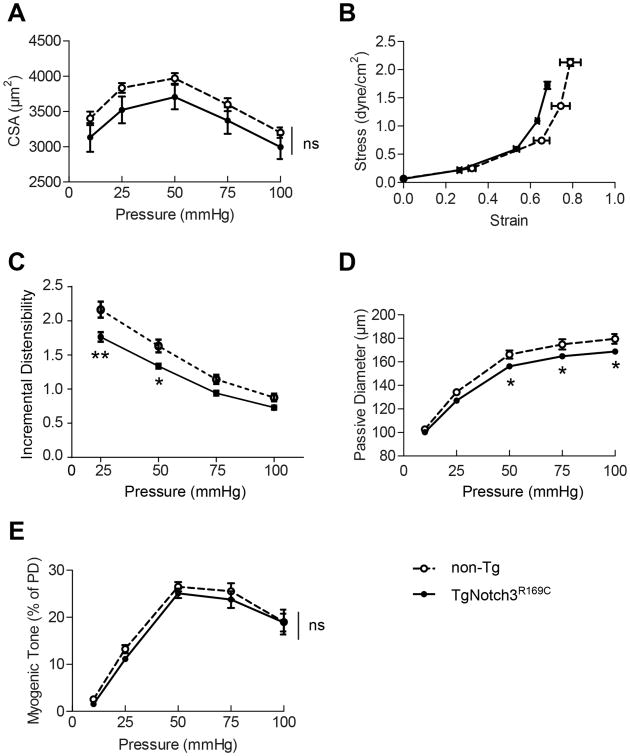
We first sought to better characterize structural and mechanical properties of cerebral arteries in TgNotch3R169C mice (Table 1). Potential causes for reductions in lumen diameter of maximally dilated arteries include inward remodeling (smaller diameter at all pressures), hypertrophy (larger cross-sectional area that encroaches on the lumen) or reductions in distensibility (increased stiffness). 3, 4 As previously reported11, the passive diameter of cerebral arteries was significantly reduced in TgNotch3R169C mice compared with non-transgenic mice, over a range of physiological pressures for the cerebral circulation (∼ 13% reduction at 50 mmHg), but not at the lowest pressure (10 mmHg). The external diameter was also significantly decreased, and the media thickness-to-lumen diameter ratio was significantly increased in TgNotch3R169C mice at 50 mmHg (Figure S1 in online-only material). In contrast, the cross-sectional area of the media was comparable between TgNotch3R169C mice and wild-type littermates at all pressures (Figure 1A). Notably, the stress-strain curves were shifted to the left and incremental distensibility was significantly reduced at 25 and 50 mmHg (Figure 1B, C). Together these data suggest that the reduction in maximal dilation of cerebral arteries in TgNotch3R169C mice is related to increased stiffness, although overt fibrosis had not been observed, even in aged mutant mice.11
Figure 1. Characterization of mechanical and functional properties of cerebral arteries in TgNotch3R169C mice.
Assessment of the media cross-sectional area (A), media stress-strain relationship (B) and incremental distensibility (C) of the P2 segment of posterior cerebral arteries at different intraluminal pressures in 6-month-old TgNotch3R169C mice and non-transgenic (non-Tg) mice (n=7–9 mice/group). Assessment of passive diameter (D) and myogenic tone (E) in 2-month-old TgNotch3R169C mice and non-Tg mice (n=6 mice/group). (*P<0.05, **P< 0.01 TgNotch3R169C vs non-Tg).
We next sought to determine the age of onset of these changes with respect to other disease manifestations. In mutant cerebral arteries, Notch3ECD accumulation is detectable in animals as young as neonates and GOM deposits are present from the age of 5 to 6 months.11 We found that the passive diameter of cerebral arteries was already significantly reduced in 2-month-old TgNotch3R169C mice compared with non-transgenic mice, although it was less reduced than that at 6 months of age (∼6% versus 13% reduction at 50 mmHg); noting that incremental distensibility was not significantly reduced at this age (Figure 1D). Interestingly, the myogenic tone of mutant arteries was unaffected at this age (Figure 1E). Thus, our results indicate that reduced maximal vasodilation in the TgNotch3R169C model is a very early and gradually progressive defect.
Elevated TIMP3 does not mediate impairment of maximal vasodilation in TgNotch3R169C mice
We next investigated the involvement of TIMP3 in the smaller lumen of TgNotch3R169C cerebral arteries. To achieve this, we used gain and loss-of-function genetic-interaction approaches (Table 1). We found that the passive diameter and stiffness of cerebral arteries was comparable in TgNotch3R169C mice with normal expression of TIMP3 (TgNotch3R169C;Timp3+/+) and TgNotch3R169C mice with reduced expression of TIMP3 (TgNotch3R169C;Timp3+/-), the latter of which are notable for their rescue of myogenic tone observed in TgNotch3R169C mice (Figure S2A-C in online-only material).15 Moreover, the passive diameter of cerebral arteries was unaffected by genetic overexpression of TIMP3 in TgBAC-TIMP3 mice, which exhibited attenuated pressure-induced myogenic constriction (Figure S2D in online-only material). Thus, excess TIMP3 does not contribute to the impairment of maximal vasodilation in the TgNotch3R169C model.
Expression of Notch3 target genes is increased in cerebral arteries of TgNotch3R169C mice
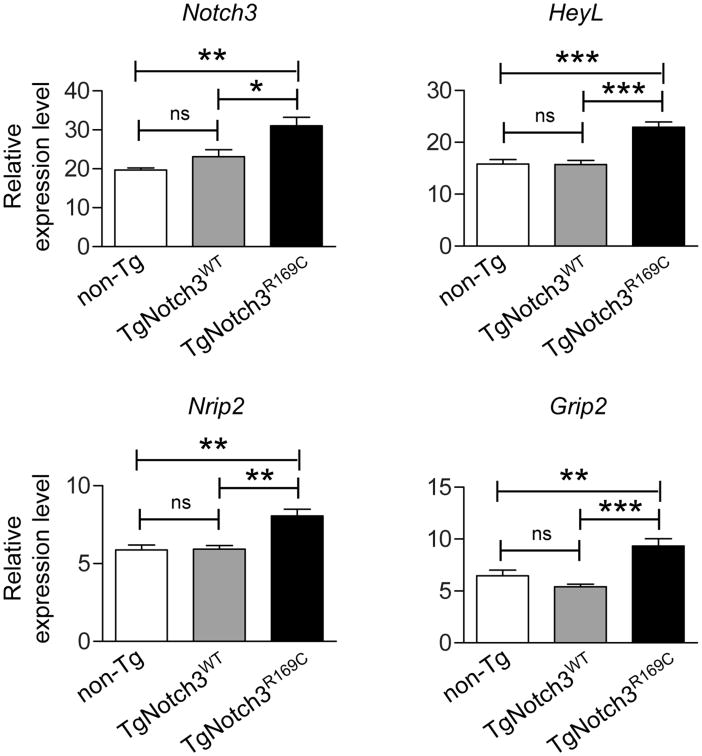
We next evaluated the contribution of Notch3 signaling. To this end, we first analyzed the level of Notch3 activity in cerebral arteries of TgNotch3R169C and non-transgenic littermate mice. To control for the potential confounding effect of Notch3 overexpression, we analyzed TgNotch3WT mice, which overexpress similar amount of rat Notch3 protein, although with the wild-type sequence, and have preserved maximal vasodilation (Table 1). Upon ligand binding, Notch receptors undergo several proteolytic cleavages that release the Notch intracellular domain, which translocates to the nucleus, where it complexes with RBPJ to form an active transcriptional complex that turns on the expression of target genes.22 We recently identified a set of genes, including Notch3, HeyL, Nrip2 and Grip2, whose expression is regulated in SMCs of adult brain arteries by Notch3 activity.21, 23 Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of dissected cerebral vessels showed that, at 2 months of age, vascular expression levels of these genes were significantly upregulated in TgNotch3R169C mice compared with TgNotch3WT and non-Tg mice, with fold changes ranging from ∼1.4 to 1.7 (Figure 2). Thus, these results indicate that expression of Notch3 target genes, an indicator of Notch3 activity, is increased by transgenic overexpression of the mutant R169C Notch3 but not by comparable overexpression of wildtype Notch3.
Figure 2. Expression of Notch3 target genes is increased in cerebral arteries of TgNotch3R169C mice.
Assessment of relative mRNA expression levels of the Notch3-regulated genes, Notch3, Nrip2, HeyL and Grip2, in dissected cerebral arteries from 2-month-old TgNotch3R169C, TgNotch3WT, and non-Tg littermate mice (n=5 biological replicates/genotype). (*P<0.05, **P<0.01, ***P<0.001).
Cerebral arteries of Notch3R170C/R170C mice display increased expression of Notch3 target genes and reduced lumen diameter
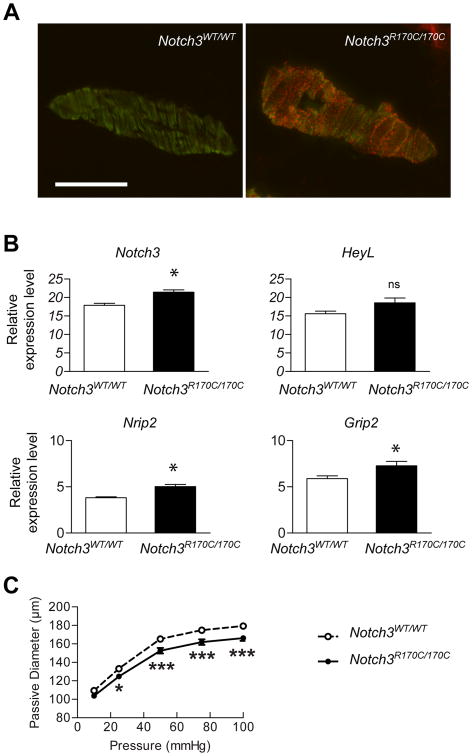
To determine whether the presence of the R169C mutation in the endogenous Notch3 locus is sufficient to produce these same alterations, we analyzed Notch3R170C/R170C mice (Table 1). At 4 months of age, Notch3R170C/R170C mice exhibited robust Notch3ECD deposition in cerebral arteries, with the extent of deposition approaching that observed in 2-month-old TgNotch3R169C mice (Figure 3A). Importantly, we found that expression levels of Notch3, Nrip2, and Grip2 were significantly upregulated (1.2–1.3-fold) in cerebral arteries of Notch3R170C/R170C mice; HeyL expression exhibited a similar trend, although this difference did not reach statistical significance (Figure 3B). Moreover, the passive diameter of cerebral arteries from 4-month-old Notch3R170C/R170C mice was significantly decreased over a range of physiological pressures, although not at the lowest pressure, compared with Notch3WT/WT littermate mice; noting that incremental distensibility was unchanged like in 2-month-old TgNotch3R169C mice (Figure 3C). Together, these data confirm that the R169C mutation causes increased expression of Notch3 target genes and impairment of maximal dilator capacity in cerebral arteries.
Figure 3. Cerebral arteries of Notch3R170C/R170C mice exhibit increased expression of Notch3 target genes and decreased maximal dilation.
A , Representative images of cerebral arteries from 4-month-old Notch3R170C/R170C and Notch3WT/WT mice co-immunostained with anti-Notch3ECD (red) and fluorescein isothiocyanate (FITC)-conjugated anti-smooth muscle α-actin (green) antibodies. Scale bar: 50 μm. B, Assessment of relative expression levels of Notch3, Nrip2, HeyL, and Grip2 mRNA in dissected cerebral arteries from 4-month-old Notch3R170C/R170C and Notch3wt/wt mice (n=5–6 biological replicates/genotype). C, The passive diameter of the P2 segment of posterior cerebral arteries was analyzed in 4-month-old Notch3R170C/R170C and Notch3WT/WT mice (n=6–8 mice/genotype). (*P<0.05, *** P<0.001 Notch3R170C/R170C vs Notch3wt/wt)
Genetic reduction of Notch activity in arterial SMCs protects against smaller lumen diameters in TgNotch3R169C mice
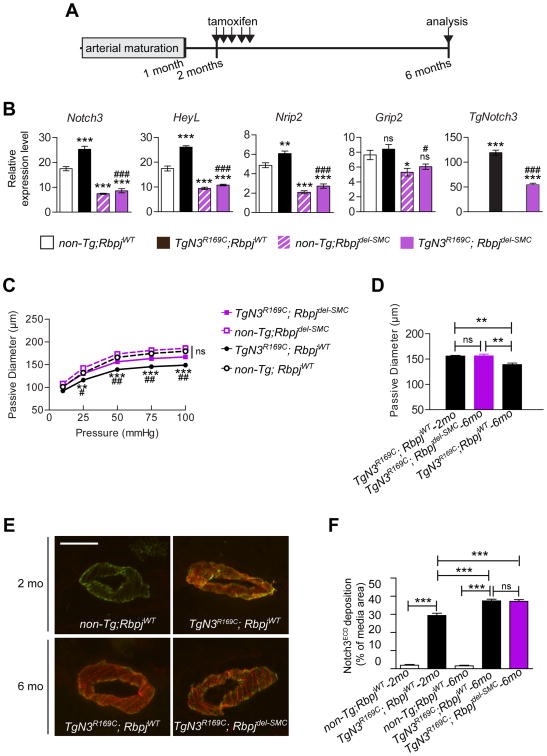
We next sought to elucidate the contribution of elevated Notch3 activity to maximal vasodilation impairment caused by the NOTCH3 R169C mutant. Our prior work established that Notch3 is the predominant Notch receptor in SMCs of cerebral arteries and that RBPJ activity in these cells is predominantly mediated by Notch3 signaling.24 Hence, we generated and analyzed TgNotch3R169C mice with a tamoxifen-inducible deletion of Rbpj in SMCs together with their control TgNotch3R169C and non-Tg littermates (Table 1). Because Notch3 activity is critically required in the postnatal period 21, deletion of Rbpj in SMCs was induced at 2 months of age (i.e., after normal completion of arterial development), and thus at the very beginning of arterial changes (Figure 4A).
Figure 4. Genetic reduction of Notch activity in SMCs protects against reductions in maximal dilation of cerebral arteries in TgNotch3R169C mice.
A , Schematic representation of the experimental design. B, TgN3R169C;Rbpjdel-SMC were analyzed at 6 months of age for relative expression levels of Notch3, Nrip2, HeyL, Grip2, and TgNotch3 mRNAs in dissected cerebral arteries. non-Tg;Rbpjdel-SMC, TgN3R169C;RbpjWT and non-Tg;RbpjWT mice served as controls (n=5 biological replicates/genotype). (*P<0.05, **P<0.01, ***P<0.001 non-Tg;RbpjWT vs. all other groups; #P<0.05, ###P<0.001 TgN3R169C;RbpjWT compared with TgN3R169C;Rbpjdel-SMC). C, Passive diameter of the P2 segment of posterior cerebral arteries was determined in 6-month-old TgN3R169C;Rbpjdel-SMC mice. Control groups used were as described in panel B (n=9–10 mice/genotype). (**P<0.01, ***P<0.001 TgN3R169C;RbpjWT compared with non-Tg;RbpjWT; #P<0.05, ## P<0.01 TgN3R169C;RbpjWT compared with TgN3R169C;Rbpjdel-SMC). D, Passive diameters of pressurized (50 mmHg) P2 segments of posterior cerebral arteries from 2-month-old TgN3R169C;RbpjWT and 6-month-old TgN3R169C;Rbpjdel-SMC and TgN3R169C;RbpjWT mice (n=6–10 mice/genotype). E, Representative images of brain arteries co-immunostained with anti-Notch3ECD (red) and FITC-conjugated anti-smooth muscle α-actin (green) antibodies. Note that non-Tg;RbpjWT and non-Tg;Rbpjdel-SMC mice do not exhibit Notch3ECD deposits. Scale bar: 50 μm. F, Quantification of Notch3ECD deposits in immunostained images of cerebral arteries from mice of the indicated ages and genotypes (n=5 mice per genotype).
qRT-PCR analyses of the Notch3-regulated genes, Notch3, HeyL, Nrip2 and Grip2, confirmed that Notch3 activity was reduced in cerebral arteries from 6-month-old TgN3R169C;Rbpjdel-SMC and non-Tg;Rbpjdel-SMC compared with TgN3R169C;RbpjWT and non-Tg;RbpjWT mice (Figure 4B).
As expected, the passive diameter of cerebral arteries was substantially reduced in 6-month-old TgN3R169C;RbpjWT mice compared with age-matched non-Tg;RbpjWT mice, indicating that tamoxifen treatment and differences in strain background did not affect the TgNotch3R169C phenotype. Importantly, SMC deletion of Rbpj in TgNotch3R169C mice (TgN3R169C;Rbpjdel-SMC) from 2 to 6 months of age significantly attenuated the reduction in passive diameter, whereas Rbpj deletion in non-Tg mice (non-Tg;Rbpjdel-SMC) had no significant effect (Figure 4C). It was noteworthy that the passive diameter of cerebral arteries from 6-month-old TgN3R169C;Rbpjdel-SMC mice, in which Rbpj had been deleted from 2 months of age, was similar to that observed in 2-month-old TgN3R169C;RbpjWT mice, suggesting that reducing Notch3 signaling halts the reduction in lumen diameter (Figure 4D).
Because Notch3 activity regulates expression of the Notch3 receptor and expression of the Notch3 R169C mutant in TgNotch3R169C mice is driven by the Notch3 promoter, we assessed the extent to which SMC deletion of Rbpj affected expression of the Notch3 mutant transgene, and consequently Notch3ECD deposition. We found that expression of Notch3 mutant transgene (TgNotch3) mRNA was reduced by half in TgN3R169C;Rbpjdel-SMC mice compared with TgN3R169C;RbpjWT mice (Figure 4B). Despite this, deposition of Notch3ECD in cerebral arteries was comparable between TgN3R169C;Rbpjdel-SMC and TgN3R169C;RbpjWT mice at 6 months of age, and significantly increased compared with that in 2-month-old TgN3R169C;RbpjWT mice (Figure 4E–F). Thus, these results establish that reducing Notch3 activity in TgNotch3R169C mice protects against attenuation in maximal vasodilation, despite the continuing progression of Notch3ECD deposition, hence suggesting that this protective effect unlikely arises from a reduction in transgene overexpression and Notch3ECD deposition.
Activation of Notch3 in SMCs mimics R169C mutant Notch3-mediated structural changes in brain arteries
We then investigated whether moderately elevating Notch3 activity in arterial SMCs was sufficient to recapitulate arterial changes as observed in TgNotch3R169C mice. To this end, we developed a transgenic mouse model in which Notch3 becomes permanently activated in SMC, upon tamoxifen treatment (TgNotch3ΔEAct-SMC, Table 1). Previous reports have shown that truncated version of Notch receptors, including Notch3, in which the extracellular domain has been deleted (Notch3ΔE) behaves as constitutively activated receptor.25 We generated transgenic mice carrying such truncated version of Notch3 under the control of the well-characterized arterial SMC-specific SM22α promoter,24 in which expression of the TgNotch3ΔE transgene is repressed by a floxed stop codon-beta geo cassette, and obtained 2 lines (B and C) that we bred with the tamoxifen-inducible SMC Cre line, SMMHC-CreERT2 (Figure S3A–C in online-only material). The SM22α promoter was chosen to achieve weak expression of the transgene and thus moderate activation of Notch3.24
In the absence of tamoxifen, transgenic mice expressed β-galactosidase in brain arteries, albeit at a low level, but not the active Notch3 (Figure S3D in online-only material). Upon tamoxifen treatment, the stop codon-beta geo cassette is excised (Figures 5A and S3E in online-only material), allowing expression of Notch3ΔE in cerebral arteries, with an estimated mRNA expression level less than 20% that of endogenous murine Notch3 mRNA in both lines (Figure 5B–C). Also, the Notch3-regulated genes Notch3, HeyL, and Nrip2 were upregulated in both TgNotch3ΔE(B)Act-SMC and TgNotch3ΔE(C)Act-SMC mice, with slightly higher upregulation in the TgNotch3ΔE(C)Act‐SMC line, whereas upregulation of Grip2 occurred only in TgNotch3ΔE(C)Act-SMC mice (Figure 5B–C). These findings confirm that Notch3 signaling is activated in the cerebral arteries of both lines and suggest that Notch3 activation in the TgNotch3ΔE(C)Act-SMC mice is slightly more pronounced, consistent with the higher expression of β-galactosidase and Notch3ΔE in the TgNotch3ΔE(C) line (Figure S3D in online-only material). Notably, the increase in Notch3-regulated genes was between 1.3- and 1.6-fold in TgNotch3ΔEAct-SMC mice—the same range as that in cerebral arteries from TgNotch3R169C mice—suggesting a comparable level of Notch3 activation in these two models. A further histological analysis of semi-thin sections of cerebral arteries from TgNotch3ΔEAct-SMC mice showed no overt alteration of SMCs in either line (Figure S4 in online-only material).
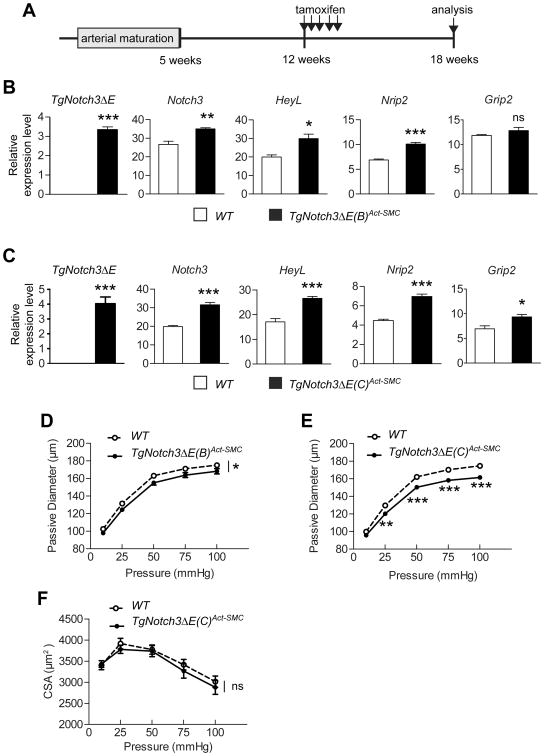
Figure 5. Elevated Notch3 activity mimics R169C Notch3 mutant-induced structural changes.
A , Schematic representation of the experimental design. TgNotch3ΔE(B)Act-SMC (B, D) and TgNotch3ΔE(C)Act-SMC (C, E) were analyzed at 18 weeks of age for relative expression levels of Notch3, Nrip2, HeyL, Grip2 and TgNotch3ΔE mRNAs in dissected cerebral arteries (B, C) and passive diameter of the P2 segment of the posterior cerebral artery (D, E). n=5 biological replicates/genotype in (B, C), and n=11–12 mice/genotype in (D, E). F, Assessment of the media CSA in TgNotch3ΔE(C)Act-SMC and WT mice (n=11–12 mice/genotype). *P<0.05, **P<0.01, ***P<0.001 TgNotch3ΔEAct-SMC vs WT.
Importantly, we found that the passive diameter of cerebral arteries was significantly reduced in TgNotch3ΔEAct-SMC mice of both lines, with a more pronounced reduction in TgNotch3ΔE(C)Act-SMC mice, compared with WT littermates (Figure 5D–E). Cross-sectional area of the media was comparable between TgNotch3ΔE(C)Act-SMC and WT mice (Figure 5F). Moreover, the stress-strain curve was slightly shifted to the left and incremental distensibility was significantly reduced at 25 mmHg in TgNotch3ΔE(C)Act-SMC. mice (Figure S5 in online-only material). Notably, resting arterial blood pressure was not altered in TgNotch3ΔE(C)Act-SMC mice (Figure S6 in online-only material). Thus, these results establish that moderate activation of Notch3 in arterial SMCs of cerebral arteries is sufficient to recapitulate structural and mechanical changes observed in TgNotch3R169C mice. Collectively, our results suggest that the R169C Notch3 mutation increases Notch3 activity, and thereby causes reductions in maximal vasodilator capacity.
Discussion
A reduction in maximal dilation of cerebral arteries is an important feature in the well-established TgNotch3R169C mouse model of CADASIL, a genetic paradigm of cSVDs. Because of the pathological importance of such alteration in general and in particular in CADASIL owing to its occurrence in the context of unaltered blood pressure and compromised CBF hemodynamics, we thought it especially important to understand its mechanism. There are several findings arising from the present study. First, cerebral arteries of TgNotch3R169C mice display altered mechanical and structural properties consistent with an increased stiffness, although no overt change in the composition of arterial wall had been detected, at least by optical microscopy analysis.11 Based on Poiseuille's law, the magnitude of reduction in arterial caliber is expected to have significant effects on vascular resistance, thus influencing local blood flow. Importantly, these alterations are very similar to those reported in comparable cerebral arteries (second-order branches of posterior cerebral arteries and distal segments of the middle cerebral artery) of stroke-prone spontaneously hypertensive rats and hypertensive transgenic rats overexpressing mouserenin.4,26 Such reductions in lumen diameter can reduce both submaximal and maximal vasodilation.27 Second, we unexpectedly found that an elevated level of TIMP3 does not contribute to these alterations, despite its key role in mutant Notch3-induced cerebrovascular dysfunction. Third, using two distinct mouse models, we established that the archetypal R169C Notch3 mutation is associated with reduced maximal dilation of cerebral arteries and increased Notch3 activity. Fourth, we identified increased Notch3 activity as a heretofore-unrecognized regulator of cerebral artery structure and mechanics and provided evidence for a causal relationship between increased Notch3 signaling and smaller lumen diameter of cerebral arteries in the TgNotch3R169C CADASIL model.
Prior to this work, CADASIL-associated NOTCH3 mutations have not been convincingly shown to alter Notch3 activity, apart from some uncommon mutations (present in about 5% of families) located in or around the ligand-binding domain of Notch3 that unambiguously abrogate Notch3 signaling.28,29 Moreover, it was still unclear whether altered Notch3 signaling plays a role in disease manifestations. Here, we showed for the first time that R169C, an archetypal CADASIL mutation, increases Notch3 signaling activity in cerebral arteries. Importantly, the observation of unchanged Notch3 signaling activity in TgNotch3WT mice, taken together with the finding of similarly increased Notch3 activity in the Notch3R170C knock-in model, precludes the possibility of an effect of the mutation in the context of Notch3 overexpression. It is possible that the increase in Notch3 activity, which appears to be moderate in these two mouse models, has thus far gone essentially unnoticed because prior assays were inappropriate or insufficiently sensitive. The vast majority of CADASIL-associated NOTCH3 mutations lead to an odd number of cysteine residues within Notch3ECD and the R169C mutation is located in the N-terminus of Notch3ECD, a mutation hotspot.8 Notably, this mutation has been identified in more than 35 CADASIL families worldwide and is associated with a typical phenotype.28 We thus surmise that other CADASIL mutations may similarly increase Notch3 activity.
The mechanism of increased Notch3 activity in cerebral arteries of TgNotch3R169C and Notch3R170C/R170C mice is puzzling. Previous reports have shown that the R169C mutation does not affect Notch3 signaling in cultured cells.9,10 However, it is noteworthy that extracellular deposition of Notch3ECD was not present in these in vitro assays. Recently, we showed that Notch3ECD accumulation in CADASIL occurs independently of ligand binding, suggesting that it may instead arise from a defect in Notch3 receptor trafficking.28,29 In Drosophila, misregulation of endosomal trafficking of the Notch receptor had been linked to its aberrant activation30, a possibility that warrants investigation in CADASIL.
Our observation that genetic reduction of Notch3 signaling in TgNotch3R169C mice protects these mice against reductions in vascular lumen diameter supports a causative role of increased Notch3 signaling in this type of structural change. However, interpretation of this genetic loss-of-function study is complicated by the fact that any pharmacological or genetic manipulation aimed at decreasing Notch3 activity inevitably decreases the expression of the Notch3 receptor, given that Notch3 expression is regulated by Notch3 signaling.21 In fact, we found that TgNotch3R169C mice in which we genetically reduced Notch3 activity (TgN3R169C;Rbpjdel-SMC) exhibited approximately a 50% reduction in the expression of the Notch3R169C transgene. Nevertheless, our data suggest that reduced transgene expression is not capable of accounting for the protection against changes in arterial diameter because it would also be expected to have an effect on Notch3ECD deposition, yet Notch3ECD deposition progressed similarly in TgNotch3R169C mice with elevated(TgN3R169C;RbpjWT) or reduced Notch3 activity (TgN3R169C;Rbpjdel-SMC). More importantly, our gain-of-function experiments demonstrated that activation of Notch3 in brain arteries on the same order of magnitude as occurs in TgNotch3R169C mice is sufficient to recapitulate R169C Notch3 mutant-induced arterial changes. Therefore, our data collectively indicate that structural changes of cerebral arteries in TgNotch3R169C mice are not related to the extent of Notch3ECD deposition, and provide support for a causative role of increased Notch3 activity, although we cannot rule out the possibility that other factors may be in play.
As discussed above, the R169C mutation and all similar mutations are predicted to be associated with increased Notch3 activity and smaller lumen diameters, whereas mutations in the ligand-binding domain of Notch3 are predicted to be associated with reduced Notch3 activity and to protect patients against these changes (noting that CADASIL mutations are present in a heterozygous state, Notch3 activity is predicted to be half-reduced in these latter patients). If hypertension is present, reductions in lumen diameter of cerebral arteries are both protective of downstream microvessels and detrimental. However, in the absence of hypertension like in the TgNotch3R169C model, it seems that these changes would only be detrimental. Therefore, the R169C mutation and all similar mutations are thus predicted to be associated with a more severe phenotype than those in the ligand-binding domain. Consistent with this prediction, our prior genotype-phenotype correlation study has revealed that CADASIL patients carrying a mutation in the mutational hotspot appear to have more severe cognitive decline than those with a mutation in the ligand-binding domain.28 Further analysis of the pathological weight of such vascular changes on stroke and cognitive decline in CADASIL could be valuable, although such studies are limited by the lack of an appropriate experimental model.
Although the molecular details of the mechanism responsible for Notch3-mediated structural changes remain unsettled, our prior work showed that Notch3 activation promotes the robust formation of actin stress fibers in cultured SMCs.20 Reductions in lumen diameter of maximally dilated arteries can be induced ex vivo by prolonged exposure of isolated arterial segments to vasoconstrictors. Importantly, studies using this paradigm have highlighted a role of the SMC actin cytoskeleton in the initial stage of the remodeling process, showing that smaller lumen diameter is associated with SMC actin polymerization and is reversed by actin depolymerization, with the possible involvement of the small GTPases, Rho/Rac/Cdc42.31 Further study will be necessary to elucidate the role of SMC actin polymerization in Notch3-induced lumen reduction and to establish mechanisms responsible for this effect.
Perspectives
CADASIL is a paradigmatic cSVD that commonly occurs in normotensive individuals. TgNotch3R169C mice, a well-established model of CADASIL, develop reduction in lumen diameter of cerebral arteries in the absence of chronic hypertension, and the magnitude of the reduction is in the same range as that seen with chronic hypertension. This change, which reduces vasodilator capacity, may adversely affect local cerebral blood flow and thereby contributes to the disease phenotype, yet the underlying molecular mechanisms have remained unknown. The current study highlights an association between an unsuspected increase in Notch3 activity and the R169C archetypal CADASIL mutation, and uncovers a previously unknown role of increased Notch3 activity in structural changes that lead to smaller lumen diameter, that is blood pressure-independent. We speculate that increased Notch3 activity and reduced vasodilator capacity are common features of many other CADASIL mutations. Further in-depth studies are needed to understand how increased Notch3 activity affects structure of cerebral arteries.
Supplementary Material
Novelty and Significance.
What Is New?
The R169C CADASIL-associated Notch3 mutation is linked to increased Notch3 activity in cerebral arteries.
Increased Notch3 activity is sufficient to reduce maximal dilation of cerebral arteries, independent of increases in arterial blood pressure.
Our findings provide evidence that reduction in maximal dilation of cerebral arteries induced by archetypal Notch3 mutants in CADASIL is mediated by increased Notch3 activity.
What Is Relevant?
Mutations in the Notch3 receptor are responsible for CADASIL, the most frequent genetic cause of stroke and vascular dementia, yet it is still unclear whether altered Notch3 signaling plays a role in disease manifestations. The well-established TgNotch3R169C mouse model exhibits unaltered blood pressure, but develops structural changes of cerebral arteries that reduce vasodilator capacity through an unknown mechanism.
Our findings have important implications for the pathogenesis of CADASIL and the mechanisms that regulate pathological changes in structure of cerebral arteries that reduce vasodilator capacity.
Summary
This study establishes that the R169C archetypal CADASIL mutation is associated with changes in structure of cerebral arteries that reduce vasodilator capacity and increased Notch3 activity in cerebral arteries of two different mouse models. Conditional reduction of Notch activity in SMCs protects mice expressing the R169C mutation against these changes; conversely, conditional activation of Notch3 in SMCs recapitulates R169C mutant Notch3-induced structural arterial changes. Our findings provide new insight into the pathogenesis of CADASIL, supporting the concept that increased Notch3 activity mediates pathological changes in structure of brain arteries in CADASIL. This study uncovers an unsuspected role of Notch3 signaling in vascular remodeling that occurs independent of increases in blood pressure.
Acknowledgments
We are grateful to Charles Fouillade for technical assistance, Stephan Offermanns for providing the SMMHC-CreERT2 line, Mieke Dewerchin for providing the Notch3R170C/WT line, Tasuku Honjo for providing the Rbpjflox/wt line, Heidi Stoehr for providing the Timp3+/- mice, University Paris Denis Diderot-site Villemin (Suzanne Orville & Frédéric Baudin) for animal housing, and SEAT transgenic mouse facilities (Cécile Gouget and Caroline Martin).
Source of funding: This work was supported by grants from the National Research Agency, France (ANR Genopath 2009-RAE09011HSA and ANR Blanc 2010-RPV11011HHA) to AJ and the Fondation Leducq (Transatlantic Network of Excellence on the Pathogenesis of SVD of the Brain) to AJ and FMF. FMF was supported by the NIH (HL113863, NS096465) and the Department of Veteran's Affair's (BX001399).
Footnotes
Disclosure/Conflict of Interest: Dr. JOUTEL owns two families of patents: “Gene involved in CADASIL, method of diagnosis and therapeutic application” licensed to Athena diagnostics, and “Immunological treatment of CADASIL”.
References
- 1.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007–1021. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 3.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension. 1996;28:505–506. [PubMed] [Google Scholar]
- 4.Hajdu MA, Baumbach GL. Mechanics of large and small cerebral arteries in chronic hypertension. Am J Physiol. 1994;266:H1027–1033. doi: 10.1152/ajpheart.1994.266.3.H1027. [DOI] [PubMed] [Google Scholar]
- 5.Rizzoni D, Porteri E, Boari GEM, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 6.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 7.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 8.Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, Cruaud C, Maciazek J, Weissenbach J, Bousser MG, Bach JF, Tournier-Lasserve E. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350:1511–1515. doi: 10.1016/S0140-6736(97)08083-5. [DOI] [PubMed] [Google Scholar]
- 9.Haritunians T, Boulter J, Hicks C, Buhrman J, DiSibio G, Shawber C, Weinmaster G, Nofziger D, Schanen C. CADASIL Notch3 mutant proteins localize to the cell surface and bind ligand. Circ Res. 2002;90:506–508. doi: 10.1161/01.res.0000013796.73742.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallays G, Nuyens D, Silasi-Mansat R, Souffreau J, Callaerts-Vegh Z, Van Nuffelen A, Moons L, D'Hooge R, Lupu F, Carmeliet P, Collen D, Dewerchin M. Notch3 Arg170Cys knock-in mice display pathologic and clinical features of the neurovascular disorder cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Arterioscler Thromb Vasc Biol. 2011;31:2881–2888. doi: 10.1161/ATVBAHA.111.237859. [DOI] [PubMed] [Google Scholar]
- 11.Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, Lemaire-Carrette B, Domenga V, Schedl A, Lacombe P, Hubner N. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120:433–445. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabertrand F, Krøigaard C, Bonev AD, Cognat E, Dalsgaard T, Domenga-Denier V, Hill-Eubanks DC, Brayden JE, Joutel A, Nelson MT. Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc Natl Acad Sci USA. 2015;112:E796–805. doi: 10.1073/pnas.1420765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monet-Leprêtre M, Haddad I, Baron-Menguy C, Fouillot-Panchal M, Riani M, Domenga-Denier V, Dussaule C, Cognat E, Vinh J, Joutel A. Abnormal recruitment of extracellular matrix proteins by excess Notch3 ECD: a new pathomechanism in CADASIL. Brain. 2013;136:1830–1845. doi: 10.1093/brain/awt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kast J, Hanecker P, Beaufort N, Giese A, Joutel A, Dichgans M, Opherk C, Haffner C. Sequestration of latent TGF-β binding protein 1 into CADASIL-related Notch3-ECD deposits. Acta Neuropathol Commun. 2014;2:96. doi: 10.1186/s40478-014-0096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capone C, Cognat E, Ghezali L, Baron-Menguy C, Aubin D, Mesnard L, Stöhr H, Domenga-Denier V, Nelson MT, Joutel A. Reducing Timp3 or vitronectin ameliorates disease manifestations in CADASIL mice. Ann Neurol. 2016;79:387–403. doi: 10.1002/ana.24573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castorena-Gonzalez JA, Staiculescu MC, Foote C, Martinez-Lemus LA. Mechanisms of the inward remodeling process in resistance vessels: is the actin cytoskeleton involved? Microcirculation. 2014;21:219–229. doi: 10.1111/micc.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umesalma S, Houwen FK, Baumbach GL, Chan SL. Roles of Caveolin-1 in Angiotensin II-Induced Hypertrophy and Inward Remodeling of Cerebral Pial Arterioles. Hypertension. 2016;67:623–629. doi: 10.1161/HYPERTENSIONAHA.115.06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu R, Lee J, Morton JS, Takawale A, Fan D, Kandalam V, Wang X, Davidge ST, Kassiri Z. TIMP3 is the primary TIMP to regulate agonist-induced vascular remodelling and hypertension. Cardiovasc Res. 2013;98:360–371. doi: 10.1093/cvr/cvt067. [DOI] [PubMed] [Google Scholar]
- 20.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fouillade C, Baron-Menguy C, Domenga-Denier V, Thibault C, Takamiya K, Huganir R, Joutel A. Transcriptome analysis for Notch3 target genes identifies Grip2 as a novel regulator of myogenic response in the cerebrovasculature. Arterioscler Thromb Vasc Biol. 2013;33:76–86. doi: 10.1161/ATVBAHA.112.251736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cognat E, Baron-Menguy C, Domenga-Denier V, Cleophax S, Fouillade C, Monet-Leprêtre M, Dewerchin M, Joutel A. Archetypal Arg169Cys mutation in NOTCH3 does not drive the pathogenesis in cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy via a loss-of-function mechanism. Stroke. 2014;45:842–849. doi: 10.1161/STROKEAHA.113.003339. [DOI] [PubMed] [Google Scholar]
- 24.Monet M, Domenga V, Lemaire B, Souilhol C, Langa F, Babinet C, Gridley T, Tournier-Lasserve E, Cohen-Tannoudji M, Joutel A. The archetypal R90C CADASIL-NOTCH3 mutation retains NOTCH3 function in vivo. Hum Mol Genet. 2007;16:982–992. doi: 10.1093/hmg/ddm042. [DOI] [PubMed] [Google Scholar]
- 25.Joutel A, Monet M, Domenga V, Riant F, Tournier-Lasserve E. Pathogenic mutations associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy differently affect Jagged1 binding and Notch3 activity via the RBP/JK signaling Pathway. Am J Hum Genet. 2004;74:338–347. doi: 10.1086/381506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn WR, Gardiner SM. Differential alteration in vascular structure of resistance arteries isolated from the cerebral and mesenteric vascular beds of transgenic [(mRen-2)27], hypertensive rats. Hypertension. 1997;29:1140–1147. doi: 10.1161/01.hyp.29.5.1140. [DOI] [PubMed] [Google Scholar]
- 27.Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arteriolar dilatation in hypertensive rats. Hypertension. 2001;37:1388–1393. doi: 10.1161/01.hyp.37.6.1388. [DOI] [PubMed] [Google Scholar]
- 28.Monet-Lepretre M, Bardot B, Lemaire B, Domenga V, Godin O, Dichgans M, Tournier-Lasserve E, Cohen-Tannoudji M, Chabriat H, Joutel A. Distinct phenotypic and functional features of CADASIL mutations in the Notch3 ligand binding domain. Brain. 2009;132:1601–1612. doi: 10.1093/brain/awp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joutel A. Pathogenesis of CADASIL: transgenic and knock-out mice to probe function and dysfunction of the mutated gene, Notch3, in the cerebrovasculature. Bioessays. 2011;33:73–80. doi: 10.1002/bies.201000093. [DOI] [PubMed] [Google Scholar]
- 30.Palmer WH, Deng WM. Ligand-Independent Mechanisms of Notch Activity. Trends Cell Biol. 2015;25:697–707. doi: 10.1016/j.tcb.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staiculescu MC, Galiñanes EL, Zhao G, Ulloa U, Jin M, Beig MI, Meininger GA, Martinez-Lemus LA. Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc Res. 2013;98:428–436. doi: 10.1093/cvr/cvt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.