Abstract
Immunopharmacotherapy offers an approach for treating cocaine abuse by specifically targeting the cocaine molecule and preventing its access to the CNS. dAd5GNE is a novel cocaine vaccine that attenuates the stimulant and the reinforcing effects of cocaine in rats. The goal of this study was to extend and validate dAd5GNE vaccine efficacy in non-human primates. Six experimentally naïve adult female rhesus monkeys (Macaca mulatta) were trained to self-administer 0.1 mg/kg/injection intravenous (i.v.) cocaine or receive candy; then 4 monkeys were administered the vaccine and 2 monkeys were administered vehicle intramuscularly, with additional vaccine boosts throughout the study. The reinforcing effects of cocaine were measured during self-administration, extinction, and reacquisition (relapse) phases. Serum antibody titers in the vaccinated monkeys remained high throughout the study. There was no change in the preference for cocaine over candy over a 20-week period in 5 of the 6 monkeys; only one of the 4 (25%) vaccinated monkeys showed a decrease in cocaine choice. All 6 monkeys extinguished responding for cocaine during saline extinction testing; vaccinated monkeys tended to take longer to extinguish responding than control monkeys (17.5 vs. 7.0 sessions). Vaccination substantially retarded reacquisition of cocaine self-administration; control monkeys resumed cocaine self-administration within 6–41 sessions and 1 vaccinated monkey resumed cocaine self-administration in 19 sessions. The other 3 vaccinated monkeys required between 57–94 sessions to resume cocaine self-administration even in the context of employing several manipulations to encourage cocaine reacquisition. These data suggest that the dAdGNE vaccine may have therapeutic potential for humans who achieve cocaine abstinence as part of a relapse prevention strategy.
Keywords: Cocaine, Vaccine, Rhesus monkeys, Self-administration, Choice Procedure, Relapse
1. Introduction
Cocaine use in the United States continues to be a significant public health problem with 1.5 million current users in 2014 (SAMHSA, 2015), yet effective behavioral or pharmacological treatments for cocaine have been elusive. In fact, despite testing well over 60 pharmacotherapies (Haney and Spealman, 2008; Karila et al., 2008; Shorter and Kosten, 2011), there are still no FDA approved medications for cocaine use disorders. Although agonist medications have demonstrated the greatest promise (Czoty et al., 2016), this pharmacologic treatment approach has not been fully endorsed (Negus and Henningfield, 2016). Immunopharmacotherapy is an alternative approach (Anton et al., 2009; Haney and Kosten, 2004; Janda and Treweek, 2011) and to that end vaccines for cocaine, and other drugs of abuse have been developed (see review by Kinsey, 2014). The basic premise of anti-addiction vaccines is that by attaching the abused drug or a chemical analog, to an immunogenic protein, antibodies specific to the abused drug are evoked, limiting the ability of the drug to cross the blood-brain-barrier, thus effectively attenuating the central nervous system (CNS) psychoactive effects associated with abuse.
In rats, active immunization using a first-generation cocaine hapten (termed GNC; 6-(2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo [3.2.1] octane-2-carbonyloxy-hexanoic acid) cocaine analog linked to a keyhole limpet hemocyanin (KLH) decreased locomotor activity (Carrera et al., 1995, 2001) and retarded relapse to cocaine self-administration (Carrera et al., 2000). Similarly, active immunization with the commercially labeled TA-CD vaccine attenuated cocaine seeking and cocaine self-administration in immunized rats that achieved sufficient antibody levels (Kantak et al., 2000, 2001). While passive immunization also has been shown to decrease cocaine-induced locomotor activity (Carrera et al., 2001) and cocaine self-administration (Mets et al., 1998) in rats, active immunization has the capacity to provide long-lasting protection without repeated administration of exogenous antibody.
The TA-CD vaccine is the only cocaine vaccine that has been evaluated in humans. Based on a Phase I study in abstinent cocaine abusers (Kosten et al., 2002) and a Phase IIa study in treatment-seeking cocaine dependent patients (Martell et al., 2005), TA-CD was well tolerated, produced dose-related increases in cocaine specific antibody levels and resulted in more cocaine negative urines among patients who achieved higher antibody levels (Martell et al., 2005). In a subsequent randomized clinical trial among cocaine-dependent methadone-maintained patients (Martell et al., 2009), greater anti-cocaine antibody levels were associated with reductions in cocaine use, but sufficient antibody levels were only achieved by 38% of the patients. Based on a controlled laboratory study in non-treatment seeking cocaine users (Haney et al., 2010), TA-CD attenuated the positive subjective effects of smoked cocaine administration only among those individuals who had the greatest antibody levels. Unfortunately, in a recent multi-site Phase III randomized controlled trial (Kosten et al., 2014), the TA-CD vaccine failed to significantly attenuate cocaine use relative to placebo.
Recent preclinical studies in mice and non-human primates have shown that high antibody titers are not necessarily related to adequate reductions in brain levels of the abused drug (McCluskie et al., 2013, 2015). Inadequate antibody affinity may result in inadequate function and account for the lack of clinical efficacy of previous anti-drug vaccines, such as several for nicotine (e.g., Hatsukami et al., 2011; Tonstad et al., 2013) and TA-CD for cocaine (Kosten et al., 2014). More recently, promising vector-based gene therapies have been developed for cocaine (Anker et al., 2012; Gao and Brimijoin, 2009; Koob et al., 2011). Hicks et al. (2011) demonstrated in mice that administration of a first-generation cocaine vaccine that links a cocaine hapten (GNC) to the capsid proteins of a disrupted adenovirus (Ad) (dAd5GNC) increased serum anti-GNC antibody titers and when mice were administered cocaine (25 or 50 μg, i.v.), those vaccinated with dAd5GNC demonstrated dramatically blunted cocaine-induced hyperactivity, reduced brain levels of cocaine, and correspondingly increased peripheral levels of cocaine. Subsequently, a third-generation cocaine hapten (termed GNE) was developed (Cai et al., 2013a,b) that when coupled to the Ad capsid proteins created dAd5GNE. Rats vaccinated with dAd5GNE showed rapid and sustained antibody titer levels, and when given cocaine, vaccinated rats showed reduced brain levels of cocaine and reduced cocaine-induced locomotor activity compared to control rats (Wee et al., 2012). While vaccinated and control rats acquired cocaine self-administration at the same rate, under a progressive ratio schedule vaccinated rats self-administered fewer cocaine doses compared to control rats and response-independent “priming” doses of cocaine during extinction failed to reinstate cocaine responding in vaccinated rats (Wee et al., 2012). The promising results of these two studies in small animal models provide preliminary evidence that the dAd5GNE vaccine may be effective for cocaine use disorders.
Non-human primate models have been used extensively in the development of immunotherapy for HIV/AIDS (Hatziioannou and Evans, 2012) and hepatitis C virus (Akari et al., 2009) and to evaluate potential pharmacotherapies for drug abuse for the translation of potential treatments to humans (see reviews by Weerts et al., 2007; Howell, 2008). Despite this, few studies have utilized non-human primates for evaluating potential immunotherapies for drug abuse. In fact, only one previous study published over 40 years ago (Bonese et al., 1974) tested immunotherapy using a non-human primate model of i.v. heroin self-administration in a single monkey. More recently, other studies have examined anti-addiction vaccines in non-human primates for nicotine (Desai and Bergman, 2015; McCluskie et al., 2013, 2015) and the dAd5GNE vaccine for cocaine (Hicks et al., 2014; Maoz et al., 2013), but none examined drug self-administration. Using PET imaging, Maoz et al. (2013) showed that administration of the dAd5GNE vaccine to rhesus monkeys resulted in high anti-cocaine titers; in that study titers above 4 × 105 substantially reduced cocaine occupancy of the dopamine transporter in the brain by sequestering cocaine in the peripheral blood. In a subsequent study in rhesus monkeys, not only did dAd5GNE vaccination successfully prevent i.v. cocaine and its metabolites from entering the CNS without delaying peripheral metabolism, there was no evidence of cocaine-related toxicity to major peripheral organs (Hicks et al., 2014). The goal of the present study was to determine whether the dAd5GNE vaccine would alter the reinforcing effects of i.v. cocaine self-administration in rhesus monkeys. A concurrent choice procedure between i.v. cocaine and a non-drug alternative reinforcer (candy) was employed since choice procedures have proven advantageous for evaluating potential pharmacotherapies for drug abuse in both preclinical and clinical research (Banks and Negus, 2012; Haney and Spealman, 2008).
2. Material and methods
2.1 Animals
Six experimentally naïve female monkeys (Macaca mulatta) participated in this 28-month study; at the beginning of the study they were 3–4 years old and weighed 4.0–5.4 kg. Monkeys received a daily ration of approximately 7–9 chow (105–135 grams; High protein monkey diet #5047, 3.37 Kcal/g; LabDiets®, PMI Feeds, Inc., St. Louis, MO), a piece of fruit, a chewable vitamin, and occasional treats to maintain relatively stable body weight increases for young adult monkeys. Monkeys were weighed monthly and at the end of the study they weighed 5.0–8.3 kg. Each monkey lived in two non-human primate cages (61.5 cm wide × 66.5 cm deep × 88 cm high each; Hazleton Systems, Inc., Aberdeen, MD) connected by 40 cm × 40 cm openings maintained in the AAALAC-approved animal care facility of The New York State Psychiatric Institute. Water was freely available from spouts located on the back panels of both cages. Cages were positioned to allow all monkeys to have visual, auditory and olfactory social contact with other monkeys, the animal caretakers and other staff. Other forms of environmental enrichment included music, cartoon videos, television, a puzzle box, a mirror, various tactile and chew toys (rotated regularly), and the operant procedures during experimental sessions. The onset and duration of menstruation was recorded for female monkeys each day on a calendar for the duration of the study so we could determine any changes in menstrual cycle length or absence of normal cycling as a function of study condition. The room lights were illuminated from 0700 to 1900. All aspects of animal maintenance and experimental procedures complied with the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals and the ARRIVE guidelines, and were approved by the New York State Psychiatric Institute Animal Care and Use Committee.
2.2 Training and apparatus
Once monkeys were acclimated to the environment and staff, monkeys were gradually trained to sit in customized wheel-mounted primate chairs (Primate Products Inc., Redwood City, CA). The primate chair was then wheeled to a workstation where a response panel was mounted on the wall, facing the monkey; this is where all experimental sessions (described below) were conducted.
Each response panel had six session lights (CM 1820, 24 v, Chicago Miniature, Buffalo Grove, IL) evenly spaced around the outside edges of the panel and 2 Lindsley levers (BRS-LVE, Beltsville, MD) mounted at the bottom of the panel, one for drug and one for candy, with a pair of stimulus lights above each lever. For candy delivery, a pellet-dispenser (BRS-LVE model PDC-005, Beltsville, MD) was mounted on the outside of the panel. The sound of the pellet-dispenser and a steady red light above the candy lever were paired with the delivery of candy. For drug delivery, a drug pump and a saline pump (Multi-Phaser, Model: NE-1000, New Era Pump Systems, Inc.) were placed on an extension on the back of each chair. The sound of the infusion pumps and a pair of flashing green lights (20 sec; 1 sec on/1 sec off) located at monkey eye level above the lever were paired with the delivery of cocaine. For cocaine delivery, a surgically-implanted subcutaneous venous access port (VAP) was accessed using a sterile Huber needle connected to a custom-designed 3-piece sterile infusion set (Access Technologies, Skokie, IL) and the extension tubing was attached to the drug pump and the saline pump. Schedule contingencies for all sessions were controlled by customized software (Eureka Software, Cary, NC) running on two Macintosh 610 computers (Cupertino, CA) located in an adjacent area.
2.3 Design and procedures
2.3.1 Design
Initially, monkeys were trained to respond for plain chocolate M&Ms® (Mars, Hackettstown, NJ; 4.5 kcal per piece: 0.6-g carbohydrate, 0.2-g fat, 0.1-g protein) while seated in the primate chair. Then surgery was performed to insert a venous access port (VAP) and monkeys were trained to self-administer i.v. cocaine (0.1 mg/kg/injection). During this time, responding on the candy lever had no consequences. During initial training for cocaine alone, the acquisition criteria for cocaine self-administration was defined as self-administering at least 50% of the cocaine doses available (> 5 out of maximum of 10) with stable responding for 5 consecutive sessions (defined as the mean number of doses self-administered +/− 1 dose). All monkeys self-administered between 5–10 cocaine doses each session (mean of 7.3 across all monkeys). When these criteria were met, a choice procedure between candy and cocaine was introduced.
2.3.2 Cocaine self-administration phase using a choice procedure
Choice sessions were typically conducted 4–5 days per week (M-F), with monkeys placed into the primate chairs, moved to the workstation, and connected to the infusion apparatus. Typically, three monkeys had sessions simultaneously, with adjacent workstations where they had visual and auditory, but not tactile contact, with each other. The beginning of each session was signaled by the illumination of the session lights and the first response on either lever terminated the schedule opportunity on the alternate lever. Monkeys responded on one lever for cocaine (0.1 mg/kg/injection) and on the other lever for candy (10 M&Ms®): the final schedule was a fixed ratio (FR) 25 for cocaine and FR5 for candy for 5 monkeys but for one monkey (C1) that responded much less than the other monkeys the final schedule was FR5 for cocaine and FR1 for candy. Candy was used because we have previously shown that M&Ms function as a preferred non-drug reinforcer in rhesus monkeys (Foltin and Evans, 2001, 2002; Foltin et al., 2015). Cocaine reinforcement consisted of a 0.4 ml infusion of cocaine followed by 1.25 ml infusion of saline, over a 20 sec period. Completion of a single FR resulted in the delivery of cocaine, or candy, and the presentation of the stimuli paired with reinforcement. After each reinforcer delivery, the light over the lever extinguished, followed by 3 min inter-trial interval. If a monkey did not pull the lever, then no drug or candy was delivered. There were a total of 10 independent choice trials per session, with sessions lasting between 1.5–2 hours. The primary dependent measures were the number of cocaine doses self-administered and the number of candy deliveries each session. Because we wanted to be able to see if the vaccine would decrease cocaine self-administration and/or shift choice from cocaine to candy, the criteria for stable choice behavior before initial vaccine administration was defined as reliably choosing cocaine over candy for two consecutive weeks (i.e., 10 consecutive sessions). After vaccine administration, self-administration choice sessions between cocaine and candy occurred for approximately 20 weeks. At the end of each session, the catheter and port were flushed and a solution of heparin and gentamycin (in a 9:1 ratio) with sterile saline was inserted into the port to maintain patency prior to returning monkeys to their home cages.
2.3.3 Extinction and reacquisition phases
During both the extinction and reacquistion phases, monkeys continued to have 10 discrete choice trials each session (refer to Figure 1 for timeline). Approximately, 20 weeks post-immunization under the choice procedure, the extinction phase began; saline was substituted for cocaine, but in all other respects the sessions were unchanged, including the stimuli associated with each reinforcer delivery; i.e., stimulus lights continued to flash and the pump continued to make sound as it delivered saline. Extinction was conducted for a minimum of 25 sessions and continued until each monkey extinguished responding for saline and self-administered 2 or fewer saline doses for 3 consecutive sessions, representing < 20% of the doses self-administered when cocaine was available. Once these criteria were met, the reacquisition phase began; cocaine (0.1 mg/kg/injection) again available for a minimum of 20 sessions and continued until each monkey returned to cocaine self-administration levels similar to week 20 post-immunization (+/− 1 dose) for 3 consecutive sessions. For monkeys that failed to resume responding within 40 sessions, a series of manipulations were employed, including locking the candy lever so that the only reinforcer available was cocaine, reducing the FR requirement for both cocaine and candy, and increasing the dose of cocaine available to 0.2 mg/kg/injection.
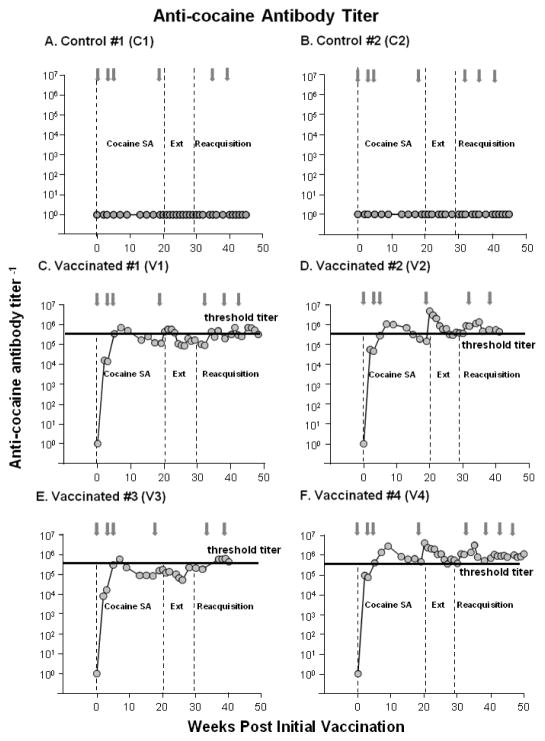
Figure 1. dAd5GNE Evoked Anti-cocaine Antibodies.
Serum anti-cocaine antibody titers as a function of weeks since the initial injection of vehicle in the 2 control monkeys (Panels A and B) or dAd5GNE in the 4 vaccinated monkeys (Panels C–F). The vaccine or vehicle was administered i.m. at week 0, 3, 5, 19, 34, 38 and monthly thereafter as indicated by the gray arrows. The dashed vertical lines indicate when the cocaine self-administration (cocaine SA; weeks 1 to 20), extinction (Ext; weeks 21–29) and reacquisition (weeks 30-end) phases were conducted post-vaccination. The horizontal black line in Panels C–F indicates the threshold of anti-cocaine antibody titers (> 4 × 105) previously shown to reduce cocaine occupancy to <20% in monkeys (see Maoz et al., 2013).
2.4 Surgical procedures
For long-term i.v. drug administration, monkeys were surgically implanted with a chronic indwelling catheter (Access Technologies, Skokie, IL) that terminated in a subcutaneous VAP (Wojnicki et al., 1994). The VAP allowed monkeys to have complete mobility in their cages and to easily leave their cages for self-administration sessions since no harness and tether was required. VAP surgeries were performed under aseptic conditions with the monkey under general anesthesia (see Cooper et al., 2013 for details on the surgical procedures). This implant allowed access to the monkey’s blood stream for at least 6 months (Wojnicki et al., 1994), and up to 2 years (Cooper et al., 2013). Monkeys had a 2-week recovery period before resuming experimental procedures. Due to the length of this study, in the event that a VAP became blocked or failed, experimental sessions were suspended and the animal would undergo surgery to repair the VAP or remove the VAP and insert another VAP in another vein.
2.5 Cocaine
Cocaine hydrochloride was obtained from Mallinckrodt, Inc. (Hazelwood, MO), and was dissolved in sterile saline for injection (U.S.P. grade). Separate stock solutions were prepared for each monkey to insure a similar injection volume across monkeys and the cocaine solution for each monkey was filtered using a 0.22 micron filter (Millipore, Billerica, MA).
2.6 Titer and biodistribution of the dAd5GNE vaccine
2.6.1 dAd5GNE vaccine
Recombinant E1a−, partial E1b−, and the E3− serotype 5 Ad vector with β-galactosidase in the expression cassette (‘Ad5’) was expanded and purified (Rosenfeld et al., 1992). The Ad5 vector was disrupted in 0.5% sodium dodecyl sulfate (56°C, 45 s). The GNE hapten was NHS-activated for coupling to the Ad proteins through the addition of (1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride in 90% dimethylformamide), 4°C overnight. The conjugation of disrupted Ad vector (“dAd5”) to the NHS activated GNE (300:1 GNE to Ad capsomere molar ratio) was carried out by incubating overnight at 4°C in phosphate-buffered saline (PBS, pH 7.4). The amount of Ad vector proteins was quantified using the bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL). The vaccine was then mixed 5:1 with Adjuplex™ adjuvant (Advance BioAdjuvants, Omaha, NE). This adjuvant was previously used in rat studies for this vaccine (Wee et al., 2012), with no observed toxic, allergic, or other adverse reactions. The adenovirus-based vaccine has been shown to be non-infectious. The vaccine was tested for potency, sterility, mycoplasma and endotoxin as a lot release criteria.
2.6.2 Immunization with dAd5GNE
The study design was to have 4 vaccinated monkeys and 2 control monkeys. After each monkey had at least 2 weeks of stable responding under the discrete trial choice procedure, investigators at the Cornell site who had no interaction with the monkeys assigned 4 monkeys to the active vaccine group and 2 monkeys to the vehicle group. The vaccine regimen began with 0.5 ml i.m. (intramuscular) injections of 100 μg of formulated dAd5GNE or the vehicle (phosphate buffered saline) at week 0, 3, 5, 19, 34, 38 and monthly thereafter until the end of the study to enable the evaluation in the context of assured high titer levels. Animals were monitored for the first three days after each injection for infection, swelling or hematoma at the site of injection and observed for any signs of distress or allergic reaction.
2.6.3 Measurement of anti-cocaine antibody titers
To determine antibody titer levels, 2 ml of whole blood were collected (usually from the VAP, or alternatively from a leg vein) at baseline, every 2 weeks until week 20 post-vaccination, and then weekly until the end of the study. The samples were allowed to clot for 5 min and then centrifuged at 10,000 g for 20 min. The isolated serum was stored at −20°C until analysis.
Wells of flat-bottomed 96-well EIA/RIA plates (Corning, New York, NY) were coated with 100 μl of 1 mg/ml cocaine hapten GNC (Carrera et al., 1995) conjugated to BSA (1:2 ratio) in carbonate buffer overnight at 4°C using the conjugation method described above. The plates were washed with 0.05% Tween 20 in PBS (PBS-Tween) and blocked with 5% dry milk in PBS for 30 min at 23°C. Two-fold serial dilutions of serum were added to each well and incubated for 90 min at 23°C. The plates were washed four times with PBS-Tween. Total IgG was detected with 100 μl of 1:2000 diluted horseradish peroxidase-conjugated goat anti-monkey IgG (Santa Cruz Biotechnology, Santa Cruz, CA) in 1% dry milk in PBS incubated for 90 min at 23°C. Peroxidase substrate (100 μl per well; Bio-Rad, Hercules, CA) was added and incubated for 15 μl per well). Absorbance was measured at 415 nm. Anti-cocaine antibody titers were calculated by interpolation of the log (OD) −log (dilution), with a cutoff value equal to twice the absorbance of background.
2.7 Data analyses
No formal statistical analyses were conducted due to the small sample that consisted of two control monkeys administered the vehicle dAd5GNE vaccine and four monkeys administered the active dAd5GNE vaccine. Instead, all data are presented for individual animals, with some descriptive statistics presented.
3. Results
3.1 dAd5GNE-evoked anti-cocaine antibody titers
dAd5GNE vaccine produced rapid increases in anti-cocaine antibody titers in the 4 vaccinated monkeys (Figure 1) with high titer levels remaining relatively stable following the 3rd administration, and all vaccinated monkeys maintaining anti-cocaine antibody titer levels of near 105 or greater throughout the study (40–50 weeks post-vaccination). Two of the vaccinated monkeys (V1 and V3) showed elevated levels of antibody throughout the study while the other two (V2 and V4) showed exceptionally high titers of anti-cocaine antibody that were maintained throughout the study. No anti-cocaine antibody titers were detected in the two control monkeys (C1 and C2) that were administered vehicle.
3.2 Menstrual cycle
During the cocaine self-administration period before vaccine administration, monkeys cycled on approximately 80% of the months (75% for control monkeys and 82% for vaccinated monkeys). After vaccine or vehicle administration, monkeys cycled on approximately 83% of the months (71% for control monkeys and 89% for vaccinated monkeys).
3.3 Cocaine self-administration phase
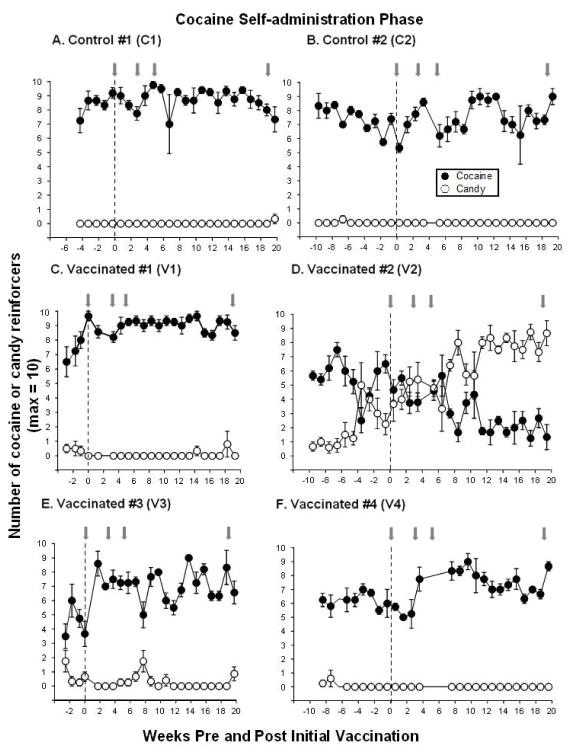
Prior to vaccine administration, each monkey demonstrated a preference for cocaine over candy (Figure 2, left side of dashed line). At baseline (the 3 weeks immediately before vaccination), monkeys self-administered an average of 5.2 to 8.7 cocaine doses (maximum of 10 trials), whereas candy was rarely chosen in 5 of the 6 monkeys. One monkey, V2 (Figure 2D), chose candy an average of 3.0±0.9 trials during the 3 weeks just prior to vaccination.
Figure 2. Cocaine Self-administration Before and After dAd5GNE Vaccination.
Mean number of cocaine (●) and candy (○) reinforcers earned each session (maximum = 10) before and for 20 weeks post-vaccination during the cocaine self-administration phase in the 2 control monkeys (Panels A and B) and 4 vaccinated monkeys (Panels C–F). Gray arrows indicate when additional boosts of dAd5GNE or vehicle were administered. The x-axis corresponds to weeks before and after the first administration of dAd5GNE vaccine (time = 0). Data before the vertical dashed line represents cocaine self-administration pre-vaccination (baseline represents the 3 weeks immediately before vaccination) and data after the vertical dashed line represents cocaine self-administration post-vaccination. Typically there were 4–5 sessions per week; data points represent the weekly mean (± S.E.M.).
After initial vaccination (week 0), monkeys continued to have the opportunity to choose between cocaine and candy for 20 weeks post-vaccination (Figure 2 post-vaccination phase indicated on right side of dashed lines). The two control monkeys showed no change (C1) or a slight increase (C2) in the number of cocaine doses self-administered relative to pre-vaccination, with no preference for candy (Figure 2A–B). Although there were elevated anti-cocaine antibody levels in all vaccinated monkeys, 3 of the 4 monkeys tended to show an increase in the number of cocaine doses self-administered and no preference for the candy alternative for 20 weeks post-vaccination. In contrast, one vaccinated monkey (V2), who had moderate selection of candy before vaccination and high titer levels (Figure 1D), switched preference away from cocaine after vaccination (Figure 2D) and by week 7 maintained a clear shift in preference from cocaine to candy for the remainder of the cocaine self-administration phase.
3.4 Saline extinction phase
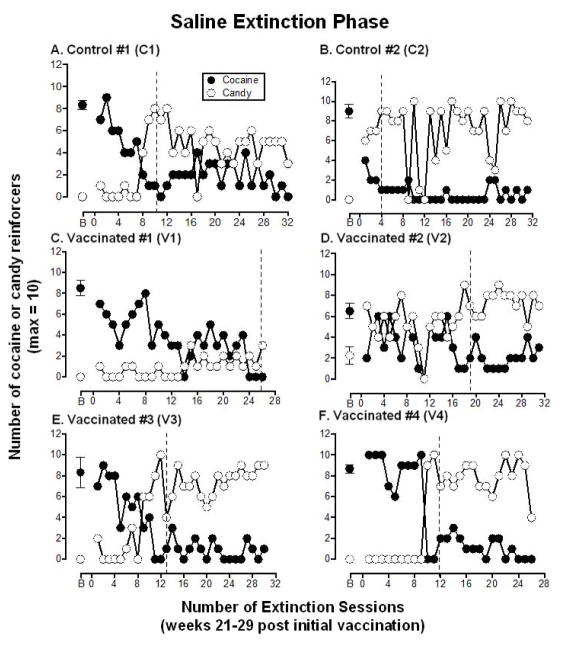
The extinction phase, when saline replaced cocaine, began in week 21 post-vaccination and continued for a minimum of 26 sessions to create a period of cocaine abstinence. Figure 3 shows the number of candy reinforcers and the number of reinforcers previously associated with cocaine that were chosen each extinction session for individual monkeys. Table 1 shows that the two control monkeys met the extinction criteria (responding for less than 2 “cocaine choices” for 3 consecutive sessions) within 4–10 sessions (mean 7.0 ± 4.2), whereas all 4 of the vaccinated monkeys took longer to meet the extinction criteria, ranging from 12–26 sessions (mean 17.5 ± 3.7). Five monkeys showed a corresponding shift in preference from the lever previously associated with cocaine to the lever associated with candy and one vaccinated monkey (V1) decreased responding on both levers.
Figure 3. Extinction of Cocaine Self-administration.
Number of “cocaine” (●) and candy (○) reinforcers earned each session (maximum = 10) during the extinction phase when saline was substituted for cocaine. These data represent week 21 to 29 post-vaccination. Additional boosts of dAd5GNE or vehicle were administered at week 19 and 34 so are not depicted in the Figure. Panels A and B show the 2 control monkeys, panels C–F show the 4 vaccinated monkeys. B = baseline and refers to the number of cocaine and candy reinforcers earned during week 20 of the cocaine self-administration phase (see Figure 2; week −1 was used for monkey V2, the one monkey who shifted preference from cocaine to candy). The dashed line indicates when extinction criteria were met for each monkey (defined as responding for ≤2 “cocaine choices” for 3 consecutive sessions). Typically there were 4–5 sessions per week; each data point (other than B) represents a single session.
Table 1.
Rate of Saline Extinction and Reacquisition of Cocaine Self-administration
| Monkey | # Days to Extinction | # Days Candy Lever Locked | # Days Cocaine FR Decreased | # Days FR Decreased & Cocaine Dose Increased | Total # Days to Reacquire Cocaine SA |
|---|---|---|---|---|---|
|
| |||||
| C1 | 10 | 0 | 0 | 0 | 6 |
| C2 | 4 | 1 | 0 | 0 | 41 |
|
| |||||
| Mean (se) | 7.0 (4.24) | 0.5 (0.71) | 0.0 (0.0) | 0.0 (0.0) | 23.5 (24.75) |
|
| |||||
| V1 | 26 | 0 | 0 | 0 | 19 |
| V2 | 19 | 5 | 19 | NA | > 61 |
| V3 | 13 | 6 | 7 | 12 | 57 |
| V4 | 12 | 15 | 23 | 26 | 94 |
|
| |||||
| Mean (se) | 17.50 (3.73) | 6.50 (3.61) | 12.25 (6.14) | 12.67 (9.20) | 57.75 (17.72) |
FR= fixed ratio; SA = self-administration; se = standard error
3.5 Cocaine reacquisition phase
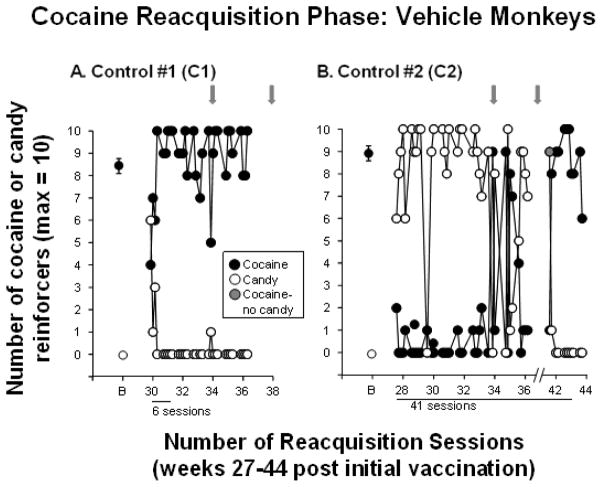
After the saline extinction phase, cocaine was again made available on the cocaine lever to examine cocaine reacquisition. Figure 4 shows that reacquisition of cocaine self-administration was rapid (6 sessions) in control monkey C1, but took substantially longer in control monkey C2. After 36 sessions the VAP for that monkey became blocked (sessions were suspended for 5 weeks), but once a new VAP was inserted stable preference for cocaine occurred within 5 days, for a total of 41 sessions to reacquire cocaine self-administration.
Figure 4. Reacquisition of Cocaine Self-administration in Control Monkeys.
Number of number of cocaine (●) and candy (○) reinforcers earned each session (maximum = 10) during the reacquisition phase when cocaine was available again in the 2 control monkeys C1 and C2. B = baseline and refers to the number of cocaine and candy reinforcers earned during week 20 of the cocaine self-administration phase (see Figure 2 A and B). Gray arrows indicate when dAd5GNE or vehicle was administered. The x-axis corresponds to weeks post-vaccination and indicates the number of sessions needed to reacquire cocaine self-administration for each monkey. For monkey C2, the VAP became blocked in week 35 and sessions were suspended (indicated by the break on the x-axis). Sessions resumed in week 41; the candy lever was locked for a single session (indicated by the gray circle) and control monkey C2 immediately returned to cocaine self-administration. Typically there were 4–5 sessions per week; each data point (other than B) represents a single session.
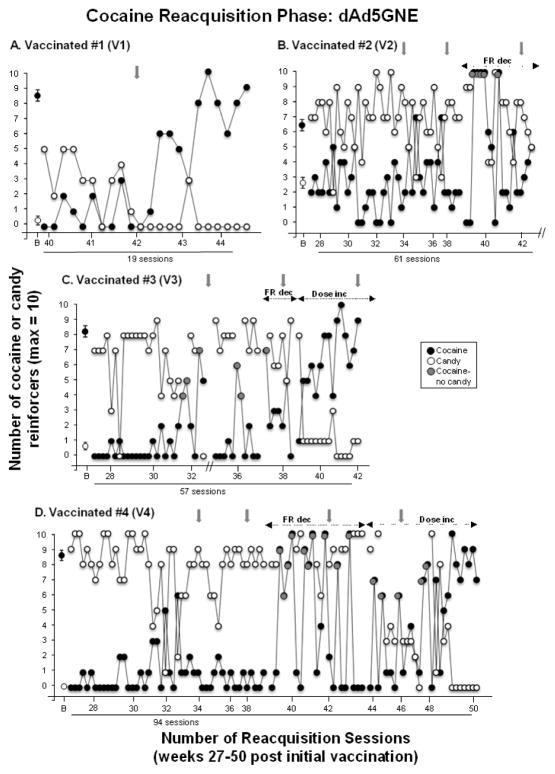
Figure 5 shows reacquisition of cocaine self-administration in the 4 vaccinated monkeys. Only one vaccinated monkey reacquired cocaine self-administration similar to control monkeys; monkey V1 reacquired responding for cocaine within 19 sessions (Figure 5A). The other 3 vaccinated monkeys did not reacquire cocaine self-administration within the first 40 sessions that cocaine was available again. For those 3 vaccinated monkeys (V2, V3 and V4; Figure 5B–D), the response requirement was subsequently decreased by 80% (FR5 cocaine; FR1 candy) and intermittently on daily sessions the candy alternative was removed, leaving monkeys with only the option to self-administer cocaine (indicated by gray symbols). Initially, these two manipulations did not increase cocaine self-administration on sessions when both options were available despite the fact that these 3 vaccinated monkeys self-administered at or near the maximal number of cocaine doses when only cocaine was available (i.e., the candy lever was locked), but on sessions when both candy and cocaine were available the vaccinated monkeys switched their preference back to candy (Figure 5B–D). Unfortunately, reacquisition of cocaine self-administration could not be determined after 61 sessions in vaccinated monkey V2 due to a failed VAP near study completion that precluded further testing.
Figure 5. Reacquisition of Cocaine Self-administration in Vaccinated Monkeys.
Number of cocaine (●) and candy (○) reinforcers earned each session (maximum = 10) during the reacquisition phase when cocaine was available again in the 4 vaccinated monkeys (V1–V4). B = baseline and refers to the number of cocaine and candy reinforcers earned during week 20 of the cocaine self-administration phase (see Figure 2 C–F; week −1 was used for V2, the one monkey who shifted preference from cocaine to candy). Gray arrows indicate when dAd5GNE or vehicle was administered. The x-axis corresponds to weeks post-vaccination and indicates the number of sessions to reacquire cocaine self-administration for each monkey. Gray circles indicate when the candy lever was locked and only cocaine was available (V2, V3 and V4). The first set of dashed arrows indicate when the response cost was reduced (FR dec) to FR5 for cocaine and FR1 for candy (V2, V3 and V4) and the second set of dashed arrows indicate when the cocaine dose available for self-administration was doubled (Dose inc) to 0.2 mg/kg/injection under the lower response requirements (V3 and V4). Vaccinated monkey V2 did not reliably reacquire cocaine self-administration after 61 sessions despite reducing the response requirement and occasionally locking the candy lever. Further testing could not be conducted due to a failed VAP near study completion (indicated by the break on the x-axis). For vaccinated monkey V3, experimental testing was interrupted due to blockage of the VAP (indicated by the break on the x-axis). Typically there were 4–5 sessions per week; each data point (other than B) represents a single session.
Lastly, for the two remaining vaccinated monkeys (V3 and V4) that failed to reacquire cocaine self-administration, the dose of cocaine available was doubled (0.2 mg/kg/injection) while under the reduced FR response requirement. Under these conditions, both monkeys reacquired cocaine self-administration after another 12–26 sessions such that reacquisition of cocaine self-administration was achieved after a total of 57 sessions for monkey V3 and 94 sessions for monkey V4. In summary, Table 1 shows that reacquisition of cocaine self-administration was achieved within 6 to 41 sessions in the 2 control monkeys, but within 19 to 94 sessions (mean = 57.8) for the 4 vaccinated monkeys. Further, despite the small sample, the number of sessions to reacquire cocaine self-administration (independent of FR schedule, removal of the candy alternative, and increased cocaine dose), increased as anti-cocaine antibody titers increased, i.e., higher titers were associated with longer time to reacquire cocaine self-administration (pearson coefficient, r=0.88).
4. Discussion
The dAd5GNE vaccine resulted in rapid (within one week) elevations in antibody titer levels in all 4 vaccinated monkeys that, with additional vaccine boosters, were maintained at high levels throughout the study. Despite these elevated titer levels, vaccine administration was only successful at attenuating on-going cocaine self-administration in one of the 4 vaccinated monkeys. With respect to saline extinction, all monkeys extinguished responding on the lever associated with cocaine; of note, in this small sample it took longer for the vaccinated monkeys to meet extinction criteria compared to the control monkeys (mean of 17.5 sessions vs. 7.0 sessions). Further, once monkeys extinguished responding for cocaine, vaccination substantially retarded reacquisition of cocaine self-administration in 3 of the 4 vaccinated monkeys and reacquisition in these monkeys was only achieved after several manipulations were implemented to encourage cocaine self-administration. Lastly, the vaccine was well tolerated, with none of the monkeys exhibiting any injection site reactions or other observable changes (e.g., body weight, food intake, temperament). Further, there was no evidence that cocaine self-administration, vaccine administration, or the combination adversely impacted menstrual cycle status. This study in non-human primates confirms and extends the previous study that examined efficacy of the dAd5GNE vaccine in reducing the reinforcing effects of cocaine in rats (Wee et al., 2012). Moreover, the present study is historically noteworthy by virtue of the fact that it is the first study in over 40 years to evaluate an anti-drug abuse vaccine on drug self-administration in non-human primates. The only previous anti-abuse vaccine study in non-human primates evaluated the effects of immunotherapy on heroin self-administration using a single rhesus monkey (Bonese et al., 1974). Only one other recent study (Desai and Bergman, 2015) examined the behavioral effects of an anti-drug vaccine in non-human primates and found that a nicotine vaccine altered the discriminative stimulus effects of nicotine in squirrel monkeys.
To enhance the clinical feasibility of immunopharmacotherapy for humans, candidate vaccines need to have a rapid onset coupled with the ability to maintain elevated titer levels in the majority of individuals with infrequent booster vaccinations. The pre-clinical evidence to date suggest that the dAd5GNE vaccine has a clear advantage over the TA-CD vaccine that in humans took as long as 13 weeks (after 4–5 vaccinations) for less than 50% of individuals to achieve sufficient antibody titer levels (Haney et al., 2010; Martell et al., 2009). In addition to elevated antibody titers, adequate antibody affinity is needed as demonstrated by a reduction in brain levels and a corresponding increase in plasma levels of the abused drug (e.g., McCluskie et al., 2013, 2015) and this has been previously demonstrated with the dAd5GNE vaccine (Hicks et al., 2011, 2014; Maoz et al., 2013). While identifying the vaccination regimen required to maintain high anti-cocaine titers and measuring antibody affinity were not the goals of the current study, it will be important to determine whether the dAd5GNE vaccine continues to induce a strong and rapid immune response when administered to humans and if the presence of high titers will be correlated with antibody affinity and a behavioral measure of efficacy, such as relapse, in humans.
The fact that dAd5GNE vaccine only reduced on-going cocaine self-administration in one vaccinated monkey (25%) is somewhat disappointing, but not entirely surprising. Similar to numerous preclinical studies in laboratory animals, human laboratory studies and clinical trials conducted in human cocaine abusers, with the exception of agonist treatments (see review by Czoty et al., 2016) whose use remains controversial (Negus and Henningfield, 2015), it is challenging to modify or disrupt ongoing cocaine use (Foltin et al., 2015; Haney and Spealman, 2008; Shorter and Kosten, 2011). However, individuals with lower levels of cocaine use at the beginning of treatment are more likely to use less cocaine during a pharmacotherapy trial (Bisaga et al., 2005). In the present study, the monkey (V2) that showed the lowest preference for cocaine pre-vaccination was the only monkey that decreased cocaine self-administration post-vaccination. Even in the previous study with the dAd5GNE vaccine in rats (Wee et al., 2012), although vaccination occurred before animals had any experience with cocaine self-administration, only a modest downward shift in the cocaine dose-response function was observed.
In the previous study with the dAd5GNE vaccine in rats (Wee et al., 2012), the rate of extinction was relatively similar between the control rats and the vaccinated rats, although control rats exhibited an extinction burst on the first day (Wee et al., 2012). In contrast, in the present study there was tendency for some vaccinated monkeys to take longer to extinguish responding on the lever previously associated with cocaine compared to control monkeys. One possible explanation for this is that the vaccine may have resulted in perseverative responding. However, this is unlikely given that vaccinated monkeys took longer to reacquire cocaine self-administration. If in fact the vaccine resulted in a position bias or perseverative responding then there should have been slower rates of extinction but faster rates of reacquisition. Without a larger sample size, caution about further speculations for these modest differences in extinction rates is warranted.
The dAd5GNE vaccine was most effective at preventing relapse to cocaine self-administration (i.e., reacquisition) once monkeys had extinguished responding for cocaine, similar to achieving abstinence in humans. However, when the candy alternative was unavailable during reacquisition, monkeys would reliably self-administer cocaine indicating the importance of having an alternative reinforcer available when testing potential treatments. Moreover, in the present study the alternative to a single dose of cocaine was 10 candies and the response cost for candy was 1/5 of the response cost for cocaine because previous research has shown that shifts in responding using choice procedures are best observed when the value of the alternative is high and the response cost low (see Foltin et al., 2015).
While the results of this study are encouraging, they need to be interpreted cautiously due to several limitations. The primary limitation is related to the sample size (2 control monkeys and 4 vaccinated monkeys) and we were unable to proceed with reacquisition testing in one vaccinated monkey due to venous port failure near the end of the study. In addition, once we failed to show a decrease in cocaine self-administration in 3 of the vaccinated monkeys, we then carried out saline extinction and reacquisition testing. Another limitation is that plasma levels of cocaine and metabolites were not measured in the present study. However, a previous study (Hicks et al., 2014) demonstrated that monkeys vaccinated with the dAd5GNE vaccine had substantially higher plasma levels of cocaine and metabolites relative to monkeys vaccinated with the vehicle, without delaying the metabolism of cocaine. Unfortunately that study did not measure cocaine self-administration, thus there is currently a lack of valuable information on the relationships between antibody affinity and cocaine pharmacokinetics to the behavioral effects of cocaine, particularly the rate of extinction or reacquisition. Further, there were a number of clinically relevant manipulations that were not evaluated in this study. For instance, we did not systematically test a range of cocaine doses. It is possible that lower cocaine doses might have been more susceptible to perturbation by the vaccine; several laboratory studies testing pharmacological interventions in humans have demonstrated reductions in cocaine self-administration when intermediate doses of cocaine were available, whereas higher doses of cocaine were relatively unaffected (e.g., Haney et al., 2006; Rush et al., 2010). We also did not systematically test higher doses of cocaine to see if monkeys would attempt to override the effects of the vaccine which is a valid clinical concern (Haney and Kosten, 2004; Shen et al., 2011), although reacquisition in the two most resistant vaccinated monkeys occurred only when the cocaine dose was doubled.
5. Conclusion
In summary, the dAd5GNE vaccine only attenuated ongoing cocaine self-administration in 25% of the monkeys, despite sustained elevated titer levels. However, after saline extinction, i.e., a period of abstinence, at least 2 of the vaccinated monkeys (50%) resisted reacquisition of cocaine self-administration relative to control monkeys even after several manipulations were conducted to encourage reacquisition. Despite the small sample size, these findings in rhesus monkeys extend the previous preclinical studies in rodents demonstrating that the dAd5GNE vaccine alters the behavioral effects of cocaine (Hicks et al., 2011; Wee et al., 2012). In addition, two other studies in rhesus monkeys indicate that the dAd5GNE vaccine prevents cocaine from entering the CNS (Hicks et al., 2014; Maoz et al., 2013), and appears to be relatively safe and devoid of toxicity to key peripheral organs (Hicks et al., 2014). Taken together, these data support the utility of using non-human primates for anti-drug abuse vaccine development. Lastly, at this time, the dAd5GNE vaccine is one of the most promising immunopharmacotherapies for treating cocaine use disorders. Given the challenge of reducing cocaine use in both humans and laboratory animals, vaccine development will be most effective within the context of a relapse prevention strategy that emphasizes incorporating alternative reinforcers.
Highlights.
Second study to evaluate immunotherapy on drug self-administration in monkeys
Vaccination attenuated ongoing cocaine self-administration in 25% of the monkeys
Vaccination retarded reacquisition of cocaine self-administration
The dAdGNE vaccine may have therapeutic potential for relapse prevention
Acknowledgments
This research was supported by grants RC2 DA028847 (SME, JBR, BPD, KDJ, SMK, RGC), T32 HL094284 (MJH), and K05 DA031749 (RWF). NIDA had no role in study design or in the decision to submit the paper for publication. NIDA also had no role in the collection, analysis and interpretation of data in the writing of the report. The authors have no financial disclosures or conflicts of interest to report. We acknowledge and appreciate the excellent technical support provided by Jean Willi and Angel Ramirez and Dr. Ziva Cooper. We also thank Dr. Amy Cassano, Dr. Moshe Shalev, Dr. Rodolfo Ricart and Girma Asfaw for the veterinary care, and Robert Scalese, Wendy Johnson, and Jon Ehrmann for their expertise in performing the VAP surgeries.
Footnotes
Disclosure/Conflict of Interest
None of the authors have any financial disclosures or conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Suzette M. Evans, Email: se18@cumc.columbia.edu.
Richard W. Foltin, Email: rwf2@cumc.columbia.edu.
Martin J. Hicks, Email: mhicks@monmouth.edu.
Jonathan B. Rosenberg, Email: jor2024@med.cornell.edu.
Bishnu P. De, Email: bpd2001@med.cornell.edu.
Kim D. Janda, Email: kdjanda@scripps.edu.
Stephen M. Kaminsky, Email: smkamins@med.cornell.edu.
Ronald G. Crystal, Email: geneticmedicine@med.cornell.edu.
References
- Akari H, Iwasaki Y, Yoshida T, Iijima S. Non-human primate surrogate model of hepatitis C virus infection. Microbiol Immunol. 2009;53:53–67. doi: 10.1111/j.1348-0421.2008.00087.x. http://dx.doi.org/10.1111/j.1348-0421.2008.00087.x. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Brimijoin S, Gao Y, Geng L, Zlebnik NE, Parks RJ, et al. Cocaine hydrolase encoded in viral vector blocks the reinstatement of cocaine seeking in rats for 6 months. Biol Psy. 2012;71:700–705. doi: 10.1016/j.biopsych.2011.11.014. http://dx.doi.org/10.1016/j.biopsych.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandez JA, et al. Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin. 2009;5:214–229. doi: 10.4161/hv.5.4.7556. http://dx.doi.org/10.4161/hv.5.4.7556. [DOI] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. http://dx.doi.org:10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, et al. Utility of lead-in period in cocaine dependence pharmacotherapy trials. Drug Alcohol Depend. 2005;77:7–11. doi: 10.1016/j.drugalcdep.2004.06.007. http://dx.doi.org/10.1016/j.drugalcdep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature. 1974;252:708–710. doi: 10.1038/252708a0. http://dx.doi:10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- Cai X, Whitfield T, Hixon MS, Grant Y, Koob GF, Janda KD. Probing active cocaine vaccination performance through catalytic and noncatalytic hapten design. J Med Chem. 2013a;56:3701–3709. doi: 10.1021/jm400228w. http://dx.doi:10.1021/jm400228w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Whitfield T, Moreno AY, Grant Y, Hixon MS, Koob GF, et al. Probing the effects of hapten stability on cocaine vaccine immunogenicity. Mol Pharm. 2013b;10:4176–4184. doi: 10.1021/mp400214w. http://dx.doi:10.1021/mp400214w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. http://dx.doi:10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci USA. 2001;98:1988–1992. doi: 10.1073/pnas.041610998. http://dx.doi:10.1073/pnas.98.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine vaccines: antibody protection against relapse in a rat model. Proc Natl Acad Sci USA. 2000;97:6202–6206. doi: 10.1073/pnas.97.11.6202. http://dx.doi:10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Foltin RW, Evans SM. Effects of menstrual cycle phase on cocaine self-administration in rhesus macaques. Horm Behav. 2013;63:105–113. doi: 10.1016/j.yhbeh.2012.10.008. http://dx.doi:10.1016/j.yhbeh.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. Evaluation of the “Pipeline: for development of medications for cocaine use disorder: A review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. http://dx.doi.org/10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Bergman J. Effects of the nanoparticle-based vaccine, SEL-068, on nicotine discrimination in squirrel monkeys. Neuropsychopharmacology. 2015;40:2207–2216. doi: 10.1038/npp.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Evans SM. The effects of D-amphetamine on responding for candy and fruit drink using a fixed ratio and a progressive ratio schedule of reinforcer delivery. Pharmacol Biochem Behav. 2001;69:125–131. doi: 10.1016/s0091-3057(01)00496-8. http://dx.doi:10.1016/S0091-3057(01)00496-8. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Evans SM. Effect of response-independent candy on responding maintained by candy using a novel model of commodity acquisition and consumption in non-human primates. Pharmacol Biochem & Behav. 2002;72:729–739. doi: 10.1016/s0091-3057(02)00746-3. http://dx.doi:10.1016/S0091-3057(02)00746-3. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Rubin E, Reed SC, Vadhan N, Balter R, et al. Development of translational preclinical models in substance abuse: Effects of cocaine administration on cocaine choice in humans and non-human primates. Pharmacol Biochem Behav. 2015;134:12–21. doi: 10.1016/j.pbb.2015.03.023. http://dx.doi:10.1016/j.pbb.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Brimijoin S. Lasting reduction of cocaine action in neostriatum—a hydrolase gene therapy approach. J Pharmacol Exp Ther. 2009;330:449–457. doi: 10.1124/jpet.109.152231. http://dx.doi:10.1124/jpet.109.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. http://dx.doi:10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Foltin RW. Effects of baclofen on cocaine self-administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology. 2006;31:1814–1821. doi: 10.1038/sj.npp.1300999. [DOI] [PubMed] [Google Scholar]
- Haney M, Kosten TR. Therapeutic vaccines for substance dependence. Expert Rev Vaccines. 2004;3:11–18. doi: 10.1586/14760584.3.1.11. http://dx.doi:10.1016/j.ddstr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. http://dx.doi:10.1016/j.ddstr.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89:392–399. doi: 10.1038/clpt.2010.317. http://dx.doi:10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatziioannou T, Evans DT. Animal models for HIV/AIDs research. Nat Rev Microbiol. 2012;10:852–867. doi: 10.1038/nrmicro2911. http://dx.doi:10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks MJ, De BP, Rosenberg JB, Davidson JT, Moreno AY, Janda KD, et al. Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol Ther. 2011;19:612–619. doi: 10.1038/mt.2010.280. http://dx.doi:10.1038/mt.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks MJ, Kaminsky SM, De BP, Rosenberg JB, Evans SM, Foltin RW, et al. Fate of systemically administered cocaine in nonhuman primates treated with the dAd5GNE anticocaine vaccine. Hum Gene Ther Clin Dev. 2014;25:40–49. doi: 10.1089/humc.2013.231. http://dx.doi:10.1089/humc.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL. Nonhuman primate neuroimaging and cocaine medication development. Exp Clin Psychopharmacol. 2008;16:446–457. doi: 10.1037/a0014196. http://dx.doi:10.1037/a0014196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda KD, Treweek JB. Vaccines targeting drugs of abuse: is the glass half-empty or half-full? Nat Rev Immunol. 2011;12:67–72. doi: 10.1038/nri3130. http://dx.doi:10.1038/nri3130. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Collins SL, Bond J, Fox BS. Time course of changes in cocaine self-administration behavior in rats during immunization with the cocaine vaccine IPC-1010. Psychopharmacology (Berl) 2001;153:334–340. doi: 10.1007/s002130000555. http://dx.doi:10.1007/s002130000555. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 2000;148:251–262. doi: 10.1007/s002130050049. http://dx.doi.org/10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, et al. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. http://dx.doi.org/10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- Kinsey B. Vaccines against drugs of abuse: where are we now? Ther Adv Vaccines. 2014;2:106–117. doi: 10.1177/2051013614537818. http://dx.doi:10.1177/2051013614537818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Hicks MJ, Wee S, Rosenberg JB, De BP, Kaminksy SM, et al. Anti-cocaine vaccine based on coupling a cocaine analog to a disrupted adenovirus. CNS Neurol Disord Drug Targets. 2011;10:899. doi: 10.2174/187152711799219334. http://dx.doi:10.2174/187152711799219334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Domingo CB, Shorter D, Orson F, Green C, Somoza E, et al. Vaccine for cocaine dependence: A randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42–47. doi: 10.1016/j.drugalcdep.2014.04.003. http://dx.doi.org:10.1016/j.drugalcdep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Rosen M, Bond J, Settles M, Roberts JSC, Shields J, et al. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20:1196–1204. doi: 10.1016/s0264-410x(01)00425-x. http://dx.doi.org/10.1016/S0264-410X(01)00425-X. [DOI] [PubMed] [Google Scholar]
- Maoz A, Hicks MJ, Vallabhjosula S, Synan M, Kothari PJ, Dyke JP, et al. Adenovirus capsid-based anti-cocaine vaccine prevents cocaine from binding to the nonhuman primate CNS dopamine transporter. Neuropsychopharmacology. 2013;38:2170–2178. doi: 10.1038/npp.2013.114. http://dx.doi:10.1038/npp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. http://dx.doi:10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. http://dx.doi:10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskie MJ, Pryde DC, Gervais DP, Stead DR, Zhang N, Benoit M, et al. Enhancing immunogenicity of a 3′ aminomethylnicotine-DT-conjugate anti-nicotine vaccine with CpG adjuvant in mice and non-human primates. Int Immunopharmacol. 2013;16:50–56. doi: 10.1016/j.intimp.2013.03.021. http://dx.doi:10.1016/j.intimp.2013.03.021. [DOI] [PubMed] [Google Scholar]
- McCluskie MJ, Thorn J, Gervais DP, Stead DR, Zhang N, Benoit M, et al. Anti-nicotine vaccines: Comparison of adjuvanted CRM 197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int Immunopharmacol. 2015;29:663–671. doi: 10.1016/j.intimp.2015.09.012. http://dx.doi:10.1016/j.intimp.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Mets B, Winger G, Cabrera C, Seo S, Jamdar S, Yang G, et al. A catalytic antibody against cocaine prevents cocaine’s reinforcing and toxic effects in rats. Proc Natl Acad Sci USA. 1998;95:10176–10181. doi: 10.1073/pnas.95.17.10176. http://dx.doi:10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. 2015;40:1815–1825. doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld MA, Yoshimura K, Trapnell BC, Yoneyama K, Rosenthal ER, Dalemans W, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. http://dx.doi:10.1016/0092-8674(92)90213-V. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Shen X, Orson FM, Kosten TR. Anti-addiction vaccines. F1000 Med Rep. 2011;3:20. doi: 10.3410/M3-20. http://dx.doi:10.3410/M3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter D, Kosten TR. Vaccines in the treatment of substance abuse. Focus (Am Psychiatr Publ) 2011;9:25–30. doi: 10.1176/foc.9.1.foc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 (HHS Publication No. SMA 15–4927, NSDUH Series H-50). Retrieved from http://www.samhsa.gov/data/
- Tonstad S, Heggen E, Giljam H, Lagerbäck PÅ, Tønnesen P, Wikingsson LD, et al. Niccine®, a nicotine vaccine, for relapse prevention: a phase II, randomized, placebo-controlled, multicenter clinical trial. Nicotine Tob Res. 2013;15:1492–1501. doi: 10.1093/ntr/ntt003. http://dx.doi:10.1093/ntr/ntt003. [DOI] [PubMed] [Google Scholar]
- Wee S, Hicks MJ, De BP, Rosenberg JB, Moreno AY, Kaminsky SM, et al. Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology. 2012;37:1083–1091. doi: 10.1038/npp.2011.200. http://dx.doi:10.1038/npp.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol. 2007;15:309–327. doi: 10.1037/1064-1297.15.4.309. http://dx.doi:10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Bacher JD, Glowa JR. Use of subcutaneous vascular access ports in rhesus monkeys. Lab Anim Sci. 1994;44:491–494. [PubMed] [Google Scholar]