Abstract
The elevated sympathetic outflow associated with hypertension is maintained by increased N-methyl-D-aspartate receptor (NMDAR) activity in the paraventricular nucleus (PVN) of the hypothalamus. Synaptic NMDAR activity is tightly regulated by protein kinases, including the Src family of tyrosine kinases. We determined whether Src kinases play a role in increased NMDAR activity of PVN neurons projecting to the rostral ventrolateral medulla (RVLM) and in elevated sympathetic vasomotor tone in spontaneously hypertensive rats (SHRs). The Src protein level in the PVN was significantly greater in SHRs than in normotensive Wistar-Kyoto (WKY) rats and was not significantly altered by lowering blood pressure with celiac ganglionectomy in SHRs. Inhibition of Src kinase activity with 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine (PP2) completely normalized the higher amplitudes of evoked NMDAR-mediated excitatory postsynaptic currents (EPSCs) and puff NMDA-elicited currents of RVLM-projecting PVN neurons in SHRs. PP2 treatment also attenuated the higher frequency of NMDAR-mediated miniature EPSCs of these neurons in SHRs. However, PP2 had no effect on NMDAR-EPSCs or miniature EPSCs of RVLM-projecting PVN neurons in WKY rats. NMDAR activity increased by a Src-activating peptide was blocked by PP2 but not by inhibition of casein kinase 2. In addition, microinjection of PP2 into the PVN not only decreased lumbar sympathetic nerve discharges and blood pressure but also eliminated the inhibitory effect of the NMDAR antagonist on sympathetic nerve activity and blood pressure in SHRs. Collectively, our findings suggest that increased Src kinase activity potentiates presynaptic and postsynaptic NMDAR activity in the PVN and sympathetic vasomotor tone in hypertension.
Keywords: Autonomic nervous system, hypertension, hypothalamus, NMDA receptor, sympathetic nerve discharges, synaptic plasticity
Introduction
Hypertension affects approximately one-third of adults in the United States and is a well-recognized risk factor for stroke, coronary artery disease, and renal failure. Although essential (primary) hypertension is the most prevalent form of hypertension, its root cause and underlying mechanisms remain poorly understood. Elevated sympathetic outflow is clearly associated with the development of essential hypertension in animal models1–3 and in hypertensive patients4–7. The paraventricular nucleus (PVN) of the hypothalamus is an important brain region controlling sympathetic outflow8–10. Presympathetic neurons in the PVN project to vasomotor neurons in the rostral ventrolateral medulla (RVLM) in the brainstem and sympathetic preganglionic neurons in the intermediolateral cell column in the spinal cord, which in turn regulate sympathetic nerve discharges11, 12. Electrolytic lesion or pharmacological inhibition of the PVN decreases arterial blood pressure (ABP) and sympathetic nerve activity in spontaneously hypertensive rats (SHRs)1, 2, 13, 14. Also, transplantation of embryonic hypothalamic tissue containing the PVN from SHRs to normotensive rats leads to hypertension in the normal rats15, 16. Although hyperactivity of PVN presympathetic neurons is a major source of elevated sympathetic outflow in SHRs1, 2, the molecular mechanisms responsible for the increased excitability of these neurons are not fully known.
Glutamate is the major excitatory neurotransmitter in the PVN, and increased N-methyl-D-aspartate receptor (NMDAR) activity is critically involved in the augmented sympathetic vasomotor tone in SHRs2, 17. The synaptic NMDAR activity of PVN presympathetic neurons is tightly regulated by phosphorylation via serine/threonine kinases and phosphatases, including casein kinase II (CK2) and calcineurin18–20. In addition, increasing the phosphorylation level of tyrosine residues in NMDARs can increase NMDAR activity21–24. The Src family of non-receptor protein tyrosine kinases has at least nine members, and Src is actively expressed in the adult hypothalamus25, 26. However, it remains unclear whether the Src kinases contribute to the increased NMDAR activity of PVN presympathetic neurons observed in SHRs.
In the present study, we determined the role of Src kinases in the increased synaptic glutamatergic input to RVLM-projecting PVN neurons in SHRs using in vivo retrograde tracing and in vitro brain slice recordings. We also studied whether Src-mediated NMDAR activity in the PVN is involved in maintaining elevated sympathetic output in SHRs. Our findings suggest that increased Src kinase activity in the PVN plays a pivotal role in the potentiation of pre- and postsynaptic NMDAR activity of PVN presympathetic neurons and in augmented sympathetic outflow in SHRs. Our study provides new insight into the molecular mechanism of the synaptic plasticity associated with sustained sympathetic outflow in hypertension.
Methods
Animal model
We used male normotensive Wistar-Kyoto (WKY) rats and SHRs (4- and 13-week-old, Harlan, Indianapolis, IN) in the present study. SHRs are the most commonly used and well-characterized animal model of essential hypertension27, 28. For the entire study, data were collected from 59 WKY rats and 77 SHRs. The experimental procedures were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals.
The detailed methods for retrograde labeling of PVN neurons, electrophysiological recordings in brain slices, celiac ganglionectomy (CGx), Western blotting, PVN microinjections and lumbar sympathetic nerve recording in vivo, and data analysis are described in Online Supplements.
Brain slice preparation and recordings
Coronal hypothalamic slices (300 µm thick) containing the PVN were obtained from FluoSphere-injected rats using a vibrating microtome. Whole-cell patch-clamp recordings were performed in labeled neurons in the PVN of the slices (Supplemental Fig. S1). Excitatory postsynaptic currents (EPSCs) were elicited by electrical stimulation through a bipolar tungsten electrode connected to a stimulator. Evoked α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-EPSCs were recorded at a holding potential of −60 mV in the presence of 10 µM bicuculline, and evoked NMDAR-EPSCs were recorded at a holding potential of +40 mV in the presence of 10 µM bicuculline and 20 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). Miniature EPSCs (mEPSCs) were recorded at a holding potential of −60 mV in the presence of 1 µM tetrodotoxin and 10 µM bicuculline. To record postsynaptic NMDAR currents, we puffed NMDA (100 µM) directly onto the recorded neuron at a holding potential of −60 mV. Because NMDARs are voltage-dependently blocked by Mg2+ at a negative holding potential and co-activated by glycine, puff NMDA-induced currents were recorded in Mg2+-free aCSF in the presence of 10 µM glycine and 1 µM tetrodotoxin.
Western immunoblotting
Hypothalamic slices were sectioned 1.08–2.12 mm caudal to the bregma, and PVN tissues were micro-punched bilaterally with a slice punch. The samples were subjected to 4–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and incubated with a mouse anti-Src antibody (1:1,000, catalog #2578064, Millipore, Bedford, MA) for 24 h. An ECL kit (ThermoFisher Scientific) was used to detect the Src protein band, which was visualized and quantified with the Odyssey Fc Imager (LI-COR Biosciences, Lincoln, NE) and normalized by the GAPDH protein band on the same blot.
PVN microinjection and recording of lumbar sympathetic nerve activity (LSNA) and ABP
Rats were anesthetized with a mixture of α-chloralose (60–75 mg/kg, ip) and urethane (800 mg/kg, ip). A small branch of the left lumbar postganglionic sympathetic nerve was isolated under an operating microscope through a retroperitoneal incision. The lumbar sympathetic nerve was cut distally to ensure that afferent activity was not recorded. The LSNA and ABP were recorded using a 1401-PLUS analog-to-digital converter and Spike2 system (Cambridge Electronic Design, Cambridge, UK). A glass microinjection pipette (tip diameter 20–30 µm) was advanced into the PVN. The location of the pipette tip and diffusion of the drugs in the PVN were determined by including 5% rhodamine-labeled fluorescent microspheres (0.04 µm; Molecular Probes) in the injection solution2, 29.
Data analysis
Data are presented as mean ± S.E.M. We used the Student t test or Mann-Whitney U test to determine the significant differences between the two groups. One-way ANOVA with Dunnett’s or Tukey’s post hoc test was used to determine the significant differences involving more than two groups. P < 0.05 was considered statistically significant.
Results
SHRs show higher Src protein level in the PVN
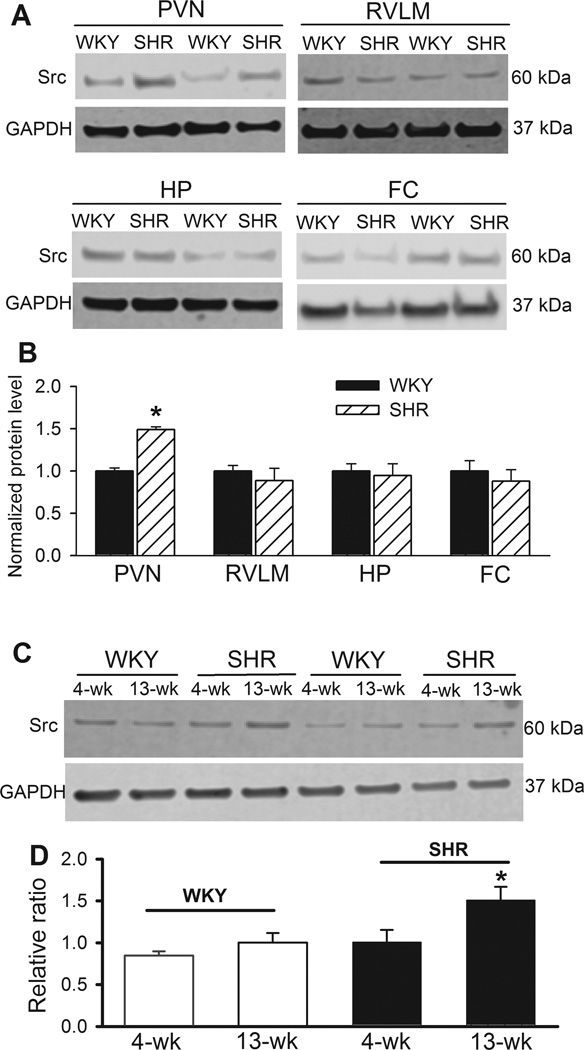
We determined the Src protein levels in the PVN, RVLM, hippocampus, and frontal cortex in WKY rats and SHRs. Western immunoblotting showed a single band of Src proteins in all of the brain tissues, and the total Src protein level in the PVN was significantly greater in SHRs than in WKY rats (n = 6 rats in each group, Fig. 1A,B). In contrast, Src protein levels in the RVLM, hippocampus, and frontal cortex did not differ significantly between WKY rats and SHRs (n = 6 rats in each group, Fig. 1A,B). Furthermore, the Src protein level did not significantly differ between 4-week-old and 13-week-old WKY rats. However, the Src protein level in 13-week-old SHRs was significantly higher than that in 4-week-old SHRs and age-matched WKY rats (n = 6 rats in each group. Fig. 1C,D).
Figure 1. Changes in Src protein levels in the PVN, rostral ventrolateral medulla, frontal cortex, and hippocampus in WKY rats and SHRs.
A and B: Representative gel images (A) and summary data (B) show the Src protein level (normalized to GAPDH) in the PVN, rostral ventrolateral medulla (RVLM), frontal cortex (FC), and hippocampus (HP) in WKY rats and SHRs (n = 6 rats in each group). C and D: Representative gel images (C) and group data (D) show the Src protein level in the PVN in 4-week-old and 13-week-old WKY and SHRs (n = 6 rats in each group). *P < 0.05, compared with WKY rats.
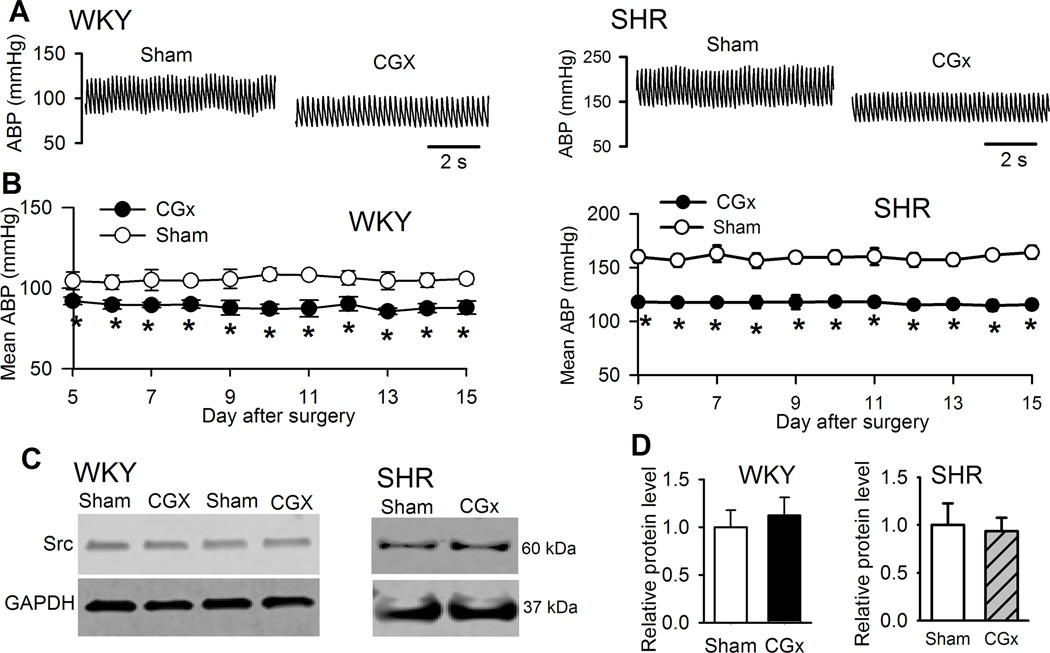
We then determined whether the higher Src protein level in the PVN of SHRs was a secondary change due to high ABP in SHRs. We performed CGx to lower ABP and then measured Src protein levels in the PVN of SHRs. CGx caused a large reduction in ABP, monitored by telemetry system, in SHRs compared with those subjected to sham surgery. CGx-induced decreases in ABP in SHRs occurred within 5 days after surgery and lasted for at least 2 weeks (Fig. 2A,B; Supplemental Fig. S2). Immunoblotting showed that the Src protein level in the PVN of SHRs did not differ significantly between rats receiving CGx and sham surgery (Fig. 2C,D). In addition, CGx induced a small decrease in ABP in WKY rats (Fig. 2A,B). The Src protein level in the PVN was not significantly altered in WKY rats subjected to CGx, compared with that in sham-operated WKY rats (Fig. 2C,D). These data suggest that Src upregulation in the PVN is independent of ABP changes in SHRs.
Figure 2. Effect of lowering ABP on the Src protein level in the PVN in WKY rats and SHRs.
A and B, Original ABP traces (A) and group data (B) show the effect of CGx and sham surgery on the mean ABP in WKY rats and SHRs (n = 6 rats in each group). C and D, Representative gel images (C) and summary data (D) show the Src protein levels (normalized to GAPDH) in the PVN in WKY rats and SHRs subjected to CGx or sham surgery (n = 6 rats in each group). *P < 0.05 compared with the sham group.
Src contributes to increased postsynaptic NMDAR currents of PVN presympathetic neurons in SHRs
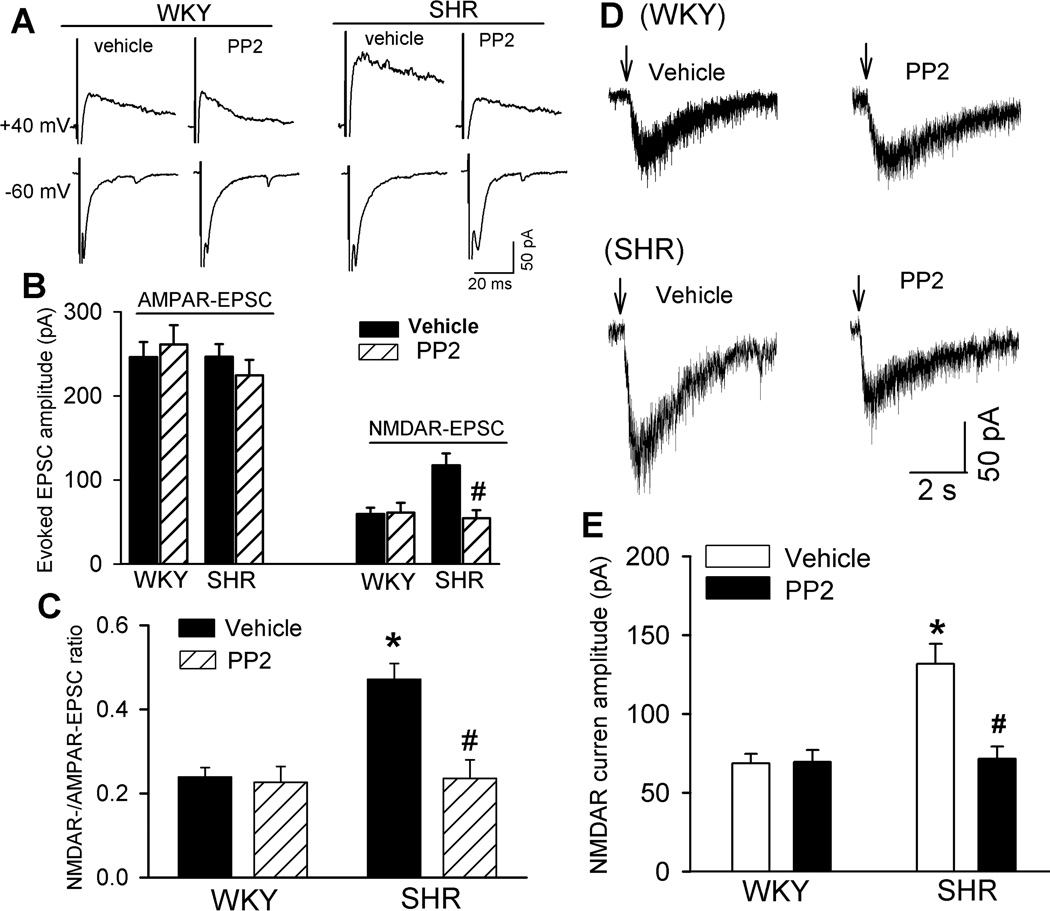
To determine the functional significance of increased Src kinase activity in regulating NMDARs of PVN presympathetic neurons in SHRs, we examined the effects of PP2, a highly selective Src kinase inhibitor30–32, on evoked AMPAR- and NMDAR-EPSCs in retrograde labeled RVLM-projecting PVN neurons. The hypothalamic slices from WKY rats and SHRs were incubated with either vehicle control (0.05% dimethyl sulfoxide, DMSO) or PP2 (10 µM) for 1–2 hours before electrophysiological recording. PP2 at 10 µM can potently inhibit the Src kinase activity in the hippocampus33. The amplitude of evoked NMDAR-EPSCs of labeled PVN neurons was significantly higher in neurons from SHRs than in those from WKY rats in vehicle-treated slices (n = 7 neurons in each group, Fig. 3A,B). PP2 treatment significantly reduced the amplitude of evoked NMDAR-EPSCs of labeled PVN neurons in SHRs but not in WKY rats (n = 7 neurons). However, PP2 treatment did not significantly alter the amplitude of evoked AMPAR-EPSCs of labeled PVN neurons in WKY rats or SHRs (n = 7 neurons in each group, Fig. 3A,B). Also, in vehicle-treated slices, the ratio of NMDAR-EPSCs to AMPAR-EPSCs was significantly higher in SHRs than in WKY rats. PP2 treatment normalized the ratio of NMDAR-EPSCs to AMPAR-EPSCs in SHRs but had no effect on this ratio in WKY rats (Fig. 3C).
Figure 3. Src is involved in increased synaptic NMDAR activity of RVLM-projecting PVN neurons in SHRs.
A, Representative current traces of evoked AMPAR-EPSCs (at −60 mV) and NMDAR-EPSCs (at +40 mV) recorded in labeled neurons from WKY rats and SHRs treated with vehicle or 10 µM PP2. B, Summary data of evoked AMPAR-EPSCs and NMDAR-EPSCs in neurons recorded from WKY rats or SHRs pretreated with vehicle or PP2 (n = 7 neurons in each group). *P < 0.05 compared with WKY rats. #P < 0.05 compared with the SHR vehicle group. C, Group data show the ratio of NMDAR-EPSCs to AMPAR-EPSCs in neurons recorded from WKY rats or SHRs treated with vehicle or PP2 (n = 7 neurons in each group). D and E, Original current traces (D) and summary data (E) show the effect of 10 µM PP2 on puff NMDA (100 µM)-elicited currents of labeled PVN neurons recorded from WKY rats (vehicle, n = 9 neurons; PP2, n = 7 neurons) and SHRs (vehicle, n = 7 neurons; PP2, n = 10 neurons) pretreated with vehicle or PP2. *P < 0.05 compared with WKY rats. #P < 0.05 compared with the SHR vehicle group.
To directly determine the role of Src kinases in the regulation of postsynaptic NMDAR activity, we examined the effect of PP2 on puff NMDA-induced currents in labeled PVN neurons. In vehicle-treated slices, puff application of NMDA (100 µM) induced significantly greater NMDAR currents in SHRs (n = 7 neurons) than in WKY rats (n = 9 neurons, Fig. 3D,E). PP2 treatment profoundly reduced NMDAR currents of labeled PVN neurons in SHRs but had no effect on puff NMDA-elicited currents in WKY rats (Fig. 3D,E). These results suggest that increased Src kinase activity critically contributes to the increased postsynaptic NMDAR activity of PVN presympathetic neurons in SHRs.
Src is involved in tonic activation of presynaptic NMDARs of PVN presympathetic neurons in SHRs
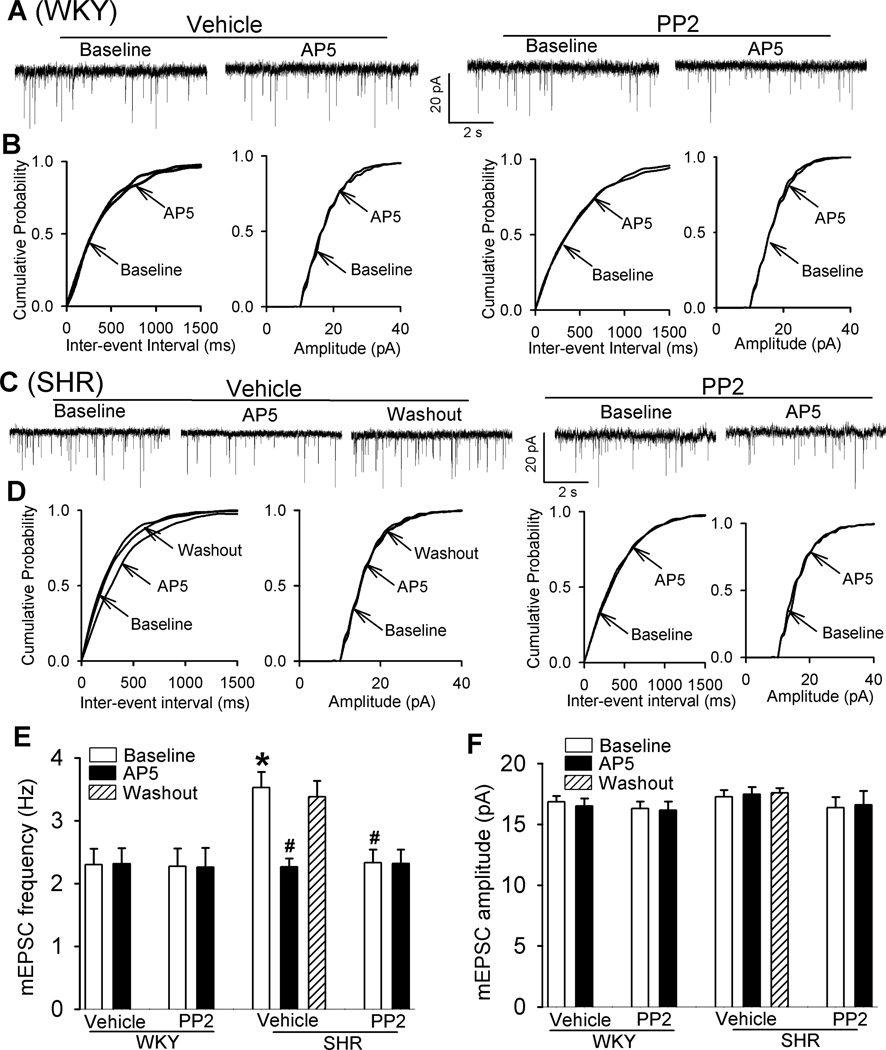
Presynaptic NMDARs in the PVN are latent and not functional in physiological conditions, but they become tonically active to increase synaptic glutamate release in SHRs17, 20. To determine whether Src kinases contribute to increased presynaptic NMDAR activity in the PVN in SHRs, we measured the frequency of mEPSCs, which reflects spontaneous quantal release of glutamate from presynaptic terminals17, 34. In these experiments, MK-801 (1 mM), an NMDAR channel blocker, was added to the pipette solution to block postsynaptic NMDAR activity35. In vehicle-treated slices, the baseline frequency of mEPSCs was significantly higher in SHRs than in WKY rats (n = 8 neurons in each group, Fig. 4A–E), but the baseline amplitude of mEPSCs did not differ significantly between the two groups. To confirm that the increase in the frequency of mEPSCs in SHRs was due to increased NMDAR activity, we bath applied the NMDAR antagonist AP5 (50 µM). In vehicle-treated slices, AP5 application significantly reduced the frequency of mEPSCs of labeled PVN neurons of SHRs, but not WKY rats (Fig. 4A–E).
Figure 4. Src contributes to increased presynaptic NMDAR activity of RVLM-projecting PVN neurons in SHRs.
A–D: Original traces and cumulative probability plots show the effect of bath application of 50 µM AP5 on mEPSCs of labeled PVN neurons recorded from WKY rats and SHRs pretreated with vehicle or 10 µM PP2. E and F, Summary data show the effects of PP2 and AP5 on the frequency and amplitude of mEPSCs in labeled PVN neurons of WKY rats (vehicle, n = 8 neurons; PP2, n = 8 neurons) and SHRs (vehicle, n = 8 neurons; PP2, n = 9 neurons). *P < 0.05 compared with WKY rats. #P < 0.05 compared with the baseline in SHR vehicle group.
PP2 treatment did not significantly change the baseline frequency or amplitude of mEPSCs of labeled PVN neurons in WKY rats. In contrast, treatment with PP2 significantly reduced the frequency of mEPSCs without changing their amplitude in SHRs (Fig. 4A–F). Furthermore, bath application of 50 µM AP5 had no effect on the frequency of mEPSCs in SHR brain slices that had been pretreated with PP2 (Fig. 4C–E). These data suggest that increased Src kinase activity plays a critical role in the enhanced presynaptic NMDAR activity of PVN presympathetic neurons in SHRs.
CK2 and Src kinases are differentially involved in the increased NMDAR activity of PVN presympathetic neurons in SHRs
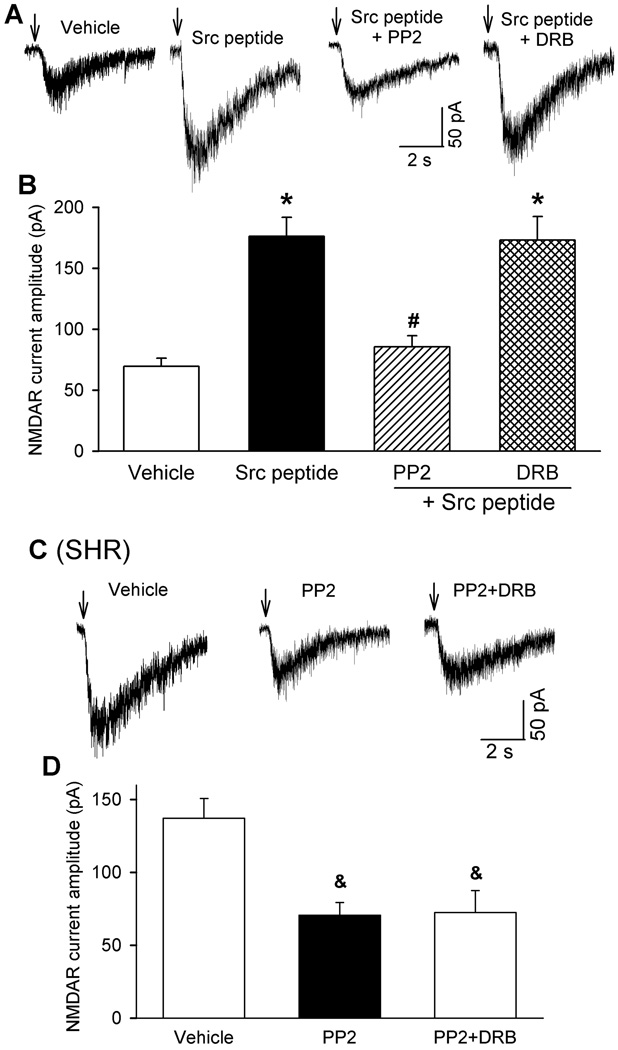
We have shown previously that CK2, a constitutively active protein serine/threonine kinase, plays a role in increased NMDAR activity of PVN presympathetic neurons in SHRs20. To determine whether CK2 and Src kinases play overlapping roles in the control of NMDAR activity in the PVN, we applied (pY)EEI, a synthetic Src-activating peptide36, 37, through the recording pipette. Intracellular dialysis of (pY)EEI (100 µM) for 15 min caused a large increase in NMDA puff-elicited currents in labeled PVN neurons of WKY rats (n = 9 neurons, Fig. 5A,B). In brain slices pretreated with PP2 (10 µM), dialysis of the (pY)EEI peptide failed to significantly alter the NMDA puff-elicited currents in labeled PVN neurons (n = 9 neurons). In contrast, in brain slices pretreated with the CK2 inhibitor DRB (100 µM), intracellular application of the Src-activating peptide still caused a large increase in NMDA puff-elicited currents in labeled PVN neurons of WKY rats (n = 11 neurons, Fig. 5A,B).
Figure 5. CK2 and Src kinases differentially potentiate NMDAR activity of RVLM-projecting PVN neurons.
A and B, Original recording traces (A) and group data (B) show the effect of intracellular dialysis of the Src-activating peptide (pY)EEI (100 µM) on puff NMDA-elicited currents of labeled PVN neurons of WKY rats pretreated with vehicle (n = 9 neurons), 10 µM PP2 (n = 9 neurons) or 100 µM DRB (n = 11 neurons). C and D, Representative traces (C) and summary data (D) show the effect of vehicle, PP2 (10 µM) alone, and PP2 plus DRB (100 µM) on puff NMDA-elicited currents of labeled PVN neurons of SHRs (n = 7 neurons in each group). *P < 0.05 compared with neurons recorded without Src peptide (vehicle). #P < 0.05 compared with neurons recorded with Src peptide alone. &P < 0.05 compared with neurons pretreated with vehicle.
Furthermore, we determined the possible interaction between CK2 and Src kinases in SHRs. Treatment with PP2 alone and PP2 plus DRB similarly reduced the amplitude of NMDA puff-elicited currents of labeled PVN neurons in SHRs (n = 7 neurons in each group, Fig. 5C,D). The amplitude of NMDAR currents of labeled PVN neurons did not significantly differ between SHR brain slices treated with PP2 alone and PP2 plus DRB. These results suggest that although both CK2 and Src kinases regulate NMDAR activity of PVN presympathetic neurons, they probably affect different phosphorylation sites of NMDARs and/or their interacting proteins.
Src-mediated NMDAR activation in the PVN plays a role in the maintenance of sympathetic vasomotor tone in SHRs
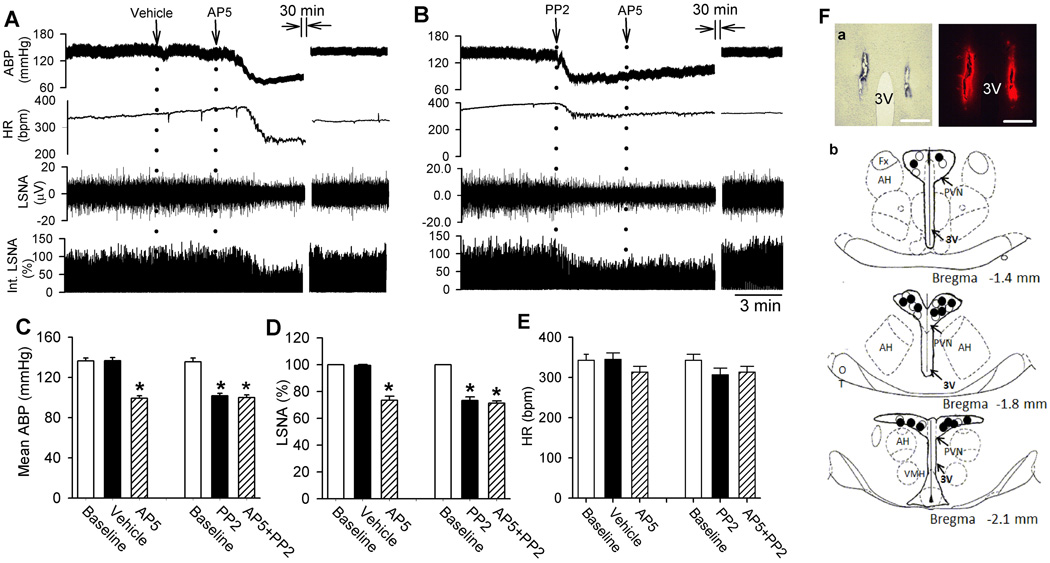
We determined the functional significance of Src-mediated NMDAR activity in the PVN in controlling sympathetic vasomotor tone in SHRs. We did not perform PP2 injection in WKY rats because blocking NMDARs in the PVN has no effect on ABP or sympathetic nerve activity in normotensive rats2. The baseline integrated LSNA (averaged over 30 s) was 0.12 ± 0.05 µVs in SHRs, which was significantly higher in SHRs than in WKY rats (0.06 ± 0.06 µVs, P < 0.05). We microinjected PP2 (40 pmol, 50 nL)38 bilaterally into the PVN, which significantly decreased the LSNA and mean ABP of SHRs (n = 7 rats, Fig. 6A–E). The LSNA and ABP started to decrease at a mean time of 3.1 ± 0.5 min after PP2 injection, and this effect lasted for 29.7 ± 1.8 min. In SHRs injected with PP2, subsequent microinjection of AP5 (1.0 nmol, 50 nL)2, 20 into the PVN failed to decrease LSNA, ABP, and HR. In contrast, microinjection of vehicle (0.5% DMSO, 50 nL) into the PVN had no significant effect on ABP and LSNA in another set of SHRs (n = 6 rats). Subsequent AP5 microinjection into the PVN significantly decreased LSNA and ABP in the SHRs receiving prior vehicle microinjection (Fig. 6A–E). These data suggest that the increased sympathetic vasomotor tone in SHRs is sustained by Src-mediated NMDAR activation in the PVN.
Figure 6. Src-mediated NMDAR activity in the PVN maintains elevated sympathetic outflow in SHRs.
A and B, Representative recording traces show the effect of bilateral microinjection of vehicle (A) or PP2 (B) and AP5 into the PVN on ABP, LSNA, and HR in SHRs. C–E, summary data show changes in mean ABP, LSNA, and HR in response to injection of AP5 after microinjection of PP2 (n = 7 rats) or vehicle (n = 6 rats) into the PVN in SHRs. F, a representative image and schematic drawing show the microinjection sites for vehicle plus AP5 (•) and PP2 plus AP5 (◦) in the PVN in SHRs. *P < 0.05 compared with the baseline control. AH, anterior hypothalamus; 3V, third ventricle; Fx, Fornix; VMH, ventromedial hypothalamus.
Discussion
The most salient finding of our study is that Src kinases critically contribute to the increased NMDAR activity, at both pre- and postsynaptic sites, of RVLM-projecting PVN neurons in a rat model of essential hypertension. Src is highly expressed in various brain regions39, 40 and can interact with postsynaptic density proteins such as PSD-9541–43. In the present study, we found that inhibition of Src kinase activity with PP2 significantly decreased the amplitude of evoked NMDAR-EPSCs and puff NMDA-induced currents in RVLM-projecting PVN neurons in SHRs but not in WKY rats. Although AMPARs can also be phosphorylated by the Src kinases44, we found that PP2 had no significant effect on the amplitude of mEPSCs or evoked AMPAR-EPSCs in PVN neurons in SHRs or WKY rats. Thus, Src regulation of AMPAR activity seems to be uninvolved in regulating glutamatergic input in the PVN in SHRs.
Src is also expressed at nerve terminals and is associated with synaptic vesicles45, 46. Presynaptic NMDARs in the hypothalamus and spinal cord are latent and not functionally active under physiological conditions18, 20. However, these receptors become tonically activated and promote synaptic glutamate release to PVN presympathetic neurons in hypertension17, 20. We found that PP2 significantly reduced the baseline frequency of mEPSCs of RVLM-projecting PVN neurons in SHRs. Furthermore, blocking NMDARs with AP5 decreased the frequency of mEPSCs in vehicle-treated, but not PP2-treated, brain slices of SHRs. Interestingly, we showed that PP2 produced no effect on puff-NMDA-elicited currents or the mEPSC frequency in normotensive WKY rats. This differential effect of Src kinase inhibition on synaptic NMDAR activity is probably due to the low basal Src kinase activity in the PVN in WKY rats. Our findings suggest that increased Src kinase activity in the PVN may facilitate glutamate release by enhancing presynaptic NMDAR activity in SHRs. The sources of endogenous glutamate for tonic activation of presynaptic NMDARs in SHRs may come from the same terminals at which the NMDARs are expressed or from excitatory interneurons in the PVN that result from increased excitability and reduced synaptic inhibition34, 47.
We showed that the Src protein level was significantly elevated in the PVN in adult SHRs compared with 4-week-old SHRs and adult WKY rats. However, the Src protein level in the RVLM, hippocampus, and frontal cortex did not significantly differ between WKY rats and SHRs, suggesting that Src upregulation is not a general phenomenon throughout all brain regions in SHRs. Because the Src protein level in the PVN was not altered by lowering ABP in SHRs with CGx, this suggests that Src upregulation in the PVN may not result from increased ABP but may instead contribute to hypertension development in SHRs. Altered tyrosine phosphorylation of NMDARs in the brain has been implicated in synaptic plasticity associated with depression48 and fear learning49. Consistent with our in vitro slice recording data, we found that microinjection of the Src kinase inhibitor PP2 into the PVN significantly decreased LSNA and ABP in SHRs. Importantly, subsequent microinjection of AP5 reduced LSNA and ABP in vehicle-injected, but not in PP2-injected, SHRs. These in vivo data suggest that Src-mediated NMDAR activity in the PVN critically contributes to the elevated sympathetic vasomotor tone in SHRs.
Increased Src kinase activity in SHRs may directly increase the phosphorylation level of certain tyrosine residues of NMDARs. For example, various tyrosine residues in GluN2A (Tyr-1292, Tyr-1325, and Tyr-1387) and GluN2B (Tyr-1252, Tyr-1336, and Tyr-1472) can be phosphorylated by Src kinases43, 50–53. However, we cannot exclude the possibility that Src kinases increase NMDAR activity by phosphorylating tyrosine residues in NMDAR-interacting proteins, such as NADH dehydrogenase subunit 254. CK2 is also involved in increased NMDAR activity of PVN presympathetic neurons in SHRs20. In this study, we found that intracellular application of a Src-activating peptide readily increased the NMDAR activity of RVLM-projecting PVN neurons. Although the potentiating effect of the Src-activating peptide on NMDAR activity was blocked by PP2, it was not affected by the CK2 inhibitor DRB. Furthermore, DRB had no further effect on NMDAR activity of PVN neurons that had already been reduced by PP2 in SHRs. Our data suggest that although both CK2 and Src kinases may contribute to increased NMDAR activity of PVN presympathetic neurons in SHRs through different phosphorylation sites, their roles are not mutually exclusive. Interestingly, PP2 has no effect on spinal NMDAR activity increased by CK255. It has been shown that CK2 phosphorylates Ser-1480 residue in GluN2B56, whereas Src kinases phosphorylate Tyr-1325 in GluN2A48. Alternatively, NMDAR phosphorylation may involve a coordinated interaction between CK2 and Src kinases. In this regard, the catalytic subunits of CK2 can be directly tyrosine-phosphorylated by the Src kinases, leading to increased CK2 activity57. The overall activity of NMDARs in the PVN likely depends on their phosphorylation levels controlled by both CK2 and Src kinases. Nevertheless, the precise interaction between CK2 and Src kinases in the regulation of NMDAR activity of PVN presympathetic neurons in hypertension remains to be delineated.
Perspectives
We present novel evidence that increased Src kinase activity in the PVN plays a pivotal role in increased pre- and postsynaptic NMDAR activity of PVN presympathetic neurons in SHRs. Our study provides new insight into the molecular mechanism underlying elevated sympathetic vasomotor tone in an animal model of essential hypertension. The prevalence of resistant hypertension has been estimated to be 20% to 30%58, making it a common and challenging clinical problem. On the basis of our findings, we suggest that Src kinases are potential targets for treating patients with neurogenic hypertension. Further studies are needed to determine the dynamic changes in the Src kinases and their various isoforms in the PVN in different animal models of hypertension and to examine the long-term effect of the Src kinase inhibition in conscious hypertensive animals.
Supplementary Material
Novelty and Significance.
1) What Is New
Src tyrosine kinase is upregulated in the hypothalamus in a rat model of hypertension
Src inhibition normalizes the increased NMDAR activity of hypothalamic presympathetic neurons in hypertensive animals
Inhibition of Src-mediated NMDAR activity reduces sympathetic vasomotor tone in hypertensive animals
2) What Is Relevant
Increased glutamatergic input and excitability of presympathetic neurons in the hypothalamus contribute elevated sympathetic output in hypertension
Src kinases may be targeted for treatment of hypertension by reducing the sympathetic vasomotor tone
3) Summary
Increased Src kinase activity augments sympathetic outflow in hypertension by potentiating glutamatergic input to hypothalamic presympathetic neurons
Acknowledgments
N/A.
Sources of Funding
This work was supported by the National Institutes of Health (Grants HL131161 and MH096086) and the N.G. and Helen T. Hawkins Endowment (to H.-L.P.).
Footnotes
Competing Interest/Disclosures
None.
References
- 1.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 2.Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- 3.Judy WV, Watanabe AM, Henry DP, Besch HR, Jr, Murphy WR, Hockel GM. Sympathetic nerve activity: role in regulation of blood pressure in the spontaenously hypertensive rat. Circ Res. 1976;38:21–29. doi: 10.1161/01.res.38.6.21. [DOI] [PubMed] [Google Scholar]
- 4.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 5.Esler M. Sympathetic nervous system: contribution to human hypertension and related cardiovascular diseases. J Cardiovasc Pharmacol. 1995;26(Suppl 2):S24–S28. [PubMed] [Google Scholar]
- 6.Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- 7.Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge : quantitative assessment in human hypertensive disease. Circulation. 1999;100:1305–1310. doi: 10.1161/01.cir.100.12.1305. [DOI] [PubMed] [Google Scholar]
- 8.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 9.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- 10.Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol. 1998;25:461–463. doi: 10.1111/j.1440-1681.1998.tb02235.x. [DOI] [PubMed] [Google Scholar]
- 11.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 12.Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res. 1998;120:164–172. doi: 10.1007/s002210050390. [DOI] [PubMed] [Google Scholar]
- 13.Ciriello J, Kline RL, Zhang TX, Caverson MM. Lesions of the paraventricular nucleus alter the development of spontaneous hypertension in the rat. Brain Res. 1984;310:355–359. doi: 10.1016/0006-8993(84)90159-8. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Nakata T, Takesako T, Itoh H, Hirata M, Kawasaki S, Hayashi J, Oguro M, Sasaki S, Nakagawa M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res. 1991;543:296–300. doi: 10.1016/0006-8993(91)90040-3. [DOI] [PubMed] [Google Scholar]
- 15.Eilam R, Malach R, Segal M. Selective elimination of hypothalamic neurons by grafted hypertension-inducing neural tissue. J Neurosci. 1994;14:4891–4902. doi: 10.1523/JNEUROSCI.14-08-04891.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eilam R, Malach R, Bergmann F, Segal M. Hypertension induced by hypothalamic transplantation from genetically hypertensive to normotensive rats. J Neurosci. 1991;11:401–411. doi: 10.1523/JNEUROSCI.11-02-00401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586:1637–1647. doi: 10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SR, Hu YM, Chen H, Pan HL. Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. J Physiol. 2014;592:215–227. doi: 10.1113/jphysiol.2013.263814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li DP, Zhou JJ, Pan HL. Endogenous casein kinase-1 modulates NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Physiol. 2015;593:4439–4452. doi: 10.1113/JP270831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye ZY, Li DP, Li L, Pan HL. Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci. 2011;31:8271–8279. doi: 10.1523/JNEUROSCI.1147-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu XM, Askalan R, Keil GJ, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 22.Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nat Neurosci. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Fang W, Li J, Zhang B, Yang Q, Yan X, Peng L, Ai H, Wang JJ, Liu X, Luo J, Yang W. Phosphorylation of Tyrosine 1070 at the GluN2B Subunit Is Regulated by Synaptic Activity and Critical for Surface Expression of N-Methyl-D-aspartate (NMDA) Receptors. J Biol Chem. 2015;290:22945–22954. doi: 10.1074/jbc.M115.663450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groveman BR, Feng S, Fang XQ, Pflueger M, Lin SX, Bienkiewicz EA, Yu X. The regulation of N-methyl-D-aspartate receptors by Src kinase. FEBS J. 2012;279:20–28. doi: 10.1111/j.1742-4658.2011.08413.x. [DOI] [PubMed] [Google Scholar]
- 25.Khan AM, Cheung HH, Gillard ER, Palarca JA, Welsbie DS, Gurd JW, Stanley BG. Lateral hypothalamic signaling mechanisms underlying feeding stimulation: differential contributions of Src family tyrosine kinases to feeding triggered either by NMDA injection or by food deprivation. J Neurosci. 2004;24:10603–10615. doi: 10.1523/JNEUROSCI.3390-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross CA, Wright GE, Resh MD, Pearson RC, Snyder SH. Brain-specific src oncogene mRNA mapped in rat brain by in situ hybridization. Proc Natl Acad Sci U S A. 1988;85:9831–9835. doi: 10.1073/pnas.85.24.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto YM, Paul M, Ganten D. Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res. 1998;39:77–88. doi: 10.1016/s0008-6363(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 28.Yamori Y. Pathogenesis of spontaneous hypertension as a model for essential hypertension. Jpn Circ J. 1977;41:259–266. doi: 10.1253/jcj.41.259. [DOI] [PubMed] [Google Scholar]
- 29.Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1110–H1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- 30.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 31.Chen JK, Capdevila J, Harris RC. Overexpression of C-terminal Src kinase blocks 14, 15-epoxyeicosatrienoic acid-induced tyrosine phosphorylation and mitogenesis. J Biol CHem. 2000;275:13789–13792. doi: 10.1074/jbc.275.18.13789. [DOI] [PubMed] [Google Scholar]
- 32.Yoshizumi M, Abe J, Haendeler J, Huang Q, Berk BC. Src and Cas mediate JNK activation but not ERK1/2 and p38 kinases by reactive oxygen species. J Biol Chem. 2000;275:11706–11712. doi: 10.1074/jbc.275.16.11706. [DOI] [PubMed] [Google Scholar]
- 33.Sanna PP, Berton F, Cammalleri M, Tallent MK, Siggins GR, Bloom FE, Francesconi W. A role for Src kinase in spontaneous epileptiform activity in the CA3 region of the hippocampus. Proc Natl Acad Sci U S A. 2000;97:8653–8657. doi: 10.1073/pnas.140219097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye ZY, Li DP, Byun HS, Li L, Pan HL. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J Neurosci. 2012;32:8560–8568. doi: 10.1523/JNEUROSCI.1346-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 2010;30:4460–4466. doi: 10.1523/JNEUROSCI.5857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wijetunge S, Hughes AD. Activation of endogenous c-Src or a related tyrosine kinase by intracellular (pY)EEI peptide increases voltage-operated calcium channel currents in rabbit ear artery cells. FEBS Lett. 1996;399:63–66. doi: 10.1016/s0014-5793(96)01177-5. [DOI] [PubMed] [Google Scholar]
- 37.Albert AP, Aromolaran AS, Large WA. Agents that increase tyrosine phosphorylation activate a non-selective cation current in single rabbit portal vein smooth muscle cells. J Physiol. 2001;530:207–217. doi: 10.1111/j.1469-7793.2001.0207l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y, Yuan N, Zhang SJ, Gao J, Shi Z, Zhou YB, Gao XY, Zhu GQ. c-Src in paraventricular nucleus modulates sympathetic activity and cardiac sympathetic afferent reflex in renovascular hypertensive rats. Pflugers Arch. 2011;461:437–446. doi: 10.1007/s00424-011-0932-7. [DOI] [PubMed] [Google Scholar]
- 39.Brugge JS, Cotton PC, Queral AE, Barrett JN, Nonner D, Keane RW. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985;316:554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- 40.Cotton PC, Brugge JS. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983;3:1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalia LV, Salter MW. Interactions between Src family protein tyrosine kinases and PSD-95. Neuropharmacol. 2003;45:720–728. doi: 10.1016/s0028-3908(03)00313-7. [DOI] [PubMed] [Google Scholar]
- 42.Du CP, Gao J, Tai JM, Liu Y, Qi J, Wang W, Hou XY. Increased tyrosine phosphorylation of PSD-95 by Src family kinases after brain ischaemia. Biochem J. 2009;417:277–285. doi: 10.1042/BJ20080004. [DOI] [PubMed] [Google Scholar]
- 43.Cheung HH, Gurd JW. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor by exogenous and postsynaptic density-associated Src-family kinases. J Neurochem. 2001;78:524–534. doi: 10.1046/j.1471-4159.2001.00433.x. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnekow A, Jahn R, Schartl M. Synaptophysin: a substrate for the protein tyrosine kinase pp60c-src in intact synaptic vesicles. Oncogene. 1990;5:1019–1024. [PubMed] [Google Scholar]
- 46.Pang DT, Wang JK, Valtorta F, Benfenati F, Greengard P. Protein tyrosine phosphorylation in synaptic vesicles. Proc Natl Acad Sci U S A. 1988;85:762–766. doi: 10.1073/pnas.85.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li DP, Byan HS, Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci. 2012;32:372–380. doi: 10.1523/JNEUROSCI.3222-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniguchi S, Nakazawa T, Tanimura A, et al. Involvement of NMDAR2A tyrosine phosphorylation in depression-related behaviour. EMBO J. 2009;28:3717–3729. doi: 10.1038/emboj.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakazawa T, Komai S, Watabe AM, et al. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon IS, Apperson ML, Kennedy MB. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc Natl Acad Sci U S A. 1994;91:3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau LF, Huganir RL. Differential tyrosine phosphorylation of N-methyl-D-aspartate receptor subunits. J Biol Chem. 1995;270:20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- 52.Yang M, Leonard JP. Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by coexpression with v-Src. J Neurochem. 2001;77:580–588. doi: 10.1046/j.1471-4159.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 53.Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- 54.Gingrich JR, Pelkey KA, Fam SR, Huang Y, Petralia RS, Wenthold RJ, Salter MW. Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondrial protein NADH dehydrogenase subunit 2. Proc Natl Acad Sci U S A. 2004;101:6237–6242. doi: 10.1073/pnas.0401413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen SR, Zhou HY, Byun HS, Chen H, Pan HL. Casein kinase II regulates N-methyl-D-aspartate receptor activity in spinal cords and pain hypersensitivity induced by nerve injury. J Pharm Exp Ther. 2014;350:301–312. doi: 10.1124/jpet.114.215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanz-Clemente A, Gray JA, Ogilvie KA, Nicoll RA, Roche KW. Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep. 2013;3:607–614. doi: 10.1016/j.celrep.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donella-Deana A, Cesaro L, Sarno S, Ruzzene M, Brunati AM, Marin O, Vilk G, Doherty-Kirby A, Lajoie G, Litchfield DW, Pinna LA. Tyrosine phosphorylation of protein kinase CK2 by Src-related tyrosine kinases correlates with increased catalytic activity. Biochem J. 2003;372:841–849. doi: 10.1042/BJ20021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.