Hypernatremia elicits multiple adaptive responses mediated via the central nervous system: the sensation of thirst, drinking (seeking and consuming fluids), changes in taste preference (water vs. salt) and neuroendocrine responses that affect the circulatory system along with sodium and water excretion by the kidneys 1. In conscious humans, hyperosmotic stimuli elevate BP and sympathetic tone 2 and this particular neurogenic response likely contributes to salt-dependent hypertension. In this issue of Hypertension, Kinsman et al. 3 suggest that the autonomic nervous system effects elicited by systemic hypernatremia result from a direct action of [NaCl] on neurons located within the organum vasculosum laminae terminalis (OVLT).
The OVLT, along with the subfornical organ (SFO) and the median preoptic nucleus (MnPO) are interconnected structures that line the anterior wall of the third ventricle (Figure) and orchestrate the behavioral and autonomic responses to hyperosmolarity4. The SFO and OVLT detect and encode the concentration of sodium present in the brain extracellular space and possibly the cerebrospinal fluid. They have fenestrated capillaries that facilitate sodium equilibration between plasma and brain extracellular fluid and render their neuropil accessible to circulating hormones such as angiotensin II. The median preoptic nucleus (MnPO) bridges the SFO and OVLT (Figure) and probably also contain sodium and osmotic pressure sensors; this imperfectly defined structure is a crucial integrative center for sodium and fluid homeostasis 5, 6.
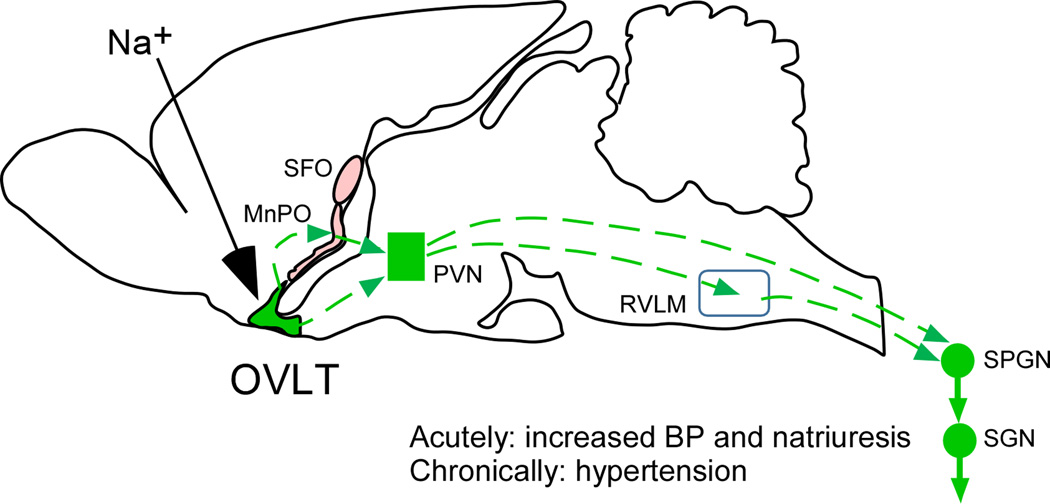
Figure 1. OVLT and salt-sensitive neurogenic hypertension.
Hypernatremia activates OVLT neurons directly or via local astrocytes. OVLT activation augments muscle sympathetic nerve activity (SNA) and reduces renal SNA, thereby elevating blood pressure and probably increasing renal natriuresis. The depicted mechanism could contribute to salt-dependent hypertension. The dashed lines represent plausible connections between OVLT neurons and sympathetic preganglionic neurons (SPGNs). Way stations may include the median preoptic nucleus (MnPO), the parvocellular division of the hypothalamic paraventricular nucleus (PVN) and the rostral ventrolateral medulla (RVLM). The SFO and MnPO also contain sodium sensors. These nuclei may orchestrate other types of adaptive responses to hypernatremia than the OVLT (e.g. drinking, taste preference etc.). Other abbreviations: SFO, subfornical organ; SGN, sympathetic ganglionic neuron.
Kinsman et al. 3 demonstrate that, in tissue slices, 56% of OVLT neurons are vigorously activated by low-grade hyperosmotic hypernatremia (2.5 to 10mM increase in [NaCle]). The inward (depolarizing) current produced by raising [NaCle] in vitro persisted under conditions of reduced synaptic transmission (TTX plus blockers of ionotropic glutamate and GABA receptors) suggesting that OVLT neurons could be intrinsically sensitive to hypernatremia. The underlying molecular mechanism is yet to be discovered. At issue is whether the OVLT detects hypernatremia via changes in osmolarity or sodium concentration and whether the sensors reside on glia or neurons.
Isolated murine OVLT neurons are depolarized by a hyperosmotic stimulus (mannitol), a response attributed to TPRPV1 channels 7. However, according to these authors, the drinking response evoked by systemic hypernatremia is only modestly reduced in TRPV1-KO mice. In the SFO, Na sensing requires a sodium channel (Nax, SCN7a) that is distantly related to the family of voltage-activated channels responsible for action potential generation in neurons but neither voltage operated not tetrodotoxin (TTX)-sensitive 8. Nax is regulated by endothelin which shifts its operating range from supraphysiological to physiological levels of [Na+e] 8. In the SFO of adult mice, Nax is primarily expressed by GFAP-positive glia 8, 9. Lactate and 5,6-epoxyeicosatrienoic acid are among the gliotransmitters that may mediate the excitatory effect of hypernatremia on SFO GABAergic and glutamatergic neurons, respectively 8. Importantly, Nax immunoreactivity and mRNA transcripts are also present in the OVLT and immediately adjacent portions of the MnPO 6, 9. According to Grob et al. 6 the ventral MnPO contains a mixture of osmosensitive (~75% of the total) and purely sodium-sensitive neurons; the latter respond to a rise in [Na+e] in the presence of TTX but are insensitive to mere hyperosmolarity. Pharmacological and histological evidence reported by Grob et al. 6 suggests that the sodium-sensing mechanism might also depend on Nax. By analogy with the work on the SFO, the Nax–dependent excitation of MnPO neurons could well be of a paracrine nature. This mode of intercellular communication is TTX-resistant and therefore compatible with Kinsman et al.’s results in the OVLT 3. In sum, the response of OVLT neurons to extracellular NaCl and the resulting autonomic effects could result from a direct effect of hyperosmolarity on these neurons or they could represent a paracrine response to [Na+e] mediated by glial Nax. In the future, deleting Nax or TRPV1 selectively from the OVLT may provide the answer.
Regardless of the precise mechanism by which hyperosmotic hypernatremia activates OVLT neurons, a key question addressed by Kinsman et al. 3 is whether these neurons respond to physiologically relevant changes in plasma [NaCl] in vivo. This is indeed the case: most OVLT neurons (72%) were vigorously activated by injecting NaCl into the carotid artery or a lateral ventricle in a dosage range that raises [Na+e] modestly (estimate: 3–8mM). These neuronal responses were accompanied by small BP rises (~10 mmHg for the highest NaCl dose) that were mimicked by injecting NaCl into the OVLT but not outside it. Interestingly, NaCl injections dorsal to the OVLT, which should have targeted the MnPO, were ineffective. The NaCl-responsive neurons located in the MnPO 6 may therefore elicit responses that are not mediated by the autonomic nervous system (e.g. drinking)10.
NaCl injection into the OVLT changed sympathetic nerve activity (SNA) in a regionally specific manner 3. These results show that a minimal rise in [NaCle] within the OVLT is sufficient to increase BP, probably by increasing SNA to muscle and skin arterioles, whilst facilitating renal sodium excretion by withdrawing renal sympathetic tone. Finally, Kinsman et al. 3 demonstrated that the OVLT circuitry must be fully functional for hyperosmotic hypernatremia to increase BP. Indeed, the rise in BP and SNA evoked by i.c.v injection of NaCl was greatly reduced by injecting the GABA-mimetic drug muscimol selectively into the OVLT. In truth, since the CVOs are all interconnected, the results of the muscimol experiment could also mean that the OVLT is a pivot for autonomic responses evoked by NaCl elsewhere (e.g. SFO and MnPO).
The interpretation of the Kinsman et al. data is subject to the limitations inherent to the use of anesthetized rodents. In unanesthetized mice, optogenetic stimulation of glutamatergic neurons located within the OVLT/MnPO elicits robust drinking and the converse occurs when local GABAergic neurons are activated 10. Drinking is likely associated with arousal and cardiovascular stimulation. The BP and SNA changes observed by Kinsman et al. 3 could be the attenuated autonomic correlates of a behavioral response whose motor and motivational components are suppressed by anesthesia. On the other hand, it could also represent an aspect of the integrated response to hypernatremia that is independent of drinking behavior. This interpretation is tempting because the autonomic response pattern elicited by activating OVLT neurons with NaCl seems well suited to facilitate renal sodium excretion during hypernatremia (increased renal artery pressure combined with reduced renal SNA).
In conclusion, OVLT neurons are activated by modest rises in plasma and/or CSF [NaCle]. OVLT neurons detect [NaCle] in a cell-autonomous manner or via the surrounding glia. The activation of OVLT neurons by NaCl elevates BP and triggers a pattern of SNA that presumably facilitates renal sodium excretion (Figure). When chronically elicited, this mechanism probably contributes to salt-dependent hypertension.
Acknowledgments
Sources of Funding: This work was supported by research grants from the National Institutes of Health (HL028785, HL HL074011).
Footnotes
Disclosures: none.
REFERENCES
- 1.Denton DA, McKinley MJ, Weisinger RS. Hypothalamic integration of body fluid regulation. Proc Natl Acad Sci. USA. 1996;93:7397–7404. doi: 10.1073/pnas.93.14.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farquhar WB, Wenner MM, Delaney EP, Prettyman AV, Stillabower ME. Sympathetic neural responses to increased osmolality in humans. Am J Physiol Heart Circ Physiol. 2006;291:H2181–H2186. doi: 10.1152/ajpheart.00191.2006. [DOI] [PubMed] [Google Scholar]
- 3.Kinsman BJ, Simmonds SS, Browning KN, Stocker SS. The organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.08372. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinley MJ, Allen AM, May CN, McAllen RM, Oldfield BJ, Sly D, Mendelsohn FA. Neural pathways from the lamina terminalis influencing cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol. 2001;28:990–992. doi: 10.1046/j.1440-1681.2001.03592.x. [DOI] [PubMed] [Google Scholar]
- 5.McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, Martelli D. The median preoptic nucleus: Front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 2015;214:8–32. doi: 10.1111/apha.12487. [DOI] [PubMed] [Google Scholar]
- 6.Grob M, Drolet G, Mouginot D. Specific na+ sensors are functionally expressed in a neuronal population of the median preoptic nucleus of the rat. J Neurosci. 2004;24:3974–3984. doi: 10.1523/JNEUROSCI.3720-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciura S, Liedtke W, Bourque CW. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: A mechanical process involving TRPV1 but not TRPV4. J Neurosci. 2011;31:14669–14676. doi: 10.1523/JNEUROSCI.1420-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiyama TY, Noda M. Sodium sensing in the subfornical organ and body-fluid homeostasis. Neurosci Res. 2016 doi: 10.1016/j.neures.2016.07.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Watanabe E, Hiyama TY, Shimizu H, Kodama R, Hayashi N, Miyata S, Yanagawa Y, Obata K, Noda M. Sodium-level-sensitive sodium channel Na(x) is expressed in glial laminate processes in the sensory circumventricular organs. Am J Physiol Regul Integr Comp Physiol. 2006;290:R568–R576. doi: 10.1152/ajpregu.00618.2005. [DOI] [PubMed] [Google Scholar]
- 10.Abbott SB, Machado NL, Geerling JC, Saper CB. Reciprocal control of drinking behavior by median preoptic neurons in mice. J Neurosci. 2016;36:8228–8237. doi: 10.1523/JNEUROSCI.1244-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]