Abstract
Objective
To determine if observation alone after nephrectomy in very low-risk Wilms tumor (defined as stage I favorable histology Wilms tumors with nephrectomy weight <550g and age at diagnosis <2 years) results in satisfactory event-free survival and overall survival, and to correlate relapse with biomarkers.
Patients and Methods
The AREN0532 study enrolled patients with very low-risk Wilms tumor confirmed by central review of pathology, diagnostic imaging, and surgical reports. After nephrectomy, patients were followed without adjuvant chemotherapy. Evaluable tumors were analyzed for WT1mutation, 1p and 16q copy loss, 1q copy gain, and 11p15 imprinting. The study was powered to detect a reduction in 4-year EFS from 87% to 75% and overall survival from 95% to 88%.
Results
A total of 116 eligible patients enrolled with a median follow up of 80 months (range: 5–97 months). Twelve patients relapsed. Estimated 4-year event-free survival was 89.7% (95% confidence interval 84.1–95.2%) and overall survival was 100%. First sites of relapse were lung (n = 5), tumor bed (n = 4), and abdomen (n = 2), with one metachronous tumor in the contralateral kidney (n = 1) at a median time of 4.3 months for those who relapsed (range 2.3–44 months). The presence of intralobar (P = 0.46) or perilobar rests (P = 1.0) were not associated with relapse (P = 0.16). 1q gain, 1p and 16q loss, and WT1 mutation status were not associated with relapse. 11p15 methylation status was associated relapse (20% relapse with loss of heterozygosity, 25% with loss of imprinting, and 3.3% relapse with retention of the normal imprinting (P = 0.011)).
Conclusions
Most patients meeting very low-risk criteria can be safely managed by nephrectomy alone with resultant reduced exposure to chemotherapy. Expansion of an observation alone strategy for low-risk Wilms tumor incorporating both clinical features and biomarkers should be considered.
Keywords: biomarkers, nephrectomy, observation, Wilms tumor
The overarching goal for children with favorable histology Wilms tumor (FHWT) is to tailor therapy to achieve a high degree of event-free survival (EFS) and overall survival whereas minimizing toxicity.1 A number of clinical and biological factors have been used to identify children with FHWT who require augmented treatment based on a predicted high risk for relapse. Prognostic factors have also been used to identify children who are candidates for reduction or elimination of therapy based on a predicted excellent EFS (time to relapse, secondary malignancy, or death) and ultimately overall survival (OS, time to death).2
Several clinical reviews suggested that patients with clinically defined very low-risk criteria could be spared treatment beyond nephrectomy.3,4 To confirm this, the fifth National Wilms Tumor Study (NWTS-5) enrolled a group of very low-risk Wilms tumor (VLRWT) patients, defined as stage I FHWT weighing <550 g in patients <2 years of age at diagnosis. However, the 3-year interim-analysis showed 2-year EFS of 86.5% that was inferior to the stopping rule set at a relapse-free survival of 90%.5 The conservative assumption then was that only 50% of patients with recurrence could be successfully salvaged with three drug chemotherapy (vincristine, dactinomycin, and doxorubicin) and site-specific radiotherapy. Of the 75 children who were treated with nephrectomy only before study closure, eight relapsed, and three developed metachronous disease in the contralateral kidney at a median of 4 months postnephrectomy. However, of these 11 children, only one died suggesting that a less conservative stopping rule for EFS might be warranted. Analysis by Shamberger et al6 of this VLRWT cohort at the 8 year mark confirmed an excellent OS (98.7%) with no further relapse or death.
A concern with the observation only strategy was that withholding chemotherapy may increase the prevalence of metachronous WT. Previous clinical trials estimated the risk of development of metachronous Wilms tumor (WT) in infants diagnosed <12 months of age to be 4% at 6 years.7 This fell to 1.5% for patients diagnosed between the ages of 12 to 23 months. Patients who develop meta-chronous WTs have an increased risk of chronic renal failure.8 As the observation-only strategy on NWTS-5 was closed early, there was insufficient power to confirm that EFS was acceptable and OS was excellent, thus preventing the widespread adoption of an observation only strategy.
Several clinical and biological risk factors for relapse were evaluated in the NWTS-5 VLRWT cohort. Histological subtype and the presence of nephrogenic rests were not associated with relapse.5 Combined loss of heterozygosity 1p and 16q was examined in the full NWTS-5 VLRWT cohort (treated and observed) and was found to rarely occur (n = 2/141).9 However, both loss of imprinting (LOI) and loss of heterozygosity (LOH) of 11p15, and WT1 mutations were associated with poorer EFS in VLRWT patients managed by observation alone,10,11 although these findings require validation in an independent cohort. Lastly, several studies from Europe and North America have both demonstrated and validated the prognostic impact of 1q gain in patients with FHWT who were treated with adjuvant chemotherapy.12,13,14 The impact of 1q gain has not been previously examined in patients with VLRWT.
The primary objective of this study was therefore to validate the hypothesis that nephrectomy only is appropriate therapy for VLRWT. Secondary aims were to validate 11p15 methylation as a prognostic factor associated with relapse for VLRWT patients, to examine the ability of 1q gain to be correlated with relapse in VLRWT, and to determine the frequency of metachronous WT in the defined cohort.
METHODS
Clinical Samples
All patients were first enrolled on the Children’s Oncology Group (COG) biology and classification study AREN03B2, where real-time central expert review of institutional pathology, operative notes related to the nephrectomy, and diagnostic imaging confirmed the status of VLRWT before enrollment on the therapeutic AR-EN0532 study. Enrollment was required within 30 days of nephrectomy. VLRWT continued to be defined as Stage I FHWT with a nephrectomy weight of <550 grams, in patients <2 years of age at diagnosis. Lymph node sampling was required. The absence of a high-risk predisposition syndrome15 was added as an exclusion criteria in August 2009, 2.9 years after the beginning of accrual. The following predisposition syndromes were exclusion criteria for this study: aniridia, Beckwith-Wiedemann syndrome, isolated hemi-hypertrophy, Simpson-Golabi-Behmel syndrome, Denys-Drash syndrome, or other associated genito-urinary anomalies, multicentric WT, unilateral WT with contralateral nephrogenic rest(s) in a child under 2 years of age, and diffuse hyperplastic perilobar nephroblastomatosis. We retrospectively confirmed that none of the relapsed patients registered on this study before the addition of the predisposition syndromes to the exclusion criteria had any one of these higher risk predisposition syndromes.
Relapsed patients remained on study utilizing therapy appropriate to the stage and site of relapse. Those who had abdominal relapse were treated with regimen DD4A (Vincristine, Doxorubicin, and Dactinomycin for 24 weeks with abdominal radiation – see supplementary file 1, http://links.lww.com/SLA/A996) similar to the regimen previously published5,16 regardless of the extent of resection of the recurrent disease at relapse. Patients who had pulmonary relapse were treated with the AREN0532 (Supplementary file 2, http://links.lww.com/SLA/A997) regimen DD4A and pulmonary radiotherapy was recommended concurrent with the beginning of chemotherapy. Patients who failed because of the development of metachronous tumors were treated with a partial nephrectomy or biopsy and chemotherapy with regimen DD4A and subsequent renal sparing surgery at 6 to 12 weeks. These patients did not receive local radiation therapy if the margins were clear. Patients who had other distant relapses, for example to liver or brain, were to be treated with regimen DD4A plus local radiotherapy for positive margins.
The NIH central Institutional Review Board (IRB) facilitated local institutional review board approvals where regulatory agreements existed. In all other locations the local institutional research ethics boards approved the study before patient enrollment. Authorization for participation was obtained from the legal guardians of the patient as all participants were under the age of 2 years at enrollment. Biological specimens were obtained from the initial nephrectomy, snap-frozen, shipped, and stored at –80°C at the COG reference laboratory. DNA was extracted by the reference laboratory and provided to the Ann & Robert H. Lurie Children’s Hospital of Chicago (LCH) for biomarker analysis. These latter studies were approved by the LCH IRB.
Methylation Analysis at 11p15
Analysis of the extent of methylation of the paternally imprinted H19 differentially methylated region (imprint control region 1) and the maternally imprinted KvDMR1 (imprint control region 2) on chromosome 11p1517 was performed using a methylation-sensitive enzyme HpaII and another enzyme (MspI) that cuts irrespective of methylation as previously described.11 Retention of imprinting (ROI) was defined as 30% to 70% methylation of both H19 and KvDMR1; LOI was defined by 80% to 100% methylation of H19 and 30% to 70% methylation of KvDMR1; LOH was defined by 80% to 100% methylation of H19 and 0% to 20% methylation of KvDMR1. Five patients had no tissue available and three tumors with values outside any of these ranges were not scored.
WT1 Mutation Analysis
WT1 point mutations and small insertions/deletions in tumor DNA was examined by sequence analysis of PCR products from all 10 exons, including the flanking intronic sequence as previously described.8 Quantitative real-time PCR was utilized to identify deletions encompassing one or more exons, as previously described.11
Chromosomal Copy Number Changes Involving 1q, 1p, and 16q
Multiplex Ligation-Dependent Probe Amplification (MLPA) was performed using a synthetic probe mixture containing four probes for each of 1q, 1p, and 16q, and six control probes, as previously described in detail.12 Analysis of the MLPA PCR products was performed on an Applied Biosystems 3100-Avant genetic analyzer (Applied Biosystems). After separation by capillary electrophoresis, peaks corresponding to each probe were identified by GeneMapper analysis (Applied Biosystems) and subject to quality control steps and normalization as previously described.12 Classification of gain or loss for a chromosomal region was determined if at least 2 markers were gained or lost, respectively.
Statistics
The study was monitored by an independent data safety monitoring board. Predetermined stopping rules included a 4-year EFS less than 85%, a 4-year OS less than 95%, and a metachronous tumor rate exceeding 7%. The study was powered based on the assumption that a true failure rate greater than 25% (long-term EFS less than 75%) would be unacceptable. Thus, 115 patients were needed to have a 95% power to detect this deficit (testing at the 15% level of statistical significance, one-sided). This sample size also provided about 95% power (testing at the 15% level of statistical significance, one-sided) to detect a reduction in the overall survival (from 95% to 88%). The study was not powered to test the validation of the prognostic biomarkers. Associations between prognostic biomarkers and disease progression/relapse (because no death and secondary malignancy was observed) are tested at the 0.02 (0.10/5) level to account for multiple testing. Statistical methods used included Fisher exact test and Kaplan-Meier estimates of the OS (time from enrollment to death) and EFS (time from enrollment to relapse, secondary malignancy, death) curves. Data frozen on December 31, 2014 were used.
RESULTS
After prospective centralized review, 120 patients were enrolled on the AREN0532 therapeutic study between October 2006 and August 2013. Four of these patients were later found to be ineligible because of lack of lymph node sampling (n = 3) or inadequate consent procedures (n = 1). Approximately, 108 patients had adequate tumor tissue for biological prognostic analysis. Patients were recruited from COG institutions in the United States, Canada, Australia, New Zealand, and Israel.
Clinical Description of the Cohort
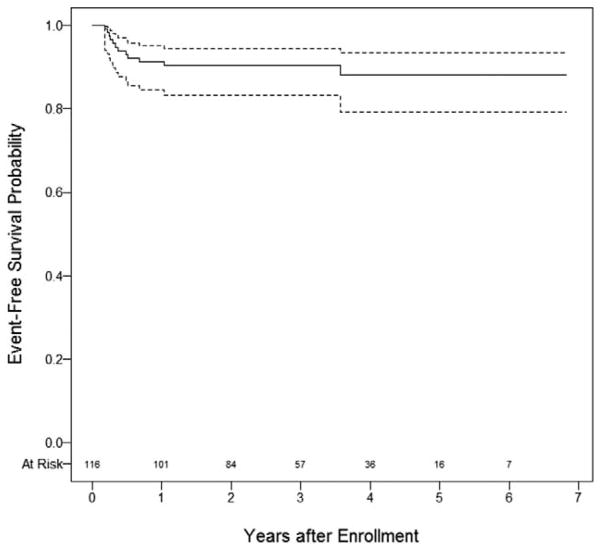
As of December 31, 2014, the median follow-up was 80 months (range: 5 – 97 months). Children were diagnosed at a median age of 11.5 months (range: 0.1 – 23 months). The estimated 4-year EFS was 89.7% [95% confidence interval (CI) 84.1–95.2%] (Figure 1). Twelve patients suffered relapse; the median time from enrollment to first relapse was 4.3 months (range of 2.3 – 44 months). The OS was 100%. No patient experienced a secondary malignancy. The EFS was the same as relapse-free survival. The clinical characteristics of the 12 patients who relapsed are described in Table 1. Relapses occurred in the lung (n = 5), tumor bed (n = 4), and abdomen (n = 2). Baseline and relapse CT scans for patients with recurrence were reviewed and there was no evidence of missed lesions at diagnosis to account for relapse. Similarly, no distinctive concerns were raised on re-review of the operative notes for those with local relapse. Only 1/116 patients developed a metachronous tumor at the median 80 months follow-up. The patient specific postrelapse therapy is indicated in Table 1. All but one relapsed patient followed protocol stipulated therapy.
FIGURE 1.
Event free survival for very low-risk Wilms tumor patients managed by observation alone after nephrectomy (solid line; dashed lines 95% confidence intervals).
TABLE 1.
Clinical Characteristics of Very Low-risk Wilms Tumor Patients Managed by Observation Alone Postnephrectomy Who Subsequently Relapsed
| UPN | Age at Diagnosis (Months) | Tumor Weight at Diagnosis (Grams) | Histology at Diagnosis | ILN at Diagnosis | PLN at Diagnosis | Time to Relapse (Days)* | Site of First Relapse | Symptoms at Relapse | Chemotherapy | Radiotherapy |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | 250 | Mixed | None | None | 68 | Lung | No | DD4A | Whole lung |
| 78 | 9 | 261 | Blastemal | None | None | 138 | Lungs | No | DD4A | Whole lung |
| 154 | 5 | 508 | Blastemal | None | None | 376 | Retroperitoneum | No | DD4A | Flank |
| 165 | 14 | 505 | Blastemal | None | None | 120 | Lung | Yes | DD4A | Whole lung |
| 399 | 10 | 271 | Mixed | None | None | 91 | Retroperitoneum | No | DD4A | Flank |
| 434 | 3 | 254 | Blastemal | None | None | 94 | Right renal fossa | No | DD4A | Delayed Flank |
| 450 | 15 | 178 | Epithelial tubular | None | None | 80 | Contra lateral kidney | No | DD4A | Flank |
| 476 | 12 | 252 | Mixed | None | None | 186 | Right renal fossa/Lungs | No | DD4A | Flank/Whole lung |
| 500 | 20 | 490 | Mixed | Present-multiple | None | 178 | Abdomen | Yes | DD4A | Whole abdomen |
| 508 | 5 | 270 | Mixed | Present | None | 1305 | Lung | No | DD4A | No |
| 756 | 13 | 540 | Mixed | Present | None | 107 | Lung | No | DD4A | Whole lung |
| 800 | 23 | 169 | Mixed | Present | None | 246 | Retroperitoneum | Yes | DD4A | abdomen |
Time to relapse from time of enrolment.
DD4A indicates vincristine, dactinomycin, doxorubicin × 24 weeks; ILN, intralobar, nephrogenic rests; PLN, perilobar nephrogenic rests; UPN, unique patient number.
No deaths have been reported to date although one child has had four relapses and was reported to have active disease at the most recent follow up. That child had initial blastemal predominant histology at first relapse and subsequently was found to have ana-plastic histology. He has been treated with chemotherapy and autologous bone-marrow transplant.
HISTOLOGY
Pathologic features of the tumors which relapsed are provided in Table 1. This validates the previous study: neither the predominant baseline histologic subtype (epithelial, stromal, blastemal, mixed, P = 0.16) nor the presence of intralobar (P = 0.46), or perilobar (P = 1.0) nephrogenic rests were associated with relapse. Pathology reports of relapse specimens were submitted to COG by the institution but central review of slides was not required, although seven sites did so. All initial relapses were reported to be offavorable histology except for the multiply relapsed patient who developed anaplasia.
Molecular Correlation With Outcome
The majority of patients had sufficient tissue submitted to assess biological predictors of outcome (n = 108). The presence of 1q gain, 1p loss, and/or 16q loss and mutation of WT1 were not statistically associated with relapse (Table 2). Of interest, only three tumors (3%) had both 1p and 16q loss and only six (5.5%) of tumors had gain of 1q compared with previously published overall frequencies of 4.7% and 27%, respectively, in all WT pateints.9,12 In contrast, the methylation status of 11p15 was correlated with relapse (P = 0.011) (Table 3). The 11p15 methylation status of the patients who relapsed is shown in Table 4. For the most part, genetic or epigenetic abnormalities were confined to LOH or LOI 11p15. Of note, few epithelial predominant WT (6/44) had LOH or LOI of 11p15.
TABLE 2.
Molecular Biomarkers of LOH 16q, LOH 1p, Gain of 1q, and WT1 Mutations are not Associated With Relapse in Very Low-risk Patients With Wilms Tumor Managed With Observation Alone Postnephrectomy
| Molecular Status | Relapse No, N (%) | Relapse Yes, N (%) | Total (N) | P* |
|---|---|---|---|---|
| LOH 16q loss NO | 91 (89.2) | 11 (10.8) | 102 | 1.000 |
| LOH 16q loss YES | 7 (87.5) | 1 (12.5) | 8 | |
| Total | 98 (89.1) | 12 (10.9) | 110 | |
| LOH 1p loss NO | 95 (89.6) | 11 (10.4) | 106 | 0.374 |
| LOH 1p loss YES | 3 (75) | 1 (25) | 4 | |
| Total | 98 (89.1) | 12 (10.9) | 110 | |
| 1q gain NO | 94 (89.5) | 11 (10.5) | 105 | 0.505 |
| 1q gain YES | 5 (83.3) | 1 (16.7) | 6 | |
| Total | 99 (89.1) | 12 (10.1) | 111 | |
| WT1 NO | 87 (89.7) | 10 (10.3) | 97 | 0.647 |
| WT1 YES | 12 (85.7) | 2 (14.3) | 14 | |
| Total | 99 (89.2) | 12 (10.8) | 111 |
P values are based on Fisher exact test.
LOH indicates loss of heterozygosity; WT1 NO, WT1 mutation absent; WT1 YES, WT1 mutation present.
TABLE 3.
11p15 Status is Associated With Relapse in Very Low-risk Patients With Wilms Tumor Managed With Observation Alone Postnephrectomy
| 11p15 LOH | Relapse No | Relapse Yes | Total | P* |
|---|---|---|---|---|
| 11p15 LOH | 32 (80%) | 8 (20%) | 40 | 0.011 |
| 11p15 LOI | 6 (75%) | 2 (25%) | 8 | |
| 11p15 ROI | 58 (96.7%) | 2 (3.3%) | 60 | |
| Total | 96 (88.9%) | 12 (11.1%) | 108 |
P values are based on Fisher exact test.
LOH indicates loss of heterozygosity; LOI, loss of imprinting; ROI, retention of imprinting.
TABLE 4.
Molecular Characteristics of Very Low-risk Wilms Tumor Patients Managed by Observation Alone Postnephrectomy Who Subsequently Relapsed
| UPN | 11p15 Status | 1 WT1 Mutation | MLPA 1p Loss | LOH 1p Loss | MLPA 16q Loss | LOH 16q Loss | MLPA 1q Gain | LOH 1p and 16q Loss |
|---|---|---|---|---|---|---|---|---|
| 1 | LOH | No | No | No | No | No | No | No |
| 78 | LOH | No | No | No | No | No | No | No |
| 154 | LOH | No | No | No | No | No | No | No |
| 165 | LOI | No | No | No | No | No | No | No |
| 399 | LOH | No | No | No | No | No | No | No |
| 434 | LOH | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 450 | ROI | No | No | No | No | No | No | No |
| 476 | LOH | No | No | No | No | No | Yes | No |
| 500 | ROI | No | No | No | No | No | No | No |
| 508 | LOH | No | No | No | No | No | No | No |
| 756 | LOH | Yes | No | No | No | No | No | No |
| 800 | LOI | No | No | No | No | No | No | No |
MLPA indicates multiplex ligation-dependent probe amplification; LOH, loss of heterozygosity; LOI, loss of imprinting; ROI, retention of imprinting; UPN, unique patient number.
DISCUSSION
We describe with high confidence the validation of an effective strategy of nephrectomy only for patients with VLRWT and confirm that the normal imprinting pattern of 11p15 (ROI, LOH, or LOI) is associated with an especially low risk of relapse in these untreated patients. We were not able to replicate WT1 mutations as an independent risk factor for relapse.11 We confirm that the presence of nephrogenic rests, and histologic subtype are not associated with relapse,5,11 although LOH or LOI 11p15 was very uncommon in epithelial predominant WT, as also noted by Gadd.18 We further confirm that 1p and 16q copy number loss is uncommon in VLRWT. We establish that copy gain of 1q is likewise uncommon in VLRWT. There was no association of these biomarkers with relapse but these results may be because of insufficient statistical power. Lastly, we confirm that the risk for development of metachronous WTis very low in patients with VLRWT who lack evidence of an underlying syndrome.
Different clinical management approaches have been used for patients with VLRWT, all of which achieve outstanding OS. The strategy employed in the current study was observation only after nephrectomy and a protocol directed approach to relapse, which resulted in 4-year EFS of 89.7% and OS of 100%. The rationale for the observation-alone approach was to avoid toxicity associated with chemotherapy, including sinusoidal obstruction syndrome associated with dactinomycin in young infants19,20 and the complications of central venous catheters,21 especially in very young children with respect to risk for thrombosis and infection. A second approach is to administer vincristine/dactinomycin to patients with VLRWT, as is done for other patients with stage I FHWT. Data from NWTS-5 demonstrated that when patients with VLRWT were treated with vincristine/dactinomycin, the 5-year EFS and OS were 97% and 99%, respectively.6 A third approach is to give 10 weeks of vincristine alone, as published by the United Kingdom Children’s Cancer Group.22 This latter strategy resulted in a 93% (95 % CI, 87–96) 4-year EFS for stage I FHWT children less than 2 year of age. It does in fact very closely approximates the same 4-year EFS risk as the observation alone strategy we report (90%). The EFS for children between 2 and 4 years of age on the UK study was 87% (95 % CI, 76–93%) and those over 4 years 71% (95 % CI, 58–81%). A decision analysis to compare these three approaches showed equipoise in terms of OS.23 Whereas, the EFS improved by increasing the number of chemotherapy agents used after nephrectomy, toxicity also increased. Our current study confirmed prospectively that observation alone with chemotherapy and radiation postrelapse achieves an excellent OS.
Careful discussion with families about the risks and benefits of each of these strategies to achieve an excellent OS is merited. The observation only strategy avoids complications of a central line and chemotherapy but exposes approximately 10% of patients to the late effects of anthracyclines (albeit a relatively low dose) and radiation24–26 (as if they had originally presented with stage III or IV disease), that they may not have received had they been delivered standard vincristine/dactinomycin therapy or the vincristine alone strategy. The prospect of an increased risk of relapse and intensity of treatment prompted some clinicians and parents to avoid enrollment on this study.27 Attempts to expand an observation only strategy to a larger cohort of patients will need to take into account the impact of this concern in estimating accrual.
The above concerns may be alleviated by an ability to predict which infants with VLRWT have a greater risk of relapse. We first described11 and now verify the association between 11p15 LOH and LOI and relapse. These genetic and epigenetic alterations can be identified using a methylation-sensitive PCR assay with rapid turnaround time. 11p15 LOH or LOI identified a group of patients with a 20% to 25% risk of recurrence, as opposed to a 3% risk of recurrence in patients who had neither LOH nor LOI. These results suggest that a reasonable strategy would be to administer adjuvant chemotherapy to patients with VLRWT if they have 11p15 LOH or LOI. The trade-off is that even in this 11p15 LOH/LOI group, the majority would not have relapsed with the observation only strategy and thus are “over-treated” with chemotherapy. On the other hand, the OS is excellent even in the presence of LOH and LOI and the argument could therefore be made for continuing to follow all patients with VLRWT with observation only, in particular if the safety of reducing salvage intensity were to be studied. Either way, an observation approach for those without LOH or LOI yields a very low risk of relapse.
It is of interest that abnormal 11p15 methylation is present at the same frequency regardless of age in the tumors of patients with WT. However, the presence of abnormally methylated 11p15 was not associated with relapse in patients who receive adjuvant chemotherapy. This may be because of the fact that abnormal methylation of loci at 11p15 reflects multiple underlying pathogenetic abnormalities assumed to be unrelated, including gain of IGF2 and loss of WT1, as previously discussed.11 It is of further interest that the known predictors of outcome overall in patients with FHWT (1p, 16q loss, and 1q gain) are infrequent in patients less than 24 months of age. This suggests the possibility that many VLRWT may arise because of mechanisms that are not currently recognized. This is supported by the presence of the unique category of epithelial predominant WT in young infants who lack many of the histologic features commonly associated WT (including nephrogenic rests). Our ability to identify a group of tumors that lack 11p15 methylation abnormalities may therefore be best characterized as removing “typical” WT, leaving a group that overall has a better outcome. It is important to remember that this residual group remains heterogeneous, and some will continue to relapse.
Future directions should consider expanding the observation only strategy to a larger group of patients by eliminating tumor-nephrectomy weight limits and expanding the age range. Perlman et al11 have previously reviewed the rationale for restricting expansion to age 4 years based on United Kingdom Children’s Cancer Group data. Adherence to the current strategy of excluding patients with known predisposition syndromes would seem essential, especially in reducing the risk of metachronous relapse and the consequent devastating late impact of renal insufficiency or failure. Similarly, multiple studies have shown the negative prognostic significance of positive lymph nodes in WT28 and clinicians should continue to insist on adequate lymph node sampling as a predicate to observation alone. On the other hand, expansion of the age and weight criteria will likely result in some patients not having a central line inserted at nephrectomy and thus require a second anesthetic if found not to meet very low criteria allowing observation alone. The balance of these risks and benefits, and the use of our LOH or LOI 11p15 observations may assist clinicians and parents in their decision making. The COG renal tumors committee is currently considering these issues in designing the upcoming therapeutic trials. Given the excellent OS and the risk of late effects of salvage, consideration is also being given to reducing the recommended intensity of salvage therapy for those who do relapse in an effort to reduce late toxicity.
We conclude that observation only for very low-risk FH WT is an effective and safe strategy and confirm that 11p15 ROI is associated with a low risk of relapse. The risk of metachronous relapse appears very low. Incorporation of these findings into future COG trials is underway.
Supplementary Material
Acknowledgments
E. J. P. received R21 grant money from the NIH relevant to this study.
E. A. M., C. V. F., and J. A. K. received support for travel to meetings relevant to this study from the U10 grants cited above administered through the COG. J. A. K. has a grant pending in application from COG.
Y. Y. C., J. T., P. E. G., J. R. A., and J. S. D.’s institution received grant money relevant to this study from the U10 grants cited above administered through the COG. J. R. A. is currently employed at Merck but the manuscript was completed before this employment. J. S. D. holds a patent with St. Jude’s Research Hospital for the invention of telomerase antibody for which he receives royalties.
Funding: The study is supported by grants U10CA180886, U10CA180899, U10CA098543, U10CA098413, and U24CA114766 from the National Cancer Institute, National Institutes of Health (NIH), to support the Children’s Oncology Group (COG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to acknowledge the statistical contribution of Qi An, Children’s Oncology Group (COG) Data Center, University of Florida, Gainesville, Florida.
The authors also wish to thank the parents and children who enrolled on this study and the investigators of the COG and the many pathologists, surgeons, pediatricians, radiation oncologists, diagnostic imagers, and other health professionals who manage the children entered on the COG renal tumor studies.
Footnotes
Clinical Trials.gov identifier: NCT00352534.
Disclosure: Presented in part at the 2015 American Society of Clinical Oncology annual meeting, Chicago, IL, May 31, 2015.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
References
- 1.Dome JS, Fernandez CV, Mullen EA, et al. Children’s Oncology Group’s 2013 blueprint for research: renal tumors. Pediatr Blood Cancer. 2013;60:994–1000. doi: 10.1002/pbc.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dome JS, Graf N, Geller JI, et al. Advances in Wilms tumor treatment and biology: progress through international collaboration. J Clin Oncol. 2015;33:2999–3007. doi: 10.1200/JCO.2015.62.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassady JR, Tefft M, Filler RM, et al. Considerations in the radiation therapy of Wilms’ tumor. Cancer. 1973;32:598–608. doi: 10.1002/1097-0142(197309)32:3<598::aid-cncr2820320312>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Green DM, Jaffe N. The role of chemotherapy in the treatment of Wilms’ tumor. Cancer. 1979;44:52–57. doi: 10.1002/1097-0142(197907)44:1<52::aid-cncr2820440110>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Green DM, Breslow NE, Beckwith JB, et al. Treatment with nephrectomy only for small, stage I/favorable histology Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol. 2001;19:3719–3724. doi: 10.1200/JCO.2001.19.17.3719. [DOI] [PubMed] [Google Scholar]
- 6.Shamberger RC, Anderson JR, Breslow NE, et al. Long-term outcomes for infants with very low-risk Wilms tumor treated with surgery alone in National Wilms Tumor Study-5. Ann Surg. 2010;251:555–558. doi: 10.1097/SLA.0b013e3181c0e5d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppes MJ, Arnold M, Beckwith JB, et al. Factors affecting the risk of contralateral Wilms tumor development: a report from the National Wilms Tumor Study Group. Cancer. 1999;85:1616–1625. doi: 10.1002/(sici)1097-0142(19990401)85:7<1616::aid-cncr26>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Ritchey ML, Green DM, Thomas PR, et al. Renal failure in Wilms’ tumor patients: a report from the National Wilms’ Tumor Study Group. Med Pediatr Oncol. 1996;26:75–80. doi: 10.1002/(SICI)1096-911X(199602)26:2<75::AID-MPO1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Grundy PE, Breslow NE, Li S, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23:7312–7321. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 10.Sredni ST, Gadd S, Huang CC, et al. Subsets of very low-risk Wilms tumor show distinctive gene expression, histologic, and clinical features. Clin Cancer Res. 2009;15:6800–6809. doi: 10.1158/1078-0432.CCR-09-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlman EJ, Grundy PE, Anderson JR, et al. WT1 mutation and 11P15 loss of heterozygosity predict relapse invery low-risk Wilms tumors treated with surgery alone: a Children’s Oncology Group study. J Clin Oncol. 2011;29:698–703. doi: 10.1200/JCO.2010.31.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gratias EJ, Jennings LJ, Anderson JR, et al. Gain of 1q is associated with inferior event-free and overall survival in patients with favorable histology Wilms tumor: a report from the Children’s Oncology Group. Cancer. 2013;119:3887–3894. doi: 10.1002/cncr.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segers H, van den Heuvel-Eibrink MM, Williams RD, et al. Gain of 1q is a marker of poor prognosis in Wilms’ tumors. Genes Chromosomes Cancer. 2013;52:1065–1074. doi: 10.1002/gcc.22101. [DOI] [PubMed] [Google Scholar]
- 14.Hing S, Lu YJ, Summersgill B, et al. Gain of 1q is associated with adverse outcome in favorable histology Wilms’ tumors. Am J Pathol. 2001;158:393–398. doi: 10.1016/S0002-9440(10)63982-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott RH, Stiller CA, Walker L, et al. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet. 2006;43:705–715. doi: 10.1136/jmg.2006.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1998;16:237–245. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 17.Ludgate JL, Le Mee G, Fukuzawa R, et al. Global demethylation in loss of imprinting subtype of Wilms tumor. Genes Chromosomes Cancer. 2013;52:174–184. doi: 10.1002/gcc.22017. [DOI] [PubMed] [Google Scholar]
- 18.Gadd S, Huff V, Huang CC, et al. Clinically relevant subsets identified by gene expression patterns support a revised ontogenic model of Wilms tumor: a Children’s Oncology Group Study. Neoplasia. 2012;14:742–756. doi: 10.1593/neo.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czauderna P, Katski K, Kowalczyk J, et al. Venoocclusive liver disease (VOD) as a complication of Wilms’ tumour management in the series of consecutive 206 patients. Eur J Pediatr Surg. 2000;10:300–303. doi: 10.1055/s-2008-1072380. [DOI] [PubMed] [Google Scholar]
- 20.Bisogno G, de Kraker J, Weirich A, et al. Veno-occlusive disease of the liver in children treated for Wilms tumor. Med Pediatr Oncol. 1997;29:245–251. doi: 10.1002/(sici)1096-911x(199710)29:4<245::aid-mpo2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Journeycake JM, Buchanan GR. Catheter-related deep venous thrombosis and other catheter complications in children with cancer. J Clin Oncol. 2006;24:4575–4580. doi: 10.1200/JCO.2005.05.5343. [DOI] [PubMed] [Google Scholar]
- 22.Pritchard-Jones K, Kelsey A, Vujanic G, et al. Older age is an adverse prognostic factor in stage I, favorable histology Wilms’ tumor treated with vincristine monochemotherapy: a study by the United Kingdom Children’s Cancer Study Group, Wilm’s Tumor Working Group. J Clin Oncol. 2003;21:3269–3275. doi: 10.1200/JCO.2003.01.062. [DOI] [PubMed] [Google Scholar]
- 23.Frazier AL, Shamberger RC, Henderson TO, et al. Decision analysis to compare treatment strategies for Stage I/favorable histology Wilms tumor. Pediatr Blood Cancer. 2010;54:879–884. doi: 10.1002/pbc.22396. [DOI] [PubMed] [Google Scholar]
- 24.Cotton CA, Peterson S, Norkool PA, et al. Early and late mortality after diagnosis of Wilms tumor. J Clin Oncol. 2009;27:1304–1309. doi: 10.1200/JCO.2008.18.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange JM, Takashima JR, Peterson SM, et al. Breast cancer in female survivors of Wilms tumor: a report from the national Wilms tumor late effects study. Cancer. 2014;120:3722–3730. doi: 10.1002/cncr.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Termuhlen AM, Tersak JM, Liu Q, et al. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57:1210–1216. doi: 10.1002/pbc.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez CV, Li N, Mullen EA, et al. Barriers to the enrollment of children in the Children’s Oncology Group study of very low-risk Wilms tumor: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2011;33:521–523. doi: 10.1097/MPH.0b013e31821b8dd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrlich PF, Anderson JR, Ritchey ML, et al. Clinicopathologic findings predictive of relapse in children with stage III favorable-histology Wilms tumor. J Clin Oncol. 2013;31:1196–1201. doi: 10.1200/JCO.2011.41.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.