Abstract
It has been proposed that membrane microdomains, caveolae, in vascular cells are critical for signal transduction and downstream functions induced by angiotensin II (AngII). We have tested our hypothesis that caveolin-1 (Cav1), a major structural protein of vascular caveolae, plays a critical role for development of vascular remodeling by AngII via regulation of epidermal growth factor receptor (EGFR) and vascular endothelial adhesion molecule-1 (VCAM-1). Cav1−/− and control Cav+/+ mice were infused with AngII for 2 weeks to induce vascular remodeling and hypertension. Upon AngII infusion, histological assessments demonstrated medial hypertrophy and perivascular fibrosis of aorta and coronary and renal arteries in Cav1+/+ mice compared with sham-operated Cav1+/+ mice. AngII-infused Cav1+/+ mice also showed a phenotype of cardiac hypertrophy with increased heart weight to body weight ratio compared with control Cav1+/+ mice. In contrast, Cav1−/− mice infused with AngII showed attenuation of vascular remodeling but not cardiac hypertrophy. Similar levels of AngII-induced hypertension were found in both Cav1+/+ and Cav1−/− mice as assessed by telemetry. In Cav1+/+ mice, AngII enhanced tyrosine-phosphorylated EGFR staining in the aorta, which was attenuated in Cav1−/− mice infused with AngII. Enhanced Cav1 and VCAM-1 expression was also observed in aorta from AngII-infused Cav1+/+ mice but not in Cav1−/− aorta. Experiments with vascular cells further provided a potential mechanism for our in vivo findings. These data suggest that Cav1, and presumably caveolae, in vascular smooth muscle and the endothelium plays a critical role in vascular remodeling and inflammation independent from blood pressure or cardiac hypertrophy regulation.
Keywords: Hypertension, Angiotensin II, Signal Transduction, Fibrosis, Hypertrophy
Subject codes: [15] Hypertrophy, [97] Other Vascular biology, [115] Remodeling, [128] ACE/Angiotension receptors, [149] Hypertension - basic studies.
Introduction
Hypertension is a disease marked by chronic vascular dysfunction and inflammation facilitating cardiovascular remodeling and subsequent end-organ damage, which contribute to increased rates of morbidity and mortality 1, 2. Past investigation in hypertensive patients and animal models has given evidence that mechanisms facilitating elevations in blood pressure and end-organ damage should be independent, at least partially 3. One of the key contributors to hypertension and the hypertensive response is the renin-angiotensin system and specifically, the vasoactive peptide angiotensin II (AngII) 4. In mouse models of AngII-induced hypertension, multiple distinct mechanisms involving endothelial cells (ECs) 5, vascular smooth muscle cells (VSMCs) 6, 7, adventitial fibroblasts 8 or bone marrow-derived cells 9 appear to mediate cardiovascular remodeling and/or end-organ damage but not hypertension. Our recent findings suggest that transactivation of epidermal growth factor receptor (EGFR) mediated by a caveolae-localized metalloproteinase, ADAM17, is required for cardiovascular remodeling independent from blood pressure regulation 7, 10. However, this mechanism may or may not be limited to VSMCs 11.
Caveolin-1 (Cav1) is a major structural component of caveolae, which are cholesterol-rich membrane microdomains that act as signaling platforms in facilitating specific signal transduction events including those activated by the AngII type 1 receptor 12, 13. Cav1 is expressed in both vascular smooth muscle and the endothelium, and is implicated in several cardiovascular diseases including atherosclerosis, dilated cardiomyopathy, pulmonary hypertension and abdominal aortic aneurysm 13–15. Regarding hypertension, it has been well documented that Cav1 inhibits endothelial nitric oxide synthase activity and contributes to maintenance of myogenic tone in the vasculature 13. However, there are numerous conflicting reports utilizing Cav1 deficient mice which may not support a direct role for Cav1 in blood pressure regulation in hypertension (reviewed in 14). This could be due to vascular compensation associated with hypertrophic arterial remodeling, impaired endothelium-dependent hyperpolarization and or contribution of the 129SVJ strain 14. In addition, ligation-induced carotid artery neointimal hyperplasia is enhanced in Cav1 deficient mice 16. However, endothelial inflammatory activation such as induction of vascular endothelial adhesion molecule-1 (VCAM-1) and atherosclerosis are attenuated in ApoE Cav1 double deficient mice 17, which appears to involve endothelial Cav1 18. Regarding cardiac remodeling, Cav1 deficient mice have been reported to develop cardiac hypertrophy and fibrosis, however there is conflicting data concerning the left ventricular wall thickness and cardiac function 14. Moreover, very limited information is available regarding how the lack of Cav1 alters blood pressure and hypertensive cardiovascular remodeling in mouse models of hypertension such as those induced by AngII.
Based on the above information, we have tested our novel hypothesis that deletion of Cav1 will attenuate hypertensive vascular remodeling (vascular hypertrophy and perivascular fibrosis) independent from hypertension and cardiac hypertrophy in mice infused with AngII. Associated signaling mechanisms such as vascular EGFR transactivation and VCAM-1 induction have also been studied.
Methods
An expanded Methods section is available in the online-only Data Supplement.
Animal Experiments
All animal procedures were performed with prior approval of the Temple University Institutional Animal Care and Use Committee and in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. 8–10 week male Cav1−/− and Cav1+/+ (C57Bl6) mice were infused with AngII (1 μg/kg/min) for 2 weeks via implanted osmotic mini-pump or sham-operated for the implant 10.
Cell Experiments
VSMCs were prepared from thoracic aorta of male Sprague-Dawley rats using the explant method as previously described 19. Sprague-Dawley rat aortic endothelial cells (RAECs) were purchased from Cell Biologics. THP-1 human monocyte cells were obtained from ATCC.
Statistical Analysis
Data are presented as mean±SEM or SD where appropriate. Differences between the multiple groups were analyzed by 1-way or 2-way ANOVA, followed by the Tukey’s post hoc test. Statistical significance was set at p<0.05.
Results
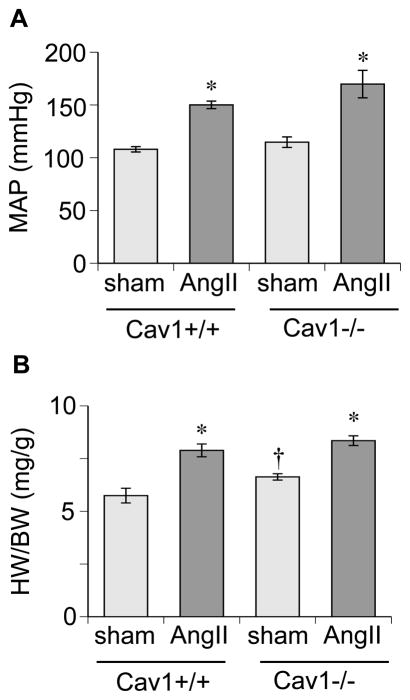
Compared to sham-operated Cav1+/+ mice, AngII infused Cav1+/+ mice showed a marked elevation in mean blood pressure assessed by telemetry. AngII infused Cav1−/− mice exhibited increased mean arterial blood pressure similar to that observed in Cav1+/+ with AngII infusion, whereas diastolic blood pressure responded higher than in control mice (Figure 1A and Online Table S1). Heart weight to body weight ratio and echocardiogram were used to assess cardiac hypertrophy. Both Cav1+/+ and −/− mice infused with AngII showed a phenotype of cardiac hypertrophy including enhanced LV volume (Figure 1B and Online Table S1). In addition, sham-operated Cav1−/− mice exhibited greater values in the heart weight ratio, IVSd, IVSs and LVPWs compared with Cav1+/+ mice.
Figure 1.
Cav1 silencing does not alter hypertension or cardiac hypertrophy in mice infused with AngII. Cav1−/− and control +/+ mice were infused with AngII (1 μg/kg/min) via osmotic mini pump for 2 weeks or sham operated for pump implantation. A: Mean arterial pressure (MAP) was evaluated by telemetry. B: Heart weight (HW) to body weight (BW) ratio was evaluated. Mean±SEM (n=5–6). *p<0.05 compared with control saline infusion. †p<0.05 compared with control Cav1+/+ mice.
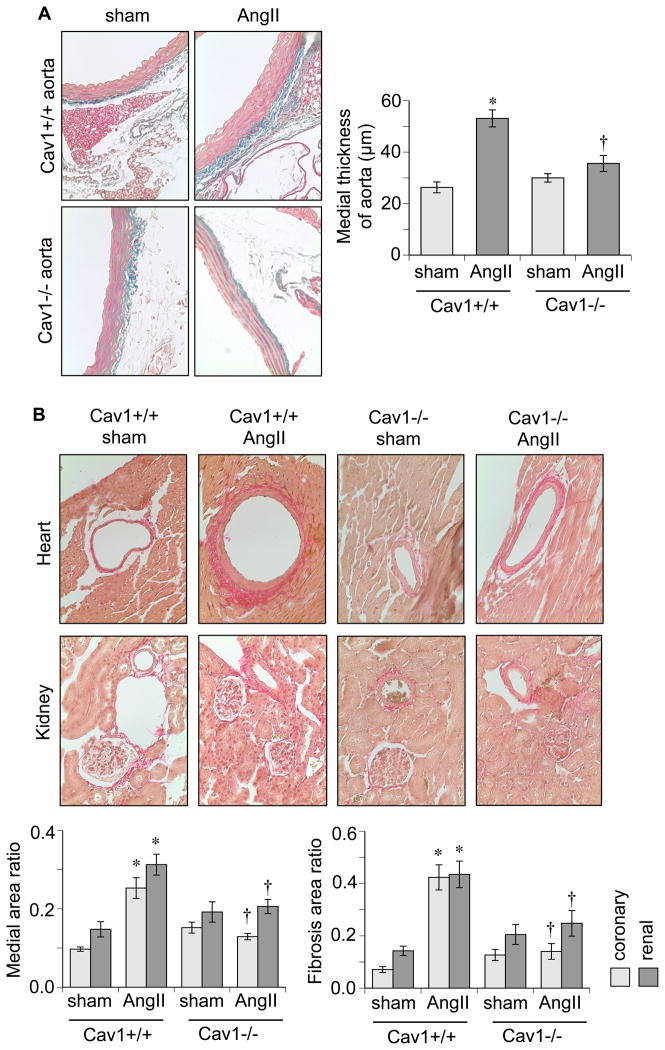
While Cav1−/− mice developed cardiac hypertrophy in response to AngII, vascular alterations had differing results. AngII-infused Cav1+/+ mice exhibited increased medial thickness in the aorta, which was attenuated in Cav1−/− mice (Figure 2A). Cav1+/+ mice also developed marked medial hypertrophy and perivascular fibrosis in hearts and kidneys in response to AngII. However, Cav1−/− mice showed attenuation of these responses to AngII (Figure 2B).
Figure 2.
AngII-induced vascular remodeling is attenuated in Cav1 deficient mice. A: Representative staining of the thoracic aorta (100x) and quantification of the medial thickness are presented. B: Representative staining of the coronary and renal arteries (200x), quantification of medial area to internal arterial area of the arteries, and quantification of perivascular fibrosis area to vascular area of the arteries are presented. Mean±SEM (n=4–6). *p<0.05 compared with control saline infusion. †p<0.05 compared with control Cav1+/+ mice.
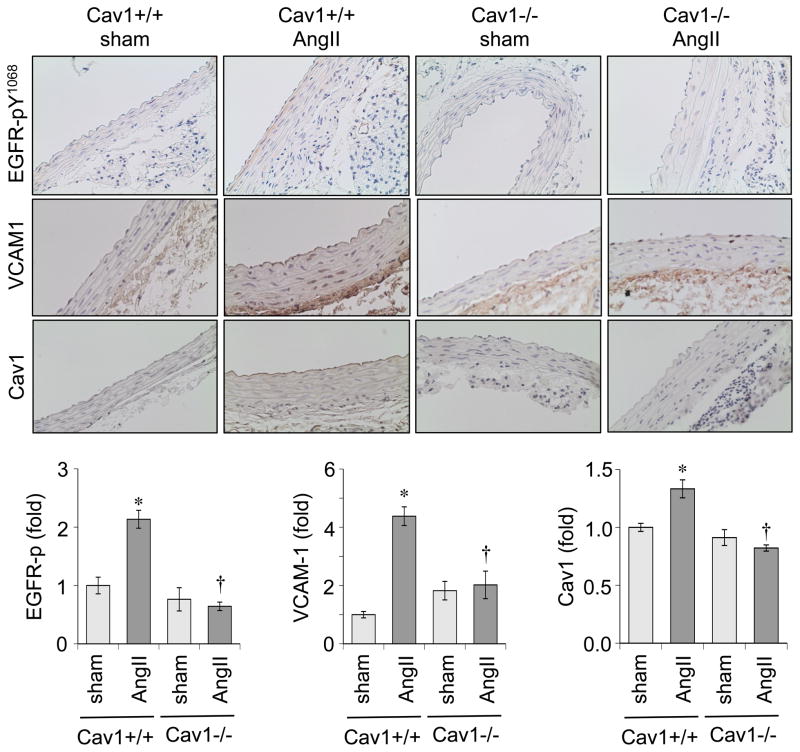
To investigate a potential signaling mechanism that is critical for AngII-induced vascular remodeling, we performed immunohistochemistry with aortic sections. AngII infused Cav1+/+ mice showed induction of phosphorylated EGFR at Tyr1068 (an auto-phosphorylation site), which was attenuated in Cav1−/− mice. AngII infusion also increased Cav1 staining in the endothelium and medial layers of the aortas in Cav1+/+ mice, which was attenuated in Cav1−/− mice. In addition, AngII infused Cav1+/+ mice showed increased VCAM-1 staining in the endothelium and adventitia which was attenuated in Cav1−/− mice infused with AngII (Figure 3).
Figure 3.
Vascular EGFR activation and VCAM-1 induction in response to AngII infusion was attenuated in Cav1 deficient mice. Aorta sections were immuno-stained with antibodies as indicated. The staining intensity was quantified in medial layers (EGFR-p and Cav1) or adventitia (VCAM-1). Mean±SEM (n=4). *p<0.05 compared with control saline infusion. †p<0.05 compared with control Cav1+/+ mice.
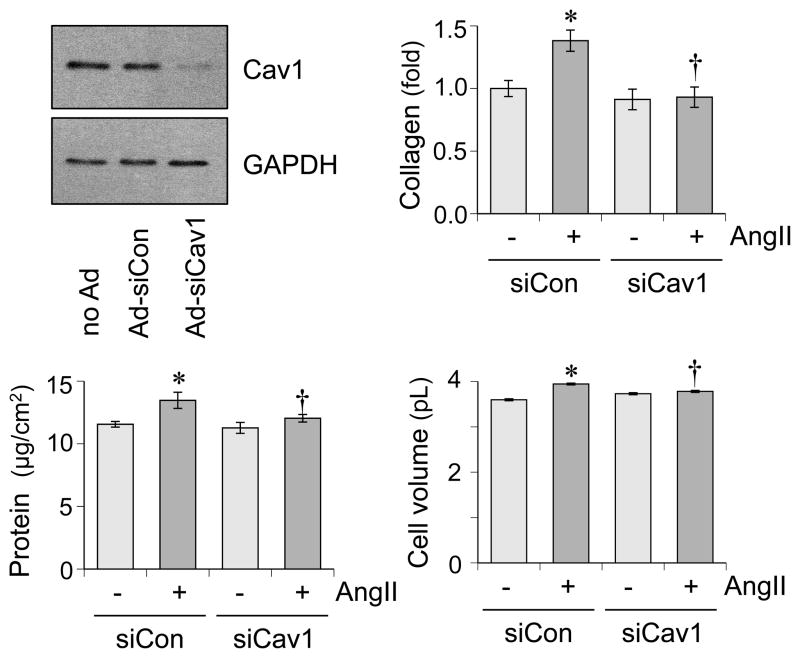
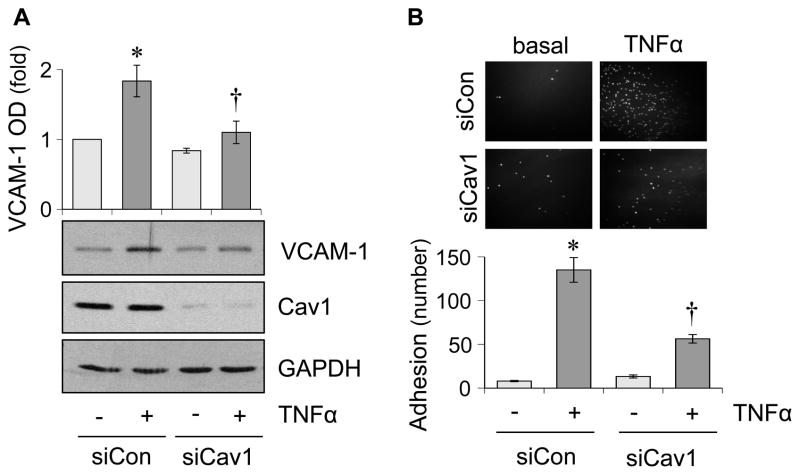
To support our in vivo findings, we have utilized cultured rat aortic VSMCs and ECs. AngII-induced fibrotic and hypertrophic responses are attenuated in VSMCs pretreated with Cav1 silencing adenovirus (Figure 4). Mitochondrial reactive oxygen species (ROS) appear critical for hypertension and cardiac hypertrophy induced by AngII 20. MitoTimer reporter, which encodes a mitochondria-targeted protein producing irreversible red fluorescence when oxidized 21, was used to determine if Cav1 in VSMC is critical for mitochondrial ROS production. AngII-induced mitochondrial ROS production is attenuated by a mitochondria-specific ROS scavenger, mitoTempo in VSMCs. Upon Cav1 silencing in VSMCs, both basal and AngII-induced mitochondrial ROS production are enhanced (Online Figure S1). Therefore, mitochondrial ROS production appears independent from the mechanism by which Cav1 silencing inhibits AngII-induced vascular remodeling, while it may participate to hypertension and cardiac hypertrophy. In RAECs, tissue necrosis factor-α (TNFα) but not AngII is able to induce VCAM-1 expression in ECs. Cav1 silencing is able to partially reduce TNFα-induced VCAM-1 expression (Figure 5A). Moreover, Cav1 silencing prevented leucocyte adhesion to RAECs in response to TNFα (Figure 5B).
Figure 4.
Cav1 silencing attenuates AngII-induced VSMC remodeling in vitro. VSMCs were pretreated with adenovirus encoding Cav1 silencing siRNA or control non-silencing siRNA for 48 hours and were stimulated with AngII (100 nmol/L) for 48 hours. Extracellular collagen accumulation, total cell protein and cell volume were evaluated. Means ± SEM (n=4). *p<0.05 compared with basal. †p<0.05 compared with control AngII stimulation.
Figure 5.
Cav1 silencing attenuates TNFα-induced VCAM-1 induction and leucocyte adhesion in endothelial cells. A. RAECs were pretreated with adenovirus encoding Cav1 silencing siRNA or control non-silencing siRNA for 48 hours and were stimulated with TNFα (10 ng/mL) for 6 hours. The cell lysates were analyzed by immunoblotting as indicated. B. RAECs transduced with Cav1 siRNA or control siRNA were stimulated with TNFα (10 ng/mL) for 6 hours and then incubated with THP-1 monocytes for 30 min. Means ± SEM (n=4). *p<0.05 compared with basal. †p<0.05 compared with control TNFα stimulation.
Discussion
Here we report that Cav1 is a critical mediator of hypertensive vascular remodeling and inflammation in mice infused with AngII. Previous studies by others and our group have highlighted the critical roles Cav1 plays in mediating AngII-induced signal transduction in vitro 12, 19, whereas its role in AngII-induced pathophysiological effects has been unclear. The present study builds a new concept that vascular Cav1 specifically mediates hypertensive vascular remodeling without altering hypertension. The vascular protective role of Cav1 silencing appears consistent with past reports utilizing Cav1−/− mice in models of atherosclerosis 17, abdominal aortic aneurysm 15, and brain microvascular hypertrophy 22. In the present study, prevention of vascular remodeling in Cav1−/− mice infused with AngII correlated with reductions in EGFR activation in the vasculature. In addition, we found that Cav1 silencing has a protective effect against AngII-mediated increases in hypertrophy and collagen synthesis in vitro in VSMCs. We have previously studied the mechanism of VSMC signaling through Cav1 and demonstrated that Cav1 is a requisite component of ADAM17 activation and gene induction 15. We have also shown that ADAM17 activation is required for EGFR transactivation in VSMCs 23. Inhibition of ADAM17 or EGFR activity reduced hypertrophic and fibrotic responses to AngII in VSMCs 7. The mechanism of Cav1 acting via ADAM17-dependent EGFR activation is further supported by reduction of AngII-induced EGFR activation in Cav1−/− aorta. Moreover, AngII-induced vascular remodeling was attenuated with EGFR inhibitor or vascular ADAM17 silencing in vivo 7, 10 .
A recent study further suggests a critical link between Cav1 and EGFR. Hypoxia inducible factor-1 was shown to up-regulate Cav1 leading to EGFR transactivation at caveolae in cancer cells 24. AngII-induced vascular remodeling was attenuated in VSMC specific hypoxia inducible factor-1α deficient mice 6. AngII can induce hypoxia inducible factor-1α in VSMCs, which also increases ADAM17 gene induction 25. Cav1 was induced in the vasculature in response to AngII in the present and past studies 26. Therefore, it is intriguing to speculate that AngII promotes several feed forward mechanisms including Cav1 induction to amplify the EGFR signaling pathway and subsequent vascular remodeling.
We are aware of the reported baseline phenotypes of Cav1−/− mice where enhanced arterial hypertrophy or fibrotic responses such as in lung and kidney have been reported 13, 14. However, we did not observe any significant difference in baseline medial thickness in the aortas and only marginal enhancement of vascular medial area and perivascular fibrosis area in −/− mice. While we do not have any mechanistic explanation for these discrepancies, it may be due to the genetic background of C57Bl/6 strain and/or the relatively young age at analysis. A protective role for Cav1 against fibrosis has been reasoned through its negative alterations on tissue growth factor-β (TGFβ) activation and function 27. In contrast, a recent study using Cav1 deficient fibroblasts demonstrates that Cav1 promotes extracellular matrix remodeling and stiffness 28. Since TGFβ is critical for the AngII-induced cardiovascular fibrotic response 29, further clarification is desired for the potential cross-talk between Cav1 and TGFβ in mediating perivascular fibrosis in hypertension.
In support of several previous reports 14, 30, we did not notice any alterations in blood pressure in sham-operated mice of both Cav1+/+ and Cav1−/− genotypes. Increased systolic and mean blood pressure was found in both Cav1+/+ and Cav1−/− mice treated with AngII compared to control mice with no differences between the AngII groups. These data are consistent with past reports infusing L-NAME 31 or AngII plus L-NAME 30 in Cav1−/− mice. However, enhanced blood pressure responses have been reported with L-NAME plus AngII infusion 32 and high fat diet 33 in Cav1−/− mice whereas reduced blood pressure response has been reported with AngII infusion in Cav1+/− mice 34. In the present study, enhanced diastolic blood pressure was also observed in Cav1−/− mice in response to AngII infusion, which may involve the role of Cav1 in promoting AngII desensitization in arterial contraction 33.
A decrease in vascular remodeling should reduce the high blood pressure response to AngII. However, in our 2 week AngII infusion model with both genetic (Cav1 or ADAM17 deletion 7) and pharmacological (erlotinib or 4-phenylbutyrate 10) interventions, we have observed a suppression of vascular remodeling and no reduction in hypertension. One potential explanation is the relatively short duration of our studies. In mice with vascular hypoxia-inducible factor-1 silencing, AngII-induced vascular remodeling is attenuated at 4 weeks whereas a reduction of blood pressure is far more noticeable at 4 weeks than 2 weeks 6. Alternatively, the high concentration of AngII used in our protocol may maintain hypertension even with the reduction in vascular remodeling. Many published articles using a dose of AngII similar to ours indicate near maximal hypertensive responses occurring within a few days 35, 36. A study has looked at the time course of vascular remodeling in response to AngII in mice. The study shows that gradual arterial hypertrophy becomes noticeable at day 3 and keeps developing for 4 weeks 37, suggesting that vascular remodeling is irreverent for the establishment of hypertension with high dose of continued AngII infusion. By contrast, 4-phenylbutyrate reduced both cardiac fibrosis and hypertension induced by AngII with 60% less infusion rate 38 than our studies. In addition, enhanced mitochondrial ROS production from VSMCs and presumably from ECs in response to AngII may also mediate hypertension in Cav1−/− mice infused with AngII.
At 2 months of age and older, Cav1−/− mice of different genetic backgrounds exhibited several pathophysiological cardiac phenotypes including cardiac hypertrophy, decreased contractility and or dilated cardiomyopathy 39, 40. Our Cav1−/− mice at 10 week of age show moderate cardiac hypertrophy with preserved contractility, which is consistent with a past publication analyzing cardiac function of 6–8 week old Cav1−/− mice 41. Several mechanisms for the basis of cardiac hypertrophy in Cav1−/− mice have been reported including an endothelium-dependent mechanism. Endothelial specific Cav1 re-expression in Cav1−/− mice rescues the cardiac phenotype 13, 42. AngII type 1 receptor blocker attenuates cardiac pathology of Cav1−/− mice suggesting a contribution of AngII 43. Enhanced nitrosative stress 44 and fibroblast ERK activation 40 are also observed in hearts of Cav1−/− mice. In the present study, AngII infusion enhanced Cav1−/− mouse cardiac hypertrophy to the levels seen in wild-type mice with AngII infusion. However, in a previous study in Cav1−/− mice infused with AngII plus L-NAME, no significant enhancement was observed in heart weight to body weight ratio 30. While we have no mechanistic explanation for this discrepancy, it is possible that L-NAME treatment helps to reduce nitrosative stress in Cav1−/− mice. Lack of an accelerated hypertrophic response in Cav1−/− compared with wild type mice with AngII infusion likely involves a common signaling mechanism of the AngII type 1 receptor in mediating both base-line and AngII-induced cardiac hypertrophy. Alternatively, an additional signaling mechanism such as enhanced mitochondrial ROS could be involved in cardiac hypertrophy induced by AngII in Cav1−/− mice. It may also involve distinct genetic backgrounds of Cav1−/− mice as mixed B6;129S background mice were used in the AngII plus L-NAME study.
Little is known about the role of endothelial Cav1 in AngII-induced pathophysiology. Cav1 has been proposed to mediate AngII-induced uncoupling of endothelial nitric oxide synthase to cause oxidative stress in endothelial cells 34. The present study further suggests a role for endothelial Cav1 in mediating vascular inflammation in hypertension via VCAM-1 induction. Our findings are consistent with a reported attenuation of TNFα-induced lymphocyte adhesion to microvascular ECs 45 by Cav1 silencing. Cav1 silencing also attenuated TNFα-induced VCAM-1 induction in human umbilical vein ECs 46. In addition, endothelial VCAM-1 induction in atherosclerosis was attenuated in Cav1−/− mice 17. In ECs, nuclear factor κ-B activation is critical for TNFα-induced VCAM-1 induction 47. Nuclear factor κ-B inhibition is likely involved for suppression of VCAM-1 induction in ECs according to the literature 46.
Taken together, these data indicate the mechanism of suppression of AngII-induced vascular remodeling with Cav1 silencing should involve inhibition of VSMC ADAM17/EGFR activation and suppression of endothelial inflammation via inhibition of VCAM-1 induction (Online Figure S2). VCAM-1 conditional knockout mice are available. Further inclusion of such mice as well as a rescue experiment with an EGFR agonist could be tested to support our conclusion. In addition, limitations of the present study include the lack of assessment of the developmental relationship between vascular remodeling and hypertension with lower AngII infusion, as well as tibia length normalization of the heart weights in our assessments on cardiac hypertrophy.
Perspective
Our findings highlight the complexity and critical role of Cav1 in mediating hypertensive vascular remodeling and inflammatory signaling. We propose Cav1 is a needed component for EGFR transactivation contributing to hypertensive vascular remodeling. Our results also indicate a role for Cav1 in vascular inflammation as we noted a requirement for Cav1 in endothelial VCAM-1 induction. Vascular remodeling precedes end-organ damage in hypertension. In addition to atherosclerosis, Cav1 may serve as a novel therapeutic target in hypertension. Vascular specific targeting of Cav1 in hypertensive patients could provide a viable avenue in the treatment of this threatening disease. However, there is still more to uncover about Cav1. Specifically, a better understanding of how Cav1 contributes to normal physiological function compared to pathophysiological in cells and tissues is a needed area of research before viable treatment options may be introduced.
Supplementary Material
Novelty and Significance.
What is new?
Analyses of blood pressure and vascular pathology in the heart, kidney and aorta with intervention established a role for Cav1 in AngII-induced vascular remodeling independent of hypertension or cardiac hypertrophy in mice.
The concept of vascular Cav1 in mediating the EGFR pathway and VCAM-1 and subsequent vascular hypertrophy, fibrosis and inflammation was presented.
What is relevant?
Results indicating prevention of vascular remodeling but not hypertension by Cav1 silencing provide a foundation to seek a potential add-on therapy to current pressure lowering treatments for hypertension.
The vascular dominant Cav1 signal transduction highlights the importance of vascular signal transduction for subsequent tissue dysfunction in hypertension.
Summary.
In AngII-infused Cav1 deficient mice, perivascular fibrosis and vascular hypertrophy were prevented compared with infused wild type mice. AngII infusion showed vascular EGFR activation and induction of Cav1 and VCAM-1, which were attenuated in Cav1 deficient mice. Cultured vascular cells were utilized to confirm the direct contribution of vascular Cav1 in hypertensive cellular pathophysiology.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health grants, HL128324 (S.E. and V.R.), HL133248 (S.E.) and F31HL127971 (S.J.F.), and American Heart Association grants, 13GRNT17060036 (S.E.), 16GRNT30410007 (S.E.), 16GRNT30130013 (V.R.), 16POST3051004 (T.K.), and 15POST25550083 (K.J.E.).
Footnotes
Disclosures
None.
References
- 1.Briet M, Schiffrin EL. Treatment of arterial remodeling in essential hypertension. Curr Hypertens Rep. 2013;15:3–9. doi: 10.1007/s11906-012-0325-0. [DOI] [PubMed] [Google Scholar]
- 2.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weir MR. Effects of renin-angiotensin system inhibition on end-organ protection: can we do better? Clin Ther. 2007;29:1803–1824. doi: 10.1016/j.clinthera.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected] Pharmacol Rev. 2015;67:754–819. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor−/− mice. Circ Res. 2011;108:574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imanishi M, Tomita S, Ishizawa K, Kihira Y, Ueno M, Izawa-Ishizawa Y, Ikeda Y, Yamano N, Tsuchiya K, Tamaki T. Smooth muscle cell-specific Hif-1alpha deficiency suppresses angiotensin II-induced vascular remodelling in mice. Cardiovasc Res. 2014;102:460–468. doi: 10.1093/cvr/cvu061. [DOI] [PubMed] [Google Scholar]
- 7.Takayanagi T, Forrester SJ, Kawai T, Obama T, Tsuji T, Elliott KJ, Nuti E, Rossello A, Kwok HF, Scalia R, Rizzo V, Eguchi S. Vascular ADAM17 as a Novel Therapeutic Target in Mediating Cardiovascular Hypertrophy and Perivascular Fibrosis Induced by Angiotensin II. Hypertension. 2016;68:949–955. doi: 10.1161/HYPERTENSIONAHA.116.07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poduri A, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Cassis LA, Daugherty A. Fibroblast Angiotensin II Type 1a Receptors Contribute to Angiotensin II-Induced Medial Hyperplasia in the Ascending Aorta. Arterioscler Thromb Vasc Biol. 2015;35:1995–2002. doi: 10.1161/ATVBAHA.115.305995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Montaniel KR, Saleh MA, Xiao L, Chen W, Owens GK, Humphrey JD, Majesky MW, Paik DT, Hatzopoulos AK, Madhur MS, Harrison DG. Origin of Matrix-Producing Cells That Contribute to Aortic Fibrosis in Hypertension. Hypertension. 2016;67:461–468. doi: 10.1161/HYPERTENSIONAHA.115.06123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takayanagi T, Kawai T, Forrester SJ, Obama T, Tsuji T, Fukuda Y, Elliott KJ, Tilley DG, Davisson RL, Park JY, Eguchi S. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension. 2015;65:1349–1355. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrester SJ, Kawai T, O'Brien S, Thomas W, Harris RC, Eguchi S. Epidermal Growth Factor Receptor Transactivation: Mechanisms, Pathophysiology, and Potential Therapies in the Cardiovascular System. Annu Rev Pharmacol Toxicol. 2016;56:627–653. doi: 10.1146/annurev-pharmtox-070115-095427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ushio-Fukai M, Alexander RW. Caveolin-Dependent Angiotensin II Type 1 Receptor Signaling in Vascular Smooth Muscle. Hypertension. 2006;48:797–803. doi: 10.1161/01.HYP.0000242907.70697.5d. [DOI] [PubMed] [Google Scholar]
- 13.Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman A, Sward K. The role of caveolin-1 in cardiovascular regulation. Acta Physiol (Oxf) 2009;195:231–245. doi: 10.1111/j.1748-1716.2008.01907.x. [DOI] [PubMed] [Google Scholar]
- 15.Takayanagi T, Crawford KJ, Kobayashi T, Obama T, Tsuji T, Elliott KJ, Hashimoto T, Rizzo V, Eguchi S. Caveolin-1 is critical for abdominal aortic aneurysm formation induced by angiotensin II and inhibition of lysyl oxidase. Clin Sci (London, England : 1979) 2014;126:785–794. doi: 10.1042/CS20130660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan GS, Jasmin JF, Schubert W, Frank PG, Lisanti MP. Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry. 2004;43:8312–8321. doi: 10.1021/bi049609t. [DOI] [PubMed] [Google Scholar]
- 17.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Hernando C, Yu J, Suarez Y, Rahner C, Davalos A, Lasuncion MA, Sessa WC. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takaguri A, Shirai H, Kimura K, Hinoki A, Eguchi K, Carlile-Klusacek M, Yang B, Rizzo V, Eguchi S. Caveolin-1 negatively regulates a metalloprotease-dependent epidermal growth factor receptor transactivation by angiotensin II. J Mol Cell Cardiol. 2011;50:545–551. doi: 10.1016/j.yjmcc.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2013;19:1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laker RC, Xu P, Ryall KA, Sujkowski A, Kenwood BM, Chain KH, Zhang M, Royal MA, Hoehn KL, Driscoll M, Adler PN, Wessells RJ, Saucerman JJ, Yan Z. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J Biol Chem. 2014;289:12005–12015. doi: 10.1074/jbc.M113.530527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umesalma S, Houwen FK, Baumbach GL, Chan SL. Roles of Caveolin-1 in Angiotensin II-Induced Hypertrophy and Inward Remodeling of Cerebral Pial Arterioles. Hypertension. 2016;67:623–629. doi: 10.1161/HYPERTENSIONAHA.115.06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott KJ, Bourne AM, Takayanagi T, Takaguri A, Kobayashi T, Eguchi K, Eguchi S. ADAM17 silencing by adenovirus encoding miRNA-embedded siRNA revealed essential signal transduction by angiotensin II in vascular smooth muscle cells. J Mol Cell Cardiol. 2013;62:1–7. doi: 10.1016/j.yjmcc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Roche O, Xu C, Moriyama EH, Heir P, Chung J, Roos FC, Chen Y, Finak G, Milosevic M, Wilson BC, Teh BT, Park M, Irwin MS, Ohh M. Hypoxia promotes ligand-independent EGF receptor signaling via hypoxia-inducible factor-mediated upregulation of caveolin-1. Proc Natl Acad Sci U S A. 2012;109:4892–4897. doi: 10.1073/pnas.1112129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obama T, Takayanagi T, Kobayashi T, Bourne AM, Elliott KJ, Charbonneau M, Dubois CM, Eguchi S. Vascular induction of a disintegrin and metalloprotease 17 by angiotensin II through hypoxia inducible factor 1alpha. Am J Hypertens. 2015;28:10–14. doi: 10.1093/ajh/hpu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seto SW, Krishna SM, Yu H, Liu D, Khosla S, Golledge J. Impaired acetylcholine-induced endothelium-dependent aortic relaxation by caveolin-1 in angiotensin II-infused apolipoprotein-E (ApoE−/−) knockout mice. PLoS One. 2013;8:e58481. doi: 10.1371/journal.pone.0058481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gvaramia D, Blaauboer ME, Hanemaaijer R, Everts V. Role of caveolin-1 in fibrotic diseases. Matrix Biol. 2013;32:307–315. doi: 10.1016/j.matbio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibanez T, Pellinen T, Echarri A, Cerezo A, Klein-Szanto AJ, Garcia R, Keely PJ, Sanchez-Mateos P, Cukierman E, Del Pozo MA. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-L-arginine methyl ester and angiotensin II. Endocrinology. 2010;151:1236–1246. doi: 10.1210/en.2009-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desjardins F, Lobysheva I, Pelat M, Gallez B, Feron O, Dessy C, Balligand J-L. Control of blood pressure variability in caveolin-1-deficient mice: role of nitric oxide identified in vivo through spectral analysis. Cardiovasc Res. 2008;79:527–536. doi: 10.1093/cvr/cvn080. [DOI] [PubMed] [Google Scholar]
- 32.Pojoga LH, Yao TM, Opsasnick LA, Siddiqui WT, Reslan OM, Adler GK, Williams GH, Khalil RA. Cooperative Role of Mineralocorticoid Receptor and Caveolin-1 in Regulating the Vascular Response to Low Nitric Oxide-High Angiotensin II-Induced Cardiovascular Injury. J Pharmacol Exp Ther. 2015;355:32–47. doi: 10.1124/jpet.115.226043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czikora I, Feher A, Lucas R, Fulton DJ, Bagi Z. Caveolin-1 prevents sustained angiotensin II-induced resistance artery constriction and obesity-induced high blood pressure. Am J Physiol Heart Circ Physiol. 2015;308:H376–385. doi: 10.1152/ajpheart.00649.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobysheva I, Rath G, Sekkali B, Bouzin C, Feron O, Gallez B, Dessy C, Balligand JL. Moderate caveolin-1 downregulation prevents NADPH oxidase-dependent endothelial nitric oxide synthase uncoupling by angiotensin II in endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:2098–2105. doi: 10.1161/ATVBAHA.111.230623. [DOI] [PubMed] [Google Scholar]
- 35.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TA, Le TH, Pennathur S, Koller B, Coffman TM. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57:577–585. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Q, Ishibashi M, Hiasa K, Tan C, Takeshita A, Egashira K. Essential role of vascular endothelial growth factor in angiotensin II-induced vascular inflammation and remodeling. Hypertension. 2004;44:264–270. doi: 10.1161/01.HYP.0000138688.78906.6b. [DOI] [PubMed] [Google Scholar]
- 38.Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32:1652–1661. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y-Y, Liu Y, Stan R-V, Fan L, Gu Y, Dalton N, Chu P-H, Peterson K, Ross J, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, Pereira de Souza A, Kitsis RN, Russell RG, Weiss LM, Tang B, Jelicks LA, Factor SM, Shtutin V, Tanowitz HB, Lisanti MP. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003;284:C457–474. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 41.Chow AK, Daniel EE, Schulz R. Cardiac function is not significantly diminished in hearts isolated from young caveolin-1 knockout mice. Am J Physiol Heart Circ Physiol. 2010;299:H1183–1189. doi: 10.1152/ajpheart.01195.2009. [DOI] [PubMed] [Google Scholar]
- 42.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieger MH, Di Lorenzo A, Teutsch C, Kauser K, Sessa WC. Telmisartan regresses left ventricular hypertrophy in caveolin-1-deficient mice. Lab Invest. 2010;90:1573–1581. doi: 10.1038/labinvest.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wunderlich C, Schober K, Lange SA, Drab M, Braun-Dullaeus RC, Kasper M, Schwencke C, Schmeisser A, Strasser RH. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun. 2006;340:702–708. doi: 10.1016/j.bbrc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 45.Bouzin C, Brouet A, De Vriese J, Dewever J, Feron O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol. 2007;178:1505–1511. doi: 10.4049/jimmunol.178.3.1505. [DOI] [PubMed] [Google Scholar]
- 46.Pavlides S, Gutierrez-Pajares JL, Iturrieta J, Lisanti MP, Frank PG. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell Tissue Res. 2014;356:147–157. doi: 10.1007/s00441-013-1767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milstone DS, Ilyama M, Chen M, O'Donnell P, Davis VM, Plutzky J, Brown JD, Haldar SM, Siu A, Lau AC, Zhu SN, Basheer MF, Collins T, Jongstra-Bilen J, Cybulsky MI. Differential role of an NF-kappaB transcriptional response element in endothelial versus intimal cell VCAM-1 expression. Circ Res. 2015;117:166–177. doi: 10.1161/CIRCRESAHA.117.306666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.