Abstract
Infancy is a critical and immensely important period in human brain development. Subtle changes during this stage may be greatly amplified with the unfolding of different developmental processes, exerting far-reaching consequences. Studies of the structure and behavioral manifestations of the infant brain are fruitful. However, the specific functional brain mechanisms that enable the execution of different behaviors remained elusive until the advent of functional connectivity fMRI (fcMRI), which provides an unprecedented opportunity to probe the infant functional brain development in vivo. Since its inception, a burgeoning field of infant brain functional connectivity study has emerged and thrived during the past decade. In this review, we describe (1) findings of normal development of functional connectivity networks and their relationships to behaviors and (2) disruptions of the normative functional connectivity development due to identifiable genetic and/or environmental risk factors during the first 2 years of human life. Technical considerations of infant fcMRI are also provided. It is our hope to consolidate previous findings so that the field can move forward with a clearer picture toward the ultimate goal of fcMRI-based objective methods for early diagnosis/identification of risks and evaluation of early interventions to optimize developing functional connectivity networks in this critical developmental window.
Keywords: resting state, functional connectivity, early brain development, functional networks, prenatal drug exposure, premature birth, genetic risks
Introduction
The explosive growth of brain structure and function in infancy is unparalleled by any other postnatal developmental period. This rapid expansion and organization is genetically determined but is also prone to epigenetic and environmental modifications. Therefore, the early brain develops with the highest level of plasticity, which facilitates both adaptive changes, representing opportunity, and malformations, reflecting vulnerability. It is increasingly recognized that most neuropsychiatric disorders, manifested as complex combinations of cognitive, emotional, and behavioral deficits, have developmental origins that are, at least in part, rooted very early in the initial laying-out of the brain’s functional blue print (Beardslee and others 2011; Insel 2010). Therefore, a better understanding of this most plastic period of human brain development is urgently needed to pave the way for early identification of risks and interventions that have the potential to alter the developmental trajectory at the earliest, most modifiable stage (Fig. 1). In the era with increasingly more interests in prevention strategies to reduce the burden of mental disorders, the importance of early identification and preventive intervention cannot be overemphasized.
Figure 1.
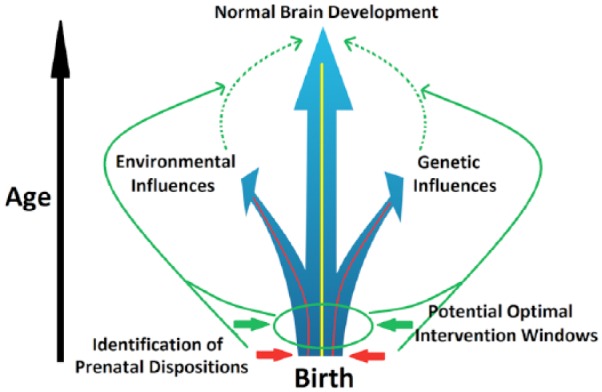
The goal of rectifying abnormal brain growth trajectories induced by genetic and/or environmental risk factors through early identification of potential prenatal dispositions and subsequent preventive intervention.
The large number of studies of pre- and postnatal structural human brain growth have provided a relatively detailed understanding of the intricate processes of the early anatomical development. Briefly, the structural basis of later neural circuits, including neurogenesis, synaptogenesis, dendritic arborization, and formation/reformation of axons, starts and greatly expands in utero (Bystron and others 2008). After birth, the brain continues to grow at a remarkable pace with its total volume doubled in the first year, followed by another 15% increase during the second (Knickmeyer and others 2008). Notably, this increase is largely accounted for by the growth of neural connections in gray matter (i.e., synapse and dendrites), long-range axons, and myelination, all of which are elements essential for the organization of distributed functional networks (Tau and Peterson 2010). Although mostly progressive, regressive development including pruning of both synapses and axons are also evident during the infancy period, thus enabling reorganization of initially established functional circuits (Levitt 2003). Together, these early structural elements establish the fundamental anatomical organization of the infant brain. However, directly linking the development of the brain’s structural elements and emergence of specific behaviors/functions, among which precursors of future problems may be hopefully identified, is challenging given the unmatched specificity levels of the two domains.
In contrast, the characterization of neural circuits, typically defined as networks of interconnected brain regions that integrate vast amounts of information and perform discrete sets of specific brain functions (Friston 2011), represents one of the most viable strategies to bridge between brain and behavior. Conceptually, in a region-specific manner, genes interact with a myriad of environmental factors to layout the determinants of neuronal birth, death, and cellular characteristics. Additionally, such gene-environmental interactions also dictate the formation and reformation of axons, dendrites, and synapses, critical elements for the building of different neural circuits with diverse configurations and functional attributes. Therefore, neural circuits represent more direct mediators of brain’s diverse functional capabilities. Their characterization during the critical infancy period may provide unprecedented insights into the brain basis of the infants’ fast-growing behavioral repertoire. Such characterization would also prove invaluable for early identification of genetically and environmentally induced alterations that enhance risks for future onset of behavioral problems and/or mental disorders (Fig. 1).
Previously hindered by the lack of appropriate experimental fMRI protocols, little progress has been made on the delineation of the infants’ functional neural circuits prior to the new century. However, the field has seen a quantum jump since the advent and popularization of the functional connectivity MRI technique (fcMRI) (Biswal and others 1995; Raichle 2010). fcMRI does not require the performance of specific tasks in the scanner, thereby removing one of the most difficult obstacles in functional neuroimaging of the infant brain. Instead, fcMRI examines the temporal correlation of spontaneous blood-oxygen level dependent (BOLD) signals in the absence of any external tasks. Based on the concept of “neurons firing together wiring together,” fcMRI thus critically queries whether and how different brain areas are synchronized to form functionally coordinated networks during resting state—essentially providing a means to depict the brain’s functional organization. Based on this exciting new technique, there have been numerous articles published on the functional development of infant (and fetal) brain in the past decade, resulting in an impressive body of work that has greatly improved our understanding of this previously “dark period” of functional brain development. Therefore, a review and consolidation of these previous findings is warranted to capitalize on these exciting and tantalizing findings to help the field move forward. Although functional connectivity can also be inferred using other modalities (e.g., electroencephalography [EEG], magnetoencephalography, near-infrared spectroscopy, etc.), studies using fcMRI will be the primary focus of this review. This review is not intended to be exhaustive but rather will focus on illustration of the potential mechanisms underlying both normal and abnormal development based on some of the most relevant discoveries. Specifically, findings describing the normative development of functional connectivity networks (FCNs) from the fetal period to the end of the second year will constitute the main body of this review. We will then describe deviations from the normative FCN development due to identifiable genetic and/or environmental risk factors (e.g., maternal mental disorders, prenatal drug exposure, and premature birth). Following that, potential technical issues for infant fcMRI study will be discussed. Finally, we will present our conclusions and suggest several future directions that deserve the field’s attention. Building on these previous findings, it is our hope that the field will move forward with a more systematic effort to tackle various risk factors that adversely affect normal early brain functional development and come up with tangible ways for early diagnosis/identification.
Normative Development of Functional Connectivity and the Behavior Associations during Infancy
Cortical Networks
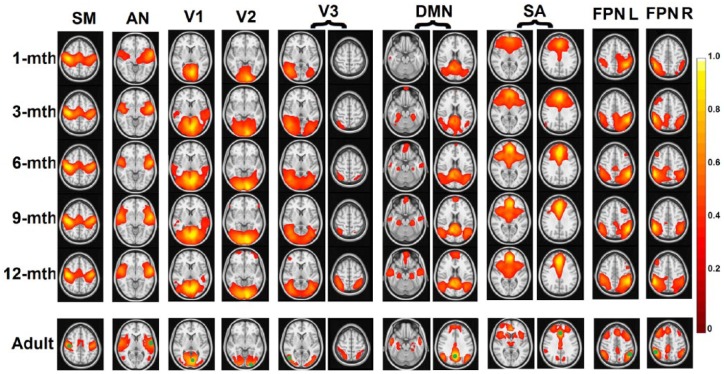
The first article that used fcMRI to characterize infant brain FCNs was published by Fransson and colleagues in 2007 (Fransson and others 2007). This report was based on data from very preterm infants (gestational age [GA] <28 weeks) scanned at term-equivalent age (GA ~41 weeks). Premature birth represents a major risk factor for potentially abnormal functional connectivity development (Kwon and others 2015; Smyser and others 2010), so this study, together with others based on preterm infants, will be discussed later in the Premature Birth section. The first fcMRI studies in typically developing, full-term infants were published by Lin and colleagues (Lin and others 2008) and Liu and colleagues in 2008 (Liu and others 2008). Symmetric brain regions of primary sensorimotor and visual networks were examined and shown to be functionally synchronized starting from birth. These findings were subsequently replicated using an independent sample by Gao and his colleagues (Gao and others 2015b). These results suggest that primary sensorimotor and visual networks are likely functioning at birth, which is highly consistent with behavioral observations. Actually, interhemisphere functional connectivity between primary functional brain regions has been demonstrated to exist even in healthy human fetuses (24-39 GA) in a challenging in vivo fetal fcMRI study (Thomason and others 2013). In contrast to primary networks, Gao and others revealed that the core regions of one of the brain’s most widely studied higher order FCNs—the default-mode network (DMN) (Raichle and others 2001), are “scattered” (i.e., not temporally synchronized) in neonates, and only become functionally connected during later development (Gao and others 2009). These contrasting maturation trajectories between the primary and higher order networks likely reflect an evolutional optimization so that primary functions are on line very early in life to ensure survival but higher order functions that inherently need more environment-based tuning undergo prolonged environmental exposure for more adaptive functioning. To test this hypothesis, Gao and others further delineated the longitudinal growth trajectories of nine cortical networks spanning both primary and higher order functions during the first year of life (Fig. 2) using a much denser sampling strategy (i.e., every 3 months during the first year) (Gao and others 2015a). The results are highly supportive of this theory and show that primary sensorimotor and auditory networks are among the first to be adult-like (which actually showed postnatal regressive growth in functional connectivity), followed by primary and secondary visual networks, then dorsal attention and DMN. In contrast, the frontoparietal executive control networks are still in a premature form at the end of the first year. This series of studies, for the first time, delineated the developmental sequence of different functional brain networks during infancy and suggested a progressive maturation from primary to higher order networks. One striking finding is that the DMN, thought to mainly govern a complex set of self-referential functions in addition to potentially other external ones (Elton and Gao 2015a; Gao and others 2013a), is one of the first higher order networks to show a well-distributed network structure by integrating distant medial frontal, medial/lateral parietal, and medial/lateral temporal regions starting at 6 months of age (Fig. 2). This finding is consistent with the rapid emergence of self-awareness during the first year of life (Amsterdam 1972) and suggests that the development of theory-of-mind-related functions associated with DMN likely serves as a foundation for other higher order functions to build on. This is consistent with the concept that social interaction lies at the core of infant cognitive and emotional development. Giving the rapid development of DMN during the first half of the first year, the report of its sensitivity to social and emotional environment is not surprising (Graham and others 2015). Actually, family social economic status (i.e., income) has been reported to show a trend of correlation with the functional connectivity pattern of DMN at 6 months of age (Gao and others 2015a), further underscoring the potential link between the social environment and DMN development. Therefore, the study of the DMN during the first year of life may serve as a window into the social/emotional development, which likely influences subsequent development of other higher order processes (Gao and others 2009).
Figure 2.
Development of the brain’s nine functional connectivity networks during the first year of life. Thresholded maps evaluated at each of the five time points are shown from the first to the fifth rows and the corresponding adult maps are shown at the bottom row (green dots show the locations of seeds). Color bar indicates correlation strength. V1 = medial occipital network; V2 = occipital pole network; V3 = lateral visual/parietal network; DM = default-mode network; SM = sensorimotor network; AN = auditory/language network; SA = salience network; FPNL/R = left/right frontoparietal networks. Adapted from Gao and others (2015a).
Overall, functional connectivity development of different networks during infancy generally follows a primary-to-higher order sequence but different FCNs demonstrate unique timings and developmental trajectories. These findings suggest that different networks likely possess different critical periods during development, and future studies are needed to more rigorously characterize such network-specific milestones and examine their behavioral associates so that clinical interventions aiming to rectify the growth of specific brain functions can be better informed.
Subcortical Networks
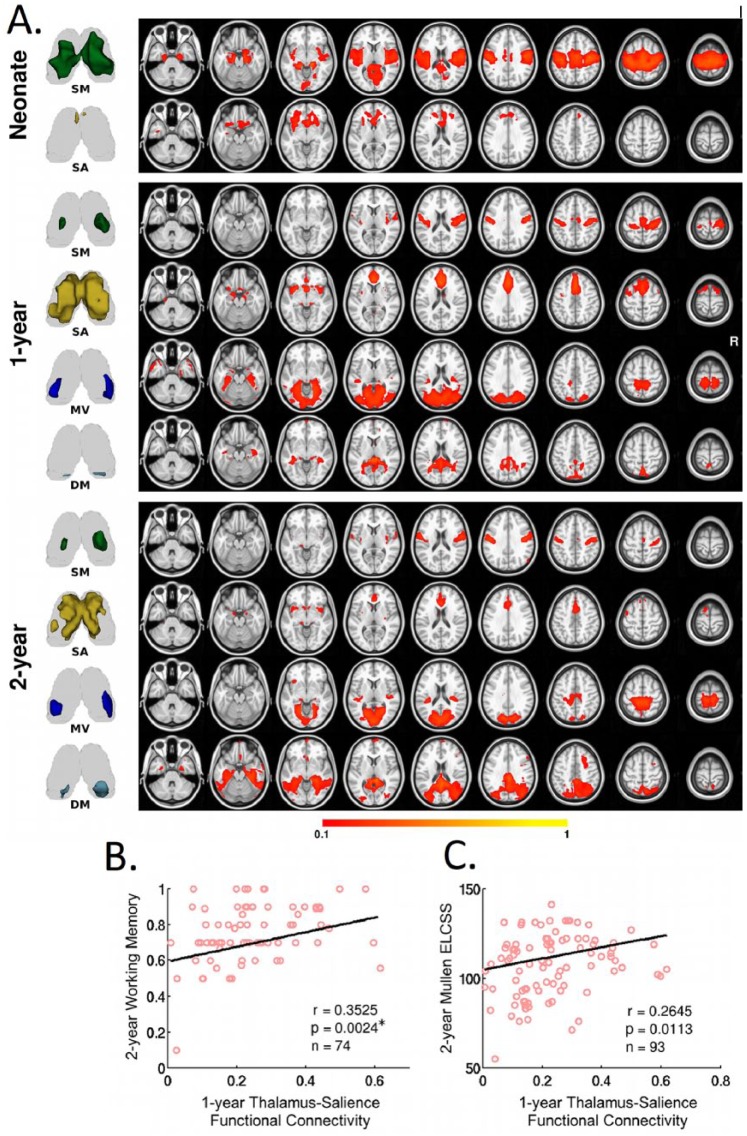
In addition to cortical networks, infant functional connectivity patterns of subcortical structures have also been characterized. The thalamus, especially, represents a critical relay center and pacemaker of the whole brain system, and is of unique importance in early brain functional development (Jones 2000). In a longitudinal study of typically developing infants, Alcauter and colleagues delineated the thalamocortical functional connectivity pattern and correlated it with later behavioral outcomes during infancy (Fig. 3) (Alcauter and others 2014). They found that neonatal thalamic functional connectivity was dominated by connections to the sensorimotor/auditory/visual networks, with a small medial anterior thalamic cluster projecting to insula and anterior cingulate cortex. The insula and anterior cingulate cortex constitute the main components of the brain’s salience network, which is thought to integrate internal and external information in order to assign salience to various events and produce a “sentient self” (Seeley and others 2007). With development, the nonspecific thalamo-primary functional network connectivity becomes more specialized, the thalamo-salience connections strengthen, and new projections to the DMN emerge, underscoring the dynamic and network-specific growth of thalamocortical functional connections. Based on a different methodology and sample (only neonates), Toulmin and others has replicated the findings of dominant thalamo-sensorimotor connectivity and the connection between anterior medial thalamus and salience network-related regions in the newborns (Toulmin and others 2015), supporting the robustness of this set of results. However, given the repeatedly reported alterations of functional connectivity by premature birth (Kwon and others 2015; Smyser and others 2010), the study design of mixing data from both term and very preterm infants in Toulmin and others may have contributed to their results and cautions should be taken when comparing results from these two studies.
Figure 3.
Development of thalamocortical functional connectivity during the first 2 years of life. (A) The 3D-rendering of each thalamic cluster is visualized alongside its cortical projection map for all three age groups. Color bar denotes connectivity strength. SM = sensorimotor; SA = salience; DM = default-mode; MV = medial visual. (B) Relationship between thalamus-salience network connectivity in 1-year-olds and working memory score in 2-year-olds. (C) Relationship between thalamus-salience network connectivity in 1-year-olds and Mullen ELCSS in 2-year-olds. Adapted from Alcauter and others (2014).
Besides delineating the growth of thalamocortical connectivity during infancy, Alcauter and others also showed that the thalamo-salience network connectivity at 1 year uniquely predicts overall cognitive and working memory performances at 2 years of age (Fig. 3B and C). This study demonstrated, for the first time, the power of fcMRI measures to predict later behavioral outcomes in infancy. This is consistent with later findings by Ball and others showing that neonatal thalamocortical structural connectivity, including those associated with salience-network regions, predicts later behavioral outcomes in preterm infants (Ball and others 2015). These findings point to the importance of the anterior-insula-centered salience network and its thalamic input in early functional development. Notably, insula is the earliest developing cortical structure (Afif and others 2007), and functional segregation of the insula has been observed as early as the neonate period, based on functional connectivity similarity measures (Alcauter and others 2015a). Moreover, a primitive “salience network” in neonates has also been observed. This is characterized by synchronous activity of anterior insula with prefrontal regions, which progressively becomes more network-like with age (red color; Fig. 4) (Alcauter and others 2015a). Taken together with the observation that this network is the only one showing robust thalamic connectivity besides primary networks in neonates (Fig. 3A), these findings support the early functioning of the salience network. Given that one of the most critical prerequisites of infants’ learning experience is “paying attention,” which is guided by the salience detection function of the salience network, it is not surprising that thalamo-salience network connectivity, which likely relays critical information for proper evaluation of the salience weighting of different events, is essential for later cognitive performance. Taken together, the salience and DMN network might represent two of the unique higher order functional networks that may profoundly influence early brain functional brain development processes. Overall, these studies suggest that subcortical areas, including but not limited to the thalamus, play critical roles in normal brain development during infancy and warrant additional investigations.
Figure 4.
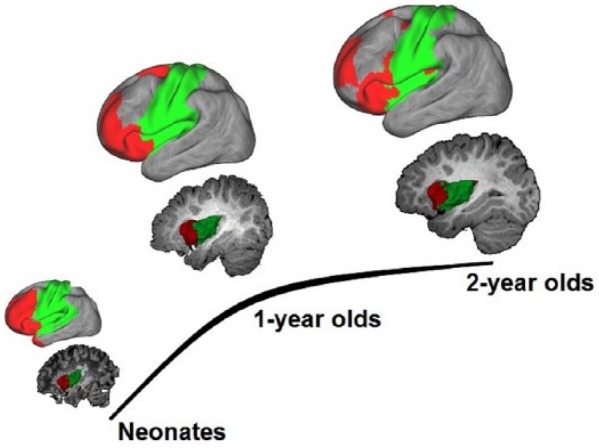
Functional segregation of the insula and associated functional networks during the first 2 years of life. Sagittal images visualize the functional parcellation of the insula into anterior (red) and posterior (green) parts based on similarity of functional connectivity patterns. The surface plots above sagittal images show the corresponding network structures of the anterior and posterior insula clusters. Adapted from Alcauter and others (2015a).
Internetwork Connectivity
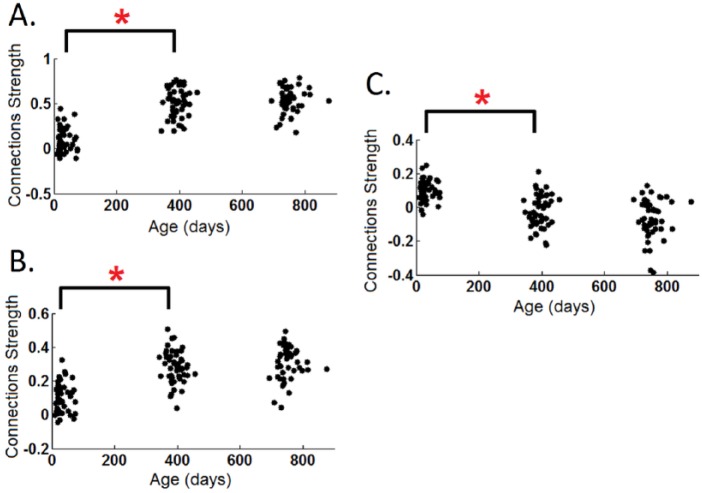
Given the critical importance of large-scale network-level interactions in both normal and abnormal adult brain functioning (Elton and Gao 2014, 2015a, 2015b; Gao and Lin 2012; Spreng and others 2010), when and how different FCNs begin to “talk” to each other during early brain development is also an important question. As an initial attempt, Gao and others examined the early developmental course of the widely reported “anti-correlation” between the dorsal attention network and the DMN (Gao and others 2013b), thought to represent potential “competing” mechanisms between contrasting brain processes (Fox and others 2005; Gao and Lin 2012). They found that such anti-correlation is absent in neonates but appears at 1 year and strengthens during the second year of life (Fig. 5). Interestingly, the “growth” of such anti-correlations coincides with the changing soothing practice in infants; starting around 6 month of age, caregivers can sooth a crying baby (i.e., temporally stopping the “internal” distressed state) by drawing his/her attention to novel toys (i.e., external attention). Therefore, the observed developmental course of the anti-correlation between the dorsal attention network and DMN provides support for the “competition” hypothesis from a developmental perspective. However, it is important not to interpret this competition as an indicator of the “task-negative” nature of the DMN; although the anti-correlation between dorsal attention and DMN persists, there is an emerging body of work showing DMN’s increased connectivity with other task-related networks (e.g., salience and executive control) during the performance of a wide variety of external tasks (Elton and Gao 2014, 2015a; Gao and Lin 2012; Gao and others 2013a; Spreng and others 2010). Actually, a separate study in adults by Gao and Lin suggests that salience and executive control networks likely act as “regulators” between the competing dorsal attention and DMN networks and will flexibly coordinate with either one to facilitate corresponding task performances (Gao and Lin 2012). Notably, developmental changes of internetwork connections is not restricted to these higher order networks but represent a universal phenomenon associated with all identifiable FCNs (Gao and others 2015b). Going beyond the infancy period, the maturing architecture of such network-level interaction mechanisms has been further demonstrated in a study of 6-year-old children, providing critical support for the prolonged development of internetwork interactions (Emerson and others 2015). This is again expected given the need of extensive environment-based tuning for the establishment of effective communication strategies between primary and higher order networks. Overall, investigations into network-level interactions during development deserve more attention and the associated behavioral significance needs to be better characterized.
Figure 5.
Development of the within-default-mode (A), within-dorsal attention (B), and their internetwork connectivity (C) during the first 2 years of life. *Denotes significant differences after FDR correction. Adapted from Gao and others (2013b).
The Integrated Whole Brain System
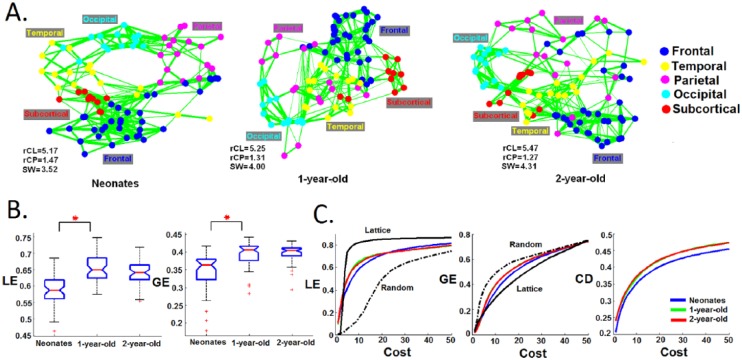
At the whole brain level, there is a growing interest in viewing the brain as an integrated system and characterizing its global properties based on graph theoretical calculations (Rubinov and Sporns 2010). Although not a perfect model, the abstraction of the whole brain functional system to a “graph (i.e., brain regions as nodes and the functional connectivity between brain regions as edges)” is theoretically appealing in that one can obtain a series of summary metrics depicting differential but fundamental aspects of the information transferring property of the whole brain. For example, the concept of a “small-world” network concisely depicts a network that possesses both local (through dense short-range connections within local neighborhood) and global efficiency (through long-range short cuts between distant nodes) in information transferring. Using this approach, Gao and others showed that the neonatal brain already exhibits a “small-world” characteristic based on functional connectivity measures (Fig. 6) (Gao and others 2011). Fransson and others observed the same topology using a voxel-based approach (Fransson and others 2011). This indicates that the newborn brain is already equipped with a relatively optimized functional organization that facilitates information transferring. However, it is also apparent that newborn brain lacks the “short-cut” long-range connections that are essential for global efficiency. These long-range connections only appear in 1-year-olds and strengthen in 2-year-olds, suggesting a developmental evolution of the brain’s functional topology toward a more efficient, globally optimized, system (Fig. 6) (Gao and others 2011).
Figure 6.
Development of the brain’s whole brain functional system based on graph theoretical measures. (A) The group mean correlation matrices at a cost of 10% are visualized using spring embedding plots for all three groups. Nodes are color coded with respect to the lobe they belong to. Each edge represents the mean connectivity strength between a pair of nodes. The rCL and rCP represent the ratio of the clustering coefficient and characteristic path length between the infants’ functional graph and those of a random graph. SW represents the small-worldness measure. (B) Statistical comparison of local (LE) and global efficiency (GE) at connectivity density of 10% based on individual subject’s correlation matrices. Red asterisks represents significant difference at P < 0.05 (FDR correction). (C) LE, GE, and mean connection distance (CD) curve across the cost range of 1% to 50%. Significant increase of LE occurs from neonates to 1-year-olds for a range of cost spanning from 2% through 21% and GE from 4% to 44% (P < 0.05, FDR corrected). The calculation was based on individual subjects and the mean values for each age group are plotted. Adapted from Gao and others (2011).
Alterations of Normative Functional Connectivity Development by Identifiable Risks
Maternal Mental Illnesses
Individuals with mental disorders are at least as likely, if not more likely, than those without psychiatric illnesses to become parents. In the United States, an estimated 65% of women diagnosed with mental illness are mothers (Seeman 2002), resulting in a large population of offspring at greater risks for later development of mental disorders/problems. Maternal mental health disorders represent a significant risk for disruption of normal brain development, due to known influences of maternal genetic factors on fetal brain growth (Satyanarayana and others 2011). However, intrauterine (e.g., disrupted hormone release) and postnatal environmental factors (e.g., poorer care giving and lower socioeconomic status) related to mental illness are also likely to contribute to the consequences, either independently or through interactions with genetic factors (Rice and Thapar 2010). Through imaging infants at the earliest stage of development, researchers can hopefully minimize some of the related postnatal environmental effects and discern new insights into the brain mechanisms underlying the genetic and intrauterine impact of maternal mental disorders.
Qiu and colleagues have conducted such a study and reported functional hyperconnectivity between the amygdala (a brain region that is primarily involved in the brain’s emotional regulation) and various limbic and medial prefrontal regions in 6-month-old infants born to mothers with depression (Qiu and others 2015). This study suggests that neural correlates of the familial transmission of the phenotypes associated with maternal depression can be detected early in infancy. In our own work (unpublished data), amygdala and thalamus functional connectivity disruptions were detected in neonates whose mothers were diagnosed with either schizophrenia or bipolar disorder, advancing the timeline for detecting neural correlates of maternal mental disorders in offspring. These findings are consistent with reports from adult patients with schizophrenia (Anticevic and others 2014) and/or adolescents at clinically high risk for developing schizophrenia (Anticevic and others 2015; Gee and others 2012), suggesting that the amygdala, thalamus, and prefrontal areas might be among the most vulnerable areas for genetic risk of schizophrenia. However, future long-term longitudinal studies are needed to characterize how such early connectivity alterations enable prediction of later behavioral problems and/or onset of mental illnesses. Moreover, the effect of medication associated with different maternal mental disorders may have contributed to the previous findings and need to be better modeled. Overall, the abnormal functional connectivity associated with genetic risks for mental disorders, particularly those observed in affected infants, provides support for future derivation of imaging-based biomarkers to identify risks and to develop very early interventions that can correct the final stages of circuit and behavioral development (Fig. 1).
Prenatal Drug Exposure
Illicit drug use among pregnant women, especially those younger than 25, is on the rise. An estimated 5% to 5.9% of all pregnant women in the United States reported using illicit drugs in 2010 to 2013, and this rate increases with decreasing age (7.4% among 18- to 25-year olds and 16.2% among 15- to 17-year olds) (Behnke and others 2013). An even greater number of pregnant women report using legal teratogenic drugs such as tobacco (16% to 17%) or alcohol (8.5%) (Ross and others 2015). In addition to adverse effects on the placenta, which delivers all essential nutrients and oxygen to the fetus, a majority of these psychotropic drugs pass through the placenta and blood-brain barrier to directly affect infant brain growth in utero. Specifically, most drugs of abuse disrupt the normal functioning of various neurotransmitters/receptors in the brain (e.g., dopamine, serotonin, GABA, norepinephrine, opioids, cannabinoids, acetetyl choline), which dictate the establishment of the primitive topology of the brain’s functional connections during the critical period of fetal development (Gaffuri and others 2012; Wu and others 2011). Neural processes vulnerable to various drugs exposures include neural progenitor proliferation and differentiation, axonal elongation, and synaptogenesis, among others (Gaffuri and others 2012; Tortoriello and others 2014; Wu and others 2011), all of which are core building blocks of developing neural circuits. Therefore, prenatal drug exposure represents another significant threat to normal functional brain development and has been linked to both short- and long-term developmental behavioral and cognitive consequences (Ackerman and others 2008; Bandstra and others 2010; Smeriglio and Wilcox 1999). Neuroimaging studies of the effects of prenatal exposure on brain structure and function are often done in later childhood or adolescence (Donald and others 2016; Li and others 2013; Roussotte and others 2012). Although informative, these findings are more likely to reflect both initial drug effects and the confounding postnatal influence of adverse environments associated with maternal drug abuse. Imaging performed in early infancy has the advantage of minimizing such confounds and provides a better depiction of the mechanisms by which functional connections are affected by gestational exposure(s).
The first study of the prenatal drug effects on neonatal brain development was recently reported by Grewen and others (2014). This study focused on the effects of prenatal cocaine exposure on neonatal brain structure. However, unlike animal models, in human studies women who use cocaine during pregnancy are also likely to use other drugs such as nicotine, alcohol, and marijuana. Therefore, researchers compared neonates exposed to cocaine and other drugs to a group of drug-naive infants and to a group exposed to a similar profile of other drugs including alcohol, nicotine, marijuana, antidepressants, but without cocaine. This enabled detection of both drug-common and cocaine-specific effects. Prenatal cocaine was associated with reduced prefrontal gray matter volume compared with both non-cocaine drug-exposed and drug-free control groups, after controlling for covariates (birth weight, gestational age at scan, gender, total brain volume). Salzwedel and others then published the first study of prenatal drug exposure effects on brain functional connectivity in the same neonate sample (Salzwedel and others 2015). Results revealed hyperconnectivity between the amygdala and a medial prefrontal cluster that was specific to prenatal cocaine exposure, after controlling for relevant covariates and other drug exposures (Fig. 7). Functional connectivity disruptions between the insula and medial prefrontal/sensorimotor areas were observed in both drug-exposed groups, suggesting a nonspecific vulnerability to multiple drugs for this connection. These modified functional connections may, at least partly, contribute to the long-lasting behavioral consequences previously reported in children with prenatal cocaine exposure. Indeed, in a follow-up study (unpublished work), more dramatic cocaine-specific functional connectivity disruptions were observed among thalamo-cortical connections. More importantly, greater alterations were related to lower overall cognitive and fine motor scores measured at 3 months of age. These findings underscore the predictive power of early functional connectivity measures for later behavioral outcomes and support the exciting possibility of using early functional neuroimaging methods for the identification of risks for future behavioral problems.
Figure 7.
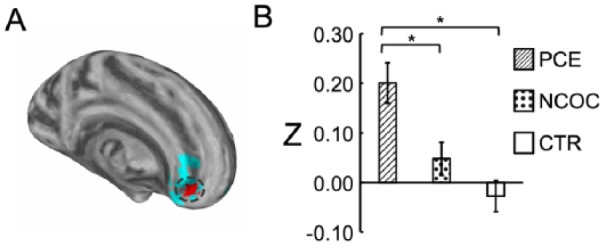
Disruption of amygdala functional connectivity by prenatal cocaine exposure. (A) Visualization of the subcluster (highlighted in red) that shows cocaine specific disruptions in functional connectivity within a big cluster showing overall drug-common effects (blue). (B) Post hoc comparison of functional connectivity by group within the detected subcluster. (*) indicate significant (P ≤ 0.05 Dunn-Sidak corrected) pairwise differences between groups. Data plotted as mean ± SEM. Adapted from Salzwedel and others (2015).
In a more recent study of the effects of prenatal marijuana exposure on neonatal functional connectivity, both marijuana-specific and drug-common effects were again observed (Grewen and others, 2015). Marijuana-specific connectivity disruptions were revealed for dorsal striatum and anterior insula seed regions, which all have high in utero Type 1 cannabinoid receptor (CB1R) expression. This early departure from typical network development may contribute to the deficits in motor and visual-spatial activity, integration and coordination (Willford and others 2010), attention (Goldschmidt and others 2012), and social-emotional stability (Gray and others 2005) reported in children and adolescents prenatally exposed to marijuana (Fried and Smith 2001, Fried and others 2003).
Taken together, these studies, for the first time, provide compelling evidence that prenatal exposure to different drugs alters early brain structural growth and the orchestration of functional networks. These drug-related alterations likely arise from disrupted prenatal programming, since postnatal exposures were minimized is this sample of newborns. These results also indicate that individual drugs likely differentially affect discrete functional neural circuits. In the future, a longitudinal and more systematic study with a larger sample size that is capable of discriminating the effects of various drugs during development is needed. Moreover, the potential drug-drug interaction effects deserve more attention, since polydrug use is the norm rather than the exception in most women who use and abuse substances during pregnancy.
Premature Birth
The exact causes for premature birth are still poorly understood but likely involve a complex set of genetic, biological, and environmental factors (Terao 1996). The rate of infants affected by premature birth is growing, partly due to the increased survival rate attributable to advances in perinatal and neonatal care. However, the last few weeks of gestation is a particularly critical period when most of the brain’s structural elements undergo an accelerated pace of development (Tau and Peterson 2010). Therefore, the transition from the womb to the outside environment during this critical period may adversely affect infants’ brain growth. Indeed, up to 40% of very premature infants (born at <30 GA) may develop motor deficits (Holsti and others 2002), and an estimated 30% to 60% experience long-term cognitive impairments and social/emotional difficulties (Anderson and others 2004; Taylor and others 2004). As mentioned above, Fransson and others published the first fcMRI study on very preterm infants scanned at term-equivalent age (Fransson and others 2007). They identified five FCNs including three primary (i.e., visual, sensorimotor, and auditory) and two higher order networks located at the anterior and posterior parts of the brain, respectively. However, since no term controls were included in this study, the effects of premature birth on infant brain development were beyond the scope of that study. A later study by Smyser and others examined the longitudinal development of FCNs in a cohort of very preterm infants and compared the data acquired at term-equivalent age with those from term controls (Smyser and others 2010). They reported functional connectivity developing from local to bilaterally symmetric connections even prior to term-equivalent age. Noticeably, premature infants demonstrate lower correlation values and limited distribution, especially among long-range thalamocortical connections. Consistent with Smyer and others, another study (Kwon and others 2015) has shown disrupted language network lateralization based on functional connectivity measures in very preterm infants compared with term controls. Notably, studies have extended findings of functional connectivity alterations related to premature birth from term-equivalent age to childhood (Damaraju and others 2010) and adolescence (Mullen and others 2011; Schafer and others 2009), together with corroborating behavioral findings (Anderson and others 2004; Taylor and others 2004). These results underscore the far-reaching impact of this risk factor. Despite the seemingly converging findings pointing to abnormal functional connectivity development associated with premature birth, there are also reports showing negative findings (Doria and others 2011; Lee and others 2013). Therefore, future work is needed to more rigorously examine the effects of premature birth on the brain’s functional development. Importantly, the observed alterations likely reflect consequences of a combination of genetic/environmental factors leading to premature birth and/or the vastly different postnatal environments surrounding premature infants compared with the womb. Indeed, a study of effects of neonatal intensive care unit stay on the development of premature infants have shown that private room setup was associated with lower language scores and a trend for lower motor scores compared with open ward care (Pineda and others 2014). The authors suggest that their results underscore the importance of sensory exposure during this sensitive period. On the other hand, Smith and others have shown that exposure to a greater number of stressors (e.g., vascular access/heel stick, radiology/diagnostic study, intubation, extubation, etc.) is associated with altered functional connectivity in the temporal lobe on top of structural alterations (Smith and others 2011). Therefore, further effort is warranted to more systematically examine the effect of environmental factors on the developing premature brain to guide better postnatal care for this vulnerable group. Overall, premature birth represents a significant threat to normal brain and behavioral development and viable objective ways (e.g., fcMRI-based) for the early detection of abnormality represents one of the highest priorities given the large and increasing number of infants affected (Blencowe and others 2013).
Other Factors
In addition to the risks described above, many other factors may also adversely affect the developing brain during infancy. Among them, autism spectrum (ASD) disorder may represent one of the most salient forms since it has been implicated that alterations of brain structures in ASD subjects occurs during early infancy (Wolff and others 2012). For functional connectivity, one study based on near-infrared spectroscopy has shown that infants at risk for autism (with at least one older sibling with autism) demonstrated altered connectivity pattern starting from 3 months of age, which waxed and waned during the first year of life (Keehn and others 2013). Furthermore, another study directly targeting at toddlers with a diagnosis of autism showed disrupted synchronization of language areas (Dinstein and others 2011). Given the early occurrence of ASD and its devastating consequences, more studies are urgently needed to better understand its brain basis and investigate ways for early diagnosis. Besides autism, researchers have also shown neonatal functional connectivity alterations associated with Down’s syndrome (Imai and others 2014), among others.
The postnatal environment also represents a significant source of variation affecting normal functional brain development. The delicate process of infants’ social and emotional development, which lies in the core of early functional growth with critical influences on overall intellectual and cognitive development, requires a nurturing and loving environment to reach the greatest potential. Consistent with this notion, early life stress, manifested as interparental conflict, has been shown to result in aberrant functional connectivity within the DMN, which subsequently is associated with greater negative infant emotionality (Graham and others 2015). More generally, disrupted caregiving and parenting behaviors due to different causes (e.g., depression, substance abuse, etc.) have been generally shown to affect normal functional connectivity development (Moses-Kolko and others 2014). Consistently, social economic status (e.g., maternal education and income) has been shown to directly correlate with the development of the motor and DMN networks during infancy (Gao and others 2015a).
Technical Considerations with fcMRI in Infants
Technical Issues
Currently, the trend for imaging healthy normal infants is to perform the scan during natural sleep without sedation, which is safer and more acceptable to the infants/family and also practically less complicated. However, given a higher likelihood of subtle motion in naturally sleeping infants than cooperative awake adults, proper steps have to be taken to minimize motion artifacts beyond the standard rigid-body motion correction and regression of motion parameters. Proposed by Power and others, data scrubbing represents an appealing way to remove the volumes contaminated by subtle motion (Power and others 2013). However, the by-product of disrupted temporal continuity introduced by scrubbing deserves further investigation. Regardless of which motion correction procedure is applied, we stress that residual motion parameters (e.g., frame-wise displacement) should be compared across subgroups of interest and/or included in statistical models to further control motion artifacts. The other frequently encountered issue in infant fcMRI studies is the procedure of global signal regression (GSR), which has been widely debated in adult literature (Fox and others 2009; Murphy and others 2009). The use of GSR in infant studies is potentially advantageous given it has the ability to reduce confounds associated with physiological parameters (Chang and Glover 2009), which are typically difficult to monitor in naturally sleeping infants. In our previous experiences, GSR mainly shifts the distribution of correlation values but does not appear to alter the relative differences across groups (Gao and others 2013a; Gao and others 2013b). Nonetheless, it is still recommended that results obtained with GSR be compared with those without GSR and/or other normalization strategies (e.g., post hoc standardization; Yan and others 2013) to minimize the possibility of spurious results after GSR. Moreover, the negative correlation coefficients after GSR should be interpreted accordingly, given the known correlation distribution shift. Overall, similar to adult studies, the entire preprocessing pipeline for infant fcMRI data should be better optimized and standardized to facilitate comparisons across groups and replication of results, which is critical for the field to move forward. In this review, we specifically emphasized replicable findings where possible to highlight those potentially more robust results.
Sleeping Stage
An important consideration for imaging naturally sleeping infants is how different stages of sleep during imaging across subjects confound our ability to assess brain functional development in infants. In theory, this difficulty could be overcome using MR-compatible EEG to simultaneously acquire MR and EEG signals. Practically, it is a daunting task. First, since most studies prefer not to use sedation and subjects are imaged during natural sleep, additional equipment setup, EEG, will substantially increase experimental complexity and chances of subjects waking up as well as difficulty for subject recruitment. As a result, most studies focusing on normal brain development do not monitor sleep stages during MRI imaging sessions. Nevertheless, available data suggest that the majority of changes in functional networks occur during the deepest stages of sleep in adults (Horovitz and others 2008; Horovitz and others 2009). Given the fact that most of the infant imaging protocols take ~30 minutes, the minor variations in sleep stage during the half-hour scan should not exert major effects for infant fcMRI studies. However, it is good practice to start scanning infants immediately after they fall asleep and fix the sequence of scans across subjects so that the fcMRI scan will be acquired at approximately the same time after sleep for all subjects. If properly controlled, the relatively homogenous sleeping state across infant samples may actually provide a better context for the study of intrinsic functional networks considering the known intersubject variability of “resting-state” functional connectivity in awake adults due to potentially different cognitive/emotional states, metabolic rates, and so on (Laumann and others 2015).
Frequency
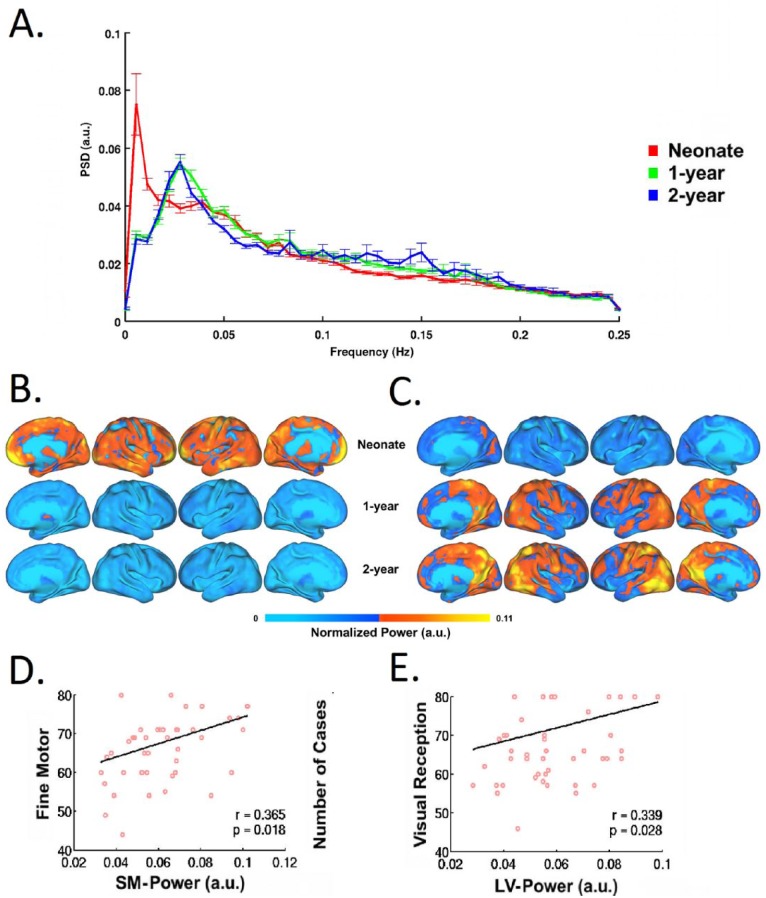
To date, most of infant fcMRI studies have focused on the frequency band of <0.1 Hz for the delineation of FCNs. This selection of frequency range is not driven physiologically but more empirically. Smith-Collins and others recently reported that a substantial proportion of signal power resides beyond this range and meaningful functional connectivity patterns can be observed using extended frequency bands in neonates (Smith-Collins and others 2015). In fact, the frequency distribution of BOLD signals and its changes across development likely reflect underlying maturation of either structural elements (e.g., axons, myelination, etc.), functional processes (e.g., neural-vessel coupling), or both. The delineation of this process, by itself, is interesting. For example, Alcauter and others have shown that the peak frequency of BOLD signal shifts to a higher value during the first year of life (Fig. 8), and the power at peak-frequency for sensorimotor and visual networks in 1-year-olds correlates with motor and visual reception scores (Alcauter and others 2015b). This underscores the importance of frequency-related properties of the BOLD signal in early brain development, which deserve more attention in future investigations.
Figure 8.
Frequency shift of spontaneous BOLD signals during infancy and its behavioral associations. (A) Average power spectral density (PSD) of spontaneous BOLD signal across of whole brain gray matter the three age groups. Error bars denote standard error of the mean for each frequency point. (B) Spectral power at 0.0056 Hz (i.e., the neonatal peak frequency) for each age group visualized on brain surfaces. (C) Spectral power at 0.0278 Hz (i.e., the 2-year-olds’ peak frequency) for each age group visualized on brain surfaces. (D) Significant positive correlation between the spectral power for the sensorimotor (SM) network and the Mullen Fine Motor Scale score in 1-year-olds. (E) Significant positive correlation between the spectral power for the lateral visual (LV) network and the Mullen Visual Perception Scale score in 1-year-olds. Adapted from Alcauter and others (2015b).
Conclusions and Future Directions
In conclusion, numerous efforts from the past decade on functional connectivity study of the infant brain enabled a substantially improved understanding of the normative functional brain development process during infancy. For cortical networks, a maturation sequence from primary to higher order networks with the default-mode network highlighted as one of the earliest maturing higher order networks was observed. Subcortical areas are also establishing cortical connectivity during infancy. Among them, the thalamo-salience network connectivity uniquely predicts later cognitive performances. Between networks, “cross-network” interactions are also evolving during infancy and highlight an emerging “competing” mechanism between the default-mode network and the dorsal attention network. Finally, at the whole brain level, the neonatal brain already demonstrates an optimized topology qualifying as a “small-world” but global efficiency shows dramatic age-dependent improvement stressing the continued optimization of the brain’s functional topology for more efficient information transferring. In addition to the delineation of the normative trajectories, infant fcMRI studies in different at-risk or diseased infant populations showed intriguing functional connectivity alterations associated with various risk and/or pathological factors, underscoring the plastic and modifiable nature of infant functional connectivity. These studies point to the exciting possibility of fcMRI-based objective ways for early diagnosis and/or identification of risks to facilitate the earliest possible intervention.
In the future, more systematic efforts should be put forth to rigorously test such possibilities and come up with tangible ways to improve clinical diagnosis and identification of risks among infants affected (Fig. 1). During this process, there are numerous efforts needed but we will list several of the urgent ones. First, it is necessary to not only image infants but also follow them to older ages so behavioral problems and/or clinical diagnosis (except for autism) can be identified/made more reliably. Only this approach would allow the detection of robust fcMRI-based biomarkers with prediction powers. Second, replication studies are critical to test the robustness of any potential biomarkers. Particularly, the robustness of functional connectivity predictors against different scanners, experimental setups, and ethnic populations should be tested. Technically, a set of normative infant-specific functional atlases based on functional connectivity signatures might prove to be very useful given the dramatic functional changes between infants and adults. The structural-functional relationship during infancy needs to be better stratified, especially between functional and structural connectivity (Honey and others 2009). Moreover, given recent fcMRI discoveries of hybrid categorical-dimensional models for different development disorders (Elton, Alcauter, and Gao, 2014; Elton, Di Matino, Hazlett, Gao, 2015), similar explorations in the study of other developmental risk factors are warranted. Finally, as infant fcMRI data continue to accumulate, more efforts are needed to develop/apply advanced data mining algorithms to better discern the “hidden” connectivity features with the most robust prediction powers for long-term behavioral outcomes. Overall, highly interdisciplinary efforts are needed, more than ever before, to move the field forward toward our final goal of helping those youngsters that have to face difficult genetic and/or environmental challenges from the inception of their lives.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health (R21NS088975 to WG; R03DA036645 to WG and KG; R01MH064065, R01HD05300 to JHG); Foundation of Hope for Research and Treatment of Mental Illness Award to WG; and Cedars-Sinai Institutional Support to WG.
References
- Ackerman JP, Llorente AM, Black MM, Ackerman CS, Mayes LA, Nair P. 2008. The effect of prenatal drug exposure and caregiving context on children’s performance on a task of sustained visual attention. J Dev Behav Pediatr 29:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afif A, Bouvier R, Buenerd A, Trouillas J, Mertens P. 2007. Development of the human fetal insular cortex: study of the gyration from 13 to 28 gestational weeks. Brain Struct Funct 212:335–46. [DOI] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Gilmore JH, Gao W. 2015a. Consistent anterior-posterior segregation of the insula during the first 2 years of life. Cereb Cortex 25:1176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Goldman BD, Reznick JS, Gilmore JH, and others. 2015b. Frequency of spontaneous BOLD signal shifts during infancy and correlates with cognitive performance. Dev Cogn Neurosci 12:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, and others. 2014b. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci 34:9067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam B. 1972. Mirror self-image reactions before age two. Dev Psychobiol 5:297–305. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group. 2004. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics 114:50–7. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, and others. 2013. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex 24:3116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, and others. 2015. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry 72:882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Pazderova L, Chew A, Tusor N, Merchant N, Arichi T, and others. 2015. Thalamocortical connectivity predicts cognition in children born preterm. Cereb Cortex 25:4310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Mansoor E, Accornero VH. 2010. Prenatal drug exposure: infant and toddler outcomes. J Addict Dis 29:245–58. [DOI] [PubMed] [Google Scholar]
- Beardslee WR, Chien PL, Bell CC. 2011. Prevention of mental disorders, substance abuse, and problem behaviors: a developmental perspective. Psychiatr Serv 62:247–54. [DOI] [PubMed] [Google Scholar]
- Behnke M, Smith VC; Committee on Substance Abuse; Committee on Fetus and Newborn. 2013. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics 131:e1009–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–41. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, and others. 2013. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 10(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. 2008. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 9:110–22. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. 2009. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage 47:1448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Phillips JR, Lowe JR, Ohls R, Calhoun VD, Caprihan A. 2010. Resting-state functional connectivity differences in premature children. Front Syst Neurosci 4. doi: 10.3389/fnsys.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, and others. 2011. Disrupted neural synchronization in toddlers with autism. Neuron 70:1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KA, Ipser JC, Howells FM, Roos A, Fouche JP, Riley EP, and others. 2016. Interhemispheric functional brain connectivity in neonates with prenatal alcohol exposure: preliminary findings. Alcohol Clin Exp Res 40:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, and others. 2011. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A 107:20015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Alcauter S, Gao W. 2014. Network connectivity abnormality profile supports a categorical-dimensional hybrid model of ADHD. Hum Brain Mapp 35:4531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Di Martino A, Hazlett HC, Gao W. 2015. Neural connectivity evidence for a categorical-dimensional hybrid model of autism spectrum disorder. Biol Psychiatry. Epub Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Gao W. 2014. Divergent task-dependent functional connectivity of executive control and salience networks. Cortex 51:56–66. [DOI] [PubMed] [Google Scholar]
- Elton A, Gao W. 2015a. Task-positive functional connectivity of the default mode network transcends task domain. J Cogn Neurosci 27:2369–81. [DOI] [PubMed] [Google Scholar]
- Elton A, Gao W. 2015b. Task-related modulation of functional connectivity variability and its behavioral correlations. Hum Brain Mapp 36:3260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Short SJ, Lin W, Gilmore JH, Gao W. 2015. Network-level connectivity dynamics of movie watching in 6-year-old children. Front Hum Neurosci 9:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. 2009. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. 2011. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex 21:145–54. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, and others. 2007. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A 104:15531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Smith AM. 2001. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol 23:1–11. doi:10.1016/ S0892-0362(01)00161-1. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. 2003. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 25:427–36. doi: 10.1016/S0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Friston KJ. 2011. Functional and effective connectivity: a review. Brain Connect 1:13–36. [DOI] [PubMed] [Google Scholar]
- Gaffuri AL, Ladarre D, Lenkei Z. 2012. Type-1 cannabinoid receptor signaling in neuronal development. Pharmacology 90:19–39. [DOI] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, and others. 2015a. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb Cortex 25:2919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith J, Gilmore J, Lin W. 2015b. Development of human brain cortical network architecture during infancy. Brain Struct Funct 220:1173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Alcauter S, Lin W. 2013a. The dynamic reorganization of the default-mode network during a visual classification task. Front Syst Neurosci 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen D, Zhu H, and others. 2011. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One 6:e25278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W. 2013b. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex 23:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W. 2012. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp 33:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, and others. 2009. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci U S A 106:6790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Karlsgodt KH, van Erp TG, Bearden CE, Lieberman MD, Belger A, and others. 2012. Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophr Res 134:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Willford JA, Severtson SG, Day NL. 2012. School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol Teratol 34:161–7. doi: 10.1016/j.ntt.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Carpenter S, Fair DA. 2015. Early life stress is associated with default system integrity and emotionality during infancy. J Child Psychol Psychiatry 56:1212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KA, Day NL, Leech S, Richardson GA. 2005. Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol 27:439–48. doi: 10.1016/j.ntt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Grewen K, Burchinal M, Vachet C, Gouttard S, Gilmore JH, Lin W, and others. 2014. Prenatal cocaine effects on brain structure in early infancy. Neuroimage 101:114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen KM, Salzwedel AP, Gao W. 2015. Functional connectivity disruption in neonates with prenatal marijuana exposure. Front Hum Neurosci. 2015. November 4; 9: 601. doi: 10.3389/fnhum.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsti L, Grunau RV, Whitfield MF. 2002. Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr 23:9–15. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, and others. 2009. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106:2035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, and others. 2009. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci U S A 106:11376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, and others. 2008. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp 29:671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Watanabe H, Yasui K, Kimura Y, Shitara Y, Tsuchida S, and others. 2014. Functional connectivity of the cortex of term and preterm infants and infants with Down’s syndrome. Neuroimage 85(Pt 1):272–8. [DOI] [PubMed] [Google Scholar]
- Insel TR. 2010. Rethinking schizophrenia. Nature 468:187–93. [DOI] [PubMed] [Google Scholar]
- Jones E. 2000. The thalamus. Cambridge, England: Cambridge University Press. [Google Scholar]
- Keehn B, Wagner JB, Tager-Flusberg H, Nelson CA. 2013. Functional connectivity in the first year of life in infants at-risk for autism: a preliminary near-infrared spectroscopy study. Front Hum Neurosci 7:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, and others. 2008. A structural MRI study of human brain development from birth to 2 years. J Neurosci 28:12176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Scheinost D, Lacadie C, Sze G, Schneider KC, Dai F, and others. 2015. Adaptive mechanisms of developing brain: cerebral lateralization in the prematurely-born. Neuroimage 108:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen MY, and others. 2015. Functional system and areal organization of a highly sampled individual human brain. Neuron 87:657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Morgan BR, Shroff MM, Sled JG, Taylor MJ. 2013. The development of regional functional connectivity in preterm infants into early childhood. Neuroradiology 55(Suppl 2):105–11. [DOI] [PubMed] [Google Scholar]
- Levitt P. 2003. Structural and functional maturation of the developing primate brain. J Pediatr 143:S35–45. [DOI] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Ellen Lynch M, Hamann S, Peltier S, and others. 2013. Prenatal cocaine exposure alters functional activation in the ventral prefrontal cortex and its structural connectivity with the amygdala. Psychiatry Res 213:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Zhu Q, Gao W, Chen Y, Toh CH, Styner M, and others. 2008. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol 29:1883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WC, Flax JF, Guise KG, Sukul V, Benasich AA. 2008. Functional connectivity of the sensorimotor area in naturally sleeping infants. Brain Res 1223:42–9. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE. 2014. In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. J Neuroendocrinol 26:665–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, and others. 2011. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage 54:2563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. 2009. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, and others. 2014. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J Pediatr 164:52–60.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2013. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage 76:439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BF, and others. 2015. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry 5:e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. 2010. Two views of brain function. Trends Cogn Sci 14:180–90. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, and others. 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Thapar A. 2010. Estimating the relative contributions of maternal genetic, paternal genetic and intrauterine factors to offspring birth weight and head circumference. Early Hum Dev 86:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EJ, Graham DL, Money KM, Stanwood GD. 2015. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology 40:61–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Rudie JD, Smith L, O’Connor MJ, Bookheimer SY, Narr KL, and others. 2012. Frontostriatal connectivity in children during working memory and the effects of prenatal methamphetamine, alcohol, and polydrug exposure. Dev Neurosci 34:43–57. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–69. [DOI] [PubMed] [Google Scholar]
- Salzwedel AP, Grewen KM, Vachet C, Gerig G, Lin W, Gao W. 2015. Prenatal drug exposure affects neonatal brain functional connectivity. J Neurosci 35:5860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana VA, Lukose A, Srinivasan K. 2011. Maternal mental health in pregnancy and child behavior. Indian J Psychiatry 53:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, and others. 2009. Alterations in functional connectivity for language in prematurely born adolescents. Brain 132:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, and others. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman M. 2002. Women with schizophrenia as parents. Primary Psychiatry. http://primarypsychiatry.com/women-with-schizophrenia-as-parents/
- Smeriglio VL, Wilcox HC. 1999. Prenatal drug exposure and child outcome. Past, present, future. Clin Perinatol 26:1–16. [PubMed] [Google Scholar]
- Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, and others. 2011. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol 70:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Collins AP, Luyt K, Heep A, Kauppinen RA. 2015. High frequency functional brain networks in neonates revealed by rapid acquisition resting state fMRI. Hum Brain Mapp 36:2483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ. 2010. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 20:2852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. 2010. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53:303–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. 2010. Normal development of brain circuits. Neuropsychopharmacology 35:147–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Minich NM, Klein N, Hack M. 2004. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc 10:149–63. [DOI] [PubMed] [Google Scholar]
- Terao T. 1996. Causes of premature birth and its prevention. Nihon Sanka Fujinka Gakkai Zasshi 48:660–5. [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, Anderson AL, and others. 2013. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med 5:173ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoriello G, Morris CV, Alpar A, Fuzik J, Shirran SL, Calvigioni D, and others. 2014. Miswiring the brain: Delta9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J 33:668–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmin H, Beckmann CF, O’Muircheartaigh J, Ball G, Nongena P, Makropoulos A, and others. 2015. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc Natl Acad Sci U S A 112:6485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willford JA, Chandler LS, Goldschmidt L, Day NL. 2010. Effects of prenatal tobacco, alcohol and marijuana exposure on processing speed, visual-motor coordination, and interhemispheric transfer. Neurotoxicol Teratol 32:580–8. doi: 10.1016/j.ntt.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, and others. 2012. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry 169:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CS, Jew CP, Lu HC. 2011. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol 6:459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Craddock RC, Zuo XN, Zang YF, Milham MP. 2013. Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. Neuroimage 80:246–62. [DOI] [PMC free article] [PubMed] [Google Scholar]