Abstract
The effect of clinic-based intensive hypertension treatment on ambulatory blood pressure (BP) is unknown. The goal of the Systolic Blood Pressure Intervention Trial (SPRINT) Ambulatory BP Ancillary Study was to evaluate the effect of intensive versus standard clinic-based BP targets on ambulatory BP. Ambulatory BP was obtained within 3 weeks of the 27 month study visit in 897 SPRINT participants. Intensive treatment resulted in lower clinic systolic BP (mean difference between groups = 16.0 mmHg (95% CI: 14.1 to 17.8 mmHg)), nighttime systolic BP (mean difference = 9.6 mmHg (95% CI: 7.7 to 11.5 mmHg)), daytime systolic BP (mean difference = 12.3 mmHg (95% CI: 10.6 to 13.9 mmHg)), and 24 hour systolic BP (mean difference = 11.2 mmHg (95% CI: 9.7 to 12.8 mmHg)). The night/day systolic BP ratio was similar between the intensive (0.92 ± 0.09) and standard treatment groups (0.91 ± 0.09). There was considerable lack of agreement within participants between clinic systolic BP and daytime ambulatory systolic BP with wide limits of agreement on Bland-Altman plots. In conclusion, targeting a systolic BP of less than 120 mmHg, as compared with less than 140 mmHg, resulted in lower nighttime, daytime, and 24 hour systolic BP, but did not change the night/day systolic BP ratio. Ambulatory BP monitoring may be required to assess the effect of targeted hypertension therapy on out of office BP. Further studies are needed to assess whether targeting hypertension therapy based on ambulatory BP improves clinical outcomes.
Keywords: hypertension, ambulatory blood pressure monitoring, circadian rhythm, goals, blood pressure
Introduction
Ambulatory blood pressure (BP), typically measured over a 24 hour period, is a strong and independent predictor of hypertension-related adverse cardiovascular and renal outcomes.1–5 A key feature of ambulatory BP compared to traditional clinic-based measurement is the ability to assess BP throughout the day and night in the setting of usual activities, rather than at a single time point in the clinician’s office. Observational studies consistently demonstrate that nighttime BP is a better predictor of clinical outcomes than daytime and clinic-based BP.2–4, 6
All large randomized trials in hypertension have utilized clinic-based BP to determine eligibility and to target antihypertensive drug therapy. The impact of clinic-based hypertension treatment on ambulatory BP is less well studied; a meta-analysis of clinical trials that evaluated ambulatory BP at baseline and after an intervention found that for every 10 mmHg decrease in clinic systolic BP, ambulatory systolic BP decreases by 4.2 mmHg.7 Even less is known about the effect of targeting different levels of clinic BP on measures of ambulatory BP. In the Hypertension Optimal Treatment (HOT) trial, which targeted three different levels of clinic diastolic BP, there was no difference in 24 hour ambulatory BP between treatment arms.8 This may relate to the small differences in achieved clinic diastolic BP between the three arms in this study. Data on the impact of targeting different levels of clinic systolic BP on ambulatory BP are limited. If lower treatment goals are more broadly incorporated into clinical practice, it will be important to understand the effect of intensive clinic-based BP lowering strategies on ambulatory BP.
The Systolic Blood Pressure Intervention Trial (SPRINT) was a large multicenter, randomized, controlled, trial in 9361 persons with a systolic BP of 130 mm Hg or higher and an increased cardiovascular risk, but without diabetes or prevalent stroke. SPRINT demonstrated significant reductions in cardiovascular events (25%) and mortality (27%) with treatment of clinic systolic BP to a target of less than 120 mm Hg (intensive-treatment) compared with a target of less than 140 mm Hg (standard-treatment). We measured ambulatory BP in a subset of participants in the SPRINT study at selected clinical sites. The goal of this analysis was to evaluate the difference in nighttime systolic BP, as well as other ambulatory BP derived parameters (daytime systolic BP, 24 hour systolic BP, night-day systolic BP ratio, and 24 hour BP variability) between the intensive and standard clinic-based BP treatment groups in SPRINT.
Methods
The design and main results of SPRINT have been published.9, 10 SPRINT was a two-arm, multicenter, randomized trial. Participants met all the following criteria: an age of at least 50 years, a clinic systolic BP of 130 to 180 mm Hg (with the acceptable upper limit for clinic systolic BP decreasing as the number of pre-trial antihypertensive medications increased), and an increased risk of cardiovascular events defined by one or more of the following: clinical or subclinical cardiovascular disease (CVD) other than stroke; chronic kidney disease; a 10-year risk of CVD of 15% or greater on the basis of the Framingham risk score; or an age of 75 years or older. Patients with diabetes mellitus, prior stroke, symptomatic heart failure within the past 6 months, or left ventricular ejection fraction less than 35% were excluded. Eligible participants were randomly assigned to a clinic systolic BP target of less than 120 mm Hg (the intensive-treatment group) or less than 140 mm Hg (the standard-treatment group). During a median 3.26 years of follow up, the mean clinic systolic BP was 121.5 mm Hg in the intensive-treatment group and 134.6 mm Hg in the standard-treatment group.9
Consecutive SPRINT participants at 15 clinical sites were approached to participate in the ambulatory BP ancillary study at the 27 month follow up visit. The protocol was approved by the Institutional Review Board at each of the participating sites. Eligible SPRINT participants willing to participate in this ancillary study provided informed consent for ambulatory BP monitoring (ABPM). Exclusion criteria were: a) arm circumference >50cm, b) shift worker or work regularly at night, c) history of breast cancer requiring mastectomy or radiation on the non-dominant arm (to avoid frequent BP measurements in patients with lymphedema), and d) end-stage renal disease.
Clinic BP was obtained using an automated measurement device (HEM-907 XL, Omron Healthcare, Lake Forest, IL). Clinic staff was instructed to set the monitor to automatically wait five minutes and then obtain three measurements at one minute intervals with the participant alone in the exam room. The mean of the three BP measurements was used for these analyses. Clinical and laboratory data were obtained at the 24 and 27 month study visits.
Ambulatory BP was measured within 3 weeks of the 27 month study visit using SpaceLabs Medical Model 90207 monitors using a 24 hour protocol based on the recommendations from the British Hypertension Society and previous studies which used similar criteria.11–14 Briefly, the monitor was placed on the participant’s non-dominant arm, recorded BP every 30 minutes, and was set so that the readings were not displayed. Written instructions regarding the procedure were provided to participants and they were asked to keep a record of antihypertensive medication dosing. A recording was deemed acceptable if there were at least 14 readings between 6:00 AM and 12:00 midnight and at least 6 readings between 12:00 midnight and 6:00 AM.13, 14 Clinical site staff were trained to follow a standard manual of procedures for obtaining clinic and ambulatory BP.
As recommended by the European Society of Hypertension, nighttime systolic BP was defined as the average of all systolic BP readings during the 1 AM to 6 AM window; daytime systolic BP was defined as the average of all systolic BP readings during the 9 AM to 9 PM window.15 The 6 AM to 9 AM and 9 PM to 1AM windows are not included to avoid the transition periods between wake and sleep time, although in other studies in the field only 2 hours were excluded for analyses.16 Participants were categorized by the night/day ambulatory systolic BP ratio: extreme dippers (<0.8), dippers (≥0.8 and ≤0.9), non-dippers (>0.9 and ≤1) and reverse dippers (>1). BP variability was defined for each participant by the weighted average of the daytime and nighttime standard deviation.17 Secondary analyses defined BP variability by calculating average real variability (ARV).18
Statistical analyses
The difference in nighttime systolic BP between treatment groups was evaluated using linear regression with adjustment for clinical site. In secondary analyses, we adjusted for age, sex, race, eGFR, smoking (current, former, or never smoker), alcohol use (unknown, nondrinker: <1 drink per month, light drinker: 1 drink per month to <3 drinks per week, moderate drinker: ≥3 drinks per week but <2 drinks per day, or heavy drinker: ≥2 drinks per day), evening dosing of hypertension medications (nighttime and 24-hour ABPM measures), and morning dosing of hypertension medications (daytime and 24-hour ABPM measures) as covariates. We tested for interaction between treatment group and the pre-specified subgroups for SPRINT: previous chronic kidney disease (CKD, eGFR based on the MDRD study equation <60 ml/min/1.73m2), sex, race (black versus nonblack), previous CVD, and baseline systolic BP tertiles (<133 mmHg, 133 to <145 mmHg, or ≥145 mmHg). Similar analyses were conducted for the secondary outcomes of 24-hour systolic BP, daytime systolic BP, night/day systolic BP ratio, and BP variability. The BP variability metrics (SD and ARV) were both log-transformed prior to regression modeling; therefore, effect estimates represent multiplicative effects on the mean for these measures. We examined the concordance between clinic and ambulatory BP measures by calculating Spearman correlations, and graphically using Bland-Altman plots.19
Based on prior ABPM studies, we assumed a standard deviation for nighttime systolic BP between 12 and 16 mmHg.1, 2 Assuming an alpha level of 0.05, we estimated that 400 participants per group would provide 80% power to detect a 3 mmHg difference in nighttime systolic BP between treatment groups.
Results
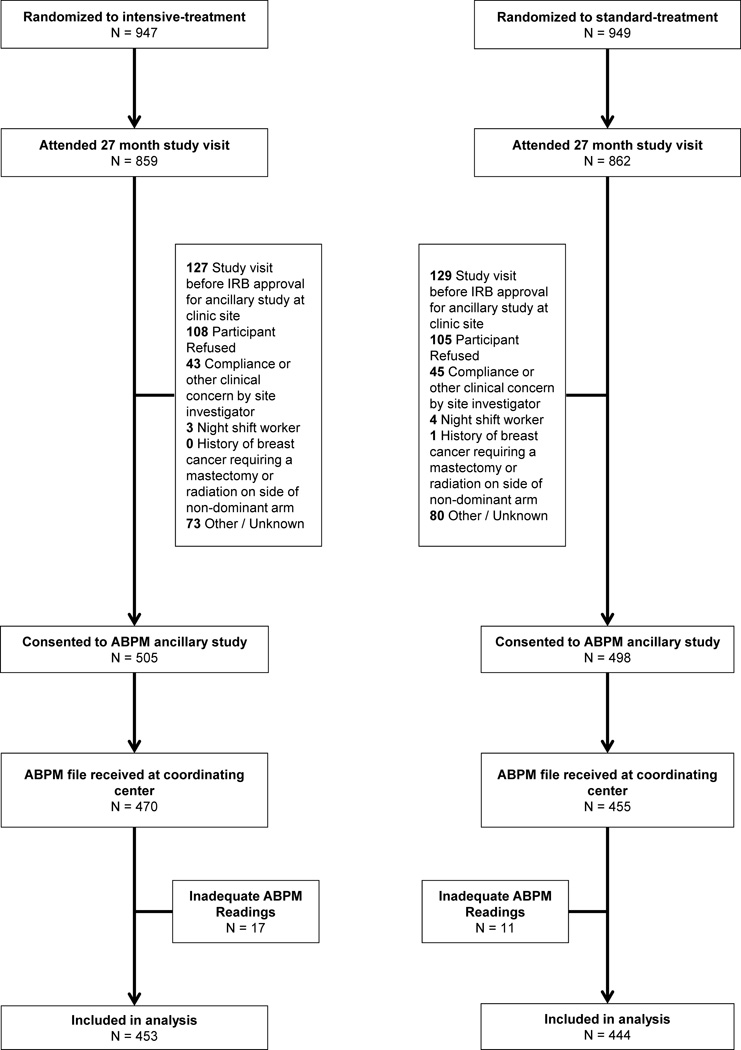
Acceptable ambulatory BP recordings were obtained in 897 SPRINT participants (Figure 1). The median number of days between the 27 month study visit and ambulatory BP measurement was 0 (interquartile range 0 to 6 days). Characteristics of participants who underwent ABPM by treatment group are shown in Table 1. At the time of ABPM, mean age of the participants was 71.5 years, 29% were female, and 28% were black. There were no significant differences in baseline demographic characteristics, Framingham risk, or history of CVD between treatment groups. The estimated glomerular filtration rate (eGFR) (mean 67.3 vs 73.4 ml/min/1.73m2; P <0.001) and urine albumin-to-creatinine ratio were lower (median 7.9 vs 10.6 mg/g; P <0.001) in the intensive-treatment group compared to the standard-treatment group at the 24 month study visit. As expected, participants in the intensive-treatment group were on more antihypertensive medications at the 27 month study visit. A greater percentage of participants in the intensive-treatment group took an antihypertensive medication at night (6 PM to 2 AM; 39% vs 31% in the standard-treatment group; P = 0.026); a similar difference was noted for morning dosing (4 AM to 10 AM; 77% vs 62% in the standard-treatment group; P <0.001).
Figure 1.
SPRINT indicates Systolic Blood Pressure Intervention Trial; ABPM, Ambulatory blood pressure monitoring.
TABLE 1.
Characteristics of SPRINT participants in the ABPM ancillary study
| Intensive-treatment | Standard-treatment | ||
|---|---|---|---|
| Variable | N = 453 | N = 444 | p-value |
| Age (years, 27M) | 71.5 ± 9.3 | 71.5 ± 9.7 | 0.898 |
| Female sex | 132 (29.1) | 125 (28.2) | 0.801 |
| Race / Ethnicity | 0.502 | ||
| White | 300 (66.2) | 304 (68.5) | |
| Black | 127 (28.0) | 124 (27.9) | |
| Hispanic | 13 (2.9) | 8 (1.8) | |
| Other | 13 (2.9) | 8 (1.8) | |
| Body Mass Index (kg/m2, 24M) | 29.6 ± 5.7 | 29.4 ± 5.5 | 0.570 |
| Smoking status (24M) | 0.999 | ||
| Never smoker | 208 (46.0) | 204 (45.9) | |
| Former smoker | 203 (44.9) | 200 (45.0) | |
| Current smoker | 41 (9.1) | 40 (9.0) | |
| Alcohol use (Baseline) | 0.098 | ||
| Non-drinker | 171 (37.7) | 178 (40.1) | |
| Light drinker | 91 (20.1) | 89 (20.0) | |
| Moderate drinker | 117 (25.8) | 99 (22.3) | |
| Heavy drinker | 43 (9.5) | 60 (13.5) | |
| Unknown | 31 (6.8) | 18 (4.1) | |
| History of CVD (Baseline) | 94 (20.8) | 101 (22.7) | 0.520 |
| Experienced CVD event before ABPM study | 15 (3.3) | 14 (3.2) | 1.000 |
| eGFR (ml/min/1.73 m2, 24M) | 67.3 ± 20.2 | 73.4 ± 21.1 | <0.001 |
| Urine albumin/Cr (mg/g, 24M) | 7.9 (4.9 to 15.2) | 10.6 (6.1 to 28.4) | <0.001 |
| Total cholesterol (mg/dL, 24M) | 183.4 ± 39.9 | 178.4 ± 38.6 | 0.058 |
| HDL cholesterol (mg/dL, 24M) | 52.7 ± 17.2 | 53.5 ± 16.7 | 0.462 |
| Fasting HDL cholesterol only (mg/dL, 24M) | 52.8 ± 17.1 | 54 ± 16.7 | 0.336 |
| LDL cholesterol (mg/dL, 24M) | 107.9 ± 34.6 | 101.9 ± 32.6 | 0.008 |
| Fasting LDL cholesterol only (mg/dL, 24M) | 108.7 ± 34.5 | 102.6 ± 32.7 | 0.013 |
| Total triglycerides (mg/dL, 24M) | 100 (70.8 to 142) | 93 (63 to 145) | 0.193 |
| Fasting total triglycerides only (mg/dL, 24M) | 100 (71 to 141.5) | 89 (62 to 139) | 0.020 |
| 10-year Framingham CVD risk (%, Baseline) | 20.8 ± 11.1 | 20.8 ± 10.6 | 0.988 |
| Number of antihypertensive medications (27M) | 2.9 ± 1.2 | 1.8 ± 1.1 | <0.001 |
| Beta-blockers | 182 (40.2) | 125 (28.2) | <0.001 |
| Calcium channel blockers | 271 (59.8) | 145 (32.7) | <0.001 |
| ACE inhibitors | 163 (36.0) | 128 (28.9) | 0.028 |
| Angiotensin receptor blockers | 190 (41.9) | 141 (31.8) | 0.002 |
| Vasodilators | 26 (5.7) | 10 (2.3) | 0.013 |
| Alpha-blockers | 47 (10.4) | 33 (7.4) | 0.156 |
| Diuretics | 342 (75.5) | 188 (42.4) | <0.001 |
Values are n (%), mean ± standard deviation, or median (25th percentile to 75th percentile). (Baseline) Data collected at randomization visit. (24M) Data collected at 24 month annual visit. (27M) Data collected at 27M study visit. (eGFR) Estimated glomerular filtration rate based on MDRD study equation.
In this study population, clinic systolic BP at the 27 month follow-up visit was 119.7 ± 12.8 mmHg in the intensive-treatment group, and 135.5 ± 13.8 mmHg in the standard-treatment group (Table 2). Nighttime systolic BP was lower in the intensive-treatment group compared to the standard-treatment group (115.7 ± 14.6 vs 125.5 ± 14.6 mmHg), as were daytime systolic BP (126.5 ± 12.3 vs 138.8 ± 12.6 mmHg) and 24 hour systolic BP (122.7 ± 12.0 vs 134.0 ± 11.8 mmHg). The adjusted difference in systolic BP between the intensive-treatment and standard-treatment groups was greater when measured in clinic (16.4 mmHg) versus nighttime BP (9.8 mmHg), daytime BP (12.1 mmHg), or 24 hour BP (11.2 mmHg) (Table 3). There were no differences between treatment groups with regard to dipping status or night-day systolic BP ratio (Tables 2 and 3). BP variability was lower in the intensive-treatment group when assessed by both weighted day-night standard deviation and absolute real variability (Table 3).
TABLE 2.
Clinic and Ambulatory Blood Pressure (BP) Results
| Intensive-treatment | Standard-treatment | Standard - Intensive | |
|---|---|---|---|
| Variable | Mean ± SD | Mean ± SD | Mean Difference (95% CI) |
| Baseline clinic systolic BP | 136.35 ± 15.40 | 138.00 ± 14.75 | 1.65 (−0.32, 3.63) |
| Baseline clinic diastolic BP | 75.80 ± 11.84 | 76.17 ± 12.10 | 0.37 (−1.20, 1.94) |
| 27 month clinic systolic BP | 119.67 ± 12.84 | 135.48 ± 13.77 | 15.81 (14.00, 17.61) |
| 27 month clinic diastolic BP | 65.94 ± 10.50 | 73.64 ± 12.22 | 7.70 (6.2, 9.25) |
| Nighttime systolic BP | 115.70 ± 14.64 | 125.51 ± 14.58 | 9.81 (7.89, 11.72) |
| Nighttime diastolic BP | 63.40 ± 9.46 | 68.50 ± 10.80 | 5.10 (3.77, 6.43) |
| Daytime systolic BP | 126.52 ± 12.32 | 138.78 ± 12.57 | 12.27 (10.64, 13.90) |
| Daytime diastolic BP | 72.03 ± 8.51 | 78.56 ± 10.68 | 6.53 (5.26, 7.79) |
| 24 hour systolic BP | 122.69 ± 11.99 | 133.97 ± 11.81 | 11.29 (9.73, 12.84) |
| 24 hour diastolic BP | 68.80 ± 7.98 | 74.71 ± 10.01 | 5.91 (4.72, 7.09) |
| Night-day systolic BP ratio | 0.92 ± 0.09 | 0.91 ± 0.09 | −0.009 (−0.021, 0.003) |
| Night-day diastolic BP ratio | 0.88 ± 0.11 | 0.88 ± 0.10 | −0.007 (−0.021, 0.006) |
| Median (IQR) | Median (IQR) | Ratio (95% CI) | |
| Weighted day-night variability (SD) | 10.52 (9.21 to 12.40) | 11.39 (9.57 to 13.69) | 1.08 (1.04, 1.12) |
| 24 hour systolic BP variability (ARV) | 9.61 (8.45 to 10.98) | 10.21 (8.88 to 11.65) | 1.06 (1.03, 1.09) |
| N (%) | N (%) | ||
| Dipping status | p=0.278 | ||
| Riser | 77 (17.0) | 63 (14.2) | |
| Non-dipper | 180 (39.7) | 168 (37.8) | |
| Dipper | 157 (34.7) | 160 (36.0) | |
| Extreme dipper | 39 (8.6) | 53 (11.9) |
(SD) Standard Deviation. (ARV) Average Real Variability. (IQR) Interquartile Range. Nighttime defined based on narrow clock time (1:00 am to 6:00 am). Daytime was also based on narrow clock time (9:00 am and 9:00 pm).
TABLE 3.
Mean differences for clinic and ambulatory blood pressure (BP) between intensive-treatment and standard-treatment groups
| Primary Analysis | Secondary Analysis | |||
|---|---|---|---|---|
| Variable | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value |
| 27 month clinic systolic BP | 15.95 (14.14, 17.77) | <0.001 | 16.35 (14.50, 18.20) | <0.001 |
| Nighttime systolic BP | 9.59 (7.68, 11.51) | <0.001 | 9.77 (7.83, 11.70) | <0.001 |
| Daytime systolic BP | 12.26 (10.63, 13.88) | <0.001 | 12.12 (10.44, 13.81) | <0.001 |
| 24 hour systolic BP | 11.21 (9.65, 12.76) | <0.001 | 11.18 (9.56, 12.80) | <0.001 |
| Night-day systolic BP ratio | −0.011 (−0.023, 0.001) | 0.082 | −0.009 (−0.021, 0.004) | 0.162 |
| Weighted day-night variability (SD) | 1.08 (1.04, 1.11) | <0.001 | 1.06 (1.03, 1.10) | <0.001 |
| 24 hour systolic BP variability (ARV) | 1.05 (1.03, 1.08) | <0.001 | 1.05 (1.02, 1.08) | 0.002 |
(SD) Standard Deviation. (ARV) Average Real Variability. Estimates denote mean effect for standard-treatment group based on general linear model. Variability metrics (SD and ARV) were modeled on log-scale, therefore estimates represent multiplicative effects on the mean. Primary analyses only adjust for clinic site. Secondary analyses also adjust for age, sex, race/ethnicity, eGFR, smoking status, and alcohol use. Nighttime systolic BP also adjusted for nighttime dosing of antihypertensive medications (between 6pm and 2am), while daytime systolic BP was also adjusted for dosing of antihypertensive medications between 4am and 10am. All other ABPM measures were adjusted for antihypertensive medication use between 6pm and 2am and/or between 4am and 10am.
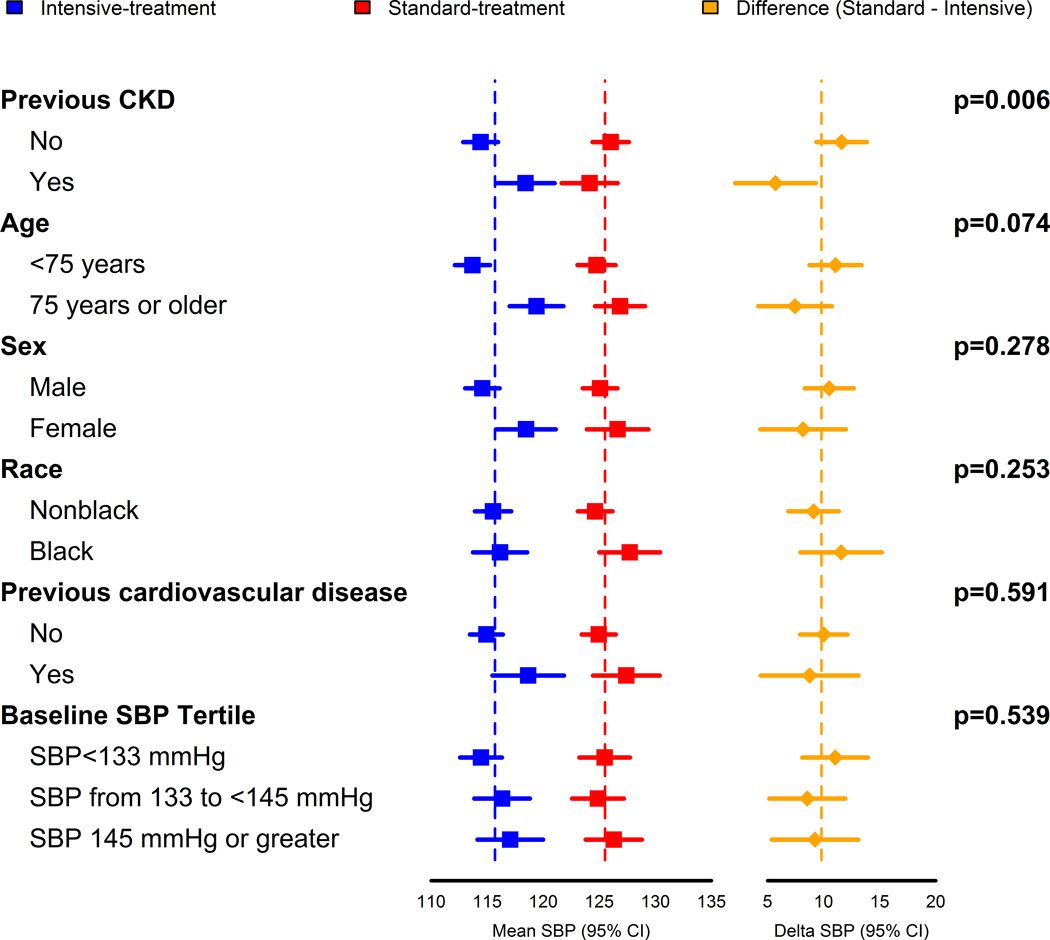
The difference in nighttime systolic BP between the intensive-treatment and standard-treatment groups was smaller in participants with CKD at the SPRINT baseline visit, and also smaller for participants 75 years of age or older, although the interaction between age and treatment group was not statistically significant (Figure 2). The difference in nighttime BP between treatment groups was consistent for subgroups defined by sex, race, prior CVD, and baseline systolic BP. Similar results by subgroups were observed for daytime and 24hr ambulatory BP, except that the difference between treatment groups was also smaller among females and participants 75 years or older (Supplemental Figures S1 and S2).
Figure 2.
Nighttime readings defined as being between 1:00 am and 6:00 am. Delta SBP denotes the mean difference in systolic blood pressure between the standard-treatment group and the intensive-treatment group. CKD indicates chronic kidney disease; SBP, systolic blood pressure.
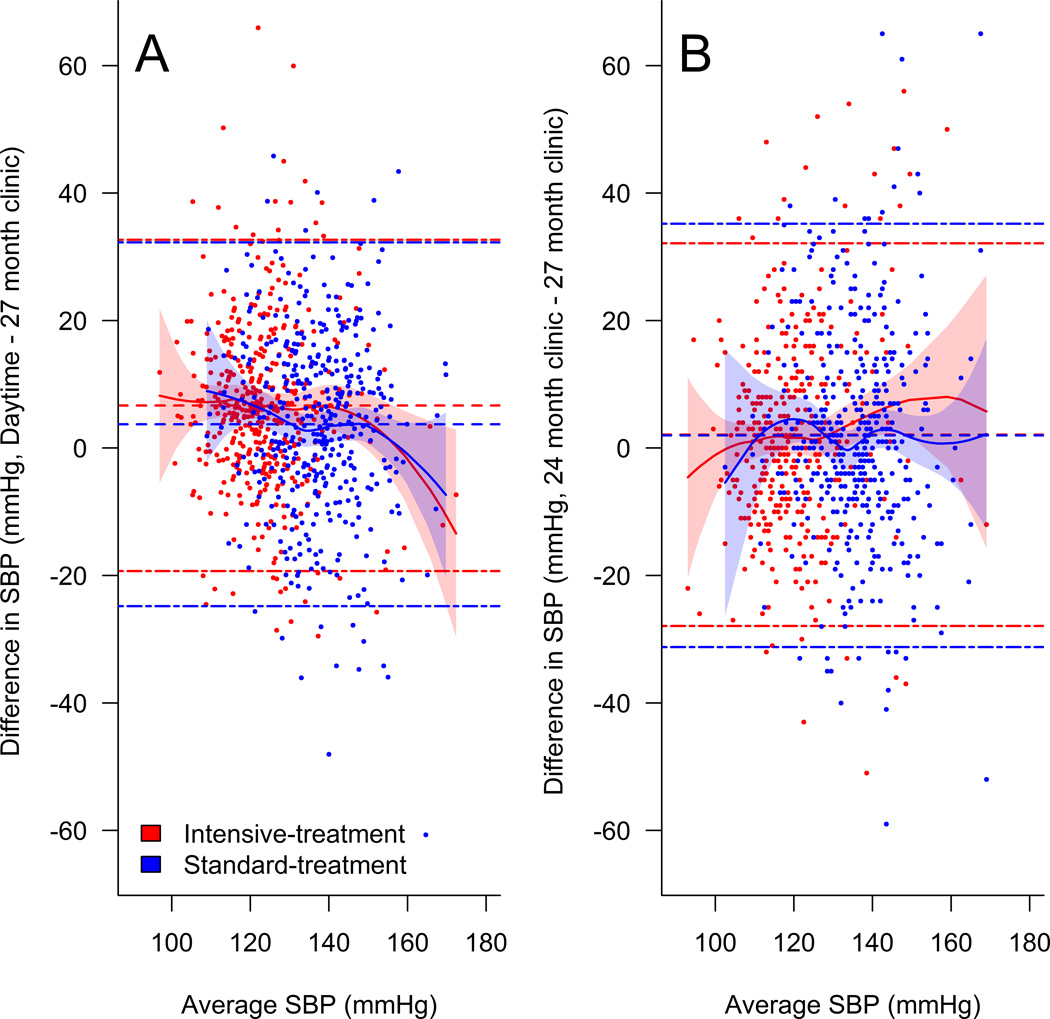
There was a moderate agreement between clinic systolic BP at the 27 month visit and daytime ambulatory systolic BP (Spearman correlation = 0.56, p<0.001). However, Bland-Altman plots indicated poor agreement with limits of agreement ranging from −19.3 to 32.7 mmHg for the intensive-treatment group and −24.8 to 32.3 mmHg for the standard-treatment group (Figure 3a). Similar levels of agreement were observed between clinic systolic BP at the 24 month and 27 month study visits, with limits of agreement of −27.9 to 32.1 mmHg for the intensive-treatment group and −31.2 to 35.2 mmHg for the standard-treatment group (Figure 3b).
Figure 3.
Daytime readings defined as being between 9:00 am and 9:00 pm. Single dash lines represent mean difference in BP and dash-dot lines denote limits of agreement (±1.96 × SD of difference). Solid lines denote estimated regression fit based on local polynomial regression with 95% point-wise confidence intervals (shaded areas). SBP indicates systolic blood pressure.
Discussion
Our results demonstrate that intensive clinic-based hypertension treatment lowers nighttime systolic BP, daytime systolic BP, and 24 hour systolic BP compared to standard clinic-based hypertension treatment. The difference in ambulatory BP between groups was less than the difference measured by clinic BP. Additionally, there was no difference in diurnal change in BP between groups.
The SPRINT ambulatory BP ancillary study results are consistent with previous reports indicating that interventions targeting clinic BP reduce clinic BP more than 24 hour ambulatory BP, and daytime ambulatory BP more than nighttime ambulatory BP. In the SYMPLICITY HTN-2 Trial, renal sympathetic denervation resulted in a 32 mmHg decrease in clinic systolic BP at 6 months, but only an 11 mmHg decrease in 24 hour ambulatory systolic BP.20 In the 3A observational study, clinic systolic BP decreased by 19 mmHg one year after antihypertensive intensification, while 24 hour ambulatory systolic BP decreased by only 10 mmHg. All prior results are based on baseline (pre-treatment) and follow-up (post-treatment) clinic and ambulatory BP measurements in observational studies and non-treat to target randomized trials. The Hypertension Optimal Treatment (HOT) trial did measure ambulatory BP in a substudy; however, it showed no difference in 24 hour ambulatory diastolic or systolic BP between randomized diastolic BP groups. The sample size for the HOT ambulatory BP substudy was relatively small, and there were only small differences in clinic BP between treatment arms.8 The SPRINT ambulatory BP results, therefore, represent the best demonstration of the impact of intensive clinic BP lowering therapy on ambulatory BP.
There are a number of important implications of our results. This study confirms that there was a significant BP difference between the intensive-treatment and standard-treatment groups using an independent technique of measuring BP. Our results show that SPRINT achieved a significant difference in nighttime and daytime systolic BP. Results were consistent across most subgroups, although the difference in ambulatory BP between treatment groups was lower among participants with CKD, those 75 years of age or older, and females. ABPM may be of more benefit in patients with these characteristics given the smaller impact of intensive treatment on ambulatory BP, which may increase the likelihood of discordance between clinic and ambulatory BPs. It’s interesting to note that daytime ambulatory systolic BP was 6.85 mmHg higher than clinic systolic BP in the intensive-treatment group, compared to 3.30 mmHg higher in the standard-treatment group. This finding suggests that ABPM may be more important when implementing intensive clinic-based hypertension therapy in order to assess for higher BP outside the office compared to in the clinical setting. This profile of BP, commonly referred to as masked hypertension, is associated with increased risk for hypertension related adverse outcomes.1–5
In SPRINT, intensive lowering of clinic BP resulted in significant reductions in ambulatory BP, cardiovascular events, and all-cause mortality.9 Therefore, at least indirectly, SPRINT suggests that reductions in ambulatory BP are associated with improved clinical outcomes. The reduction in cardiovascular events and all-cause mortality may be due in part to the reduction in nighttime BP and BP variability observed with intensive lowering of clinic BP in the current study. The decrease in BP variability may be due to the lower clinic BP target or the increased use of antihypertensive medications associated with lower BP variability such as chlorthalidone and calcium channel blockers.21 The effect of the intensive clinic BP target on ambulatory BP may also be due in part to increased utilization of these long acting medications. Surprisingly, there was no difference in the diurnal change in BP between treatment groups. Recent studies indicate that nighttime hypertension itself, rather than diurnal change in BP is associated with adverse outcomes.22, 23
The United States Preventive Services Task Force (USPSTF) recently made a grade A recommendation for measurement of ambulatory BP in patients with elevated clinic BP to confirm the diagnosis of hypertension prior to initiating treatment.24, 25 This recommendation is based on observational studies demonstrating that approximately 25% of patients with elevated clinic BP have normal BP outside the clinic, known as white-coat hypertension; patients with white-coat hypertension are at low risk for adverse outcomes.26 As mentioned previously, it remains unknown whether patients with white-coat hypertension benefit from antihypertensive therapy because nearly all hypertension trials have not included measurement of ambulatory BP at baseline.
Of note, clinic BP was lower than daytime ambulatory BP in SPRINT. This could be due to the careful guideline based measurement of clinic BP in SPRINT, use of an automated device, and a lower white-coat effect since participants were coming to a known environment and staff and were allowed to rest alone for 5 minutes prior to BP measurement.27 Our results reinforce the concept that ambulatory BP is required to assess the burden of hypertension during the course of patients’ usual activities in their environment, and cannot be reliably estimated by clinic BP readings. Finally, while we measured BP outside the research setting with ABPM, the BP achieved in the routine clinic setting remains unknown. Given that BP is not measured per American Heart Association recommendations in most clinics, understanding the achieved BP in the routine clinic setting during the treat to target phase is critically important to implementing SPRINT results.
Our study has important limitations. Ambulatory BP was not measured at the baseline visit in SPRINT. This limits our ability to assess how intensive and standard treatment strategies affect ambulatory and nighttime BP within individuals, and to identify patient characteristics associated with ambulatory BP responses to treatment. Ambulatory BP was assessed in only a subset of SPRINT participants; however, the subjects who did and did not participant in the SPRINT ABPM ancillary study had similar baseline characteristics (Supplemental Table S1) and only 5% were excluded due to compliance concerns. While there is robust epidemiologic literature documenting the predictive value of nighttime BP, whether lowering nighttime BP improves outcomes is not resolved.6, 13 SPRINT was not designed to lower nighttime BP specifically, and therefore, our findings are post-hoc, and do not fully resolve this issue. Finally, ambulatory BP was only assessed at one visit and BP categorization, such as white-coat hypertension, may vary over time in up to 25% of patients.28
While the importance of ABPM is increasingly being recognized, several issues that are important to fully leverage ABPM to improve hypertensive patients’ outcomes remain unresolved. It is unknown whether treating patients with normal clinic BP and elevated ambulatory BP, known as masked hypertension, reduces risk for cardiovascular and renal disease; similarly, it is unknown whether withholding therapy for patients with elevated clinic BP and normal ambulatory BP (white-coat hypertension) is safe. Additionally, in patients with elevated clinic and ambulatory BP, it is unknown whether a treatment strategy targeting ambulatory BP reduces adverse outcomes compared to a conventional strategy targeting clinic BP. Another important issue that our data cannot address relates to evening dosing of antihypertensive medications, which has been shown to reduce nighttime BP and risk for CVD.29, 30 While nighttime BP was lower in the intensive-treatment group and a greater percent of intensive-treatment versus standard-treatment participants took an antihypertensive medication in the evening (39% vs 31%), the effect of evening dosing with either intensive or standard clinic-based BP targets was not assessed. Further studies are needed to assess whether targeting hypertension therapy based on ambulatory BP reduces adverse outcomes compared to clinic-based therapy, and whether evening dosing of antihypertensive therapy reduces risk for CVD.
Perspectives
This is the first study to demonstrate the effect of intensive and standard clinic-based systolic BP targets on ambulatory BP. Compared to standard treatment, intensive treatment of clinic BP resulted in lower nighttime, daytime, and 24 hour ambulatory BP as well as BP variability, but did not alter the diurnal BP pattern. However, there was a greater difference in clinic BP than ambulatory BP; additionally, the within participant agreement between clinic and ambulatory BP was low. These results highlight the importance of ambulatory BP to assess the true burden of hypertension during treatment. Finally, given the increasing awareness of ambulatory BP for defining hypertension related risk for adverse outcomes, the SPRINT ambulatory BP results will inform the design of hypertension trials that move beyond clinic BP to evaluate the potential benefits of new treatment strategies.
Supplementary Material
Novelty and Significance.
What is New?
This is the first study to demonstrate the effect of intensive and standard clinic-based systolic BP targets on ambulatory BP.
What is Relevant?
These results highlight the importance of ambulatory BP to assess the true burden of hypertension during treatment.
Given the increasing awareness of ambulatory BP for defining hypertension related risk for adverse outcomes, the SPRINT ambulatory BP results will inform the design of hypertension trials that move beyond clinic BP to evaluate the potential benefits of new treatment strategies.
Summary
Targeting a systolic BP of less than 120 mmHg, as compared with less than 140 mmHg, resulted in lower nighttime, daytime, and 24 hour systolic BP, but did not change the night/day systolic BP ratio. Further studies are needed to assess whether targeting hypertension therapy based on ambulatory BP improves clinical outcomes.
Acknowledgments
For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: SPRINT Acknowledgment.
Sources of Funding/Support: The SPRINT ambulatory blood pressure ancillary study was supported by grants R03DK100530 (PED), K12HD043451 (MKW), K23DK091521 (DER), and R03DK105314 (DER) from the National Institutes of Health (NIH) and funds from the University of Minnesota Chronic Kidney Disease Research Fund. The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NHLBI, the U.S. Department of Veterans Affairs, the U.S. Department of Health and Human Services, the University of Minnesota, or the United States Government.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
Role of the Funder/Sponsor: All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. Scientists at the National Institutes of Health participated in the design of the study and as a group had one vote on the steering committee of the trial.
Dr Cushman reports receipt of institutional grants from Eli Lilly and Boerhinger-Ingelheim and unpaid consulting and steering committee work for Takeda.
Footnotes
Trial Registration: clinicaltrials.gov identifier: NCT01835249
Disclosures: The remaining authors report no conflicts of interest.
References
- 1.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, Santucci C, Reboldi G. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 2.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 3.Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 4.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O'Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 5.Minutolo R, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, Nappi F, Stanzione G, Conte G, De Nicola L. Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: A multicenter prospective cohort study. Am J Kidney Dis. 2014;64:744–752. doi: 10.1053/j.ajkd.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Parati G. Office compared with ambulatory blood pressure in assessing response to antihypertensive treatment: A meta-analysis. J Hypertens. 2004;22:435–445. doi: 10.1097/00004872-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Omboni S, Parati G, Clement DL, Haley WE, Rahman SN, Hoogma RP. Twenty-four hour ambulatory blood pressure in the Hypertension Optimal Treatment (HOT) study. J Hypertens. 2001;19:1755–1763. doi: 10.1097/00004872-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 9.The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosius WT, Sink KM, Foy CG, et al. SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien E, Coats A, Owens P, Petrie J, Padfield PL, Littler WA, de Swiet M, Mee F. Use and interpretation of ambulatory blood pressure monitoring: Recommendations of the British Hypertension Society. BMJ. 2000;320:1128–1134. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drawz PE, Alper AB, Anderson AH, et al. Chronic Renal Insufficiency Cohort Study Investigators. Masked hypertension and elevated nighttime blood pressure in CKD: prevalence and association with target organ damage. Clin J Am Soc Nephrol. 2016;11:642–652. doi: 10.2215/CJN.08530815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabbai FB, Rahman M, Hu B, et al. African American Study of Kidney Disease and Hypertension (AASK) Study Group. Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol. 2012;7:1770–1776. doi: 10.2215/CJN.11301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parati G, Stergiou G, O'Brien E, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–1366. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 16.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 17.Hansen TW, Thijs L, Li Y, et al. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. doi: 10.1161/HYPERTENSIONAHA.109.140798. [DOI] [PubMed] [Google Scholar]
- 18.Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–511. doi: 10.1097/01.hjh.0000160205.81652.5a. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Comparing methods of measurement: Why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 20.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): A randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 21.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, Levitan EB, Whelton PK, Cushman WC, Louis GT, Davis BR, Oparil S. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: A cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuspidi C, Facchetti R, Bombelli M, Sala C, Negri F, Grassi G, Mancia G. Nighttime blood pressure and new-onset left ventricular hypertrophy: Findings from the Pamela population. Hypertension. 2013;62:78–84. doi: 10.1161/HYPERTENSIONAHA.111.00682. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Lloret S, Toblli JE, Cardinali DP, Malateste JC, Milei J. Nocturnal hypertension defined by fixed cut-off limits is a better predictor of left ventricular hypertrophy than non-dipping. Int J Cardiol. 2008;127:387–389. doi: 10.1016/j.ijcard.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Piper MA, Evans CV, Burda BU, Margolis KL, O'Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192–204. doi: 10.7326/M14-1539. [DOI] [PubMed] [Google Scholar]
- 25.Siu AL U.S. Preventative Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778–786. doi: 10.7326/M15-2223. [DOI] [PubMed] [Google Scholar]
- 26.Bobrie G, Chatellier G, Genes N, Clerson P, Vaur L, Vaisse B, Menard J, Mallion JM. Cardiovascular prognosis of "masked hypertension" detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA. 2004;291:1342–1349. doi: 10.1001/jama.291.11.1342. [DOI] [PubMed] [Google Scholar]
- 27.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Grant FC, Kaczorowski J. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: Randomised parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muxfeldt ES, Fiszman R, de Souza F, Viegas B, Oliveira FC, Salles GF. Appropriate time interval to repeat ambulatory blood pressure monitoring in patients with white-coat resistant hypertension. Hypertension. 2012;59:384–389. doi: 10.1161/HYPERTENSIONAHA.111.185405. [DOI] [PubMed] [Google Scholar]
- 29.Minutolo R, Gabbai FB, Borrelli S, Scigliano R, Trucillo P, Baldanza D, Laurino S, Mascia S, Conte G, De Nicola L. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in ckd: An 8-week uncontrolled trial. Am J Kidney Dis. 2007;50:908–917. doi: 10.1053/j.ajkd.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the mapec study. Chronobiol Int. 2010;27:1629–1651. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.