Abstract
Background & Aims
In 2015, a national Egyptian health issue survey was conducted to describe the prevalence of hepatitis C virus (HCV) infection. In this paper, we describe the HCV burden in 2015, compare the results with the national survey conducted in 2008, and discuss the implications of the new findings on prevention of HCV in Egypt.
Methods
A multistage probability sampling approach was used, similar to the national demographic survey conducted in 2008. More than 90% of sampled individuals complied with the interview and provided blood samples.
Results
In the 15–59‐year age groups, the prevalence of HCV antibody was found to be 10.0% (95% CI 9.5–10.5) and that of HCV RNA to be 7.0% (95% CI 6.6–7.4). In children, 1–14 years old, the prevalence of HCV antibody and HCV RNA were 0.4% (95% CI 0.3–0.5) and 0.2% (95% CI 0.1–0.3) respectively. Approximately, 3.7 million persons have chronic HCV infection in the age group 15–59 in 2015. An estimated 29% reduction in HCV RNA prevalence has been seen since 2008, which is largely attributable to the ageing of the group infected 40–50 years ago during the mass schistosomiasis treatment campaigns. Prevention efforts may have also contributed to this decline, with an estimated 75% (95% CI 6–45) decrease in HCV incidence in the 0–19 year age groups over the past 20 years.
Conclusions
These findings can be used to shape future HCV prevention policies in Egypt.
Keywords: hepatitis C Egypt, incidence HCV, nation‐wide surveys, prevalence HCV
Abbreviations
- CIA
chemiluminescent microplate immunoassay
- CPHL
Central Public Health Laboratory
- DAA
direct‐acting antivirals
- EDHS
Egyptian Health Demographic Survey
- EHIS
Egyptian Health Issue Survey
- ELISA
third‐generation enzyme immunoassay
- FDA
Food and Drug Administration
- HCV
hepatitis C virus
- PPU
Pasteur‐Paris University
Key points.
There has been an approximate 30% decrease in HCV prevalence in Egypt between 2008 and 2015.
This decline is mostly related to the ageing of the initially infected cohort; this phenomenon will be seen in most countries as the bulk of worldwide HCV infections took place between 1960 and 1980.
Still, a 28% decline in incidence in younger age groups is estimated, most likely related to prevention efforts such as injection safety and awareness programmes.
Treatment has not yet demonstrated an impact on decline of HCV prevalence in Egypt.
Hepatitis C Virus (HCV) infection is a major global health challenge; it is estimated that more than 80 million people are chronically infected worldwide, with 3–4 million new infections and 350 000 deaths occurring each year because of HCV‐related complications 1, 2, 3. Egypt is the country with the highest HCV prevalence in the world; in 2008, the Egyptian Demographic Health Survey (EDHS), which was conducted on a large nationally representative sample, estimated the prevalence of HCV antibodies and HCV RNA, among the 15–59 year age group, to be 14.7 and 9.8% respectively. Based on the population census and the EDHS done in 2008, it was estimated that more than 6.8 million persons aged 15–59 years had HCV antibodies, of which more than 4.5 million individuals had active HCV infection 4. In 2015, the Egyptian Health Issues Survey (EHIS) was done to re‐estimate the prevalence of HCV infection in Egypt. In this paper, we describe the prevalence of HCV in Egypt in 2015, using measures of both HCV RNA, which indicates the burden of disease, and of HCV antibody, which provides an estimate of past infection. We also compare the results of the two national surveys conducted in 2008 and 2015 in order to estimate national changes in prevalence and incidence, and discuss the implications of these findings on the national policy for HCV prevention and treatment in Egypt.
Materials and methods
Source of data
We obtained data from the EDHS 2008 4 and EHIS 2015 5, both of which were conducted by El‐Zanaty and Associates with support from the United States Aid of International Development‐sponsored DHS‐7 project.
Sampling strategy
The national surveys in 2008 and 2015 were cross‐sectional household surveys, where sampling weights were used to provide estimates considered representative of the Egyptian population on the basis of a complex, three‐stage probability sampling approach. The two surveys provide estimates of HCV prevalence in Egypt for the country as a whole and broken down for the major administrative regions (Urban Governorates, Lower Egypt, Upper Egypt and the Frontier Governorates). In both EDHS 2008 and EHIS 2015, those aged 15–59 years were invited to participate, however, in EHIS 2015, children aged 6 months to 14 years were also included 4, 5.
Data collection
Individuals within sampled households were invited and consented for participation. Basic demographic information was collected, including: age, gender, marital status, place of residence, level of education, work status and wealth status.
Laboratory procedures
The laboratory procedures applied in 2015 were similar to those in 2008 4, 5. In 2015, consented individuals provided 7 ml of venous blood added to an EDTA vacutainer tube. In the field laboratory, the 2015 EHIS biomarker staff centrifuged the blood and transferred the serum to five microvials that were stored in liquid nitrogen tanks before being transferred to the Central Public Health Laboratory (CPHL) in Cairo. A hepatitis C testing algorithm used third‐generation enzyme immunoassay (ELISA) to determine the presence of HCV antibodies. A more specific assay, the chemiluminescent microplate immunoassay (CIA) was used to confirm HCV antibody status for ELISA‐positive samples and 5% of the ELISA‐negative samples. Quantitative real‐time PCR was used to test for HCV RNA in HCV antibody‐positive samples to confirm active infections. A quality control procedure of retesting of approximately 10% of all samples was undertaken at the CPHL and a further external quality control was done at the Theodor Bilharz Research Institute, in Cairo, by retesting approximately 5% of the samples tested at the CPHL 5. The only key difference between this procedure and that of the previous survey is that, in 2008, the quality control measure (10% of samples retested) at CPHL was not carried out.
Statistical analysis
Comparisons were carried out for HCV antibody‐ and HCV RNA‐positive tests, by estimating the absolute and relative risk reductions between the two surveys in comparable age groups. To calculate the 95% confidence interval for the risk difference, Newcombe–Wilson's method without continuity correction was applied 6. In order to estimate confidence intervals for the relative risk, we used the methods described by Armitage and Berry 7. The level of significance (P‐value) was inferred to be less than 0.05 if the corresponding 95% confidence intervals for the relative and absolute effect measures were devoid of ‘zero’ 8. HCV virus clearance was estimated by finding the absolute difference between the proportion of HCV antibody and HCV RNA‐positive participants, and dividing this by the proportion of HCV antibody‐positive participants. Comparisons of HCV clearance between combined age groups in 2008 and 2015 were done using the chi‐square test. In order to demonstrate the cohort effect, the 2008 DHS data were shifted forward; 7 years were added to each participant's age from the 2008 DHS survey and the HCV antibody and HCV RNA prevalence estimates were re‐calculated using the new numerator and denominator in each age category. The estimated number of population positive for HCV antibodies and HCV RNA was calculated by multiplying the age‐ and gender‐specific prevalence of 2015 by the population census of January 2015 categorised by age and gender.
Ethical considerations
No ethical approval was needed for the data analysis presented in this paper; the anonymised data is publically available online. El‐Zanaty and Co is the responsible party for the ethical considerations of the EDHS 2008 and EHIS 2015; verbal informed consent was obtained from all individuals aged 18 years and older and from married minors aged 15–17 years. For children less than 18 years, consent was obtained from the parent or child caretaker.
Results
In EDHS 2008, 4757 households including 12 780 individuals aged 15–59 years were identified for interview and blood testing. A total of 4662 households complied (98%), 12 008 persons were interviewed (93.9%) and out of these, 11 126 persons provided blood samples for testing (92.7%). In EHIS 2015, 7649 households including 28 079 individuals were identified. A total of 7516 households complied (98.3%), and 27 549 persons (age: 1–59 years) were interviewed (98.1%). Of the total 28 079 persons identified, 17 182 were aged 15–59 years, of which 16 671 were interviewed (97.0%) and out of these, 16 003 provided blood samples for testing (96.0%). The remaining 10 897 persons identified were children aged 1–14 years, and of these, 10 878 (99.8%) were interviewed through their caregivers and 10 044 (92.3%) children provided blood samples for testing. Children therefore represented 39.5% of the total study population (those interviewed) in EHIS 2015. The characteristics of the two populations surveyed in EDHS 2008 and EHIS 2015 are presented in Table 1. For comparison purposes, we describe the age group of 15–59 years who were targeted in both surveys.
Table 1.
Participant characteristics of Egyptian Demographic Health Surveys in 2008 compared to EHIS 2015
| 2008 | 2015 | |||
|---|---|---|---|---|
| No. | % | No | % | |
| Study population (15–59 years), total interviewed | 12 008 | 100 | 16 671 | 100 |
| Laboratory tested | ||||
| Yes | 11 126 | 92.7 | 16 003 | 95.9 |
| No | 882 | 7.3 | 668 | 4.1 |
| Households, total sampled | 4953 | 100 | 7813 | 100 |
| Interviewed | ||||
| Yes | 4662 | 94.1 | 7516 | 96.2 |
| No | 291 | 5.9 | 297 | 3.8 |
| Age group (years), total 15–59 | ||||
| 15–19 | 2151 | 17.9 | 2713 | 16.3 |
| 20–24 | 1960 | 16.3 | 2044 | 12.3 |
| 25–29 | 1635 | 13.6 | 2433 | 14.6 |
| 30–34 | 1322 | 11.0 | 2118 | 12.7 |
| 35–39 | 1209 | 10.1 | 1917 | 11.5 |
| 40–44 | 1148 | 9.6 | 1550 | 9.3 |
| 45–49 | 1044 | 8.7 | 1424 | 8.5 |
| 50–59 | 1539 | 12.8 | 2472 | 14.8 |
| Gender, total | ||||
| Males | 5718 | 47.6 | 7462 | 44.8 |
| Females | 6290 | 52.4 | 9209 | 55.2 |
| Marital status, total | ||||
| Never married | 3863 | 32.2 | 4375 | 26.2 |
| Married | 7588 | 63.2 | 11 372 | 68.2 |
| Widowed | 400 | 3.3 | 329 | 2.0 |
| Divorce/separated | 157 | 1.3 | 595 | 3.6 |
| Residence, total | ||||
| Urban | 5288 | 44.0 | 6206 | 37.2 |
| Rural | 6720 | 56.0 | 10 465 | 62.8 |
| Place of residence | ||||
| Urban governorates | 2445 | 20.4 | 2267 | 13.6 |
| Lower Egypt | 5213 | 43.4 | 8204 | 49.2 |
| Upper Egypt | 4168 | 34.7 | 6081 | 36.5 |
| Frontier governorates | 182 | 1.5 | 119 | 0.7 |
| Education | ||||
| No education | 2588 | 21.6 | 2652 | 15.9 |
| Some primary | 1084 | 9.0 | 1459 | 8.8 |
| Primary complete/some secondary | 2919 | 24.3 | 4552 | 27.3 |
| Secondary complete/higher | 5417 | 45.1 | 8008 | 48.0 |
| Wealth quintile | ||||
| Lowest | 2042 | 17.0 | 3268 | 19.6 |
| Second | 2442 | 20.3 | 3234 | 19.4 |
| Middle | 2425 | 20.2 | 3212 | 19.3 |
| Fourth | 2440 | 20.3 | 3436 | 20.6 |
| Highest | 2659 | 22.1 | 3521 | 21.1 |
There was an overall significant reduction of 32 and 29% in the prevalence of HCV antibody and HCV RNA‐positive individuals, respectively, between the DHS in 2008 and the EHIS in 2015 (Table 2). The age‐specific prevalence of HCV antibody and HCV RNA‐positive individuals in 2008 and 2015 is presented in Table 2 and Fig. 1. The pattern of increased prevalence of HCV antibody and HCV RNA‐positive persons with age was observed in both the 2008 and the 2015 survey. A statistically significant reduction in HCV antibody prevalence was observed in all age groups, and the greatest relative prevalence reduction (75%) was observed among those aged 15–19 years. A statistically significant reduction in HCV RNA‐positive individuals was observed in all except the two age groups encompassing those 20–29 years of age. The age‐specific prevalence of HCV antibody and HCV RNA‐positive individuals from 2008 shifted forward by 7 years is presented alongside the 2015 prevalence estimates (Fig. 1). The current data (EHIS 2015) for the three oldest age groups (45–59 years) show a similar HCV clearance percentage when compared to the same age groups in the shifted‐forward 2008 data (31.2% in 2015, 32.0% in the shifted 2008, P = 0.756). For the cohort of children aged 1–14 years in 2015, the overall prevalence of HCV antibody and HCV RNA‐positive individuals was 0.4% (95% CI 0.3–0.5) and 0.2% (95% CI 0.1–0.3) respectively (Table 3).
Table 2.
Prevalence of hepatitis C virus (HCV) antibody and HCV RNA positive persons (age 15–59 years) by age, gender and region (EDHS 2008 and EHIS 2015 surveys)
| Characteristic | HCV antibody positive | HCV RNA positive | ||||||
|---|---|---|---|---|---|---|---|---|
| % Positive 2008 | % Positive 2015 | Prevalence difference (95% CI) | % Prevalence reduction (95% CI) | % Positive 2008 | % Positive 2015 | Prevalence difference (95% CI) | % Prevalence reduction (95% CI) | |
| Overall prevalence | 14.7 | 10.0 | 4.7 (3.9–5.5)a | 32 (60–69)a | 9.9 | 7.0 | 2.9 (2.2–3.6)a | 29 (23–35)a |
| Age group | ||||||||
| 15–19 | 4.1 | 1.0 | 3.1 (2.2–4.1)a | 75 (64–85)a | 2.8 | 0.8 | 2.2 (1.2–2.8)a | 73 (55–83)a |
| 20–24 | 4.9 | 3.2 | 1.6 (0.4–2.9)a | 34 (10–52)a | 3.0 | 2.2 | 0.8 (−0.2 to 1.8) | 27 (−8.8 to 51) |
| 25–29 | 6.1 | 4.4 | 1.7 (0.3–3.2)a | 28 (6–45)a | 3.9 | 3.0 | 0.9 (−0.2 to 2.1) | 24 (−7 to 45) |
| 30–34 | 11.8 | 7.1 | 4.5 (2.5–6.7)a | 40 (25–51)a | 8.3 | 4.9 | 3.3 (1.6–5.2)a | 41 (23–55)a |
| 35–39 | 13.8 | 8.2 | 5.5 (3.2–7.9)a | 40 (26–52)a | 9.9 | 6.0 | 3.8 (1.9–6.0)a | 39 (22–53)a |
| 40–44 | 23.0 | 11.6 | 11.4 (8.4–14.5)a | 50 (40–58)a | 15.0 | 8.9 | 6.1 (3.5–8.7)a | 41 (26–52)a |
| 45–49 | 28.6 | 16.3 | 12 (8.6–15.5)a | 43 (33–51)a | 18.9 | 11.5 | 7.3 (4.3–10.4)a | 39 (26–50)a |
| 50–54 | 38.3 | 27.9 | 10.6 (6.3–14.8)a | 27 (17–36)a | 25.3 | 19.7 | 5.6 (1.8–9.4)a | 22 (8–34)a |
| 55–59 | 39.4 | 33.9 | 5.1 (0.4–9.8)a | 14 (2–24)a | 27.4 | 22.3 | 5.0 (0.8–9.2)a | 18 (3–31)a |
| Gender | ||||||||
| Males | 17.4 | 12.4 | 5 (3.7–6.2)a | 29 (22–35)a | 12.1 | 8.9 | 3.1 (2.1–4.3)a | 26 (18–34)a |
| Females | 12.2 | 8.1 | 4 (2.9–5.0)a | 33 (26–39)a | 7.8 | 5.5 | 2.3 (1.4–3.2)a | 30 (20–38)a |
| Work status | ||||||||
| Working for cash | 18.8 | 13.3 | 5.5 (4.1–6.8)a | 29 (23–35)a | 13.0 | 9.5 | 3.5 (2.3–4.6)a | 27 (19–34)a |
| Not working for cash | 11.3 | 7.4 | 3.9 (2.9–4.8)a | 35 (28–41)a | 7.3 | 5.1 | 2.2 (1.4–3.0)a | 30 (21–38)a |
| Region | ||||||||
| Urban governorates | 9.5 | 6.9 | 2.6 (0.98–4.21)a | 27 (11–41)a | 6.2 | 4.4 | 1.8 (0.4–3.0)a | 29 (8–45)a |
| Lower Egypt | 17.5 | 12.2 | 5.3 (4.0–6.6)a | 30 (24–36)a | 11.5 | 8.7 | 2.8 (1.7–3.9)a | 24 (16–32)a |
| Upper Egypt | 14.7 | 8.2 | 6.5 (6.9–9.5)a | 44 (37–50)a | 10.2 | 5.8 | 4.4 (3.2–5.5)a | 43 (35–50)a |
| Frontier governorates | 3.0 | 3.5 | −0.2 (−5.7 to 5.0) | −17 (−369 to 71) | 2.5 | 2.6 | −0.1 (−5.2 to 4.6) | −4 (−402 to 78) |
Statistically significant (P < 0.05).
Figure 1.
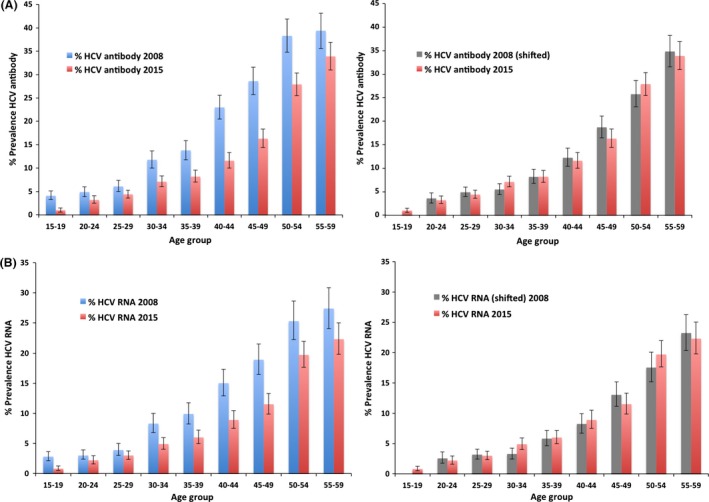
(A) Age‐specific prevalence of hepatitis C virus (HCV) antibody‐positive persons in 2008 and 2015 (left), then shifted (by 7 years) 2008 and 2015 (right). (B) Age‐specific prevalence of HCV RNA‐positive persons in 2008 and 2015 (left), then shifted (by 7 years) 2008 and 2015 (right).
Table 3.
Estimated population numbers who are hepatitis C virus (HCV) antibody and HCV RNA positive (EHIS 2015)
| Age groups (years) | Males | Females | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male population Jan 2015 | % Positive HCV antibodies | Estimated number of positive HCV antibodies | % HCV RNA positive | Estimated number of HCV RNA positive | Female population Jan 2015 | % Positive HCV antibodies | Estimated number of positive HCV antibodies | % HCV RNA positive | Estimated number of HCV RNA positive | Estimated population with HCV RNA | Estimated population with HCV antibodies | |
| 1–4 | 4 176 030 | 0.5 | 20 880 | 0.2 | 8352 | 3 871 877 | 0.4 | 15 487 | 0.3 | 11 616 | 19 968 | 36 367 |
| 5–9 | 4 790 338 | 0.5 | 23 952 | 0.2 | 8580 | 4 456 493 | 0 | 0 | 0 | 0 | 8580 | 23 952 |
| 10–14 | 4 279 072 | 1.1 | 47 070 | 0.1 | 4279 | 4 013 008 | 0.2 | 8026 | 0.2 | 8026 | 12 305 | 55 096 |
| 15–19 | 4 367 988 | 0.9 | 39 312 | 0.6 | 26 208 | 4 131 991 | 1.2 | 49 584 | 0.9 | 37 188 | 63 396 | 88 896 |
| 20–24 | 4 623 621 | 4.8 | 221 934 | 3.1 | 143 332 | 4 424 043 | 2.1 | 92 905 | 1.5 | 66 361 | 209 693 | 314 839 |
| 25–29 | 4 334 645 | 6.8 | 294 756 | 4.7 | 203 728 | 4 175 258 | 2.8 | 116 907 | 1.9 | 79 330 | 283 058 | 411 663 |
| 30–34 | 3 456 601 | 9.0 | 311 095 | 7.1 | 245 419 | 3 364 004 | 5.6 | 188 384 | 3.2 | 107 648 | 353 067 | 499 479 |
| 35–39 | 2 711 932 | 9.4 | 254 922 | 6.9 | 192 547 | 2 639 282 | 7.1 | 187 389 | 5.3 | 139 882 | 332 429 | 442 311 |
| 40–44 | 2 422 954 | 14.4 | 348 905 | 10.8 | 256 833 | 2 379 682 | 9.0 | 214 171 | 7.3 | 173 717 | 430 550 | 563 076 |
| 45–49 | 2 222 893 | 17.8 | 395 675 | 12.4 | 282 307 | 2 184 980 | 14.8 | 323 377 | 10.4 | 227 238 | 509 545 | 719 052 |
| 50–54 | 1 922 803 | 31.5 | 605 683 | 23.7 | 448 013 | 1 903 745 | 24.3 | 462 610 | 16.1 | 306 503 | 754 516 | 1 068 293 |
| 55–59 | 1 567 139 | 41.9 | 656 631 | 27.8 | 441 933 | 1 557 610 | 27.6 | 429 900 | 17.6 | 274 139 | 716 072 | 1 086 531 |
| Total | 40 876 016 | 7.5 | 3 220 815 | 5.3 | 2 261 532 | 39 101 973 | 5.3 | 2 088 740 | 3.6 | 1 431 648 | 3 693 180 | 5 309 555 |
A significant reduction of HCV antibody and HCV RNA‐positive individuals was observed when looking at the overall prevalence in the Urban Governorates and, and for Lower and Upper Egypt Governorates (Fig. 2). No significant change in prevalence of either HCV antibody or HCV RNA positivity was observed in the frontier governorates.
Figure 2.
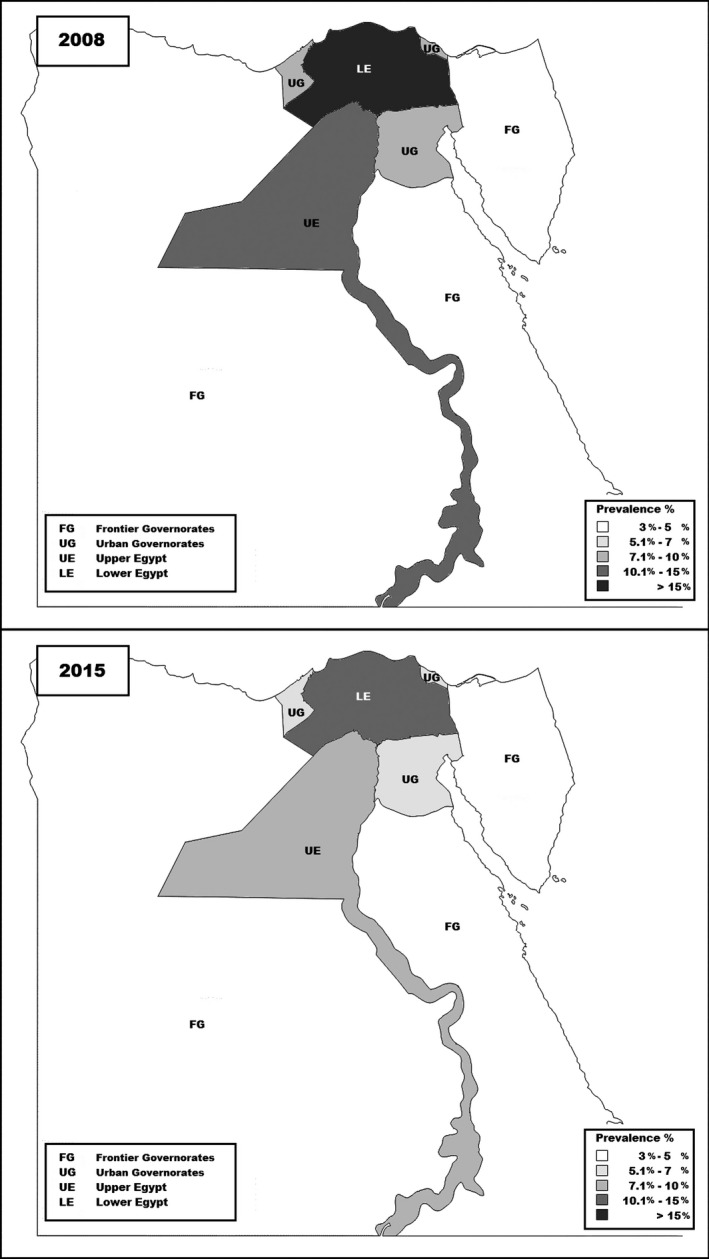
Prevalence of hepatitis C virus antibody in the 2008 and 2015 Egyptian Demographic and Health Surveys.
Table 3 shows the age‐ and gender‐specific prevalence of HCV antibody and HCV RNA‐positive persons in 2015, as well as the estimated total number of persons positive for HCV antibody and HCV RNA in Egypt at this time. In the population aged 0–59 years, we estimated a total of 3 693 180 persons with chronic HCV infection (HCV RNA positive), and 5 309 555 persons with HCV antibodies.
Discussion
In 2015, HCV still affects a substantial proportion of the Egyptian population, where it is estimated that, in the 1–59‐year age group, 5.3 million persons are positive for HCV antibodies and, of these, approximately 3.7 million (69.5%) are HCV RNA positive. This is an underestimate of the total human HCV reservoir in Egypt because older age groups (> 59 years) were not included in the EHIS 2015. This recent survey shows a similar epidemiological pattern of increased HCV antibody prevalence with age as did the EDHS 2008. This phenomenon was described in many studies 9, 10 and is because of the continuing exposure and risk of infection with age 11, while the proportion of persons with HCV infection who go on to develop severe disease and die of it remains low until 30 years after infection; the rate of progression to cirrhosis is estimated at 7% after 20 years of being infected 12. The increase in HCV antibody prevalence with age is therefore demonstrating the cumulative HCV incidence over time until HCV‐related mortality becomes manifest.
This study shows a significant reduction in the overall prevalence of HCV antibody from 14.7 to 10.0%, and HCV RNA from 9.9 to 7.0%, between 2008 and 2015 among those aged 15–59 years. The main explanation for this marked reduction in HCV prevalence is the disappearance of the group infected during the mass schistosomiasis treatment campaign with reused syringes (1960s through early 1980s) to outside the age range covered by the survey (i.e. those older than 59 years) 13, 14. Indeed, as demonstrated in Fig. 1, the age‐specific HCV antibody and HCV RNA prevalence for 2015 matches well with the 2008 prevalence estimates shifted by 7 years, suggesting that the ageing of the infected cohort, the so‐called ‘cohort effect’, is the driving mechanism underlying the HCV age distribution; this was also demonstrated in the earlier reduction of HCV antibody prevalence from 30% in 1996 14 to 14.7% in 2008 4.
In order to see whether or not a decrease in HCV incidence may have also been a contributor to the reduction in HCV prevalence, the most interesting age groups to look at are those less than 20 years of age; persons who were not affected by the mass treatment campaigns and for whom HCV‐related mortality remains low. If we assume that mother‐to‐child HCV transmission is a negligible source of infant infection, as suggested by the <0.5% HCV antibody prevalence in the 1–4‐year age group, then the HCV antibody prevalence at age 19 indicates the cumulative incidence over the past 20 years. In 2008 and 2015, the prevalence of HCV antibodies in those aged 15–19 years was 4.1 and 1.0%, respectively; the percentage of relative risk reduction was 75% (95% CI 64–85), implying a very substantial reduction in HCV incidence in the past 20 years in this age group. Furthermore, the prevalence of HCV antibody (0.4%) and HCV RNA‐positive persons (0.2%) observed in EHIS 2015 among the age group 1–14 years is low compared to several studies conducted in early 2000s, which described the HCV antibody prevalence to be ranging from 2 to 7% in children under 10 years in rural areas of the Nile Delta 15, 16, 17.
This change in incidence in the younger age groups could possibly be because of the various public health interventions implemented by the Ministry of Health and Population and its partners since 2008. In this time, several efforts have been made to promote and expand the infection prevention and control programmes beyond Ministry of Health and Population hospitals, particularly to the university hospitals. Auto disabled syringes were introduced to the routine immunisation sector in 2008 in order to promote safe injection practices among children. Safe blood transfusion activities, including policies and guidelines, have been intensified since 2009. Raising the awareness of the public, by targeting universities and schools to improve their understanding on the epidemiology and prevention of viral hepatitis, was also carried out. Pre‐service education targeting healthcare staff has been carried out since 2008 to enforce the concepts of safe healthcare and prevention of blood‐borne pathogens.
One may question whether the national treatment programme, which managed to treat more than 350 000 persons in the past 7 years 18 using pegylated interferon and ribavirin 19, has had an impact on HCV prevalence figures. In such a case, it would be expected that cured patients would have cleared HCV RNA, but kept HCV antibodies. Therefore, the impact would be seen through an increase in HCV clearance percentage in the age groups most targeted by the national treatment programme. Owing to established approximate 50% cure rates of the combination of pegylated interferon and ribavirin 20, only half of these persons (i.e. 175 000) would have cleared their HCV infection after treatment. The national treatment programme prioritises persons with more advanced forms of liver disease, and therefore, at least half of the beneficiaries of this service are in their 40s or 50s. Indeed, in a group of 3235 patients treated for HCV in a hospital run by the Ministry of Health in Cairo, between 2007 and 2011, the mean age was 41 years with a standard deviation of approximately 10 years 21. In order to see whether or not treatment has, as of yet, had an impact on lowering the prevalence of persons with HCV RNA in Egypt, we can see whether or not there has been increased clearance in the four older age groups (i.e. encompassing 40–59 years) over the past 7 years. If we assume that around half (87 500) of the total persons cured was in the 40–59‐year age group, and considering that we have estimated 2.87 million persons with HCV antibodies in the same age group in 2015 (Table 3), we would have expected to see around a 3% higher clearance percentage in this group, in comparison with 2008. However, an increase in clearance in these older age groups was not observed when comparing data from 2015 and the shifted‐forward 2008 data. Indeed, it would require around 4000 anti‐HCV‐positive persons in this age group, in each data set, in order to have enough power to demonstrate this expected 3% difference; unfortunately, the anti‐HCV population examined in this survey was much lower than that.
Treatment may show a larger impact in the near future as it is expected that emphasis will be put on an upscale of treatment of infected persons to prevent long‐term complications 22, particularly considering that new direct‐acting antiviral (DAA) drugs were approved for the treatment of HCV by the U.S. Food and Drug Administration (FDA) in late 2013, and then introduced into Egypt in late 2014. These new treatment regimens have reduced treatment duration to 12–24 weeks, decreased side effects and improved outcomes, with cure rates of 85–95% across all patient populations 23, 24.
Finally, differential migration and mortality of persons infected with HCV could have contributed to the reduced prevalence of both HCV antibody and HCV RNA‐positive persons in 2015, as long as these rates are higher than infection incidence 25, 26, 27; these topics needs to be explored further.
The prevalence of HCV antibody and HCV RNA‐positive individuals varied among governorates and was reduced in several geographical regions. Lower Egypt governorates, which are mostly rural in nature, still show a higher prevalence of HCV antibodies and HCV RNA when compared to urban governorates. This pattern of high HCV prevalence in rural areas is similar to previous multiple studies conducted in rural Lower Egypt governorates which showed a prevalence ranging from 14.4 to 18.5% 28, 29, 30. The frontier governorates did not show any significant change, however, this is possibly owing to the fact that the sample size was very low (n < 200), in both the 2008 and 2015 surveys.
Based on our findings in this study, we recommend the expansion of national health surveys in Egypt in order to include older age groups and allow further follow‐up of elderly persons who have been the most affected by HCV and who, by the cohort effect, are being pushed out of view. Furthermore, there should be continued prioritisation of prevention programmes to increase the effects we are seeing in incidence in young age groups, with a focus on interventions which promote injection safety by reducing the frequency of unnecessary injections and syringe reuse. Interventions could include introduction of single‐use materials and engineered safety devices, such as auto‐disabled or auto‐destructive syringes into the curative sector. Expansion of infection prevention and control programmes are of utmost importance, along with development of elaborate systems for delivering and renewing licenses of healthcare facilities to ensure continuity of safe procedures and application of standard precautions. Other analyses of the EHIS data should be done, using tools such as mathematical modelling, in order to properly estimate and explain changes in HCV incidence in Egypt; this could lead to further evidence‐based recommendations related to prevention and control efforts. Finally, access to treatment should be a priority, and although economic constraints are faced by the country, treatment has been shown to be cost‐effective in this context 31, 32 and focused, early treatment strategies may be effective in supporting prevention measures and reducing transmission 33.
Acknowledgements
The authors thank Omar Okasha, University of Tampere, for conducting the statistical analysis of an earlier version of the manuscript. Anna L. Funk is a scholar in the Pasteur‐Paris University (PPU) International PhD program. We thank Drs Hanaa Abu Elsood, Epidemiology and Surveillance Unit at Ministry of Health and Population, Egypt and Noha Salah at the Preventive Sector Technical Office at Ministry of Health and Population, Egypt for facilitating the work.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, Egypt Ministry of Health and Population, US Centers for Disease Control and Pasteur Institute. Maha Talaat is a contractor of the U.S. Government. This work was prepared as part of her official duties. Title 17 USCx105 provides that ‘copyright protection under this title is not available for any work of the United States Government.’ Title 17 USCx101 defines U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Financial support: No financial support was received.
Conflicts of interest: The authors do not have any disclosures to report.
Liver Int. 2017; 37: 45–53. DOI: 10.1111/liv.13186
Handling editor: Alessio Aghemo
References
- 1. Gower E, Estes C, Blach S, Razavi‐Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61: S45–57. [DOI] [PubMed] [Google Scholar]
- 2. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology 2013; 57: 1333–42. [DOI] [PubMed] [Google Scholar]
- 3. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45: 529–38. [DOI] [PubMed] [Google Scholar]
- 4. El‐Zanaty F, Way A. (2009). Egypt Demographic and Health Survey 2008. Cairo, Egypt: Ministry of Health,El‐Zanaty and Associates, and Macro International. [Google Scholar]
- 5. Ministry of Health, Egypt , El‐Zanaty and Associates , Egypt and ICF International . (2015). Egypt Health Issues Survey 2015. Cairo, Egypt and Rockville, MD: Ministry of Health and ICF International. [Google Scholar]
- 6. Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 1998; 17: 873–90. [DOI] [PubMed] [Google Scholar]
- 7. Armitage P, Berry G. Statistical Methods in Medical Research, 3rd edn London: Blackwell, 1994; 131. [Google Scholar]
- 8. Gardner MJ, Altman DG. Confidence intervals rather than P values: estimation rather than hypothesis testing. BMJ (Clin Res Ed) 1986; 292: 746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talaat M, El‐Sayed N, Kandeel A, et al Sentinel surveillance for patients with acute hepatitis in Egypt, 2001–04. East Mediterr Health J 2010; 16: 134–40. [PubMed] [Google Scholar]
- 10. Centre for Disease Control and Prevention (CDC) . Establishment of a viral hepatitis surveillance system – Pakistan, 2009–2011. MMWR Morb Mortal Wkly Rep 2011; 60: 1385–90. [PubMed] [Google Scholar]
- 11. MatheÏ C, Buntinx F, Van Damme P. Is the prevalence of hepatitis c virus (HCV) RNA in anti‐HCV–positive injection drug users positively correlated with age? J Infect Dis 2001; 184: 659–60. [DOI] [PubMed] [Google Scholar]
- 12. Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage‐specific fibrosis progression rates in chronic hepatitis C virus infection: a meta‐analysis and meta‐regression. Hepatology 2008; 48: 418–31. [DOI] [PubMed] [Google Scholar]
- 13. Breban R, Doss W, Esmat G, et al Towards realistic estimates of HCV incidence in Egypt. J Viral Hepat 2013; 20: 294–6. [DOI] [PubMed] [Google Scholar]
- 14. Frank C, Mohamed MK, Strickland GT, et al The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 2000; 355: 887–91. [DOI] [PubMed] [Google Scholar]
- 15. Habib M, Mohamed MK, Abdel‐Aziz F, et al Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology 2001; 33: 248–53. [DOI] [PubMed] [Google Scholar]
- 16. Medhat A, Shehata M, Magder LS, et al Hepatitis C in a community in Upper Egypt: risk factors for infection. Am J Trop Med Hyg 2002; 66: 633–8. [DOI] [PubMed] [Google Scholar]
- 17. Esmat G, Hashem M, El‐Raziky M, et al Risk factors for hepatitis C virus acquisition and predictors of persistence among Egyptian children. Liver Int 2012; 32: 449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waked I, Doss W, El‐Sayed M, et al The current and future disease burden of chronic hepatitis C virus infection in Egypt. Arab J Gastroentero 2014; 15: 45–52. [DOI] [PubMed] [Google Scholar]
- 19. Ford N, Singh K, Cooke GS, et al Expanding access to treatment for hepatitis C in resource‐limited settings: lessons from HIV/AIDS. Clin Infect Dis 2012; 54: 1465–72. [DOI] [PubMed] [Google Scholar]
- 20. Centre for Disease Control and Prevention (CDC) . Progress toward prevention and control of hepatitis c virus infection – Egypt, 2001–2012. MMWR Morb Mortal Wkly Rep 2012; 61: 545–9. [PubMed] [Google Scholar]
- 21. El Raziky M, Fathalah WF, Zakaria Z, et al Predictors of virological response in 3,235 chronic HCV Egyptian patients treated with peginterferon alpha‐2a compared with peginterferon alpha‐2b using statistical methods and data mining techniques. J Interferon Cytokine Res 2016; 36: 338–46. [DOI] [PubMed] [Google Scholar]
- 22. Deuffic‐Burban S, Schwarzinger M, Obach D, et al Should we await IFN‐free regimens to treat HCV genotype 1 treatment‐naive patients? A cost‐effectiveness analysis (ANRS 95141). Hepatology 2014; 61: 7–14. [DOI] [PubMed] [Google Scholar]
- 23. Lawitz E, Mangia A, Wyles D, et al Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368: 1878–87. [DOI] [PubMed] [Google Scholar]
- 24. Breban R, Arafa N, Leroy S, et al Effect of preventive and curative interventions on hepatitis C virus transmission in Egypt (ANRS 1211): a modelling study. Lancet Glob Health 2014; 2: e541–9. [DOI] [PubMed] [Google Scholar]
- 25. Negro F. Epidemiology of hepatitis C in Europe. Dig Liver Dis 2014; 46: S158–64. [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization . Hepatitis C. WHO/CDS/CSR/LYO/2003.
- 27. Espinosa M, Martn‐Malo A, Ojeda R, et al Marked reduction in the prevalence of hepatitis C virus infection in hemodialysis patients: causes and consequences. Am J Kidney Dis 2004; 43: 685–9. [DOI] [PubMed] [Google Scholar]
- 28. Abdel Aziz F, Habib M, Mohamed M, et al Hepatitis C virus infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology 2000; 32: 111–5. [DOI] [PubMed] [Google Scholar]
- 29. Stoszek SK, Abdel‐Hamid M, Narooz S, et al Prevalence of and risk factors for hepatitis C in rural pregnant Egyptian women. Trans R Soc Trop Med Hyg 2006; 100: 102–7. [DOI] [PubMed] [Google Scholar]
- 30. El Gohary A, Hassan A, Nooman Z, et al High prevalence of hepatitis C virus among urban and rural population groups in Egypt. Acta Trop 1995; 59: 155–61. [DOI] [PubMed] [Google Scholar]
- 31. Ruane PJ, Ain D, Stryker R, et al Sofosbuvir plus ribavirin for the treatment of chronic genotype 4 hepatitis C virus infection in patients of Egyptian ancestry. J Hepatol 2015; 62: 1040–6. [DOI] [PubMed] [Google Scholar]
- 32. Obach D, Deuffic‐Burban S, Esmat G, et al Effectiveness and cost‐effectiveness of immediate versus delayed treatment of hepatitis C virus‐infected patients in a country with limited resources: the case of Egypt. Clin Infect Dis 2014; 58: 1064–71. [DOI] [PubMed] [Google Scholar]
- 33. Obach D, Yazdanpanah Y, Esmat G, et al How to optimize hepatitis C virus treatment impact on life years saved in resource‐constrained countries. Hepatology 2015; 62: 31–9. [DOI] [PubMed] [Google Scholar]


