Abstract
Exercise is associated with lower rates of drug use in human populations and decreases drug self-administration in laboratory animals. Most of the existing literature examining the link between exercise and drug use has focused on chronic, long-term exercise, and very few studies have examined the link between exercise output (i.e., amount of exercise) and drug self-administration. The purpose of this study was to examine the effects of acute bouts of exercise on cocaine self-administration, and to determine whether these effects were dependent on exercise output and the time interval between exercise and drug self-administration. Female rats were trained to run in automated running wheels, implanted with intravenous catheters, and allowed to self-administer cocaine on a fixed ratio (FR1) schedule of reinforcement. Immediately prior to each test session, subjects engaged in acute bouts of exercise in which they ran for 0, 30, or 60 min at 12 m/min. Acute bouts of exercise before test sessions decreased cocaine self-administration in an output-dependent manner, with the greatest reduction in cocaine intake observed in the 60-min exercise condition. Exercise did not reduce cocaine self-administration when wheel running and test sessions were separated by 12 hours, and exercise did not reduce responding maintained by food or responding during a saline substitution test. These data indicate that acute bouts of exercise decrease cocaine self-administration in a time- and output-dependent manner. These results also add to a growing body of literature suggesting that physical activity may be an effective component of drug abuse treatment programs.
Keywords: cocaine, exercise, rat, self-administration, wheel
1. Introduction
Individuals who engage in exercise-related activities over the course of treatment for substance use disorders have better outcomes relative to individuals who do not engage in exercise-related activities (Brown et al., 2010; Weinstock et al., 2008). Preclinical studies have consistently shown that rats given free access to exercise wheels in their home cage self-administer less cocaine, methamphetamine, and other psychomotor stimulants than sedentary control rats (Aarde et al., 2015; Miller et al., 2012; Smith et al., 2008; 2011; 2012). Most preclinical studies reporting positive effects of wheel running on drug self-administration have used exercise protocols that employed long-term access to running wheels for 6 weeks or longer (e.g., Lacy et al., 2014; Smith et al., 2008; 2011; 2012); however, the positive effects of wheel running on drug self-administration are observed even in the absence of extended access (Smith and Witte, 2012) and may be observed in as little as 22 hours (Aarde et al., 2015).
Acute exercise, defined as a single, short-term bout of exercise, produces positive effects on several psychological measures that influence drug self-administration. For instance, acute bouts of exercise decrease measures of depression and anxiety (Dunn et al., 2001; Fritz and O’Connor, 2016) and increase measures of self-esteem and wellbeing (Fox, 1999). Moreover, acute bouts of exercise reduce measures of impulsivity (Wang et al., 2016) and increase measures of cognitive functioning (Kamijo et al., 2009; Nanda et al., 2013; Weng et al., 2015). Acute exercise reduces the symptoms associated with nicotine withdrawal (Abrantes et al., 2014; Bock et al., 1999; Prapavessis et al., 2014; Williams et al., 2011) and reduces craving for nicotine and methamphetamine (Elibero et al., 2011; Haasova et al., 2013; Wang et al., 2016); however, the effects of acute exercise on measures of drug intake have not been examined.
The effects of an acute bout of exercise on psychological processes relative to drug use are dependent on exercise output. For instance, light and moderate exercise decreases methamphetamine craving relative to both vigorous exercise and a sedentary control condition (Wang et al., 2016). Similarly, exercise output also determines the effects of acute exercise on measures of mood (Steptoe and Cox, 1988; Tate and Petruzzello, 1995), executive control (Labelle et al., 2013), and cognitive functioning (Loprinzi and Kane, 2015; McMorris and Hale, 2012; Tomporowski, 2003). Preclinical studies examining the effects of exercise on measures of drug intake have typically manipulated access to running wheels rather than exercise output per se. Consequently, the effects of exercise output on drug self-administration are not known.
The purpose of the present study was to examine the effects of acute bouts of exercise on cocaine self-administration and to determine whether these effects are dependent on exercise output. To this end, rats were trained in a forced-running procedure using automated running wheels rotating at a speed of 12 m/min. Forced running rather than voluntary running was selected so that exercise output could be controlled as an experimental manipulation. All subjects ran in the wheels for 0 min (sedentary control), 30 min (short duration), or 60 min (long duration) immediately prior to intravenous drug self-administration sessions. Our primary hypothesis was that acute exercise would decrease cocaine self-administration in an output-dependent (i.e., duration-dependent) manner.
2. Method
2.1. Animals
Female, Long-Evans rats (n = 16) were obtained on postnatal day 42 (PND 42) from Charles River Laboratories (Raleigh, NC). Rats were housed individually in transparent polycarbonate cages that permitted no exercise beyond normal cage ambulation. All rats were housed in a temperature- and humidity-controlled colony room maintained on a 12-hour light/dark cycle (lights on: 0500). Except during periods of food-maintained responding, food was freely available in the home cage; water was continuously available in the home cage for the duration of the study. Estrous phases were allowed to cycle normally and not monitored. All subjects were maintained in accordance with the Institutional Animal Care and Use Committee of Davidson College and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011).
2.2. Apparatus
Self-administration training and testing took place in aluminum and polycarbonate operant conditioning chambers (interior dimensions: 31 cm × 24 cm × 21 cm) obtained from Med Associates, Inc. (St. Albans, VT). Each chamber was equipped with a house light, an audio speaker, two response levers, two stimulus lights above the levers, and a food receptacle located between the two levers. A food pellet dispenser was located behind the forward wall and an infusion pump was mounted outside the chamber. Drug infusions were delivered via Tygon tubing protected by a stainless-steel spring and attached to a counter-balanced swivel at the top of the chamber. All chambers were housed in larger, sound-attenuating cabinets with an exhaust fan to circulate air and to mask extraneous noise. Experimental procedures were programmed and data were collected using software and interfacing from Med Associates, Inc.
Wheel running was experimentally controlled via automated running wheels. The wheels and motorized wheel bed were obtained from Lafayette Instrument Company (Lafayette, IN). The wheels measured 34.0 cm in diameter and 11.2 cm in width. Each wheel had polycarbonate sides with 82 aluminum rungs (4.8 mm in diameter) spaced 13.4 mm apart. The running surface of the wheels was lined with low-density polyethylene mesh to prevent tails from getting pinched while running. Six wheels could rotate simultaneously when placed in the wheel bed (exterior dimensions: 130 cm × 46 cm × 43 cm). The speed and duration of wheel rotation were programmed via a handheld LCD interface.
2.3. Exercise Training
Three days after arrival, rats began daily training on the running wheels. On the first day of exposure, rats ran at a speed of 3 m/min for 15 min. The speed increased by 1 m/min and the duration increased by 5 min each day until the terminal speed of 12 m/min and 60 min was reached after 10 days. Rats ran at this speed and duration for three consecutive days before catheter surgery and the commencement of self-administration training.
2.4. Lever-Press Training
One week after arrival and during the second week of exercise training, rats were restricted to no less than 85% of the free-feeding body weight and trained to lever press using food reinforcement. During these sessions, responding was reinforced with a single 45 mg food pellet on a fixed ratio (FR1) schedule of food presentation. Each session continued until 40 reinforcers were delivered or 2 hours elapsed, whichever occurred first. Training continued in this manner until a rat received the maximum number of reinforcers in each of four training sessions. All rats met this criterion within 7 days.
2.5. Surgery
Three weeks after arrival and one day following the completion of exercise training, rats were anesthetized with a combination of ketamine (100 mg/kg, ip) and xylazine (15 mg/kg, ip) and surgically implanted with intravenous catheters into the right jugular vein. Catheters exited the body on the dorsal surface of the scapulae. Ketoprofen (5.0 mg/kg, sc) was given immediately after surgery and the following morning as a postoperative analgesic. A solution of heparinized saline and ticarcillin (20 mg/kg, iv) was infused through the catheter daily to maintain patency and prevent infection, respectively. After 7 days, ticarcillin administration was discontinued and only heparinized saline was used to maintain catheter patency.
2.6. Self-administration training
Self-administration training commenced 4 days following surgery. Immediately prior to each training session, rats were placed on the automated running wheels at 12 m/min for 30 min. Rats were then moved directly to the operant conditioning chambers and connected to the infusion pump via the Tygon tubing. All sessions began with illumination of the house light, illumination of the stimulus light above one (active) response lever, and a non-contingent infusion of cocaine (Sigma Chemical Company, St. Louis, MO). Each response on the active response lever produced an infusion of cocaine (0.5 mg/kg/infusion) on a FR1 schedule of reinforcement. Coincident with the infusion, a tone was sounded for 5 s and the stimulus light above the response lever turned off for 20 s to signal a timeout period in which cocaine was not available. After 20 s, the light above the lever was re-illuminated and cocaine was available on the FR1 schedule of reinforcement. Responses on the second (inactive) response lever were recorded but had no programmed consequences. Training continued in this manner for 5 consecutive days.
2.7. Self-Administration Testing
Following 5 days of self-administration training, behavioral testing commenced. The exercise manipulation was performed at the beginning of the dark phase of the light/dark cycle (1700), immediately prior to each test session. Using a within-subjects design, each rat was exposed to one of the following three exercise conditions immediately before each test session: (1) a sedentary control condition, (2) a short-duration condition, or (3) a long-duration condition. In the sedentary condition, rats were taken to the testing room and placed in clean polycarbonate cages for 60 min. In the short-duration condition, rats were taken to the testing room, placed in clean polycarbonate cages for 30 minutes and then placed in the automated running wheels rotating at 12 m/min for an additional 30 min. In the long-duration condition, rats were taken to the testing room and placed in the automated running wheels rotating at 12 m/min for 60 min. Thus, in all conditions, rats were in the testing room and out of their home cage for 60 min prior to cocaine self-administration testing.
All experimental parameters during drug self-administration test sessions were identical to those described above for training sessions, with the exception that the dose of cocaine changed every three days. Tests were conducted for four doses of cocaine (0.01, 0.03, 0.1, and 0.3 mg/kg/infusion) and for saline. Doses were tested in a pseudo-random order with the stipulation that no more than two ascending or descending doses could be tested in a row. Rats were exposed to all three exercise conditions for each dose of cocaine and for saline. Each exercise condition lasted one day and the three exercise conditions were tested in sequence. The sequence of testing was randomized and counterbalanced across rats.
In order to determine whether the effects of acute exercise were dependent on the time elapsed between wheel running and drug self-administration testing, the effects of the three exercise conditions on responding maintained by 0.03 mg/kg/infusion cocaine were redetermined. This dose was selected because it produced the greatest range in mean responding across the three exercise conditions during initial testing. In these tests, the exercise manipulation (0, 30, and 60 min of wheel running) occurred 12 hours prior to cocaine self-administration. All other experimental parameters were identical to those described above.
2.8. Responding Maintained by Food
To determine whether the effects of acute exercise were specific to the cocaine stimulus, additional tests were conducted in which responding was maintained by food. Following the completion of cocaine self-administration testing, rats were restricted to no less than 85% of their free-feeding body weight and tested daily in food self-administration sessions. All experimental parameters were identical to those used during lever-press training, with the exception that no limit was placed on the maximal number of reinforcers that could be earned. All discriminative stimuli were identical to those used during cocaine self-administration. At the end of each session, the number of food pellets that remained uneaten in the food receptacle was counted to determine the number of pellets consumed by each rat. In these tests, the exercise manipulation (0, 30, and 60 min of wheel running) occurred immediately prior to each test session in a manner identical to that described for cocaine self-administration.
2.9. Data Analysis
Cocaine self-administration immediately after wheel running was analyzed via two-way, repeated-measures ANOVA, with dose of cocaine and exercise condition serving as factors. Area Under the Curve (AUC) estimates were also computed for the dose-response data using the Trapezoidal Rule. These data were analyzed via one-way ANOVA with exercise condition serving as the factor. This AUC analysis was conducted to directly compare the magnitude of the exercise effect across the three durations, which is advantageous when a curve has both ascending and descending limbs or when there is a significant interaction term (see Cooper et al., 2008). A planned comparison was performed on data obtained at the dose producing the greatest range of mean responding across the three exercise conditions. This dose was then used to test the effects of exercise when wheel running occurred 12 hr prior to cocaine self-administration. Data obtained in these tests were analyzed using a two-way, repeated-measures ANOVA, with time (0 vs. 12 hr) and exercise condition serving as factors. Data for food-maintained responding (i.e., number of responses, number of pellets consumed) and from the saline substitution test were examined via one-way ANOVA, with exercise condition serving as the factor. For all analyses, post hoc comparisons were conducted using Fischer’s Least Significant Difference Test if the omnibus test was significant. All statistical tests were two tailed and used an alpha level of .05. Effect sizes were computed where appropriate using the partial eta squared (η2) statistic or the Cohen’s d statistic.
3. Results
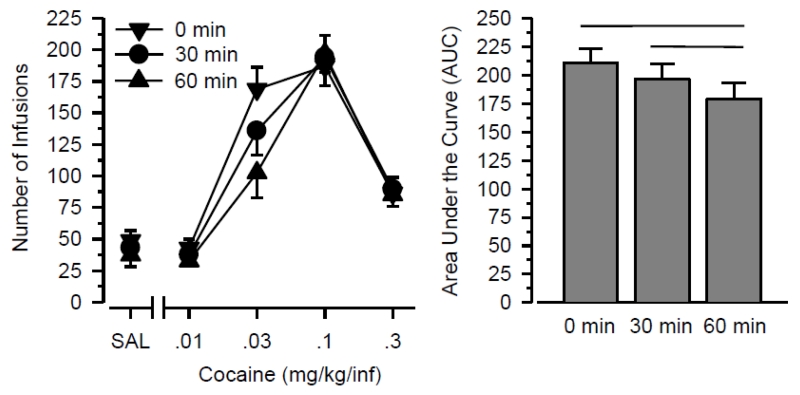
Figure 1 shows that cocaine self-administration was characterized by an inverted U-shaped dose-effect curve (main effect of dose: F3,45 = 39.615; p < .001). Cocaine self-administration decreased as a function of exercise output (main effect of exercise: F2,30 = 5.438; p = .010), and this effect varied as a function of dose (dose × exercise interaction: F6,90 = 6.236; p < .001). A significant effect of exercise was also obtained in the AUC analysis (F2,30 = 5.040; p = .013), which functionally collapses across dose. In post hoc analyses, AUC values were significantly lower in the long-duration condition than both the short-duration (p = .041) and sedentary (p = .032) conditions. AUC values were 8% lower in the short-duration condition than in the sedentary condition, but this effect was not statistically significant (p = .081). The effect size produced by the exercise manipulation was large in both the dose-response (η2 = 0.266) and AUC analysis (η2 = 0.251). In contrast, exercise did not influence responding during the saline substitution test. Similarly, exercise did not influence responding on the inactive lever under any dose condition (data not shown).
Figure 1.
Cocaine self-administration immediately following wheel running for 0, 30, or 60 min. Left Panel: Number of infusions obtained during 2-hr sessions (vertical axis) when responding was maintained by doses of cocaine and saline (horizontal axis). Right Panel: Area Under the Curve (AUC) values for the dose-effect data. Vertical lines represent the SEM; where not indicated, the SEM fell within the data point. Horizontal lines indicate significant differences between groups. All data reflect the mean of 16 rats.
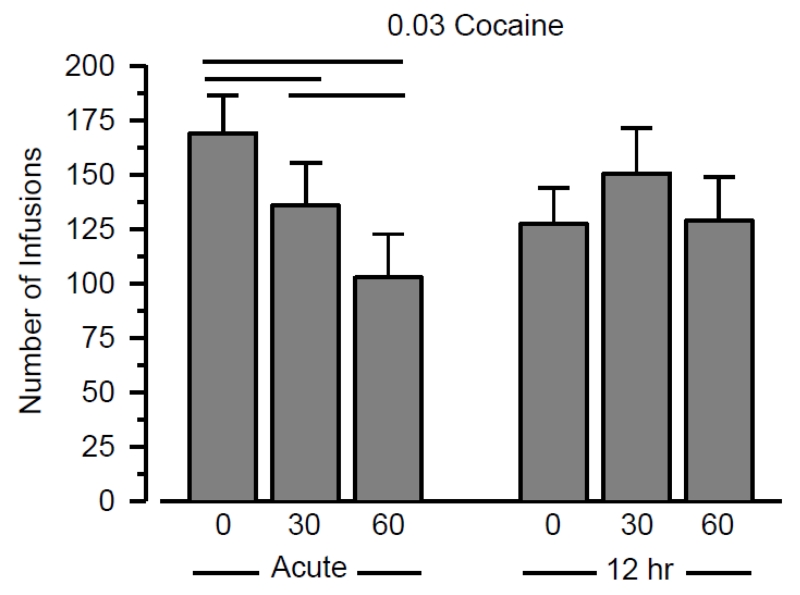
Cocaine self-administration decreased as a function of exercise output when responding was maintained by 0.03 mg/kg/infusion (Figure 2). The effect of exercise at this dose was statistically significant (F2,30 = 8.467; p < .001) and characterized by a large effect size (η2 = 0.361). All post-hoc analyses at this dose were statistically significant with moderate-to-large effect sizes between conditions. Specifically, cocaine self-administration was less in the long-duration condition than the short-duration (p = .014; d = .695) and sedentary (p = .005; d = .819) conditions, and cocaine self-administration was less in the short-duration condition than the sedentary condition (p = .044; d = .549). To determine whether the effects of exercise depended on the time between wheel running and cocaine self-administration, the effects of all three running durations were re-examined at this dose, but with the bout of wheel running occurring 12 hr prior to self-administration testing. Time was a moderating factor of exercise under these conditions (time × exercise interaction: F2,30 = 5.593; p = .009). Whereas there was a significant, output-dependent effect of exercise when wheel running occurred immediately prior to testing (see above), there was no significant effect of exercise when wheel running occurred 12 hr prior to testing (F2,30 = 1.468; p = .247; not significant).
Figure 2.
Responding maintained by 0.03 mg/kg/infusion cocaine immediately (left) or 12 hr (right) following wheel running for 0, 30, or 60 min. Vertical axis depicts number of infusions obtained during 2-hr sessions. Data on left side of panel are redrawn from Figure 1. Vertical lines represent the SEM. Horizontal lines indicates significant differences between groups. All data reflect the mean of 16 rats.
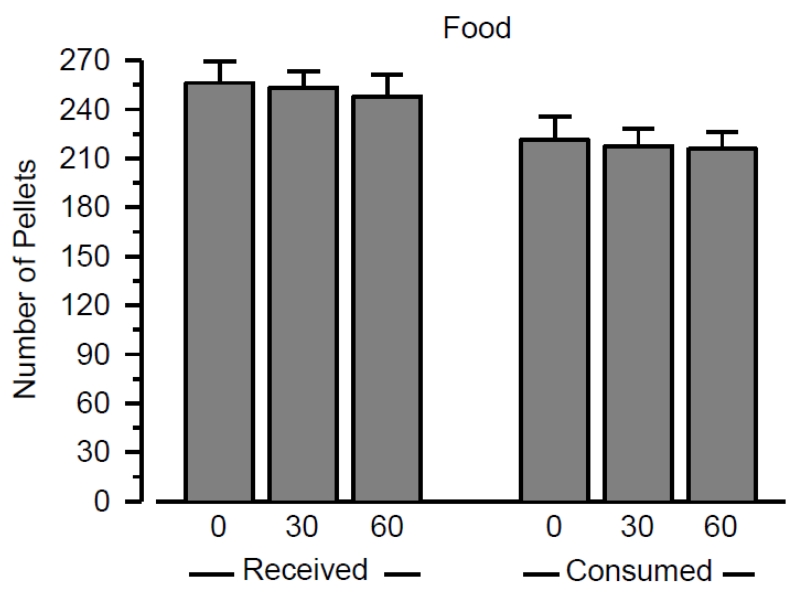
To determine whether the effects of exercise were specific to the cocaine stimulus, responding maintained by food was examined under each exercise condition (Figure 3). Acute bouts of exercise did not influence the number of pellets earned or the number of pellets consumed over the 2-hr test session.
Figure 3.
Number of food pellets received (left) and consumed (right) following wheel running for 0, 30, or 60 minutes. Vertical lines represent the SEM. All data reflect the mean of 16 rats.
4. Discussion
The primary finding of this study is that acute bouts of exercise, limited to no more than 60 min/day, significantly decreased cocaine self-administration. Moreover, this effect was directly related to exercise output, in that 60 min of exercise was significantly more effective at reducing cocaine intake than 30 minutes of exercise at a moderate dose of cocaine. These beneficial effects of acute exercise were also time-dependent and were not observed when an acute bout of exercise occurred 12 hr prior to drug exposure. Finally, the effects of acute exercise were specific to the cocaine stimulus, given that acute exercise did not alter responding maintained by food. These data suggest that acute bouts of exercise immediately before drug exposure reduce the positive reinforcing effects of cocaine in an output-dependent manner.
We know of no studies that have specifically addressed the effects of acute bouts of exercise on measures of drug intake. Previous studies have reported that acute bouts of exercise decrease cravings for methamphetamine (Wang et al., 2016) and nicotine (Roberts et al., 2015; Van Rensburg et al., 2012; 2013) and decrease the symptoms of nicotine withdrawal (Prapavessis et al., 2014; Williams et al., 2011). Preclinical studies have reported that extended periods of wheel running are not necessary to reduce cocaine self-administration (Smith and Witte, 2012) and that as little as 22 hr of access to a running wheel decreases methamphetamine, MDMA, and methylone self-administration (Aarde et al., 2015). In conjunction with the present data, these findings suggest that a long history of exercise or continuous exposure to exercise is not needed to produce rapid and significant decreases in drug intake that might be advantageous in substance-abusing populations.
A primary aim of this study was to examine the effects of exercise output on cocaine self-administration. Exercise output was manipulated by varying the duration of forced wheel running in automated running wheels. At 0.03 mg/kg/infusion cocaine, a dose that yielded the largest range of mean responding across conditions, acute exercise “dose dependently” decreased cocaine intake, with all three exercise conditions differing significantly from one another. We previously reported that chronic exercise output, as measured by total number of wheel revolutions over a 6-week period, was inversely correlated to breakpoints maintained by a high dose of cocaine (Smith et al., 2008); however, this relationship was not observed in a series of follow-up studies from our laboratory (e.g., Lacy et al., 2014; Smith et al., 2011, 2012; Smith and Pitts, 2011; 2012; Smith and Witte, 2012). Two previous studies manipulated the duration of access to running wheels and reported duration-dependent effects on measures relevant to drug intake. In those studies, access to running wheels for 1, 2, 6, or 24 hr/day during abstinence reduced subsequent reinstatement of cocaine seeking in a duration-dependent manner (Peterson et al., 2014a; 2014b); however, exercise output, per se, was not manipulated and measures of drug intake were not recorded.
A secondary aim of this study was to determine whether the effects of an acute bout of exercise were moderated by the time between wheel running and self-administration testing. The large effects observed with acute exercise at 0.03 mg/kg/infusion immediately before testing were absent when 12 hr separated wheel running from cocaine self-administration, indicating that an acute bout of exercise must occur in close temporal proximity to drug exposure to significantly decrease cocaine intake. We previously reported that six weeks of continuous access to running wheels was completely ineffective at reducing cocaine self-administration if access to the wheels was terminated on the first day of drug exposure (Smith and Witte, 2012). In that study, rats ran an average of 7.9 km/day and an average of 317 km over 6 weeks prior to cocaine exposure, but did not differ from a sedentary control group on measures of cocaine intake once wheel access was removed. Aarde and colleagues (2015) showed that only 22 hours of wheel access was sufficient to reduce responding maintained by methamphetamine, MDMA, and methylone; however, it is not known when wheel running, per se, occurred in relation to drug self-administration.
It is important to note that acute bouts of exercise did not influence rates of nonspecific responding on an inactive lever and did not influence responding during a saline substitution test. Furthermore, acute bouts of exercise did not influence responding maintained by food or the amount of food consumed, with the caveat that the discriminate stimuli signaling food were the same as those for cocaine. These findings suggest that acute bouts of exercise do not impact nonspecific rates of operant responding or consummatory behavior. Most importantly, these findings indicate that output-dependent decreases in cocaine-maintained responding cannot be attributed to exercise-related decreases in motor performance, such as might be caused by muscle fatigue.
We emphasize that only females were tested in the study, and we do not know whether males would produce the same pattern of results. Females were chosen for this study to facilitate comparisons with previous studies (e.g., Smith et al., 2008; Smith and Witte, 2012) and because of their general under-representation in preclinical biomedical research (Clayton and Collins, 2014; Klein et al., 2015). A previous study reported that concurrent access to running wheels decreased cocaine self-administration to a greater extent in females than males (Cosgrove et al., 2002); however, we have failed to observe sex differences in cocaine self-administration when running wheels were confined to the home cages (Smith et al., 2011; 2012). We are aware of only one study that used a forced exercise manipulation and took measures of drug-reinforced responding. In that study, male rats that ran 90 min/day, every day, self-administered less morphine than sedentary control rats (Hosseini et al., 2009). Collectively, these studies suggest that (1) both males and females are sensitive to exercise-based interventions and (2) both forced and voluntary exercise interventions are effective at reducing drug self-administration.
It is important to note that exercise output was only manipulated by varying the duration of wheel running, and we do not know whether other manipulations of exercise output would have similar effects on cocaine self-administration. For instance, automated running wheels typically allow both speed and duration of running to be controlled by the experimenter, and future studies should determine whether a similar relationship is observed when speed is varied but duration remains consistent. We also do not know whether longer durations of wheel running (e.g., 90 or 120 min) would lead to greater effects on cocaine-maintained responding. Although our data suggest a linear relationship between wheel running and drug self-administration, moderate-intensity exercise is more beneficial than high-intensity exercise on some measures relevant to substance use (e.g., inhibitory control; Wang et al., 2016).
A number of mechanisms may contribute to the beneficial effects of acute bouts of exercise on cocaine self-administration. For instance, acute exercise decreases measures of depression and anxiety (Dunn et al., 2001; Fritz and O’Connor, 2016; Tate and Petruzzello, 1995), both of which are comorbid risk factors of drug use. Moreover, acute exercise increases measures of cognitive function (Kamijo et al., 2009; Prapavessis et al., 2014; Weng et al., 2015), self-esteem (Fox, 1999), and inhibitory control (Wang et al., 2016), which are generally regarded as protective against drug use. Acute bouts of exercise also stimulate the release of several signaling molecules that are important for the positive reinforcing effects of cocaine and other drugs. For instance, acute bouts of exercise increase central and plasma concentrations of dopamine (Meeusen and De Meirleir, 1995; Meeusen et al., 1997; Skriver et al., 2014), which is the critical neurotransmitter mediating the positive reinforcing effects of cocaine. Furthermore, acute exercise increases the expression of tyrosine hydroxylase (Ji et al., 2014), the rate-limiting enzyme in the synthesis of dopamine. The effects of acute exercise on both dopamine and tyrosine hydroxylase are positively correlated with exercise output (Freed and Yamamoto, 1985; Ji et al., 2014), which may explain the output-dependent effects reported in the present study.
Recent surveys indicate that 48.4% of the adult population of the United States fails to meet the minimum federal recommendation for physical activity, and 25.4% of the adult population in the United States engages in no leisure time physical activity (Centers for Disease Control and Prevention, 2014). In the present study, we found that a single, brief bout of exercise may be sufficient to significantly reduce cocaine use in individuals with a limited history of physical activity. These data are particularly relevant given recent studies reporting that a long history of exercise is not necessary for exercise to have a positive impact on measures of drug self-administration (Aarde et al., 2015; Smith and Witte, 2012). Indeed, preclinical studies that have focused exclusively on the chronic, long-term effects of exercise may have led the field somewhat astray, and the present data suggest that more focus should be placed on the behavioral and neurobiological effects of acute bouts of exercise.
Highlights.
Cocaine self-administration was examined after acute bouts of wheel running
Female rats ran for 0, 30, or 60 minutes prior to cocaine self-administration
Acute bouts of wheel running decreased cocaine intake
Greater decreases in intake were observed following longer durations of running
Acknowledgements
This work was supported by the National Institutes of Health (NIDA Grants R01DA031725 and R01DA027485 to MAS). The authors thank Justin Strickland for data analytic assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Miller ML, Creehan KM, Vandewater SA, Taffe MA. One day access to a running wheel reduces self-administration of D-methamphetamine, MDMA and methylone. Drug Alcohol Depend. 2015;151:151–158. doi: 10.1016/j.drugalcdep.2015.03.016. DOI: 10.1016/j.drugalcdep.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes AM, Bloom EL, Strong DR, Riebe D, Marcus BH, Desaulniers J, Fokas K, Brown RA. A preliminary randomized controlled trial of a behavioral exercise intervention for smoking cessation. Nicotine Tob. Res. 2014;16(8):1094–1103. doi: 10.1093/ntr/ntu036. DOI: 10.1093/ntr/ntu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addict. Behav. 1999;24(3):399–410. doi: 10.1016/s0306-4603(98)00088-4. DOI: 10.1016/S0303-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Oakley JR, Ramsey SE, Kahler CW, Stuart GG, Dubreuil ME, Gordon AA. A pilot study of aerobic exercise as an adjunctive treatment for drug dependence. Ment. Health. Phys. Act. 2010;3(1):27–34. doi: 10.1016/j.mhpa.2010.03.001. DOI: 10.1016/j.mhpa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . State Indicator Report on Physical Activity. U.S. Department of Health and Human Services; Atlanta, GA: 2014. [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Troung YN, Shi YG, Woods JB. Morphine deprivation increases self-administration of the fast- and short-acting mu-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther. 2008;326(3):920–929. doi: 10.1124/jpet.108.139196. DOI: 10.1124/jpet.108.139196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: Sex differences. Pharmacol. Biochem. Behav. 2002;73(3):663–671. doi: 10.1016/s0091-3057(02)00853-5. DOI: 10.1016/S0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med. Sci. Sports Exerc. 2001;33(6 Suppl):S587–S597. doi: 10.1097/00005768-200106001-00027. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol. Behav. 1988;43(5):625–630. doi: 10.1016/0031-9384(88)90217-x. DOI: 10.1016/0031-9384(88)90217-X. [DOI] [PubMed] [Google Scholar]
- Elibero A, Van Rensburg KJ, Drobes DJ. Acute effects of aerobic exercise and hatha yoga on craving to smoke. Nicotine Tob. Res. 2011;13(11):1140–1148. doi: 10.1093/ntr/ntr163. DOI: 10.1093/ntr/ntr163. [DOI] [PubMed] [Google Scholar]
- Ensari I, Greenlee TA, Motl RW, Petruzzello SJ. Meta-analysis of acute exercise effects on state anxiety: An update of randomized controlled trials over the past 25 years. Depress. Anxiety. 2015;32(8):624–634. doi: 10.1002/da.22370. DOI: 10.1002/da.22370. [DOI] [PubMed] [Google Scholar]
- Fox KR. The influence of physical activity on mental well-being. Public Health Nutr. 1999;2(3a):411–418. doi: 10.1017/s1368980099000567. DOI: 10.1017/S1368980099000567. [DOI] [PubMed] [Google Scholar]
- Freed CR, Yamamoto BK. Regional brain dopamine metabolism: A marker for the speed, direction, and posture of moving animals. Science. 1985;229(4708):62–65. doi: 10.1126/science.4012312. DOI: 10.1126/science.4012312. [DOI] [PubMed] [Google Scholar]
- Fritz KM, O’Connor PJ. Acute exercise improves mood and motivation in young men with ADHD symptoms. Med. Sci. Sports Exerc. 2016;48(6):1153–1160. doi: 10.1249/MSS.0000000000000864. DOI: 10.1249/MSS.0000000000000864. [DOI] [PubMed] [Google Scholar]
- Haasova M, Warren FC, Ussher M, Van Rensburg KJ, Faulkner G, Cropley M, Byron-Daniel J, Everson-Hock ES, Oh H, Taylor AH. The acute effects of physical activity on cigarette cravings: Systematic review and meta-analysis with individual participant data. Addiction. 2013;108(1):26–37. doi: 10.1111/j.1360-0443.2012.04034.x. DOI: 10.1111/j.1360-0443.2012.04034.x. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. eighth ed. The National Academies Press; Washington, D.C.: 2011. [Google Scholar]
- Ji E-S, Kim C-J, Park JH, Bahn GH. Duration-dependence of the effect of treadmill exercise on hyperactivity in attention deficit hyperactivity disorder. J. Exerc. Rehabil. 2014;10(2):75–80. doi: 10.12965/jer.140107. DOI: 10.12965/jer.140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K, Hayashi Y, Sakai T, Yahiro T, Tanaka K, Nishihira Y. Acute effects of aerobic exercise on cognitive function in older adults. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2009;64(3):356–363. doi: 10.1093/geronb/gbp030. DOI: 10.1093/geronb/gbp030. [DOI] [PubMed] [Google Scholar]
- Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, Zucker I. Opinion: Sex inclusion in basic research drives discovery. Proc. Natl. Acad. Sci. USA. 2015;112(17):5257–5258. doi: 10.1073/pnas.1502843112. DOI: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle V, Bosquet L, Mekary S, Bherer L. Decline in executive control during acute bouts of exercise as a function of exercise intensity and fitness level. Brain Cogn. 2013;81(1):10–17. doi: 10.1016/j.bandc.2012.10.001. DOI: 10.1016/j.bandc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Brophy MK, Witte MA, Smith MA. Exercise decreases speedball self-administration. Life Sci. 2014;114(2):86–92. doi: 10.1016/j.lfs.2014.08.005. DOI: 10.1016/j.lfs.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi PD, Kane CJ. Exercise and cognitive function: A randomized controlled tiral examining acute exercise and free-living physical activity and sedentary effects. Mayo Clin. Proc. 2015;90(4):450–460. doi: 10.1016/j.mayocp.2014.12.023. DOI: 10.1016/j.mayocp.2014.12.023. [DOI] [PubMed] [Google Scholar]
- McMorris T, Hale BJ. Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: A meta-analytical investigation. Brain Cogn. 2012;80(3):338–351. doi: 10.1016/j.bandc.2012.09.001. DOI: 10.1016/j.bandc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20(3):160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Meeusen R, Smolders I, Sarre S, De Meirleir K, Keizer H, Serneels M, Ebinger G, Michotte Y. Endurance training effects on neurotransmitter release in rat striatum: An in vivo microdialysis study. Acta Physiol. Scand. 1997;159(4):335–341. doi: 10.1046/j.1365-201X.1997.00118.x. DOI: 10.1046/j.1365-201X.1997.00118.x. [DOI] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MR, Jr., Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend. 2012;121(1-2):90–96. doi: 10.1016/j.drugalcdep.2011.08.013. DOI: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda B, Balde J, Manjunatha S. The acute effects of a single bout of moderate-intensity aerobic exercise on cognitive functions in healthy adult males. J. Clin. Diagn. Res. 2013;7(9):1883–1885. doi: 10.7860/JCDR/2013/5855.3341. DOI: 10.7860/JCDR/2013/5855.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Abel JM, Lynch WJ. Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex Bdnf exon IV expression in rats. Psychopharmacology (Berl.) 2014a;231(7):1305–1314. doi: 10.1007/s00213-013-3321-4. DOI: 10.1007/s00213-013-3321-4. [DOI] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: Impact of sex and estrous cycle in rats. Psychopharmacology (Berl.) 2014b;231(13):2661–2670. doi: 10.1007/s00213-014-3437-1. DOI: 10.1007/s00213-014-3437-1. [DOI] [PubMed] [Google Scholar]
- Prapavessis H, De Jesus S, Harper T, Cramp A, Fitzgeorge L, Mottola MF, Ussher M, Faulkner G, Selby P. The effects of acute exercise on tobacco cravings and withdrawal symptoms in temporary abstinent pregnant smokers. Addict. Behav. 2014;39(3):703–708. doi: 10.1016/j.addbeh.2013.10.034. DOI: 10.1016/j.addbeh.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts V, Gant N, Sollers JJ, III, Bullen C, Jian Y, Maddison R. Effects of exercise on the desire to smoke and physiological responses to temporary smoking abstinence: A crossover trial. Psychopharmacology (Berl.) 2015;232(6):1071–1081. doi: 10.1007/s00213-014-3742-8. DOI: 10.1007/s00213-014-3742-8. [DOI] [PubMed] [Google Scholar]
- Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JW, Kiens B, Nielsen JB. Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiol. Learn. Mem. 2014;116:46–58. doi: 10.1016/j.nlm.2014.08.004. DOI: 10.1016/j.nlm.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121(1-2):54–61. doi: 10.1016/j.drugalcdep.2011.08.006. DOI: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Access to a running wheel inhibits the acquisition of cocaine self-administration. Pharmacol. Biochem. Behav. 2011;100(2):237–243. doi: 10.1016/j.pbb.2011.08.025. DOI: 10.1016/j.pbb.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Wheel running decreases the positive reinforcing effects of heroin. Pharmacol. Rep. 2012;64(4):960–964. doi: 10.1016/s1734-1140(12)70891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98(1-2):129–135. doi: 10.1016/j.drugalcdep.2008.05.006. DOI: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology (Berl.) 2011;218(2):357–369. doi: 10.1007/s00213-011-2321-5. DOI: 10.1007/s00213-011-2321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Witte MA. The effects of exercise on cocaine self-administration, food-maintained responding, and locomotor activity in female rats: Importance of the temporal relationship between physical activity and initial drug exposure. Exp. Clin. Psychopharmacol. 2012;20(6):437–446. doi: 10.1037/a0029724. DOI: 10.1037/a0029724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Cox S. Acute effects of aerobic exercise on mood. Health Psychol. 1988;7(4):329–340. doi: 10.1037//0278-6133.7.4.329. [DOI] [PubMed] [Google Scholar]
- Tate AK, Petruzzello SJ. Varying the intensity of acute exercise: Implications for changes in affect. J. Sports Med. Phys. Fitness. 1995;35(4):295–302. [PubMed] [Google Scholar]
- Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol. (Amst.) 2003;112(3):297–324. doi: 10.1016/s0001-6918(02)00134-8. DOI: 10.1016/S0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- Van Rensburg KJ, Taylor A, Benattayallah A, Hodgson T. The effects of exercise on cigarette cravings and brain activation in response to smoking-related images. Psychopharmacology (Berl.) 2012;221(4):659–666. doi: 10.1007/s00213-011-2610-z. DOI: 10.1007/s00213-011-2610-z. [DOI] [PubMed] [Google Scholar]
- Van Rensburg KJ, Elibero A, Kilpatrick M. Impact of aerobic exercise intensity on craving and reactivity to smoking cues. Exp. Clin. Psychopharmacol. 2013;21(3):196–203. doi: 10.1037/a0032768. DOI: 10.1037/a0032768. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhou C, Zhao M, Wu X, Chang Y. Dose-response relationships between exercise intensity, cravings, and inhibitory control in methamphetamine dependence: An ERPs study. Drug Alcohol Depend. 2016;161:331–339. doi: 10.1016/j.drugalcdep.2016.02.023. DOI: 10.1016/j.drugalcdep.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Weinstock J, Barry D, Petry NM. Exercise-related activities are associated with positive outcome in contingency management treatment for substance use disorders. Addict. Behav. 2008;33(8):1072–1075. doi: 10.1016/j.addbeh.2008.03.011. DOI: 10.1016/j.addbeh.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng TB, Pierce GL, Darling WG, Voss MW. Differential effects of acute exercise on distinct aspects of executive function. Med. Sci. Sports Exerc. 2015;47(7):1460–1469. doi: 10.1249/MSS.0000000000000542. DOI: 10.1249/MSS.0000000000000542. [DOI] [PubMed] [Google Scholar]
- Williams DM, Dunsiger S, Whiteley JA, Ussher MH, Ciccolo JT, Jennings EG. Acute effects of moderate intensity aerobic exercise on affective withdrawal symptoms and cravings among women smokers. Addict. Behav. 2011;36(8):894–897. doi: 10.1016/j.addbeh.2011.04.001. DOI: 10.1016/j.addbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]