Abstract
Purpose
To investigate local control, survival outcomes and associated prognostic factors for patients with malignant peripheral nerve sheath tumors (MPNST) treated with combined surgery and radiation therapy (RT).
Methods
We reviewed the medical records of 71 consecutive patients treated with surgery and RT for localized MPNST between 1965 and 2012. Preoperative RT was used to treat 23 patients (32%) to a median dose of 50 Gy (range, 50–60 Gy), while 48 (68%) received postoperative RT to a median dose of 64 Gy (range, 45–70 Gy).
Results
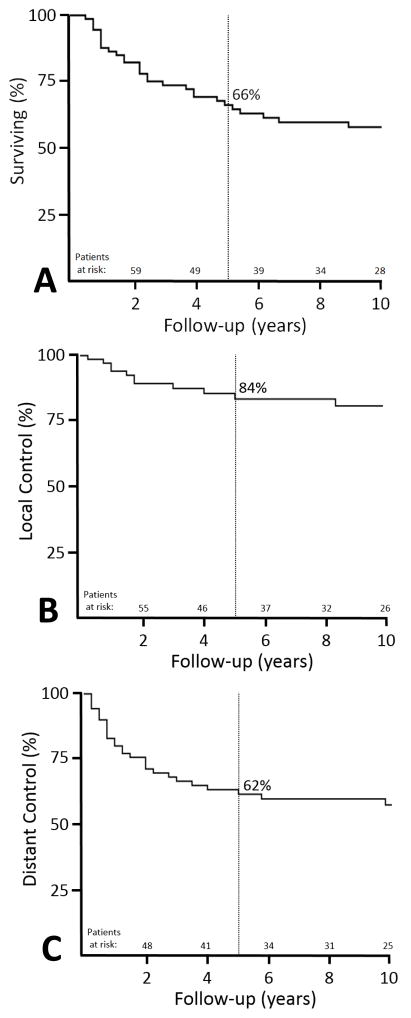
Median follow-up for living patients was 118 months (range, 21–512 months). The 5-year local control (LC), distant metastatic free survival (DMFS), and disease specific survival (DSS) rates were 84%, 62% and 66%, respectively. To identify predictors of outcome, several multivariate models were constructed: 1) Positive/uncertain surgical margin status was the only factor adversely associated local relapse at 5 years (28% vs. 5% for negative margins, P=0.02, HR 5.92, 95% CI 1.3–27.4). 2) No factors were significantly associated with DMFS. Of the 35 patients (49%) who sustained disease relapse, only 3 were ultimately salvaged. Only two patients had Grade 2 late toxicities (necrosis, fibrosis) based on CTCAE 4.03 criteria, and one patient had Grade 1 edema.
Conclusions
Combination therapy with surgery and RT provides favorable local control. Distant recurrences, however, continue to be challenging with limited salvage success at the time of relapse.
Keywords: malignant peripheral nerve sheath tumor, surgery, radiation therapy, multimodality
INTRODUCTION
Malignant peripheral nerve sheath tumors (MPNST), synonymously recognized in the literature as neurogenic sarcomas or malignant schwannomas, are rare spindle cell malignancies that account for 5% to 10% of all soft tissue sarcomas.1–4 These tumors are defined as malignancies arising from peripheral nerves or demonstrating nerve sheath differentiation. While many occur sporadically, more often MPNSTs arise in patients with neurofibromatosis type 1 (NF1) from preexisting plexiform neurofibromas. Recent studies have estimated the lifetime risk of a NF1 patient developing a MPNST ranging between 8% and 12%.5,6
Unfortunately, outcomes for patients with MPNST tend to be relatively poor. Most series report 5-year survival rates between 35% and 50%,1,7–10 and some series have reported even poorer survival among patients with NF1-associated tumors compared to those with sporadic tumors.1,8,10,11 The mainstay of treatment is complete surgical resection. However, even with aggressive surgeries that obtain negative margins, MPNSTs frequently relapse with most series reporting local recurrences occurring in 36% to 43% of their cohort.6,7,9,10
Given the poor outcomes and high relapse rates of patients with MPNST, most experts recommend a multimodality approach to local therapy. In particular, radiation therapy (RT) is advocated by many studies because of the locally aggressive nature of this disease.1,7–10,12,13 Due to the rarity of MPNST, most series lack the power to adequately analyze prognostic factors. Furthermore, nearly every study is comprised of highly heterogenous populations both treated inconsistently and including patients with metastatic and/or recurrent disease; this limits the interpretability of the current body of literature for this tumor. Given our institutional approach to soft tissue sarcomas, we have a comparatively large MPNST cohort consecutively treated using a consistent paradigm. Therefore, we analyzed our experience managing MPNST with combined modality therapy using surgery and RT to assess outcomes and the associated prognostic factors.
METHODS
We identified 71 consecutive patients with histologically confirmed, non-metastatic, malignant peripheral nerve sheath tumors treated with external beam RT and surgery as part of their definitive management at The University of Texas MD Anderson Cancer Center (MDACC) during the period from 1965 through 2012. These patients were extracted from a departmental database of patients with non-metastatic soft tissue sarcomas treated between 1960 and 2012. Patients with recurrent presentations (i.e. non-de novo presentations) or with RT-associated tumors were excluded from this analysis. Medical records were reviewed in detail after institutional review board approval. Patients underwent a full history, complete physical examination, routine blood tests, and appropriate imaging before their treatment. All MPNST diagnoses were confirmed at MDACC by pathologic review of the tissue.
Patient and Tumor Characteristics
Patient and tumor characteristics are listed in Table 1. The median age was 39 years (range, 16–88), and 55% of patients were female (n=39). The most common site for MPNST was the lower extremity (n=32, 45%), with the majority of the tumors found proximally in the thigh (n=20, 63%) followed by the leg (n=6, 19%). Of the tumors located within the trunk (n=15, 21%), the chest wall (n=5, 33%) and the axilla (n=3, 20%) were the two most common locations. Among the tumors in the upper extremity (n=14, 20%), three locations including the shoulder, upper arm, and forearm each had 4 patients (29%). Finally, 10 patients (14%) had tumor primaries of the head and neck, most commonly in the neck (n=4, 40%) and sinuses (n=3, 30%). MPNST tumors are generally considered high grade, and no tumors in the series were reported to be well-differentiated (grade 1); all were either not-specifically graded (presumed grade 3) or were noted specifically to be high grade. Tumor size was recorded in 67 patients (94%) with a median maximal dimension of 8.5 cm (range, 1.0–28.0 cm). Forty eight patients (68%) had tumors larger than 5 cm, with 21 (29%) having a tumor >10 cm. Therefore, 19 patients (27%) were considered AJCC (7th ed.)Stage IIA (T1N0M0, grade 3), while 48 patients (68%) were Stage III (T2N0M0, grade 3).
Table 1.
Patient and Tumor Characteristics of 71 Patients with MPNST
| Variable | All Patients (n=71) |
|---|---|
| Value or No. (%) | |
| Age, years | |
| Median | 39 |
| Range | 16–88 |
| Sex | |
| Female | 39 (55) |
| Male | 32 (45) |
| NF-1 Status | |
| Mutated | 26 (37) |
| Non-mutated | 45 (63) |
| Tumor Location | |
| Head & Neck | 10 (14) |
| Trunk | 15 (21) |
| Upper Extremities | 14 (20) |
| Lower Extremities | 32 (45) |
| Laterality | |
| Right | 38 (54) |
| Left | 30 (42) |
| Midline | 3 (4) |
| Maximum Tumor Dimension, cm | |
| Median | 8.5 |
| Range | 1–28 |
| Tumor size | |
| ≤ 5 cm | 19 (27) |
| > 5 cm | 48 (68) |
| Unknown | 4 (6) |
| Stage* | |
| IIA | 19 (27) |
| III | 48 (68) |
| Unknown | 4 (6) |
| Treatment Sequence | |
| Postop RT | 48 (68) |
| Preop RT | 23 (32) |
| Final Surgical Resection Margin | |
| Positive/Uncertain | 32 (45) |
| Negative | 39 (55) |
| Radiation Dose, Gy | |
| Median | 60 |
| Range | 45–70 |
| Chemotherapy | |
| Neo/Adj Only | 32 (45) |
| Both Neo/Adj & CCRT | 3 (4) |
| None | 36 (51) |
Abbreviations: RT, radiation therapy; Neo/Adj, neoadjuvant or adjuvant; CCRT, concurrent chemoradiation.
AJCC 7th edition.
Treatment Strategy
At the time of presentation to MDACC, 40 patients (56%) had gross tumor, while after undergoing excision at an outside facility 9 patients (13%) presented with positive margins, 16 (23%) had uncertain margins, and 6 (9%) had negative margins. Surgeons at MDACC determined the need for re-excision (performed in 50 patients, 70%) on the basis of prior surgical margins, location and extent of residual disease, and morbidity associated with additional surgery. Ultimately, after definitive excision of their tumor, 39 patients (55%) had negative final margins, while 15 (21%) had positive margins and 17 (24%) had uncertain margins. Given the importance of final margin status, an unknown final margin status was considered as high-risk for local recurrence as a positive margin. Therefore, local treatment disposition for patients with uncertain final margins was similar to that of patients with positive margins, and we thus combined positive/uncertain margin status for the analyses.
The decision to treat with RT and the timing of treatment was made in consultation with a multidisciplinary team. Preoperative RT was used to treat 23 patients (32%), to a median dose of 50 Gy (range, 50–60 Gy), while 48 patients (68%) received postoperative RT to a median dose of 64 Gy (range, 45–70 Gy). Only 4 patients (6%) were treated with intensity modulated radiation therapy.
The use of chemotherapy was determined at the discretion of the treating medical oncologist, and various regimens were employed (most commonly doxorubicin and ifosfamide, others include gemcitabine +/− docetaxel, dacarbazine, cisplatin, etc). A total of 35 patients (49%) were treated with chemotherapy either neoadjuvantly or adjuvantly and 3 patients (4%) received concurrent chemoradiation.
Follow-up and Statistical Analysis
The median follow-up time from the completion of RT for patients alive at last follow-up was 118 months (range, 21–512 months). Functional limb status was retrospectively evaluated at last contact and retrospectively scored using the Common Terminology Criteria for Adverse Events (CTCAE) v. 4.03.
Descriptive statistics were used to evaluate baseline characteristics, and differences between proportions of categorical data were analyzed by using Fisher’s exact test and chi-squared analyses as appropriate. Survival times were calculated from the RT completion date to the first occurrence of the event of interest. The Kaplan-Meier method was used to estimate rates of overall survival (OS), disease-specific survival (DSS), local control (LC), nodal control and distant metastatic free survival (DMFS). Log-rank tests were used to assess for significance (P ≤ 0.05) of differences between curves. The Cox proportional hazards model was used for multivariate analysis to assess the adjusted effects of numerous factors on the outcomes of interested. Significant (P ≤ 0.05) estimated hazard ratios (HR) are reported. IBM SPSS Statistics (version 22) was used for data analysis.
RESULTS
Characteristics of Patients with NF-1 versus non-NF-1 Tumors
The median age for patients with non-NF-associated MPNST was older at 45 years (range, 16–88) compared to the median age of patients with NF-associated MPNST of 37 years (range, 20–57) (P=0.03) (Table 2). There were no other significant differences related to sex (P=0.62), tumor primary location (P=0.92), or tumor size (continuous variable, P=0.18; categorical at 5 cm, P=0.53).
Table 2.
Comparing Patient and Tumor Characteristics between Sporadic MPNST and those associated with NF1
| Variable | Non-mutated (n=45) | NF-1 Mutated (n=26) | P Value |
|---|---|---|---|
| Value or No. (%) | Value or No. (%) | ||
| Age, years | |||
| Median | 45 | 37 | 0.03 |
| Range | 16–88 | 20–57 | |
| Sex | |||
| Female | 26 (57) | 14 (52) | 0.62 |
| Male | 20 (43) | 13 (48) | |
| Tumor Location | |||
| Head & Neck | 7 (15) | 3 (11) | 0.92 |
| Trunk | 10 (22) | 6 (22) | |
| Upper Extremities | 9 (20) | 5 (19) | |
| Lower Extremities | 20 (43) | 13 (48) | |
| Maximum Tumor Dimension, cm | |||
| Median | 8.0 | 9.75 | 0.18 |
| Range | 1.0–18.0 | 2.0–28.0 | |
| Tumor size | |||
| ≤ 5 cm | 12 (26) | 7 (26) | 0.53 |
| > 5 cm | 30 (65) | 20 (74) | |
| Unknown | 4 (9) | 0 (0) | |
Abbreviations: NF1, neurofibromatosis 1; RT, radiation therapy; Neo/Adj, neoadjuvant or adjuvant.
Survival
The actuarial 5-year and 10-year OS rates were 66% and 54%, respectively. There were 43 deaths (61%) among the cohort, and 30 of those were cancer-related resulting in actuarial DSS rates at 5-year and 10-years of 66% and 59%. Twenty patients (67%) died with distant metastatic disease only, while 7 patients (23%) died with concomitant distant metastases and either local or nodal relapses. The other 3 patients (9%) died with local relapses alone.
Disease Recurrence
Disease relapse developed in 35 patients (49%) resulting in a 5-year and 10-year disease free survival rate of 55% and 48%, respectively, with a median time to any failure of 16 months (range, 1–120 months). Disease relapse was not associated with treatment decade (P=0.31).
The actuarial 5-year and 10-year LC rates were 84% and 78%, respectively. Local recurrence developed in 12 patients (17%) with a median time to local failure of 20 months (range, 3–120 months). There was no association with local control outcome and site of final surgical excision having been at our institution vs an outside institution (P=0.32). Of those, the most common site of local relapse was within the treated RT field (in-field) (n=8, 67%), while 2 (17%) were considered marginal failures and 2 (17%) out-of-field. On univariate analysis, only margin status was associated with a significantly higher rate of local recurrence at 5 years (Table 3), with positive/uncertain margins remaining significantly associated with poorer local control on multivariate analysis (P=0.02, HR 5.9, 95% CI 1.3–27.4).
Table 3.
Univariate Analyses and Multivariate analyses for Local Control of MPNST
| Variable | LC at 5y | LC P Value |
|---|---|---|
| Age, years | ||
| ≤ 65 | 84 | 0.75 |
| > 65 | 75 | |
| Sex | ||
| Male | 80 | 0.43 |
| Female | 87 | |
| NF-1 Status | ||
| Mutated | 83 | 0.57 |
| Non-mutated | 85 | |
| Site | ||
| Head & Neck | 90 | 0.61 |
| Trunk | 74 | |
| Upper Extremities | 85 | |
| Lower Extremities | 85 | |
| Tumor Size | ||
| > 5 cm | 82 | 0.56 |
| ≤ 5 cm | 83 | |
| Final Margin Status* | ||
| Positive/Uncertain | 72 | 0.01 |
| Negative | 95 | |
| Treatment Sequence | ||
| Postoperative RT | 84 | 0.94 |
| Preoperative RT | 86 | |
| RT Dose | ||
| ≥60 Gy | 83 | 0.90 |
| <60 Gy | 86 | |
| Neo or Adj. Chemo | ||
| Yes | 86 | 0.34 |
| No | 81 | |
Abbreviations: LC, local control; RT, radiation therapy
Significant on multivariate modeling.
Reported in-text.
Only 1 patient (1%) developed nodal relapse for an actuarial 10-year nodal control rate of 99%, while 29 patients (41%) developed distant relapses for a 5-year and 10-year DMFS of 62% and 58%, respectively, with the most common site being the lungs (n=26, 90%). The median time to metastasis was 12 months (range 1–176 months). Tumor size was not significantly correlated with increased rates of distant relapse (cutoff of 5cm, P=0.13) (cutoff of 10cm, P=0.13). On multivariate modeling, no factors were significantly associated with an increased distant relapse rate.
Outcomes after Relapse
For the 35 patients with disease relapse, the median survival time was 13 months (range, 1–291) resulting in an actuarial 1-year and 2-year DSS rate after relapse of 66% and 34%, respectively. Four patients (11%) were ultimately salvaged and had a median survival after relapse of 99 months (range, 16–291 months).
For the 12 patients with local recurrences, the 1-year and 2-year DSS after relapse was 42% and 33%. Seven patients (58%) with local recurrences received salvage surgery and 6 patients (50%) received salvage chemotherapy. Three of the surgical procedures were amputations. Ultimately, 3 patients (25%) were successfully salvaged using either amputation (n=2) or surgical excision (n=1) with a median survival time after recurrence of 83 months (range, 16–115 months).
For the 29 patients with metastatic relapses, 11 patients (38%) had their metastases surgically excised, and 24 patients (83%) received systemic therapy. While there certainly was selection bias toward healthier patients or those with limited disease burden undergoing surgery, patients receiving surgical salvage had a longer median survival than those not having surgical resection (26 vs. 10 months, P=0.007). Ultimately, only 1 patient (3%) was successfully salvaged after metastatic relapse with 291 months of follow-up following recurrence.
Treatment complications
Seventy patients (96%) had normal limb and organ function at last contact, resulting in an actuarial10-year rate of return to normal function status of 95%. Of the 3 patients with functional deficits, two were considered grade 2 and one grade 1 using CTCAE v4.03 criteria. One patient was treated for a retroperitoneal MPNST and developed grade 2 necrosis of surrounding tissue. A second patient developed grade 2 fibrosis following treatment of a thigh primary. Finally, the last patient grade 1 ipsilateral edema of the lower extremity after receiving treatment for an inguinal primary. Notably, all three patients underwent postoperative RT to a median dose of 64 Gy (range, 60–69 Gy).
DISCUSSION
In a disease with such poor outcomes and high rates of relapse, few studies explicitly report on local control outcomes after multimodality therapy for MPNST. Our series is one of the larger studies to specifically address the outcomes associated with combined surgery and RT for the treatment of localized MPNST. We observed better than expected local control rates considering the high rates of local relapse reported in the literature. However, distant relapse risk remained quite high. When patients with MPNST recur, attempts at salvage are largely unsuccessful with most patients succumbing to their disease. Therefore, achieving upfront control with an aggressive attempt at negative margin resection and perioperative RT is essential.
While surgery is the mainstay of treatment, our data show high rates of local control with surgery in combination with RT. In fact, our 5-year local recurrence rate of 18% was notably lower than all other series, which may be explained by the fact that fewer patients in others reports received combination therapy (35–64% of patients received RT vs. 100% in the current series, Table 4).6,8,9,10,12,14,15,17 For comparison, two older series from Memorial Sloan Kettering Cancer Center (MSKCC) and Mayo Clinic reported 5-year local relapse rates of 40% and 42%, respectively.8,14 Additionally, Kahn and colleagues from the National Cancer Institute (NCI) recently reported a 5-year recurrence rate of 55% in patients not receiving RT.9 Unfortunately however, direct comparison of our results to other series is challenging because few report actuarial rates. If we instead compare our crude local recurrence rate, again our rate of 18% is markedly lower than in many series with ranges between 36% and 43%.6,7,9,10 In these series, 40% to 56% of the patients treated for MPNST did not receive RT,7,9,10 whereas our entire cohort was consistently treated with combination therapy.
Table 4.
Local recurrence rates among series treating patients with MPNSTs
| First Author | Year | No. of Patients | % receiving RT | 5-year LR | Crude LR rate |
|---|---|---|---|---|---|
| Ducatman8 | 1986 | 120 | 49 | NR | 42 |
| Hruban14 | 1990 | 43 | 35 | NR | 40 |
| Wong10 | 1998 | 134 | 54 | 49 | 43 |
| Anghileri7 | 2006 | 205 | 44 | 27 | 29 |
| Zou6 | 2009 | 140 | 49 | NR | 36 |
| Stucky12ŧ | 2012 | 175 | 63 | NR | 22 |
| LaFemina15 | 2013 | 105 | 64 | NR | 28 |
| Kahn9 | 2014 | 33 | 61 | NR | 36 |
| Present Study | 2015 | 73 | 100 | 18 | 18 |
Abbreviations: RT, radiation therapy; LR, local recurrence.
updated report on Wong et al with a notable decreased local recurrence rate attributed to increased use of aggressive multimodality therapy (better surgical clearance and increased use of adjuvant RT)
Several series have demonstrated a statistical local control benefit with the use of RT, which is particularly important given the association between local failure and DSS (HR 4.4)12, as well as the morbidity of local recurrences. Wong and colleagues showed that when RT was combined with surgical excision, the 5-year local control rate was improved (65% vs. 34% for surgery alone, P<0.001). Higher doses of RT and the use of intraoperative RT remained significantly associated with improved local control in their multivariate analysis.10 In an update of their series after the increased adoption of aggressive multimodality therapy, the local relapse rate decreased to 22% (from 42%), which the authors attributed to better surgical clearance and higher rates of adjuvant RT use;12 their updated local relapse rate (22%) was more consistent with the rate observed in our series suggesting aggressive multimodality therapy (surgical expertise and adjuvant RT) may be important for local control. Finally, a large series out of Milan reported that combination therapy with surgery and RT decreased the risk of local recurrence by about 50%, though the finding was not statistically significant.7
Despite the comparatively low local relapse rate in our series, survival outcomes remain poor. We observed an actuarial 5-year OS and DSS rate of 66%. Patient outcomes, however, are better in our series than in most with 5-year survival rates ranging from 35% to 52%,1,6,8–10 perhaps related to lower rates of local relapse (associated with DSS)12 or because we excluded patients with recurrent disease. Unfortunately, however, distant disease control remains a significant challenge for patients with MPNST. We observed a 5-year actuarial distant metastasis rate of 40% and a crude rate of 43%, with reports in the literature ranging between 36% and 42%.6,7,9,10 Some studies report tumor size and grade as significant factors associated with distant relapse.1,7,8, 9,10,12,13,14 However, grading of MPNST is not routinely performed at MDACC because it has not been shown to be of clinical utility under the French Federation of Cancer Centers.6 Therefore, MPNST are generally considered high grade given the lack of consensus regarding efficacy of grading and their uniformly poor prognosis.
While this study represents one of the largest series of combined modality local therapy for MPNST, there are several limitations to consider when interpreting the results. As with any retrospective series, there is inherent selection bias in the treatments selected, especially salvage treatments. For instance, the timing of RT is often delivered based on factors (ie. tumor size, location, previous procedures, functional outcomes) that are difficult to account for retrospectively. Additionally, the patients in this series were treated over several decades during which surgical and RT techniques have changed. Also, in an attempt to report a more homogenous cohort, we did exclude patients with recurrent disease or RT-induced tumors, which may have contributed to our favorable survival outcomes. Finally, retrospectively capturing functional deficits is challenging.
In conclusion, MPNST is a highly aggressive tumor with poor survival outcomes. However, surgical resection in combination with radiation therapy should be the standard approach for the primary tumor in patients presenting without obvious metastatic disease. Distant metastases continue to be challenging due to marginally effective systemic therapies against this disease, and salvage after relapse is poor. Upfront aggressive combined modality local therapy is well tolerated and critical to achieving ultimate disease control for patients presenting with localized MPNST.
Figure 1.
Kaplan-Meier curves showing 5-year outcomes for patients with malignant peripheral nerve sheath tumors: (A) disease specific survival (66%), (B) local control (84%), and (C) distant metastatic free survival (62%).
Acknowledgments
Supported in part by Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center.
Footnotes
Disclaimers: The authors declare no conflicts of interest.
References
- 1.Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23:8422–30. doi: 10.1200/JCO.2005.01.4886. [DOI] [PubMed] [Google Scholar]
- 2.Doorn PF, Molenaar WM, Buter J, et al. Malignant peripheral nerve sheath tumors in patients with and without neurofibromatosis. Eur J Surg Oncol. 1995;21:78–82. doi: 10.1016/s0748-7983(05)80073-3. [DOI] [PubMed] [Google Scholar]
- 3.Kleihues P, Cavenee WK International Agency for Research on Cancer. Pathology and genetics of tumours of the nervous system. Lyon: IARC Press; 2000. [Google Scholar]
- 4.Sordillo PP, Helson L, Hajdu SI, et al. Malignant schwannoma--clinical characteristics, survival, and response to therapy. Cancer. 1981;47:2503–9. doi: 10.1002/1097-0142(19810515)47:10<2503::aid-cncr2820471033>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–4. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–22. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 7.Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107:1065–74. doi: 10.1002/cncr.22098. [DOI] [PubMed] [Google Scholar]
- 8.Ducatman BS, Scheithauer BW, Piepgras DG, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–21. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Kahn J, Gillespie A, Tsokos M, et al. Radiation therapy in management of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Front Oncol. 2014;4:324. doi: 10.3389/fonc.2014.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong WW, Hirose T, Scheithauer BW, et al. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998;42:351–60. doi: 10.1016/s0360-3016(98)00223-5. [DOI] [PubMed] [Google Scholar]
- 11.Moretti VM, Crawford EA, Staddon AP, et al. Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy. Am J Clin Oncol. 2011;34:417–21. doi: 10.1097/COC.0b013e3181e9c08a. [DOI] [PubMed] [Google Scholar]
- 12.Stucky CC, Johnson KN, Gray RJ, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19:878–85. doi: 10.1245/s10434-011-1978-7. [DOI] [PubMed] [Google Scholar]
- 13.Wanebo JE, Malik JM, VandenBerg SR, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer. 1993;71:1247–53. doi: 10.1002/1097-0142(19930215)71:4<1247::aid-cncr2820710413>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Hruban RH, Shiu MH, Senie RT, et al. Malignant peripheral nerve sheath tumors of the buttock and lower extremity. A study of 43 cases. Cancer. 1990;66:1253–65. doi: 10.1002/1097-0142(19900915)66:6<1253::aid-cncr2820660627>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.LaFemina J, Qin LX, Moraco NH, et al. Oncologic outcomes of sporadic, neurofibromatosis-associated, and radiation-induced malignant peripheral nerve sheath tumors. Ann Surg Oncol. 2013;20:66–72. doi: 10.1245/s10434-012-2573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh BC, Ghosh L, Huvos AG, et al. Malignant schwannoma. A clinicopathologic study. Cancer. 1973;31:184–90. doi: 10.1002/1097-0142(197301)31:1<184::aid-cncr2820310126>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Guccion JG, Enzinger FM. Malignant Schwannoma associated with von Recklinghausen’s neurofibromatosis. Virchows Arch A Pathol Anat Histol. 1979;383:43–57. doi: 10.1007/BF00427009. [DOI] [PubMed] [Google Scholar]