Abstract
Reduced capacity to cognitively regulate emotional responses is a common impairment across major neuropsychiatric disorders. Brain systems supporting one such strategy, cognitive reappraisal of emotion, have been investigated extensively in the healthy population, a research focus that has led to influential meta-analyses and literature reviews. However, the emerging literature on neural substrates underlying cognitive reappraisal in clinical populations is yet to be systematically reviewed. Therefore, the goal of the current review was to summarize the literature on cognitive reappraisal and highlight common and distinct neural correlates of impaired emotion regulation in clinical populations. We performed a two-stage systematic literature search, selecting 32 studies on cognitive reappraisal in individuals with mood disorders (n=12), anxiety disorders (n=14), addiction (n=2), schizophrenia (n=2), and personality disorders (n=5). Comparing findings across these disorders allowed us to determine underlying mechanisms that were either disorder-specific or common across disorders. Results showed that across clinical populations, individuals consistently demonstrated reduced recruitment of the ventrolateral prefrontal cortex (vlPFC) and dorsolateral prefrontal cortex (dlPFC) during downregulation of negative emotion, indicating that there may be a core deficit in selection, manipulation and inhibition during reappraisal. Further, in individuals with mood disorders, amygdala responses were enhanced during downregulation of emotion, suggesting hyperactive bottom-up responses or reduced modulatory capacity. In individuals with anxiety disorders, however, emotion regulation revealed reduced activity in the dorsal anterior cingulate cortex (dACC) and inferior/superior parietal cortex, possibly indicating a deficit in allocation of attention. The reviewed studies thus provide evidence for both disorder-specific and common deficits across clinical populations. These findings highlight the role of distinct neural substrates as targets for developing/assessing novel therapeutic approaches that are geared towards cognitive regulation of emotion, as well as the importance of transdiagnostic research to identify both disorder specific and core mechanisms.
Keywords: fMRI, EEG, emotion regulation, depression, anxiety disorder, RDoC
1. Introduction
The combined lifetime prevalence of anxiety, mood, externalizing and substance use disorders is estimated to be 18-36% worldwide (Kessler et al., 2009). These neuropsychiatric disorders pose a substantial economic burden to the society and significant personal distress to the affected individuals and their families. A recent comprehensive meta-analysis of mortality rates indicates that mental health disorders lead to 8 million deaths annually worldwide (Walker, McGee, & Druss, 2015), with staggering short- and long-term societal costs for healthcare expenditures and impaired functioning (Kessler et al., 2009).
Across these major neuropsychiatric disorders a common cognitive impairment is a reduced capacity for emotion regulation. Persistent negative appraisal is thought to play a key role in the initiation and maintenance of depressed mood (Beck, Rush, Shaw, & Emery, 1979) and the maintenance of excessive anxiety (Amstadter, 2008), and to contribute to psychosis (Livingstone, Harper, & Gillanders, 2009). Moreover, the loss of control over drug intake in addiction has been linked to a compromised regulation of drug wanting (Goldstein & Volkow, 2011). A reduced capacity for cognitive regulation of emotion is assumed to arise from both sensitized emotional/reward brain systems and weakened brain networks for cognitive control (Etkin & Wager, 2007; Goldstein & Volkow, 2011; Hamilton et al., 2012). However, while there is mounting evidence implicating these brain systems during symptom provocation and performance of cognitive tasks in general, the literature on the functional neuroimaging studies that investigate emotion regulation per se has not been reviewed across these clinical populations.
As the effective use of emotion regulation strategies has been shown to constitute a resilience factor for mental health (Min, Yu, Lee, & Chae, 2013), correlating positively with health outcomes (Hu et al., 2014), a better understanding of the neural underpinnings of impaired emotion regulation in clinical populations would be of immense interest for both the development and the evaluation of new therapeutic interventions. Describing evidence-derived disorder-specific mechanisms for the observed deficits in emotion regulation in clinical populations may thus provide novel targets for pharmacological/cognitive-behavioral interventions or neuromodulation approaches using external [e.g., deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS)] or internal stimulation (e.g., neurofeedback guided emotion regulation). Moreover, identifying a basic mechanism of emotion regulation and demonstrating core deficits spanning different clinical populations may inform research within the Research Domain Criteria (RDoC) framework.
1.1. Cognitive regulation of emotion
Due to its efficacy in reducing emotional reactivity, cognitive reappraisal is the emotion regulation strategy most often studied in both healthy and clinical populations. It “changes the way a situation is construed so as to decrease its emotional impact” (Gross, 2002). Use of cognitive reappraisal affects the early stages of the emotion-generative process and therefore decreases both the experience and the behavioral expression of emotion, while other strategies acting later in the emotion-generative process, such as suppression, affect only behavioral expression without changing the experience of emotion (Goldin, McRae, Ramel, & Gross, 2008; Gross, 2002). Cognitive reappraisal entails both self- and situation-focused techniques. Self-focused reappraisal has also been labelled “distancing”, as participants are asked to change the emotional impact of a situation by altering its personal relevance, taking the perspective of a detached and objective observer instead of feeling involved. For example, participants are asked to “imagine you were a professional arriving at the scene” or to “imagine your loved ones were involved” to REDUCE or to INCREASE the emotional reactivity to the stimulus, respectively. Situation-focused reappraisal has also been called “reinterpretation”, as it aims at re-evaluating the situation by changing its outcomes (e.g., REDUCE: “imagine the situation to be better than initially perceived”, INCREASE: “imagine the situation to be worse than initially perceived”).
1.2. Brain systems involved in emotion regulation
The brain systems supporting cognitive reappraisal of emotion have been investigated extensively in the healthy population. A recent meta-analysis of 23 functional magnetic resonance imaging (fMRI) studies assessing co-activation patterns during reappraisal-mediated downregulation of emotion in healthy participants reported activations in the bilateral dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC), dorsal anterior cingulate cortex (dACC), premotor cortex/supplementary motor area (SMA) and inferior/superior parietal cortex (Kohn et al., 2014). A second recent meta-analysis, reviewing 44 fMRI studies on downregulation as well as 12 studies on upregulation of emotion, detected the same regulatory network during downregulation, and a less extended network including only the dACC, left dlPFC and premotor cortex during upregulation (Frank et al., 2014). Systematic modulatory effects were demonstrated in the amygdala/parahippocampal gyrus, which showed decreased activation during down- and increased activation levels during upregulation of emotion (Frank et al., 2014). Finally, results from a third recent meta-analysis, including 48 fMRI studies, converged with previous results showing extensive recruitment of a large regulatory network including the bilateral dlPFC, vlPFC, dACC, premotor cortex/SMA and inferior/superior parietal cortex during both down- and upregulation of emotion and a systematic modulatory influence on the extended amygdala (Buhle et al., 2014). Based on these findings, Buhle and colleagues proposed a key role for both the dlPFC and vlPFC, suggesting that the dlPFC supports the manipulation of appraisals in working memory, while the vlPFC supports the selection and inhibition of appraisals.
In summary, all three meta-analyses converged on describing the same regulatory network, which largely overlaps with the classic frontoparietal cognitive control network. This network includes lateral prefrontal regions (vlPFC, dlPFC) generally implicated in emotion regulation (Ochsner & Gross, 2005; Phillips, Drevets, Rauch, & Lane, 2003), but extends beyond this core network to the dACC and inferior/superior parietal cortex, known to be involved in allocating resources during processes that require goal-oriented attention (Cole & Schneider, 2007; Lueckmann, Jacobs, & Sack, 2014). These meta-analyses are in agreement with recent conceptualizations of emotion regulation as supported by a frontoparietal network, with the involvement of the dACC in allocating control (Shenhav, Botvinick, & Cohen, 2013) and the inferior/superior parietal cortex in supporting salience detection and allocating attention (Corbetta & Shulman, 2002). The target, the emotional network being modulated, is centered around the amygdala, a region that has been highlighted for its role in processing negative emotion (Costafreda, Brammer, David, & Fu, 2008).
These findings can further be extended to studies using electroencephalography (EEG), which allow high temporal resolution (msec) in tracking of emotional arousal. Specifically, an event-related potential, the late positive potential (LPP), is a composite measure indicating the emotional intensity and motivational salience of a stimulus (Hajcak, MacNamara, & Olvet, 2010). Indeed, the emotional modulation of the LPP has recently been reported to reflect recruitment of attentional networks during processing of motivationally salient stimuli (Moratti, Saugar, & Strange, 2011). While the LPP is increased during sustained attention towards salient stimuli, up- and downregulation via cognitive reappraisal increases and decreases the LPP amplitudes, respectively (Hajcak et al., 2010; Hajcak & Nieuwenhuis, 2006; Parvaz et al., 2015; Parvaz, MacNamara, Goldstein, & Hajcak, 2012).
1.3 Cognitive reappraisal in clinical populations
The goal of the current systematic review is to summarize the literature on cognitive reappraisal of emotion in select neuropsychiatric disorders and discuss the brain networks that are impaired during cognitive regulation of emotion in these clinical populations. Comparison of findings across disorders is aimed to highlight disorder-specific as well as core deficits as potential targets for interventions.
2. Methods
2.1. Study selection
We performed a two-stage systematic literature search to identify fMRI studies investigating cognitive reappraisal in clinical populations. First, we searched Medline/Pubmed using a search term comprised of the method (“fMRI” OR “magnetic resonance imaging” OR “PET” OR “positron emission tomography” OR “EEG” OR “electroencephalography” OR “LPP” OR “late positive potential”), combined with a term related to the disorder (“depression” OR “anxiety” OR “addiction” OR “dependence” OR “schizophrenia” OR “disorder” OR “patient”), and a term referring to the paradigm (“reappraisal” OR “emotion regulation”). Once data extraction was complete, we performed a second manual search for relevant papers based on the reference lists of all included papers. Studies adhering to the following criteria were included:
Studies published in English, in a peer-reviewed journal, in any year.
Studies that scanned participants during cognitive reappraisal versus a control condition.
Studies comparing adults with a DSM-III/IIIR/IV/V diagnosis to a matched control group.
Studies that reported whole brain results for the group difference.
2.2. Data extraction
Study manuscripts were reviewed for their adherence to the inclusion criteria. All fMRI and EEG studies in clinical populations with a cognitive reappraisal condition for either down- or upregulation of emotion were included. We summarized the main methods used, including: population studied, number of participants included in the clinical group, stimuli used for emotion provocation, instruction regarding reappraisal strategy and imaging modality and analysis thresholds used (Table 1). We report behavioral data emerging during emotion regulation for both the down- and upregulation conditions in the within group analyses, as well as for group differences during both conditions (Table 1, “self-report”). Other task conditions were not reported.
Table 1. Reviewed Studies.
| First author | Year | Populat ion |
n | Stimuli | Strategy | Self-report (reappraisal) |
Analysi s |
Group differences in brain response (reappraisal) |
|---|---|---|---|---|---|---|---|---|
| Mood disorders | ||||||||
| Beauregard | 2006 | MDD | 12 | sad films | self | n.s. | fMRI◇ << |
REDUCE (NEG): ↑ amygdala (R), dACC (R), insula (R), ventral temporal (R) |
| Johnstone | 2007 | MDD | 21 | pleasant/ unpleasant IAPS |
self/ situation |
n.a. | fMRI < |
REDUCE (NEG): ↓ vlPFC (L); ↑ dlPFC (R) |
| Heller | 2009 | MDD | 27 | pleasant/ unpleasant IAPS |
self/ situation |
n.a. | fMRI < |
INCREASE (POS): ↓ NAcc (L), insula (L), thalamus (R) |
| Erk | 2010 | MDD | 17 | unpleasant IAPS | self | reduction | fMRI << |
REDUCE (NEG): ↓ dlPFC (R) |
| Dillon | 2013 | MDD | 12 | unpleasant IAPS/ other negative |
self | reduction/ increase |
fMRI | n.s. |
| Wang | 2014 | MDD | 12 | pleasant/ unpleasant IAPS |
self | reduction/ increase |
fMRI < |
REDUCE (NEG/POS): ↑ amygdala (L), ↓ vlPFC (L); INCREASE (NEG/POS): ↓ amygdala (L); ↑ vlPFC (L) |
| Greening | 2014 | MDD | 19 | pleasant/ unpleasant IAPS |
self/ situation |
reduction*/ increase* |
fMRI α |
REDUCE (NEG): ↑ dlPFC (L), premotor, inferior parietal, precuneus, visual |
| Kanske | 2012 | rMDD | 23 | pleasant/ unpleasant IAPS |
self/ situation |
reduction | fMRI◇ << |
REDUCE (NEG): ↑ amygdala (L), OFC (R) |
| Smoski | 2013 | rMDD | 18 | sad faces | self/ situation |
reduction | fMRI < |
REDUCE (NEG): ↓ premotor (R); ↑ rACC (R) |
| Morris | 2012 | BD | 13 | unpleasant IAPS | self | reduction*/ increase |
fMRI◇ | REDUCE (NEG): ↑ vlPFC (R); INCREASE (NEG): ↑ vlPFC (R), dACC (R), dlPFC (R) |
| Townsend | 2013 | BD | 30 | unpleasant IAPS | situation | n.a. | fMRI◇ | REDUCE (NEG): ↓ vlPFC, dACC, dlPfC, premotor, caudate, thalamus (R), PCC, inferior parietal (L), auditory/visual |
| Kanske | 2015 | BD | 22 | pleasant/ unpleasant IAPS |
self/ situation |
reduction/ increase |
fMRI◇ << |
REDUCE (NEG/POS): ↑ amygdala, parahippocampal (R) |
| Anxiety disorders | ||||||||
| New | 2009 | PTSD | 14 | unpleasant IAPS | situation | reduction*/ increase |
fMRI < α |
REDUCE (NEG): ↓ dlPFC (L), SMA; INCREASE (NEG): ↑ SMA |
| Rabinak | 2014 | PTSD | 21 | unpleasant IAPS | self/ situation |
reduction | fMRI << |
REDUCE (NEG): ↓ dlPFC (L) |
| Woodward | 2015 | PTSD | 27 | unpleasant IAPS/ disorder relevant |
self/ situation |
n.s. | EEG | n.s. |
| Blair | 2012 | GAD | 17 | pleasant/ unpleasant IAPS |
self/ situation |
reduction/ increase |
fMRI | REDUCE (NEG)/INCREASE (POS): ↓ dACC; REDUCE (NEG): ↓ superior parietal (R) |
| Blair | 2012 | GAD/ SAD |
17 | pleasant/ unpleasant IAPS |
self/ situation |
reduction/ increase |
fMRI | REDUCE (NEG)/INCREASE (POS): ↓ dACC; REDUCE (NEG): ↓ superior parietal (R) |
| Manber- Ball |
2013 | GAD | 23 | unpleasant IAPS | self/ situation |
reduction | fMRI◇ < |
REDUCE (NEG): ↓ dlPFC, premotor (L), visual |
| Manber- Ball |
2013 | PD | 18 | unpleasant IAPS | self/ situation |
reduction | fMRI◇ < |
REDUCE (NEG): ↓ dlPFC, premotor (L), visual |
| Reinecke | 2015 | PD | 18 | accidents/ funerals |
self/ situation |
reduction | fMRI α |
REDUCE (NEG): ↓ vmPFC (R), vlPFC (R), dmPFC (R), PCC/precuneus (R), hippocampus (R), visual (R) |
| Goldin | 2009a | SAD | 15 | harsh faces/ violent scenes |
self | reduction | fMRI | REDUCE (NEG): ↓ dACC (R), mPFC (R), premotor, PCC/cuneus, posterior insula (R), inferior/superior parietal, auditory/visual; ↑ dlPFC (R), caudate |
| Goldin | 2009b | SAD | 27 | autobiographic | situation | reduction | fMRI | REDUCE (NEG) early: ↓ vlPFC, aPFC, dACC, dlPFC, SMA, inferior/superior parietal, thalamus (L), auditory (L), ventral temporal (R) |
| Blair | 2012 | SAD | 19 | pleasant/ unpleasant IAPS |
self/ situation |
reduction/ increase |
fMRI | REDUCE (NEG)/INCREASE (POS): ↓ dACC; REDUCE (NEG): ↓ superior parietal (R) |
| Ziv | 2013 | SAD | 27 | harsh faces/ criticism/ autobiographic |
self/ situation |
reduction* | fMRI | REDUCE (NEG): ↓ vlPFC (L), dACC, putamen (L), auditory/visual |
| Gaebler | 2014 | SAD | 21 | unpleasant IAPS | self | reduction/ increase |
fMRI◇ | n.s. |
| Paul | 2015 | OCD | 39 | aversive/ disorder relevant |
self/ situation |
reduction | EEG | REDUCE (NEG): ↑ LPP |
| Other disorders | ||||||||
| Albein- Urios |
2014 | CUD | 17 | unpleasant IAPS | self/ situation |
reduction | fMRI <<< |
REDUCE (NEG): ↓ vlPFC, dlPFC (L), insula (L), thalamus (R), PCC/cuneus, visual |
| Wu | 2015 | ND | 25 | pleasant/ unpleasant IAPS |
situation | reduction | EEG | n.s. |
| Morris | 2012 | Sz | 12 | unpleasant IAPS | self | reduction*/ increase |
fMRI◇ | REDUCE (NEG): ↓ vlPFC, dlPFC (L), OFC (L); INCREASE (NEG): ↑ vlPFC (R), dACC (R), dlPFC (R) |
| van der Meer |
2014 | Sz | 20 | unpleasant IAPS | self/ situation |
reduction | fMRI <<< |
REDUCE (NEG): ↓ vlPFC, caudate, thalamus, insula, parahippocampal gyrus, inferior parietal |
| Koenigsberg | 2009 | BPD | 18 | negative interpersonal |
self | reduction | fMRI < |
REDUCE (NEG): ↓ dACC (L), inferior parietal; ↑ amygdala (R), auditory (R), frontal eye field (R) |
| Schulze | 2011 | BPD | 15 | unpleasant IAPS | self | reduction | fMRI <<< |
REDUCE (NEG): ↓ vlPFC (L), dlPFC (L), pallidum (R), auditory, inferior parietal (R); ↑ insula, premotor, PCC INCREASE (NEG): ↓ PCC/precuneus, thalamus; ↑ somatosensory/visual |
| Lang | 2012 | BPD | 14 | negative scripts | self | reduction/ increase |
fMRI << |
INCREASE (NEG): ↓ dACC (L), dmPFC, PCC |
| Marissen | 2010 | BPD | 30 | pleasant/ unpleasant IAPS |
self/ situation |
n.s. | EEG | n.s. |
| Denny | 2015 | AvPD | 17 | negative interpersonal |
self | reduction | fMRI < α |
n.s. |
The n reported is the number of participants in the patient group. Cognitive reappraisal strategies were either self- (self) or situation-focused (situation). Within group behavioral results for regulation are reported (reduction = significant effect during downregulation, increase = significant effect during upregulation). Group differences in achieved regulation are marked with an asterisk (*). For neuroimaging data, brain regions with significant task*group interactions for the regulation versus watch contrast were reported for downregulation (REDUCE) and upregulation (INCREASE). Unilateral effects were marked with (L) = left and (R) = right, all other effects were observed bilaterally.
The following studies are marked in the Analysis column: studies where the task*interaction was not significant and group differences during the regulation condition were reported (marked with ◇); studies that applied ad hoc correction with a fixed cluster threshold and voxelwise thresholds: p<0.001, p<0.005 (marked with <<<); studies performing ROI analysis with multiple comparison correction (marked with <<); studies that performed an additional ROI analysis of the amygdala without multiple comparison correction (marked with α); studies using cluster-size thresholding with a lenient voxelwise threshold (p<0.01, p<0.05) (marked with an <); all other studies employing either cluster-size thresholding (p<0.05) with voxelwise threshold (p<0.005), Gaussian Random fields correction (p<0.05), or FWE corrected whole-brain correction (p<0.05) are not marked.
Abbreviations: IAPS = International Affective Picture System; NEG = negative/unpleasant, POS = positive/pleasant; LPP = Late positive potential MDD = Major Depressive Disorder, rMDD = remitted Major Depressive Disorder, BD = Bipolar Disorder, PTSD = Posttraumatic Stress Disorder, GAD = Generalized Anxiety Disorder, SAD = Social Anxiety Disorder, PD = Panic Disorder, OCD = Obsessive Compulsive Disorder, CUD = Cocaine use Disorder, ND = Nicotine Dependence, Sz = Schizophrenia, BPD = Borderline Personality Disorder, AvPD = Avoidant Personality Disorder Brain regions: aPFC = anterior prefrontal cortex, dACC = dorsal anterior cingulate, dlPFC = dorsolateral prefrontal cortex, dmPFC = dorsomedial prefrontal cortex, mPFC = medial prefrontal cortex, NAcc = nucleus accumbens, rACC = rostral anterior cingulate, OFC = orbitofrontal cortex, PCC = posterior cingulate cortex, SMA = supplementary motor area, vlPFC = ventrolateral prefrontal cortex, vmPFC = ventromedial prefrontal cortex
A list of the included studies is provided in Supplementary Material 1 (Albein-Urios et al., 2014; Beauregard, Paquette, & Lévesque, 2006; Blair et al., 2012; Davis et al., 2014; Denny et al., 2015; Dillon & Pizzagalli, 2013; Erk et al., 2010; Gaebler, Daniels, Lamke, Fydrich, & Walter, 2014; Goldin, Manber, Hakimi, Canli, & Gross, 2009; Goldin, Manber-Ball, Werner, Heimberg, & Gross, 2009; Greening, Osuch, Williamson, & Mitchell, 2014; Heller et al., 2009; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; P Kanske, Schönfelder, Forneck, & Wessa, 2015; Philipp Kanske, Heissler, Schönfelder, & Wessa, 2012; Koenigsberg et al., 2009; S. Lang et al., 2012; Manber-Ball, Ramsawh, Campbell-Sills, Paulus, & Stein, 2013; Marissen, Meuleman, & Franken, 2010; Morris, Sparks, Mitchell, Weickert, & Green, 2012; New et al., 2009; Paul, Simon, Endrass, & Kathmann, 2015; Rabinak et al., 2014; Reinecke et al., 2015; Reinecke, Thilo, Filippini, Croft, & Harmer, 2014; Schulze et al., 2011; Smoski, Keng, Schiller, Minkel, & Dichter, 2013; Townsend et al., 2013; van der Meer et al., 2014; Wang et al., 2014; Woodward et al., 2015; Wu et al., 2015; Ziv, Goldin, Jazaieri, Hahn, & Gross, 2013).
To evaluate differences in the brain responses during neuroimaging, we reported all brain regions demonstrating significant task × group interactions for the regulation (cognitive reappraisal) versus control (picture viewing) contrast (Table 1, “group differences in brain response”). If there were no brain regions showing a significant task × group interaction, we instead reported brain regions with a significant group difference during the regulation condition (as marked in all tables). Next, we evaluated the analyses thresholds used. A recent evaluation of the statistical methods typically used in the field reported that methods differ widely in their robustness (Eklund, Nichols, & Knutsson, 2015). Specifically, the authors report a drastic inflation of false positives when ad hoc procedures with a stringent cluster defining threshold (p = 0.001) and an arbitrary fixed cluster extent threshold (10 voxels) were employed. Such inflation was reduced, but still present, when cluster-size thresholding with lenient voxelwise threshold (p<0.01, p<0.05) was employed. This effect was most pronounced in within-group analyses and reduced by about 50% when analyses of group differences were considered. Inflation of results also depended on the preprocessing parameters and software packages employed; specifically, the commonly used SPM package performed reasonably well (2nd best), particularly when its default smoothing value of 8 mm was used (reducing inflation by another 50%).
We indicated in all tables the thresholds used, highlighting analyses with lenient and very lenient procedures. Following the recommendations by Eklund and colleagues (2015), first, we defined very lenient procedures as a) the use of a fixed cluster extent threshold with a given voxelwise (p<0.001, p<0.005) threshold or b) region of interest (ROI) analyses. To characterize ROI analyses (which were not directly investigated by Eklund and colleagues) and enable comparison with other whole-brain multiple correction methods, we considered the theoretical “whole-brain threshold” achieved with these methods (p<0.004 = p<0.05/mean number of 13 ROIs across the reviewed studies). Second, we defined lenient procedures as cluster-size thresholding with a lenient voxelwise threshold (p<0.01, p<0.05). Third, we indicated the studies that performed an additional ROI analysis of the amygdala without multiple comparison correction. For the main conclusions of this review we considered whether results would be altered if studies with very lenient procedures were excluded.
Finally, as findings from distinct studies may not necessarily be comparable across different studies given the use of different labelling systems, we relabeled all originally reported results using a single labeling system. To achieve this we first transformed all originally reported peak coordinates (see Supplementary Table 1 + 2) into MNI space using Brett’s Talairach to MNI algorithm (http://www.sdmproject.com/utilities/) and then relabeled all regions using the MRIcron Brodmann template (https://www.nitrc.org/projects/mricron). All reported peak coordinates in the reviewed studies were relabeled using the following delineation: vlPFC [Brodmann Area (BA) 44/45/47], dlPFC (BA 9/46), dACC (dorsal BA 24/32), ventromedial prefrontal cortex (vmPFC) (medial BA 11), orbitofrontal cortex (OFC) (lateral BA 11), anterior prefrontal cortex (aPFC) (BA 10), rostral anterior cingulate cortex (rACC) (rostral BA 24/32), inferior parietal (BA 39/40), superior parietal (BA 7), premotor cortex (lateral BA 6) and SMA (medial BA 6). All relabeling was done by the same person and terms such as “lateral” and “medial” were applied by dividing the respective Brodmann region in the middle.
3. Results
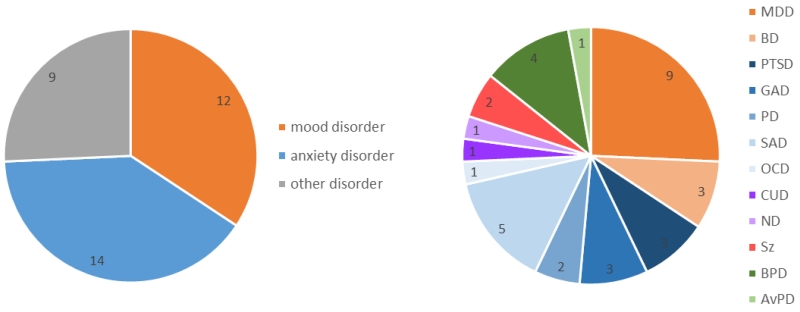
We identified 32 studies, published between 2006 and 2015, investigating different groups of clinically diagnosed individuals across a wide range of disorders (Table 1, a list of reviewed studies is provided in Supplementary Material 1). Of the selected studies, 34% were conducted in individuals with mood disorders [any mood disorder: n=12; major depressive disorder (MDD): n=9, bipolar disorder (BP): n=3], 40% in individuals with anxiety disorders [any anxiety disorder: n=14; posttraumatic stress disorder (PTSD): n=3, generalized anxiety disorder (GAD): n=3, panic disorder (PD): n=2, social anxiety disorder (SAD): n=5, obsessive compulsive disorder (OCD): n=1] and 26% in other disorders, including addiction [cocaine use disorder (CUD): n=1, nicotine dependence (ND): n=1], schizophrenia (Sz): n=2, and personality disorders [any personality disorder: n=5; borderline personality disorder (BPD): n=4, avoidant personality disorder (AvPD): n=1] (Figure 1). Addiction, schizophrenia and personality disorders were reviewed under ‘Other disorders’ given the low number of studies. Finally, only four studies employed EEG as a research tool (in PTSD, OCD, ND, BPD, table 1). The average sample size of the clinical group was n=20 (SD=6) and the smallest sample size of a clinical group was n=12.
Figure 1. Proportion of studies investigating reappraisal by diagnosis.
Of the selected studies, 34% of the selected studies were conducted in individuals with mood disorders, 40% in individuals with anxiety disorders and 26% in other disorders, including addiction, schizophrenia and personality disorders.
Abbreviations: MDD = Major Depressive Disorder, BD = Bipolar Disorder, PTSD = Posttraumatic Stress Disorder, GAD = Generalized Anxiety Disorder, PD = Panic Disorder, SAD = Social Anxiety Disorder, OCD = Obsessive Compulsive Disorder, CUD = Cocaine use Disorder, ND = Nicotine Dependence, Sz = Schizophrenia, BPD = Borderline Personality Disorder, AvPD = Avoidant Personality Disorder
All studies employed negative (or unpleasant) stimuli and included a downregulation condition. In 31% of the clinical groups positive (or pleasant) stimuli were additionally employed. In 71% of the clinical groups, pictures from the International Affective Picture System [IAPS, (P. J. Lang, Bradley, & Cuthbert, 2008)] were used to generate emotion, more often in mood disorders (83%) than in individuals with anxiety (64%) or other disorders (66%). Regarding task instructions, 34% asked participants to use a self-focused reappraisal strategy, while only 11% instructed participants to use a situation-focused reappraisal strategy, and the remaining 55% provided participants with both instructions. There was no bias in the selected instruction for any clinical group, except in borderline patients (75% self-focused strategies). Lastly, 40% of the clinical groups were also asked to upregulate their emotion (mood disorders: 66%, anxiety disorders: 36%, other: 22%) to study general deficits of emotion regulation.
The quality of statistical methods in the reviewed studies was higher than previously reported for the entire field. Generally, some 40% of recent papers do not report any multiple comparison corrections (Eklund et al., 2015), while all studies included in this review employed a multiple comparison correction, with only three studies (10%) choosing the most lenient approach (ad hoc procedures) (Albein-Urios et al., 2014; Schulze et al., 2011; van der Meer et al., 2014). These three studies were conducted in populations of the ‘other disorders’ category, limiting our ability to draw strong conclusions for this group. Overall, twenty of the included studies (65%) used suboptimal lenient or very lenient statistical methods, pointing to the importance of conducting future research using more stringent methods to replicate the current findings. Importantly, however, the inflation of results (or possibility for false positives) in the current review was effectively reduced by two factors: first, the large majority of the included studies used optimal preprocessing procedures (e.g., strong smoothing) and second, this review only considered analyses of group differences, for which the problem is substantially less severe.
3.1. Self-report on cognitive reappraisal
In 91% of the reviewed studies that assessed self-report measures during cognitive regulation of emotion, both the control and the clinical groups achieved downregulation of their emotional responses by cognitive reappraisal (Table 1). Of the studies that employed an upregulation condition, participants were successful in increasing the intensity of their emotion in 92% of studies (Table 1). Significant group differences, where healthy controls were more successful in regulation of emotion compared to the clinical group, were reported only in 16% of the studies that employed a downregulation condition and in 17% of the studies that included an upregulation condition (Table 1). There were no systematic biases regarding the employed stimuli or precise instruction for studies in which patients were less successful. In summary, the large majority of both patients and healthy controls indicated that they were able to apply cognitive reappraisal to voluntarily regulate their emotional response, independent of the stimuli or reappraisal strategy used.
3.2. Brain response during cognitive reappraisal
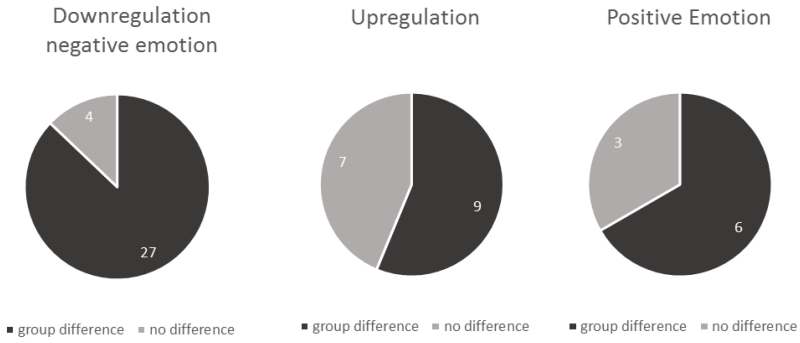
The majority of the reviewed fMRI studies on reappraisal demonstrated differences in brain activation patterns between patients and healthy controls during downregulation (87%) and upregulation (56%) of emotion (Table 1, Figure 2). Across clinical populations, patients as compared to healthy controls showed significantly different recruitment of emotional/cognitive brain networks during downregulation of emotion (Table 1: mood disorders: 83%, anxiety disorders: 92%, other disorders: 71%) and upregulation of emotion (Table 1: mood disorders: 38%, anxiety disorders: 80%, other disorders: 66%). Further, 66% of fMRI studies that investigated regulation of positive emotion found a group difference in brain responses (Table 1: mood disorders: 50%, anxiety disorders: 100%, other disorders: not investigated). The instruction regarding the employed reappraisal strategy (self-focused or situation-focused) did not seem to systematically impact brain activation patterns in any of the clinical groups (Table 1). Finally, of the four studies investigating cognitive reappraisal by EEG, the study with the largest sample of individuals with anxiety disorders found group differences during downregulation of negative emotion (Figure 3).
Figure 2. Proportion of fMRI studies reporting group differences by reappraisal instruction.
While 87% of studies reported differences during downregulation of negative emotion, only 56% found differential upregulation of emotion (both positive and negative), and 66% showed different brain activation patterns during regulation of positive emotion.
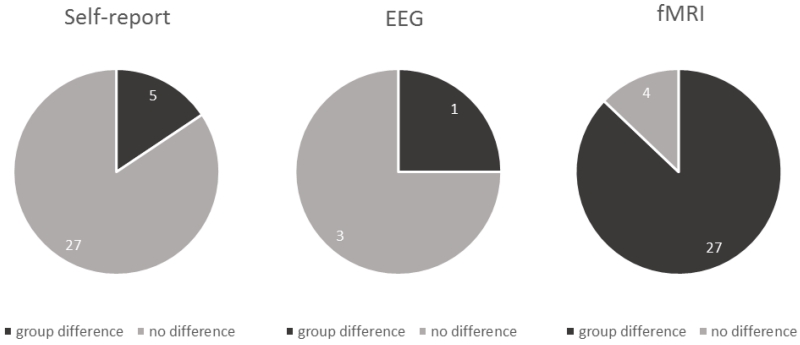
Figure 3. Proportion of studies reporting group differences (patients vs. controls) when downregulating negative emotion based on self-report, EEG, and fMRI.
Significant group differences in achieved regulation success during downregulation of negative emotion were reported in only 16% of studies based on self-report and 25% of studies employing EEG (n=1). However, 87% of studies employing fMRI reported group differences in brain activation patterns.
In summary, patients from different clinical populations demonstrated abnormal recruitment of brain networks during downregulation of negative emotion. Second, while individuals with mood disorders demonstrated abnormal brain activation patterns primarily during downregulation of negative emotion, individuals with anxiety and other disorders showed abnormalities consistently during both down- and upregulation, as well as during regulation of positive emotion. This suggests a more general deficit in engagement of the regulatory brain networks in individuals with anxiety and other disorders, compared to those with mood disorders.
3.2.1. Common findings in all clinical populations
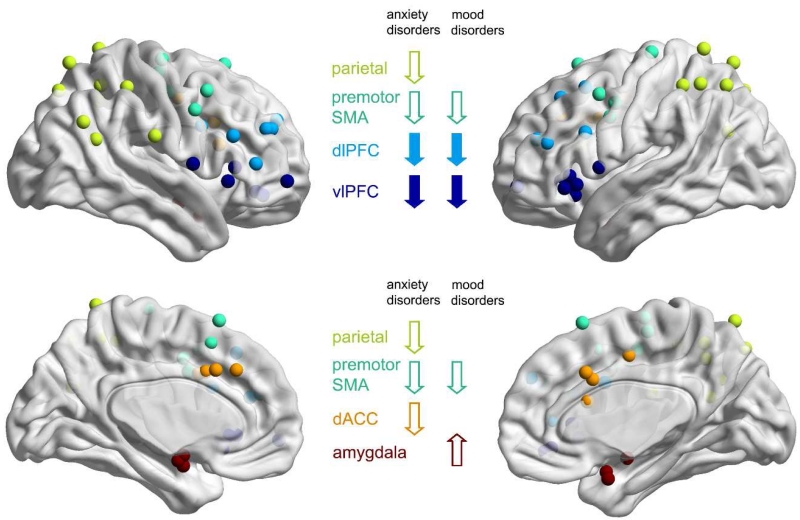
Compared to healthy controls, patients showed a consistent pattern of reduced activation of vIPFC and dlPFC (crucial nodes of the regulatory network) during downregulation of negative emotion (Table 2, see Supplementary Table 1 for the originally reported peak coordinates, see Figure 4 for a representation of reported peak coordinates in MNI space). After excluding one study that reported significant group differences in behavioral performance (Morris et al., 2012), the vlPFC demonstrated consistent reduction during downregulation of both positive and negative emotion across all clinical populations as compared to the healthy population (Table 2). This conclusion remains unchanged when excluding from this review the studies that used very lenient statistical thresholds. During upregulation of positive/negative emotion, studies consistently reported increased vlPFC activation in the clinical populations compared to healthy controls (n=3, Table 3, Supplementary Table 2). The dlPFC showed a reduced response during downregulation of negative emotion in most studies, but was somewhat less consistently implicated than the vlPFC (Table 2). Also, when excluding studies that used very lenient statistical thresholds from this review, the interpretation of the dlPFC response becomes equivocal within patients with mood disorders, with one study reporting increased dlPFC response in depressed patients (Johnstone et al., 2007) and another decreased activation levels in bipolar patients (Townsend et al., 2013). The dlPFC was also less often implicated in the upregulation of emotion, showing an increased response in two studies (Table 3). Notably, there was no modulation of the amygdala response that was consistent across clinical populations (Table 2, 3).
Table 2. Group differences during downregulation of negative emotion reported by fMRI studies.
| First author | Year | Population | n | Self-report | Analy sis |
vIPFC | dlPFC | amyg dala |
dACC | parie tal |
SMA /PM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mood disorders | |||||||||||
| Beauregard | 2006 | MDD | 12 | n.s. | ◇ << | - | - | ↑ | ↑ | - | - |
| Johnstone | 2007 | MDD | 21 | n.a. | < | ↓ | ↑ | - | - | - | - |
| Heller | 2009 | MDD | 27 | n.a. | < | - | - | - | - | - | - |
| Erk | 2010 | MDD | 17 | reduction | << | - | ↓ | - | - | - | - |
| Dillon | 2013 | MDD | 12 | reduction | - | - | - | - | - | - | |
| Wang | 2014 | MDD | 12 | reduction | < | ↓ ↓ | - | ↑ ↑ | - | - | - |
| Greening | 2014 | MDD | 19 | reduction* | α | - | - | - | - | ↑ | ↑ |
| Kanske | 2012 | rMDD | 23 | reduction | ◇ < | - | - | ↑ | - | - | - |
| Smoski | 2013 | rMDD | 18 | reduction | < | - | - | - | - | - | ↓ |
| Morris | 2012 | BD | 13 | reduction* | ◇ | ↑ | - | - | - | - | - |
| Townsend | 2013 | BD | 30 | n.a. | ◇ | ↓ | ↓ | - | ↓ | ↓ | ↓ |
| Kanske | 2015 | BD | 22 | reduction | ◇ << | - | - | ↑ ↑ | - | - | - |
| Anxiety disorders | |||||||||||
| New | 2009 | PTSD | 14 | reduction* | < α | - | ↓ | - | - | - | ↓ |
| Rabinak | 2014 | PTSD | 21 | reduction | << | - | ↓ | - | - | - | - |
| Blair | 2012 | GAD | 17 | reduction | - | - | - | ↓ | ↓ | - | |
| Blair | 2012 | GAD/SAD | 17 | reduction | - | - | - | ↓ | ↓ | - | |
| Manber- Ball |
2013 | GAD | 23 | reduction | ◇ < | - | ↓ | - | - | - | ↓ |
| Manber- Ball |
2013 | PD | 18 | reduction | ◇ < | - | ↓ | - | - | - | ↓ |
| Reinecke | 2015 | PD | 18 | reduction | α | ↓ | - | - | - | - | - |
| Goldin | 2009a | SAD | 15 | reduction | - | ↑ | - | ↓ | ↓ | ↓ | |
| Goldin | 2009b | SAD | 27 | reduction | ↓ | ↑ | - | ↓ | ↓ | ↓ | |
| Blair | 2012 | SAD | 19 | reduction | - | - | - | ↓ | ↓ | - | |
| Ziv | 2013 | SAD | 27 | reduction* | ↓ | - | - | ↓ | - | - | |
| Gaebler | 2014 | SAD | 21 | reduction | ◇ | - | - | - | - | - | - |
| Other disorders | |||||||||||
| Albein- Urios |
2014 | CUD | 17 | reduction | <<< | ↓ | ↓ | - | - | - | - |
| Morris | 2012 | Sz | 12 | reduction* | ◇ | ↓ | ↓ | - | - | - | - |
| van der Meer |
2014 | Sz | 20 | reduction | <<< | ↓ | - | - | - | ↓ | - |
| Koenigsberg | 2009 | BPD | 18 | reduction | < | - | - | ↑ | ↓ | ↓ | - |
| Schulze | 2011 | BPD | 15 | reduction | <<< | ↓ | ↓ | - | - | ↓ | ↑ |
| Lang | 2012 | BPD | 14 | reduction | << | - | - | - | - | - | - |
| Denny | 2015 | AvPD | 17 | reduction | < α | - | - | - | - | - | - |
All brain regions showing an increased (↑) or decreased (↓) brain response in patients relative to controls during downregulation of negative emotion are listed. Brain regions demonstrating a group difference during downregulation of BOTH negative and positive emotion are shown as increased (↑↑) or decreased (↓↓). Within group behavioral results are reported (reduction = significant downregulation). Group differences in achieved regulation are marked with an asterisk (*). The n reported is the number of participants in the patient group.
The following studies are marked in the Analysis column: studies where the task*interaction was not significant and group differences during the regulation condition were reported (marked with ◇); studies that applied ad hoc correction with a fixed cluster threshold and voxelwise thresholds: p<0.001, p<0.005 (marked with <<<); studies performing ROI analysis with multiple comparison correction (marked with <<); studies that performed an additional ROI analysis of the amygdala without multiple comparison correction (marked with α); studies using cluster-size thresholding with a lenient voxelwise threshold (p<0.01, p<0.05) (marked with an <); all other studies employing either cluster-size thresholding (p<0.05) with voxelwise threshold (p<0.005), Gaussian Random fields correction (p<0.05), or FWE corrected whole-brain correction (p<0.05) are not marked.
Abbreviations: vlPFC = ventrolateral prefrontal cortex, dlPFC = dorsolateral prefrontal cortex, dACC = dorsal anterior cingulate, SMA = supplementary motor area, PM = premotor cortex MDD = Major Depressive Disorder, rMDD = remitted Major Depressive Disorder, BD = Bipolar Disorder, PTSD = Posttraumatic Stress Disorder, GAD = Generalized Anxiety Disorder, SAD = Social Anxiety Disorder, PD = Panic Disorder, OCD = Obsessive Compulsive Disorder, CUD = Cocaine use Disorder, ND = Nicotine Dependence, Sz = Schizophrenia, BPD = Borderline Personality Disorder, AvPD = Avoidant Personality Disorder
Figure 4. Observed group differences during downregulation of negative emotion reported by fMRI studies.
All peak coordinates of brain regions showing either an increased or a decreased brain response in patients relative to controls during downregulation of negative emotion (as reported in table 2) are represented in MNI space. The regions are color coded according to the labelling used throughout this review. If the original coordinates were reported in Talairach space, they were converted using Brett’s algorithm (http://www.sdmproject.com/utilities/). The main findings of the current review are indicated by arrows (filled arrows = common findings across all clinical populations).
Table 3. Group differences during upregulation of emotion reported by fMRI studies.
| First author | Year | Population | n | Self-report | Anal ysis |
vlPFC | dlPFC | amyg dala |
dACC | parie tal |
SMA /PM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mood disorders | |||||||||||
| Beauregard | 2006 | MDD | 12 | n.s. | ◇ << | - | - | - | - | - | - |
| Johnstone | 2007 | MDD | 21 | n.a. | < | - | - | - | - | - | - |
| Heller | 2009 | MDD | 27 | n.a. | < | - | - | - | - | - | - |
| Dillon | 2013 | MDD | 12 | increase | - | - | - | - | - | - | |
| Wang | 2014 | MDD | 12 | increase | < | ↑ ↑ | - | ↓ ↓ | - | - | - |
| Greening | 2014 | MDD | 19 | increase* | α | - | - | - | - | - | - |
| Morris | 2012 | BD | 13 | increase | ◇ | ↑ | ↑ | - | ↑ | - | - |
| Kanske | 2015 | BD | 22 | increase | ◇ << | - | - | - | - | - | - |
| Anxiety disorders | |||||||||||
| New | 2009 | PTSD | 14 | increase | < α | - | - | - | - | - | ↑ |
| Blair | 2012 | GAD | 17 | increase | - | - | - | ↓ | - | - | |
| Blair | 2012 | GAD/SAD | 17 | increase | - | - | - | ↓ | - | - | |
| Blair | 2012 | SAD | 19 | increase | - | - | - | ↓ | - | - | |
| Gaebler | 2014 | SAD | 21 | increase | ◇ | - | - | - | - | - | - |
| Other disorders | |||||||||||
| Morris | 2012 | Sz | 12 | increase | ◇ | ↑ | ↑ | - | ↑ | - | - |
| Schulze | 2011 | BPD | 15 | increase | <<< | - | - | - | - | - | - |
| Lang | 2012 | BPD | 14 | increase | << | - | - | - | ↓ | - | - |
All brain regions showing an increased (↑) or decreased (↓) brain response in patients relative to controls during upregulation of EITHER positive or negative emotion are listed. Brain regions demonstrating a group difference during upregulation of BOTH negative and positive emotion are shown as increased (↑↑) or decreased (↓↓). Within group behavioral results are reported (increase = significant upregulation). Group differences in achieved regulation are marked with an asterisk (*). The n reported is the number of participants in the patient group.
The following studies are marked in the Analysis column: studies where the task*interaction was not significant and group differences during the regulation condition were reported (marked with ◇); studies that applied ad hoc correction with a fixed cluster threshold and voxelwise thresholds: p<0.001, p<0.005 (marked with <<<); studies performing ROI analysis with multiple comparison correction (marked with <<); studies that performed an additional ROI analysis of the amygdala without multiple comparison correction (marked with α); studies using cluster-size thresholding with a lenient voxelwise threshold (p<0.01, p<0.05) (marked with an <); all other studies employing either cluster-size thresholding (p<0.05) with voxelwise threshold (p<0.005), Gaussian Random fields correction (p<0.05), or FWE corrected whole-brain correction (p<0.05) are not marked.
Abbreviations: vlPFC = ventrolateral prefrontal cortex, dlPFC = dorsolateral prefrontal cortex, dACC = dorsal anterior cingulate MDD = Major Depressive Disorder, rMDD = remitted Major Depressive Disorder, BD = Bipolar Disorder, PTSD = Posttraumatic Stress Disorder, GAD = Generalized Anxiety Disorder, SAD = Social Anxiety Disorder, PD = Panic Disorder, OCD = Obsessive Compulsive Disorder, CUD = Cocaine use Disorder, ND = Nicotine Dependence, Sz = Schizophrenia, BPD = Borderline Personality Disorder, AvPD = Avoidant Personality Disorder
3.2.2. Findings specific to mood disorders
Individuals with mood disorders consistently showed enhanced amygdala response during downregulation of positive/negative emotion (Table 2, Supplementary Table 1, Figure 4), and blunted amygdala response during upregulation of either emotion relative to healthy controls (Table 3, Supplementary Table 2). This conclusion remains unchanged when excluding studies with very lenient statistical thresholds. Interestingly, one study also reported a blunted nucleus accumbens response during upregulation of positive emotion (Table 1). In patients with mood disorders there was also some evidence for a reduced response in SMA/premotor regions during downregulation of negative emotion, although two studies reported a reduced and one study reported an increased response (Table 2). Other regions of the cognitive control network, such as the dACC and inferior/superior parietal regions, showed an activation pattern that was similar to healthy controls, with only one study reporting increased and one study reporting decreased activation levels in these regions (Table 2). The typical general activation pattern observed in patients with mood disorders during downregulation of negative emotion was thus a reduced vlPFC/dlPFC response and, most consistently, increased activation level in the amygdala (Table 2).
3.2.3. Findings specific to anxiety disorders
In contrast to individuals with mood disorders, those with anxiety disorders showed a very consistent reduction in the dACC and inferior/superior parietal cortex during downregulation of negative emotion (Table 2, Supplementary Table 1, Figure 4), and in the dACC during upregulation of positive emotion relative to healthy controls (Table 3, Supplementary Table 2). This pattern was also observed when excluding studies that used very lenient statistical thresholds. In some studies this pattern extended into the premotor cortex/SMA as well (Table 2). However, unlike individuals with mood disorders, those with anxiety disorders did not show enhanced amygdala responses during downregulation (both negative and positive) (Table 2, 3), even when the amygdala was included in a ROI analysis without correction for multiple comparisons (New et al., 2009; Rabinak et al., 2014; Reinecke et al., 2015). In fact, only one study reported increased activation levels during downregulation in any region, specifically showing that both the caudate and the dlPFC activity were increased during downregulation of negative emotion in patients with anxiety disorders as compared to healthy controls (Goldin et al, 2009a). All other studies reported decreased activation levels for all implicated regions, including the dlPFC (n=4). While the effects varied in the extent of the network involved, the general activation patterns thus showed reduced vlPFC, dlPFC, SMA/premotor, dACC, and inferior/superior parietal cortex activation during downregulation of negative emotion in this population (Table 2). The only EEG study performed in anxiety disorders extended this finding by reporting increased LPPs during downregulation of negative emotion, possibly indicating a decreased capacity for recruitment of the attentional networks during regulation due to increased emotional intensity and salience of the emotionally provoking stimuli in patients relative to healthy controls (Table 1).
3.2.4. Findings specific to other disorders
Consistent with the other clinical groups, individuals with cocaine use disorder, schizophrenia and borderline personality disorder showed reduced vlPFC and dlPFC responses during downregulation of negative emotion compared to healthy controls (Table 2). Further, schizophrenia patients showed reduced recruitment of the inferior parietal cortex, similar to individuals with anxiety disorders, and increased vlPFC/dlPFC responses during upregulation of negative emotion as observed in individuals with mood disorders (Table 3). Lastly, individuals with borderline personality disorder showed enhanced amygdala response during downregulation of negative emotion, as typical for individuals with mood disorders, but also decreased recruitment of the dACC/inferior parietal cortex as in individuals with anxiety (Table 2, 3). However, the number of studies conducted in other disorders was small and four out of seven studies used very lenient statistical thresholds (Table 2). Only a tentative interpretation of these results is therefore possible.
4. Discussion
In summary, we found that the large majority of patients were able to apply cognitive reappraisal to voluntarily regulate their emotional responses. However, while there were no clear differences between the clinical groups and the healthy controls with regards to subjectively reported emotion levels, neuroimaging results highlighted both core as well as disorder-specific deficits in activation patterns, indicating differential recruitment of the emotional/regulatory brain networks in clinical populations.
4.1. Common or disorder-specific deficits
The reviewed studies provide evidence for both common and disorder-specific deficits in the recruitment of brain regions during cognitive reappraisal in clinical populations as compared to healthy controls. The core pattern observed during cognitive reappraisal in patients was a reduction of activation levels in the vlPFC and dlPFC, two regions that have been consistently implicated in healthy participants as key nodes of the regulatory cognitive control network, for having a role in supporting the selection, manipulation and inhibition of appraisals (Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014). We thus found evidence that a key aspect of the basic mechanisms supporting manipulation of emotional information is impaired across clinical populations, possibly reducing the capacity of patients to support the construction and reevaluation of appraisals during regulation of emotion.
Apart from this common core deficit, we additionally observed disorder-specific abnormalities for each clinical group, especially for individuals with mood and anxiety disorders. During downregulation of negative emotions, individuals with mood disorders showed enhanced activation levels in the amygdala, a core region of the emotion network (Costafreda et al., 2008). This hyperactivity of the amygdala during emotion regulation might be attributed to increased bottom-up responses, as has been observed during emotion appraisal in depressed patients (Chen, Suckling, Lennox, Ooi, & Bullmore, 2011; Delvecchio, Sugranyes, & Frangou, 2012; Groenewold, Opmeer, de Jonge, Aleman, & Costafreda, 2013; Hamilton et al., 2012); alternatively it could also be attributed to the reduced modulatory capacity of regulatory networks during cognitive reappraisal. Importantly, however, this aspect of emotion regulation was only impaired in individuals with mood disorder. In comparison, individuals with anxiety disorders showed reduced activation levels in the dACC and inferior/superior parietal cortex during both up- and downregulation of negative and positive emotion, providing evidence for a deficit in a different aspect of emotion regulation. In the reviewed studies, reduced recruitment of the dACC and inferior/superior parietal cortex during downregulation of negative emotion generally co-occurred. Among the many possible interpretations of this effect, an interpretation that takes this co-occurrence into account therefore invokes a reduced recruitment of the frontoparietal network that is needed to support the allocation of resources during processes that require goal-oriented attention regions (Cole & Schneider, 2007; Corbetta & Shulman, 2002; Lueckmann et al., 2014; Shenhav et al., 2013). This tentative interpretation suggests that in individuals with anxiety disorders specifically the allocation of attention and control may be impaired. Importantly and unlike individuals with mood disorders, those with anxiety disorders did not show enhanced amygdala response during cognitive reappraisal, and unlike individuals with anxiety disorders, those with mood disorders did not show reduced recruitment of the parietal regions/dACC involved in allocation of attention and control, pointing to an interesting double-dissociation of impairment.
Considering previous literature, the apparent lack of a deficit in downregulating the amygdala response in patients with anxiety disorders is particularly surprising. In anxiety disorders, hyperactivation of the amygdala during anxiety provocation has been proposed to be a core feature (Brühl, Delsignore, Komossa, & Weidt, 2014; Etkin & Wager, 2007; Sartory et al., 2013), similar to that described in patients with mood disorders (Chen et al., 2011; Delvecchio et al., 2012; Groenewold et al., 2013; Hamilton et al., 2012). However, consistent with our results, the literature also provides some evidence in support of a weaker and less consistent amygdala hyperactivation in anxiety than in mood disorders, specifically in depression. First, several meta-analyses studies on symptom provocation in individuals with anxiety disorders did not report a hyperactive amygdala (Ramage et al., 2013; Rotge et al., 2008). Second, a recent meta-analysis reported that gray matter loss in the amygdala is greater in individuals with depression, as compared to anxiety disorders, externalizing disorders, or individuals with bipolar disorder (Goodkind et al., 2015). Third, a recent model on depression has proposed that unbalanced activation patterns are driven unidirectionally by hyperactivity originating in limbic structures, including the amygdala, stressing the particular importance of the hyperactive amygdala in depression (Hamilton et al., 2012).
Overall, the current results also provide evidence that deficits may be not only disorder-specific, but in part dependent on the task employed. Of particular interest here is the observed reduction of activation in the dACC during emotion regulation, specifically in individuals with anxiety disorders. This finding may be somewhat unexpected, given that the dACC has consistently been reported to be hyperactive during emotion appraisal in patients with anxiety (Hilbert, Lueken, & Beesdo-Baum, 2014; Ramage et al., 2013; Sartory et al., 2013). However, the dACC is a multifaceted region, implicated in numerous ways in both cognitive and emotional processing (Etkin, Egner, & Kalisch, 2011). Recent research provides support to the notion that the recruitment of the dACC changes depending on the task employed. A recent meta-analysis found that individuals with anxiety disorders showed dACC hyperactivity during emotional, but not during purely cognitive tasks (Ramage et al., 2013). Further, a systematic review reported that individuals with anxiety disorders demonstrated dACC hyperactivation during symptom provocation, while tasks requiring the monitoring of behavior were characterized by a blunted dACC response (Hilbert et al., 2014). In conclusion, recruitment of the dACC seems to be dependent on the task context, with individuals with high anxiety showing either enhanced or reduced recruitment of this region depending on the specific context. We therefore tentatively conclude that the observed hypoactivation of the dACC and inferior/superior parietal cortex during emotion regulation may indicate reduced recruitment of attentional network particularly during emotion regulation, as opposed to emotional arousal, potentially indicative of a specific deficit in individuals with high anxiety.
In summary, the conclusion that there may be disorder-specific deficits in emotion regulation, as evident by hyperactivation of the amygdala in mood disorders and hypoactivation of the dACC/parietal cortex in anxiety, is supported by the literature.
4.2. Comparison with findings in the healthy population
Across clinical populations, patients demonstrated reduced recruitment of the vlPFC and dlPFC during emotion regulation, two regions which have been described as being core nodes of the regulatory network in healthy participants (Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014). In individuals with anxiety disorders this pattern extended into SMA/premotor regions, which have also been consistently implicated as central nodes of the regulatory network in healthy individuals (Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014). Further, disorder-specific deficits in individuals with anxiety disorders implicated other regions of the regulatory network as described in the healthy population, such as the dACC and parietal cortex (Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014), while the disorder-specific deficit observed in mood disorders implicated the amygdala, a core region of the affective network as described in healthy participants (Buhle et al., 2014; Frank et al., 2014). Overall, the brain regions showing differential recruitment in patients, as presented in this review (Figure 4), thus strikingly resemble the networks described in previous meta-analyses on emotion regulation in the healthy population (e.g. Figure 1 in Buhle et al., 2014). However, the differences between patient populations indicate that in different clinical populations different subdomains of emotion regulation may be affected (e.g., impaired attention allocation with increased anxiety vs. reduced modulatory capacity in mood disorders). To further understand these important differences as well as the shared basic mechanisms, future research would ideally be conducted transdiagnostically.
4.3. Brain versus Behavior
Another general finding of this review is that while there were consistent differences in brain activation patterns between the clinical groups and healthy controls during cognitive regulation of emotion, self-report data did not support these neuroimaging findings. There are several possible explanations. First, differences between groups may be subtle and therefore not measurable with the instruments employed for self-report during fMRI (unfortunately, most studies did not report the specific instructions provided to the participants for the rating procedure, e.g., the anchors provided, design of rating scale, hence this issue cannot be presently resolved). Second, there may be a compromised capacity for accurate self-awareness in both clinical and control groups, as has been suggested for individuals with addiction (Goldstein et al., 2009; Moeller et al., 2010). Third, there may be social desirability effects. Importantly, however, the consistency of the brain results across studies provides strong support for measurable group differences between patients and controls. While differences in recruitment of brain networks may, at least in part, reflect compensatory responses in patients, the reported group differences also support a reduced, rather than increased, recruitment of the self-regulatory networks generally implicated in successful emotion regulation in healthy participants.
4.4. Implications
The general finding of both common and disorder-specific deficits has important implications for the selection of neural targets when assessing new therapeutic approaches. Given the recent growing interest in using neuroimaging as a tool to assess therapeutic mechanisms (Konova, Moeller, & Goldstein, 2013; Zilverstand, Parvaz, Moeller, & Goldstein, 2016) and develop novel brain-based therapies, such as neuromodulation approaches using external stimulation devices (Marin, Camprodon, Dougherty, & Milad, 2014; Wani, Trevino, Marnell, & Husain, 2013) or emotion regulation trainings guided by neurofeedback (Sarkheil et al., 2015; Zilverstand, Sorger, Sarkheil, & Goebel, 2015), these findings are crucially informative. The general finding that deficits underlying the effective use of therapeutic strategies, such as cognitive reappraisal, are both disorder-specific and common, implies that neural targets for interventions need to be tailored to both the patient group and the particular impaired mechanism.
Specifically, while a common deficit in the construction and reevaluation of appraisals, as indicated by reduced activation levels in the vlPFC/dlPFC, demonstrates the impairment of a core mechanism of cognitive emotion regulation across clinical populations, the reported disorder-specific abnormalities indicate that additional aspects of compromised cognitive control need to be addressed per clinical group. Specifically, whereas it may be necessary to additionally target increased negative emotionality, as indexed by hyperactivity of the amygdala in individuals with mood disorders, the dACC and inferior/superior parietal regions may be additional targets for alleviating deficits in attentional allocation in individuals with anxiety disorders. In summary, under the assumption that disorder-specific deficits reveal additional mechanisms underlying compromised cognitive control, targeting only the vlPFC/dlPFC would not capitalize on the possibility for tailoring approaches to the specific weaknesses of each clinical group, potentially leading to incomplete recovery. In general, we therefore propose that for successful development of brain-based interventions, it is necessary to address both core deficits as well as disorder-specific mechanisms. To achieve this, neuroimaging may be employed as a tool to first assess disorder-specific mechanisms, which cannot be extrapolated based on research in healthy individuals.
Finally, a common deficit across different clinical applications, found within the core regulatory network of emotion regulation as described in healthy participants, has important implications for conducting future research within an integrative research framework, such as the RDoC approach. The present results suggest that emotion regulation deficits can be described as involving a common basic mechanism, which spans the healthy population as well as different clinical populations, and as such can be studied with a transdiagnostic focus.
5. Conclusions and future perspectives
In conclusion, we demonstrated that it is relevant and necessary to compare neuroimaging studies across clinical populations, providing compelling evidence for both common and disorder-specific neural targets of emotion regulation using cognitive reappraisal. Given the relatively low number of studies in clinical populations, clearly additional studies are needed before specific recommendations can be made for the development of brain-based therapeutic interventions. Further, it will be important to optimize the statistical methods to increase robustness of findings in future research. The observed findings also reveal insufficient research using EEG as a neuroimaging tool, and paucity of neuroimaging studies on reappraisal in individuals with addiction, and those with other externalizing disorders, schizophrenia and a range of personality disorders. In a larger context, the identified core deficit may point to a basic mechanism that may be dysregulated across a wide range of mental disorders, warranting further research within the RDoC approach. Overall, results support the merit of systematic reviews of neuroimaging results across clinical populations as a first step towards developing novel brain-based therapies.
Supplementary Material
Highlights.
A systematic review of 32 neuroimaging studies on cognitive reappraisal in patients
Lower vlPFC/dlPFC activation is a core deficit in downregulation across patients
Amygdala hyperactivity is a specific deficit in downregulation in mood disorders
dACC/parietal hypoactivity specific deficit in downregulation in anxiety disorders
Implications: neural targets for therapeutic interventions need to be tailored
Acknowledgements
This work was supported by a fellowship from the Netherlands Organisation for Scientific Research (Rubicon 446-14-015 to A.Z.) and by grants from the National Institute on Drug Abuse (1F32DA033088 to M.A.P. and R.Z.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albein-Urios N, Verdejo-Román J, Asensio S, Soriano-Mas C, Martínez-González JM, Verdejo-García A. Re-appraisal of negative emotions in cocaine dependence: Dysfunctional corticolimbic activation and connectivity. Addiction Biology. 2014;19:415–426. doi: 10.1111/j.1369-1600.2012.00497.x. doi:10.1111/j.1369-1600.2012.00497.x. [DOI] [PubMed] [Google Scholar]
- Amstadter A. Emotion regulation and anxiety disorders. Journal of Anxiety Disorders. 2008;22(2):211–221. doi: 10.1016/j.janxdis.2007.02.004. doi:10.1016/j.janxdis.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Paquette V, Lévesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17(8):843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. doi:10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. The Guilford Press; New York: 1979. [Google Scholar]
- Blair KS, Geraci M, Smith BW, Hollon N, DeVido J, Otero M, Pine DS. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biological Psychiatry. 2012;72(6):476–82. doi: 10.1016/j.biopsych.2012.04.013. doi:10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder—A meta-analytic review resulting in a new neurofunctional model. Neuroscience & Biobehavioral Reviews. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. doi:10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Ochsner KN. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. doi:10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-H, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disorders. 2011;13(1):1–15. doi: 10.1111/j.1399-5618.2011.00893.x. doi:10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37(1):343–60. doi: 10.1016/j.neuroimage.2007.03.071. doi:10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nature Reviews Neuroscience. 2002;3(3):215–229. doi: 10.1038/nrn755. doi:10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. doi:10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Davis TS, Mauss IB, Lumian D, Troy AS, Shallcross AJ, Zarolia P, McRae K. Emotional reactivity and emotion regulation among adults with a history of self-harm: Laboratory self-report and functional MRI evidence. Journal of Abnormal Psychology. 2014;123(3):499–509. doi: 10.1037/a0036962. doi:10.1037/a0036962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvecchio G, Sugranyes G, Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychological Medicine. 2012;43:553–569. doi: 10.1017/S0033291712001432. doi:10.1017/S0033291712001432. [DOI] [PubMed] [Google Scholar]
- Denny BT, Fan J, Liu X, Ochsner KN, Guerreri S, Mayson SJ, Koenigsberg HW. Elevated amygdala activity during reappraisal anticipation predicts anxiety in avoidant personality disorder. Journal of Affective Disorders. 2015;172:1–7. doi: 10.1016/j.jad.2014.09.017. doi:10.1016/j.jad.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Pizzagalli DA. Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Research - Neuroimaging. 2013;212(2):99–107. doi: 10.1016/j.pscychresns.2013.01.001. doi:10.1016/j.pscychresns.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols T, Knutsson H. Can parametric statistical methods be trusted for fMRI based group studies? 2015. arXiv:1511.01863. [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2010;30(47):15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. doi:10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. doi:10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. doi:10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Sabatinelli D. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience & Biobehavioral Reviews. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. doi:10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Gaebler M, Daniels J, Lamke J-P, Fydrich T, Walter H. Behavioural and neural correlates of self-focused emotion regulation in social anxiety disorder. Journal of Psychiatry & Neuroscience. 2014;39(4):249–258. doi: 10.1503/jpn.130080. doi:10.1503/jpn.130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural Bases of Social Anxiety Disorder. Emotional Reactivity and Cognitive Regulation During Social and Physical Threat. Archives of General Psychiatry. 2009;66(2):170. doi: 10.1001/archgenpsychiatry.2008.525. doi:10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural Mechanisms of Cognitive Reappraisal of Negative Self-Beliefs in Social Anxiety Disorder. Biological Psychiatry. 2009;66(12):1091–1099. doi: 10.1016/j.biopsych.2009.07.014. doi:10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–86. doi: 10.1016/j.biopsych.2007.05.031. doi:10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig a D. B., Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends in Cognitive Sciences. 2009;13(9):372–80. doi: 10.1016/j.tics.2009.06.004. doi:10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews. Neuroscience. 2011;12(11):652–69. doi: 10.1038/nrn3119. doi:10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Etkin A. Identification of a Common Neurobiological Substrate for Mental Illness. JAMA Psychiatry. 2015;72(4):305. doi: 10.1001/jamapsychiatry.2014.2206. doi:10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening SG, Osuch EA, Williamson PC, Mitchell DGV. The neural correlates of regulating positive and negative emotions in medication-free major depression. Social Cognitive and Affective Neuroscience. 2014;9(5):628–37. doi: 10.1093/scan/nst027. doi:10.1093/scan/nst027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold N. a., Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neuroscience and Biobehavioral Reviews. 2013;37(2):152–163. doi: 10.1016/j.neubiorev.2012.11.015. doi:10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/s0048577201393198. doi:10.1017.S0048577201393198. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-Related Potentials, Emotion, and Emotion Regulation: An Integrative Review. Developmental Neuropsychology. 2010;35(2):129–155. doi: 10.1080/87565640903526504. doi:10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective & Behavioral Neuroscience. 2006;6(4):291–297. doi: 10.3758/cabn.6.4.291. doi:10.3758/CABN.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. The American Journal of Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. doi:10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22445–22450. doi: 10.1073/pnas.0910651106. doi:10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert K, Lueken U, Beesdo-Baum K. Neural structures, functioning and connectivity in Generalized Anxiety Disorder and interaction with neuroendocrine systems: A systematic review. Journal of Affective Disorders. 2014;158:114–126. doi: 10.1016/j.jad.2014.01.022. doi:10.1016/j.jad.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Hu T, Zhang D, Wang J, Mistry R, Ran G, Wang X. Relation between emotion regulation and mental health: a meta-analysis review. Psychological Reports. 2014;114(2):341–362. doi: 10.2466/03.20.PR0.114k22w4. doi:10.2466/03.20.PR0.114k22w4. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to Regulate: Counterproductive Recruitment of Top-Down Prefrontal-Subcortical Circuitry in Major Depression. Journal of Neuroscience. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. doi:10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: The influence of regulation strategy, habitual regulation use, and emotional valence. NeuroImage. 2012;61(3):686–693. doi: 10.1016/j.neuroimage.2012.03.089. doi:10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- Kanske P, Schönfelder S, Forneck J, Wessa M. Impaired regulation of emotion: neural correlates of reappraisal and distraction in bipolar disorder and unaffected relatives. Translational Psychiatry. 2015;5:e497. doi: 10.1038/tp.2014.137. doi:10.1038/tp.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Wang PS. The global burden of mental disorders: An update from the WHO World Mental Health (WMH) Surveys. Epidemiologia E Psichiatria Sociale. 2009;18(1):23–33. doi: 10.1017/s1121189x00001421. doi:10.1017/S1121189X00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise KG, Pizzarello S, Siever LJ. Neural Correlates of the Use of Psychological Distancing to Regulate Responses to Negative Social Cues: A Study of Patients with Borderline Personality Disorder. Biological Psychiatry. 2009;66:854–863. doi: 10.1016/j.biopsych.2009.06.010. doi:10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird a. R., Fox PT, Habel U. Neural network of cognitive emotion regulation - An ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. doi:10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Goldstein RZ. Common and distinct neural targets of treatment: Changing brain function in substance addiction. Neuroscience and Biobehavioral Reviews. 2013;37:2806–2817. doi: 10.1016/j.neubiorev.2013.10.002. doi:10.1016/j.neubiorev.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainesville, Florida, USA: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lang S, Kotchoubey B, Frick C, Spitzer C, Grabe HJ, Barnow S. Cognitive reappraisal in trauma-exposed women with borderline personality disorder. NeuroImage. 2012;59(2):1727–34. doi: 10.1016/j.neuroimage.2011.08.061. doi:10.1016/j.neuroimage.2011.08.061. [DOI] [PubMed] [Google Scholar]
- Livingstone K, Harper S, Gillanders D. An Exploration of Emotion Regulation in Psychosis. Clinical Psychology and Psychotherapy. 2009;16(5):418–430. doi: 10.1002/cpp.635. doi:10.1002/cpp.635. [DOI] [PubMed] [Google Scholar]
- Lueckmann HC, Jacobs HIL, Sack AT. The cross-functional role of frontoparietal regions in cognition: internal attention as the overarching mechanism. Progress in Neurobiology. 2014;116:66–86. doi: 10.1016/j.pneurobio.2014.02.002. doi:10.1016/j.pneurobio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Manber-Ball T, Ramsawh HJ, Campbell-Sills L, Paulus MP, Stein MB. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychological Medicine. 2013;43(7):1475–1486. doi: 10.1017/S0033291712002383. doi:10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M-F, Camprodon JA, Dougherty DD, Milad MR. Device-Based Brain Stimulation To Augment Fear Extinction: Implications for Ptsd Treatment and Beyond. Depression and Anxiety. 2014;31:269–278. doi: 10.1002/da.22252. doi:10.1002/da.22252. [DOI] [PubMed] [Google Scholar]
- Marissen MAE, Meuleman L, Franken IHA. Altered emotional information processing in borderline personality disorder: an electrophysiological study. Psychiatry Research. 2010;181(3):226–32. doi: 10.1016/j.pscychresns.2009.10.006. doi:10.1016/j.pscychresns.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Min JA, Yu JJ, Lee CU, Chae JH. Cognitive emotion regulation strategies contributing to resilience in patients with depression and/or anxiety disorders -- licenta. Comprehensive Psychiatry. 2013;54(8):1190–1197. doi: 10.1016/j.comppsych.2013.05.008. doi:10.1016/j.comppsych.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133(5):1484–1493. doi: 10.1093/brain/awq066. doi:10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratti S, Saugar C, Strange BA. Prefrontal-Occipitoparietal Coupling Underlies Late Latency Human Neuronal Responses to Emotion. Journal of Neuroscience. 2011;31(47):17278–17286. doi: 10.1523/JNEUROSCI.2917-11.2011. doi:10.1523/JNEUROSCI.2917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Sparks A, Mitchell PB, Weickert CS, Green MJ. Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Translational Psychiatry. 2012;2(3):e90. doi: 10.1038/tp.2012.16. doi:10.1038/tp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, Charney DS. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biological Psychiatry. 2009;66(7):656–664. doi: 10.1016/j.biopsych.2009.05.020. doi:10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. doi:10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Konova AB, Proudfit GH, Dunning JP, Malaker P, Moeller SJ, Goldstein RZ. Impaired Neural Response to Negative Prediction Errors in Cocaine Addiction. Journal of Neuroscience. 2015;35(5):1872–1879. doi: 10.1523/JNEUROSCI.2777-14.2015. doi:10.1523/JNEUROSCI.2777-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, MacNamara A, Goldstein RZ, Hajcak G. Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(4):730–740. doi: 10.3758/s13415-012-0107-9. doi:10.3758/s13415-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Simon D, Endrass T, Kathmann N. Altered emotion regulation in obsessive-compulsive disorder as evidenced by the late positive potential. Psychological Medicine. 2015:1–11. doi: 10.1017/S0033291715001610. doi:10.1017/S0033291715001610. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biological Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. doi:10.1016/S0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Rabinak C. a., MacNamara A, Kennedy AE, Angstadt M, Stein MB, Liberzon I, Phan KL. Focal and Aberrant Prefrontal Engagement During Emotion Regulation in Veterans With Posttraumatic Stress Disorder. Depression and Anxiety. 2014;31:851–861. doi: 10.1002/da.22243. doi:10.1002/da.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage AE, Laird AR, Eickhoff SB, Acheson A, Peterson AL, Williamson DE, Fox PT. A coordinate-based meta-analytic model of trauma processing in posttraumatic stress disorder. Human Brain Mapping. 2013;34(12):3392–3399. doi: 10.1002/hbm.22155. doi:10.1002/hbm.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke A, Filippini N, Berna C, Western DG, Hanson B, Cooper MJ, Harmer C. Effective emotion regulation strategies improve fMRI and ECG markers of psychopathology in panic disorder: Implications for psychological treatment. Translational Psychiatry. 2015;5:e673. doi: 10.1038/tp.2015.160. doi:10.1038/tp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke A, Thilo K, Filippini N, Croft A, Harmer CJ. Predicting rapid response to cognitive-behavioural treatment for panic disorder: The role of hippocampus, insula, and dorsolateral prefrontal cortex. Behaviour Research and Therapy. 2014;62:120–128. doi: 10.1016/j.brat.2014.07.017. doi:10.1016/j.brat.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Rotge J-Y, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, Aouizerate B. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. Journal of Psychiatry & Neuroscience : JPN. 2008;33(5):405–12. [PMC free article] [PubMed] [Google Scholar]
- Sarkheil P, Zilverstand A, Kilian-Hütten N, Schneider F, Goebel R, Mathiak K. fMRI feedback enhances emotion regulation as evidenced by a reduced amygdala response. Behavioural Brain Research. 2015;281:326–332. doi: 10.1016/j.bbr.2014.11.027. doi:10.1016/j.bbr.2014.11.027. [DOI] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schürholt B, Lebens M, Seitz RJ, Schulze R. In Search of the Trauma Memory: A Meta-Analysis of Functional Neuroimaging Studies of Symptom Provocation in Posttraumatic Stress Disorder (PTSD) PLoS ONE. 2013;8(3):e58150. doi: 10.1371/journal.pone.0058150. doi:10.1371/journal.pone.0058150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Domes G, Krüger A, Berger C, Fleischer M, Prehn K, Herpertz SC. Neuronal Correlates of Cognitive Reappraisal in Borderline Patients with Affective Instability. Biological Psychiatry. 2011;69(6):564–573. doi: 10.1016/j.biopsych.2010.10.025. doi:10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. doi:10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Keng SL, Schiller CE, Minkel J, Dichter GS. Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. Journal of Affective Disorders. 2013;151(1):171–177. doi: 10.1016/j.jad.2013.05.073. doi:10.1016/j.jad.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JD, Torrisi SJ, Lieberman MD, Sugar C. a, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biological Psychiatry. 2013;73(2):127–35. doi: 10.1016/j.biopsych.2012.06.030. doi:10.1016/j.biopsych.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Swart M, van der Velde J, Pijnenborg G, Wiersma D, Bruggeman R, Aleman A. Neural correlates of emotion regulation in patients with schizophrenia and non-affected siblings. PloS One. 2014;9(6):e99667. doi: 10.1371/journal.pone.0099667. doi:10.1371/journal.pone.0099667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ER, McGee RE, Druss BG. Mortality in Mental Disorders and Global Disease Burden Implications. JAMA Psychiatry. 2015;72(4):334–341. doi: 10.1001/jamapsychiatry.2014.2502. doi:10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Feng Z, Zhou D, Lei X, Liao T, Zhang L, Li J. Dissociable self effects for emotion regulation: a study of chinese major depressive outpatients. BioMed Research International. 2014;2014:390865. doi: 10.1155/2014/390865. doi:10.1155/2014/390865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani A, Trevino K, Marnell P, Husain MM. Advances in brain stimulation for depression. Annals of Clinical Psychiatry. 2013;25(3):217–224. [PubMed] [Google Scholar]
- Woodward SH, Shurick AA, Alvarez J, Kuo J, Nonyieva Y, Blechert J, Gross JJ. A psychophysiological investigation of emotion regulation in chronic severe posttraumatic stress disorder. Psychophysiology. 2015;52(5):667–678. doi: 10.1111/psyp.12392. doi:10.1111/psyp.12392. [DOI] [PubMed] [Google Scholar]
- Wu L, Winkler MH, Wieser MJ, Andreatta M, Li Y, Pauli P. Emotion regulation in heavy smokers: experiential, expressive and physiological consequences of cognitive reappraisal. Frontiers in Psychology. 2015 Oct;6:1–11. doi: 10.3389/fpsyg.2015.01555. doi:10.3389/fpsyg.2015.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Moeller SJ, Goldstein RZ. Cognitive interventions for addiction medicine: Understanding the underlying neurobiological mechanisms. Progress in Brain Research. 2016;224:xv–xx. doi: 10.1016/bs.pbr.2015.07.019. doi:10.1016/bs.pbr.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Sorger B, Sarkheil P, Goebel R. fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Frontiers in Behavioral Neuroscience. 2015 Jun;9:1–12. doi: 10.3389/fnbeh.2015.00148. doi:10.3389/fnbeh.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv M, Goldin PR, Jazaieri H, Hahn KS, Gross JJ. Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biology of Mood & Anxiety Disorders. 2013;3(1):20. doi: 10.1186/2045-5380-3-20. doi:10.1186/2045-5380-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.