Abstract
Background
Food allergy is a growing health problem with very limited treatment options. Investigation of the immunological pathways underlying allergic sensitization to foods in humans has been greatly constrained by the limited availability of intestinal tissue and gut-resident immune cells. While mouse models have offered insights into pathways of food sensitization, differences between rodent and human immune physiology limit the extension of these findings to our understanding of human disease.
Objective
To develop a strategy for the generation of mice with humanized adaptive immune systems, complete with tissue engraftment by human mast cells that are competent to mount specific IgE-mediated responses and drive systemic anaphylaxis upon ingestion challenge.
Methods
Non-obese diabetic (NOD) severe combined immunodeficient (SCID) mice lacking the cytokine receptor common gamma chain (γc−/−) and carrying a human stem cell factor (SCF) transgene were engrafted with human hematopoietic stem cells (HSC). The impact of peanut (PN) feeding and IgE neutralization on the development of immune responses, mast cell homeostasis and anaphylactic food allergy was assessed in these animals.
Results
NSG SCF (huNSG) mice exhibited robust engraftment with functional human T and B lymphocytes and human mast cells were found in significant numbers in their tissues, including the intestinal mucosa. Following gavage feeding with PN they mounted specific antibody responses, including PN-specific IgE. When enterally challenged with PN, they exhibited mast cell mediated systemic anaphylaxis, as indicated by hypothermia and increases in plasma tryptase levels. Anti-IgE (omalizumab) treatment ablated this anaphylactic response.
Conclusions
huNSG mice provide a novel tool for studying food allergy and IgE-mediated anaphylaxis.
Keywords: IgE, mast cells, anaphylaxis, humanized mice, tryptase, peanut allergy
INTRODUCTION
Food allergy is a rising health challenge with a lack of effective treatments. As such, new tools are required to probe the earliest cellular and molecular signals driving allergic food sensitization and those responsible for maintaining the response. Furthermore, innovative approaches are needed for the preclinical evaluation of treatment strategies emerging from basic research. While substantial advances in delineating pathways of allergic sensitization to foods and effector mechanisms of hypersensitivity have recently been made in human subjects, progress has been constrained by the limited availability of the key tissues required to analyze local intestinal responses (e.g. intestinal mucosa, mast cells and gut-associated lymphoid tissue).
Mouse models have been applied with significant success but, for the most part, have required non- or less physiologic methods of sensitization (i.p. priming or using adjuvant) or genetic manipulations to enhance sensitivity. In addition, inherent differences in the immune physiology of rodents and humans limit the interpretation of findings from such models. Notably, the high affinity IgE receptor, FcεRI, displays a wider distribution on human leukocytes, including antigen-presenting cells; and soluble CD23 (FcεRII) complexed to IgE can stimulate IgE synthesis in human but not mouse B cells via CD211, 2. The differences in antibody Fc biology extend to the interaction between IgG and FcγRIIb, which exerts a critical brake on degranulation and anaphylaxis and is generally stronger in mice than humans,3, 4 with human skin mast cells expressing primarily activating FcγRIIa5.
We reasoned that a model in which rodents harbor a fully humanized adaptive immune system capable of generating food-specific IgE following allergen ingestion, as well as fostering the development of the human innate effector cells of anaphylaxis, would provide such a tool. Here we describe the conditions for the generation of such mice, their physiological response to peanut (PN) allergen and their utility as a source of human mast cells. Furthermore, we demonstrate the impact of omalizumab-mediated IgE neutralization on PN-induced anaphylaxis.
METHODS
Mice
NSG SCF (NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(PGK1-KITLG*220)441Daw/SzJ) mice were purchased from The Jackson Laboratory, and bred in a biosafety level 2 facility at Boston Children’s Hospital. NSG mice were maintained in autoclaved cages with Sulfatrim oral suspension (Sulfamethoxazole/Trimethoprim, HiTech Pharmacal) in the sterilized drinking water. All procedures were carried out under protocols approved by the local institutional animal care and use committee.
Stem cells
Cord blood-derived flow-sorted human CD34+ hematopoietic stem cells were purchased from AllCells. Such cells are obtained under Institutional Review Board (IRB)- or Human Subject Committee-approved protocols. Cells (5×104–105) were injected intravenously via the retro-orbital sinus into 3–6 week old mice. Engraftment was monitored in samples of peripheral blood using flow cytometry monthly for the four month engraftment period preceding allergen sensitization.
Flow Cytometry
Cells were stained with the following antibodies: Brilliant Violet 421-conjugated anti-human CD45 (clone HI30), PE-Cy7 anti-mouse CD45 (30-F11), Alexa Fluor 700 anti-CD3 (HIT3a), FITC anti-CD4 (OKT4), PE-Dazzle594 anti-IL-4 (MP4-25D2), Alexa Fluor 647 anti-Foxp3 (259D), PerCP-Cy5.5 anti-CD127 (A019D5), PE-Cy7 anti-CD25 (BC96), PerCP-Cy5.5 anti-IFNγ (4S.B3), PE anti-IL-10 (JES3-19F1), APC anti-CD19 (HIB19), PE-Cy7 anti-HLA-DR (L243), Brilliant Violet anti-c-Kit (104D2), and PE anti-FcεRI (AER-37 (CRA-1)) were purchased from Biolegend. APC anti-IgE (Ige21) was obtained from Affymetrix eBioscience. Anti-mouse CD16/32 (clone 93) and TruStain FcX Fc receptor blocking solution (both Biolegend) were used to prevent non-specific binding. Dead cells were excluded using fixable viability dye eFluor 780 (Affymetrix eBioscience). Intracellular cytokine staining was performed after a 4hr stimulation at 37°C with 500ng/ml ionomycin, 500ng/ml phorbol 12,1 3-dibutyrate and 1µg/ml brefeldin A (all Sigma-Aldrich). Coordinate analysis of transcription factors and cytokine production was performed using BD Biosciences Cytofix and Cytoperm reagents as previously described6. Intestinal leukocyte isolation was performed according to established protocols7.
Food allergen sensitization and anaphylaxis
After four months of stem cell engraftment, mice were sensitized by intragastric feeding with 22.5mg (5mg protein) Skippy creamy peanut butter (Hormel Foods) in 250µl 0.2M sodium bicarbonate pH 8.0 weekly for eight weeks. Control mice were sham-sensitized with sodium bicarbonate alone. Allergen challenge was performed by gavage feeding with 350mg peanut butter suspended in 0.2M sodium bicarbonate. Temperature measurements were performed using microchip transponders implanted subcutaneously 48hrs prior to challenge, as we have previously described8. Omalizumab (αIgE) was administered weekly by i.p. injection 48hrs prior to PN feedings at 120mg/kg. Dosing was calculated to correspond to the 10mg/kg used in humans, based upon surface area conversion recommendations from the Food and Drug Administration and other sources9, 10 and was designed to optimize the potential for IgE neutralization rather than an attempt to mimic therapeutic use in human patients.
ELISAs
PN-specific IgE was quantified by ELISA, capturing IgE onto plates coated with 3µg/ml anti-human IgE (clone G7–18, BD Biosciences), and detecting with biotinylated PN extract (200ng/ml, see reference11). A standard curve was constructed using PN-allergic patient sera that had been previously quantified by Immunocap. Tryptase measurements were performed by ELISA as previously described12.
Statistics
Data were plotted and analyzed in Prism 5.0f (GraphPad Software, Inc.). Temperature curves were analyzed using repeated measures two-way ANOVA, with matching for each individual mouse across the time course. Tryptase and IgE values were log transformed prior to analysis by ANOVA with Bonferroni post-tests. Data are represented as mean±SEM, with an individual point for each mouse where applicable.
RESULTS
PN-fed huNSG mice exhibit PN-specific IgE responses, PN-induced systemic anaphylaxis and intestinal mast cell expansion
A number of murine models of food allergy have been developed by our group and others to probe mechanisms underlying both allergic sensitization to foods and the induction of tolerance8, 13–18. One such model, in which an atopic tendency including susceptibility to food allergy is conferred by targeted insertion of a gene expressing an activated form of the IL-4 receptor α-chain, revealed the importance of IgE antibodies and mast cells in mediating food anaphylaxis8. Although there is a great deal of overlap in the pathways leading to immunological sensitization and tolerance in humans and rodents there are inherent differences that limit the application of findings from animal models to human disease. However, the direct study of human intestinal immune responses to food allergens has been hindered by the lack of available tissue. Food allergic subjects are generally otherwise healthy and have no clinical indications for endoscopy and biopsy, which would be necessary to obtain primary tissue for investigational purposes. We reasoned that a compromise would be to reconstitute immunodeficient mice with human hematopoetic progenitor that would develop into the human adaptive immune cells capable of generating Th2 and IgE responses as well as the effector innate immune cells (mast cells) of immediate hypersensitivity. Such humanized mice would permit us to test food allergy mechanisms and specifically the role of IgE antibodies and mast cells in a humanized setting.
A number of approaches have been described for the generation of humanized mice, generally using immunodeficient mice reconstituted with human PBMC or stem cells19–23. We elected to use a stem cell repopulating model, in which CD34+ cord blood hematopoietic stem cells (5×104) are injected into NOD SCID common gamma chain (yc)−/− (NSG) mice and develop into a human immune system. We predicted that, in addition to reconstituting full T and B cell adaptive immune function, it would be necessary in an allergy model for the recipient mice to be populated with functional human mast cells. Mast cells are dependent on stem cell factor (SCF), which binds to c-Kit, for their expansion and survival in tissues so we selected NSG mice with a human membrane-bound stem cell factor (SCF) transgene (NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(PGK1-KITLG*220)441Daw/SzJ) as recipients. SCF expression in this strain had previously been shown by Takagi et al. to support functional mast cell development and enhanced immune engraftment without requiring irradiation21, 24.
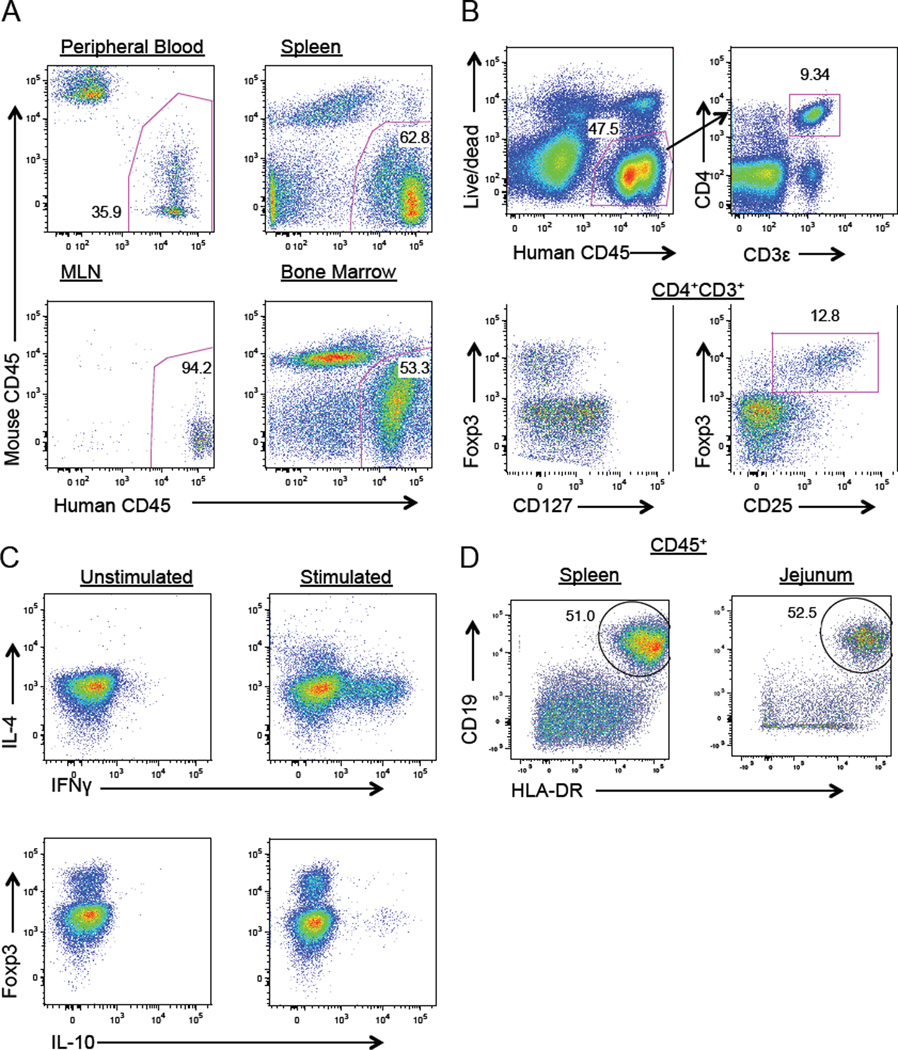
This approach provided robust engraftment and, after 12–20 weeks, human CD45+ leukocytes were predominant in the peripheral blood, spleen, mesenteric lymph nodes, bone marrow and small intestinal lamina propria (Fig 1 and data not shown). Adaptive immune lymphocytes were exclusively of human origin. Human CD45+ cells included CD4+ effector T cells exhibiting Th1 (IFNγ+) and Th2 (IL-4+) T-helper phenotypes following stimulation as well as Foxp3+CD127lowCD25+ regulatory T cells. An IL-10+Foxp3− population of T cells was observed as well. The B cell compartment was also well reconstituted with abundant CD19+HLA-DR+ B cells in the spleen and jejeunum.
FIG 1.
A. Flow cytometry identifying human leukocytes in peripheral blood, spleen, mesenteric lymph nodes (MLN) and bone marrow. B. Intracellular staining of splenic CD4+CD3+ T cells identifying regulatory T cells (Foxp3+CD127lowCD25+) and C. T helper subsets (IFNγ+ or IL-4+). D. Identification of CD19+HLA-DR+ B lymphocytes in the spleen and small intestinal lamina propria. Data are representative of three independent experiments.
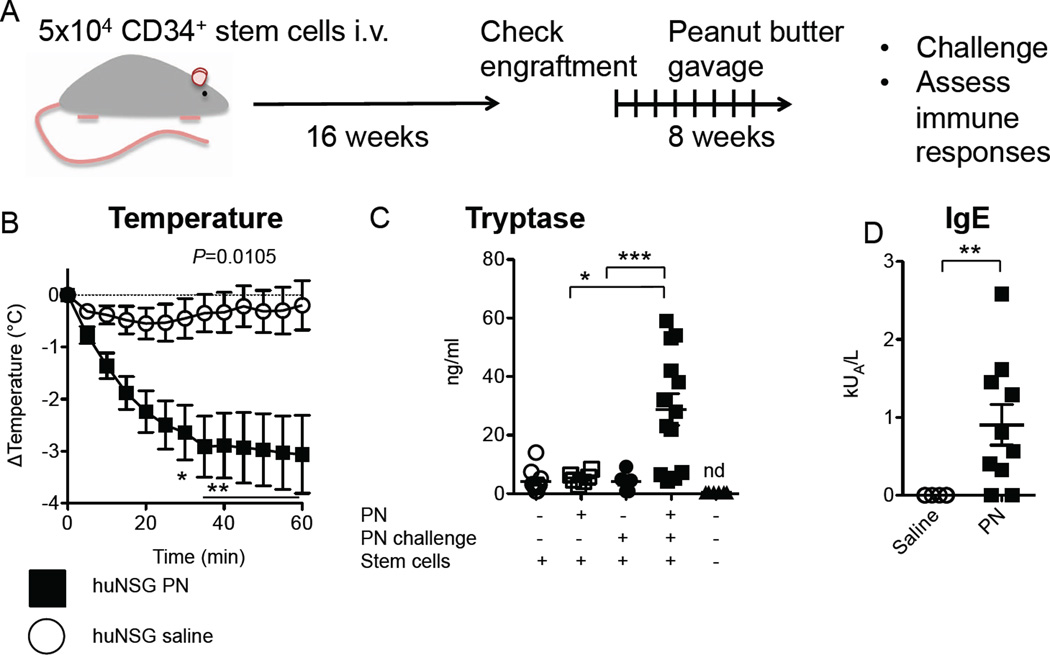
When repeatedly fed small doses of commercial peanut butter (22.5mg, 5mg protein content) by gavage weekly for eight weeks (Fig 2A), the mice with human immune systems developed anaphylactic sensitivity to the PN. Upon enteral challenge with a high dose of PN (350mg, 80 mg protein), most animals displayed a rapid and sustained loss of core body temperature (3.4°C± 2.1°C SD at nadir), indicative of the vasodilation and peripheral blood flow diversion characteristic of anaphylaxis (Fig 2B). In human subjects undergoing anaphylaxis tryptase levels are often elevated, reflecting mast cell degranulation25. PN-sensitized and challenged but neither mock-sensitized nor unchallenged mice exhibited marked increases in human tryptase in serum 1hr after PN challenge (Fig 2C). PN-allergic humanized mice made PN-specific human IgE in their serum, with a maximal response around 2.5 kU/L (Fig 2D). Neither human tryptase nor human IgE were detected in the sera of mice that did not receive HSC.
FIG 2.
Peanut (PN) allergy in humanized mice. A. Experimental design. SCF-transgenic NSG mice are engrafted with human CD34+ hematopoietic stem cells (5×104) by i.v. injection. After 4 months, mice are sensitized by weekly gavage feedings with PN (22.5mg) for 2 months. B. Core body temperature after enteral PN challenge in mice sensitized as in A and then challenged with 350mg PN (n=6–10), P=0.0105 by repeated measures 2-way ANOVA. C. Serum levels of human tryptase. *P<0.05, ***P<0.001 by Bonferroni post-test on ANOVA of log-transformed values. D. Serum PN-specific IgE levels.
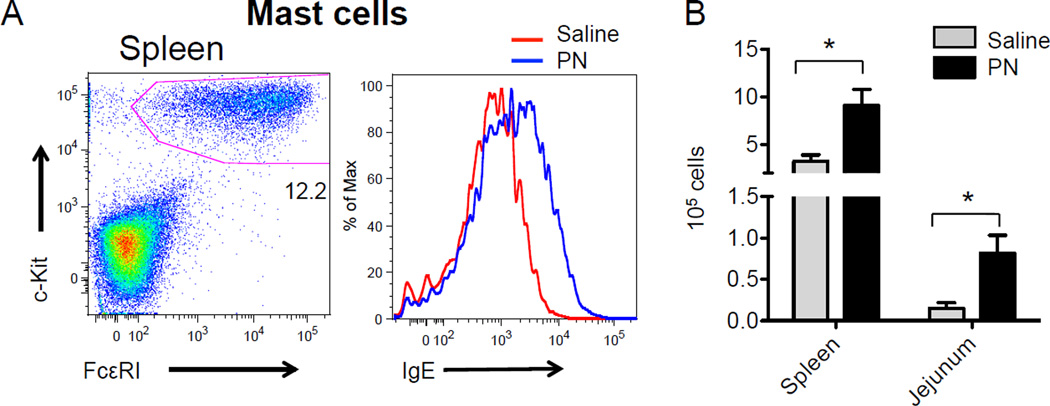
The tissues of the huNSG mice were efficiently reconstituted with human mast cells and these cells were brightly positive for human IgE (Fig 3A). A significant mast cell expansion was evident in the jejunum of PN-fed mice, concordant with murine models of food allergy (Fig 3B). Mast cell counts were also elevated in the spleen following PN sensitization. Histological examination revealed that hematopoietic stem cell transfer gave rise to robust human mast cell engraftment in the small intestine (Fig E1). Comparison with literature-reported values for human mast cell counts in biopsy samples suggested that mast cell densities in these huNSG mice might be somewhat elevated relative to healthy human tissues (Table E2) although it is possible that some of these differences might be due to variations in the tissue fixation and staining techniques used in the cited studies.
FIG 3.
A. Flow cytometry for total human IgE on FcεRI+c-Kit+ splenic mast cells. B. Human FcεRI+c-Kit+ mast cell numbers in the spleen and jejunal lamina propria as assessed by flow cytometry. Data are representative of three independent experiments.
Anaphylaxis in PN-fed huNSG mice is mediated exclusively by engrafted human mast cells and human IgE, with no contribution from host murine mast cells
While these findings were strongly consistent with PN anaphylaxis mediated by human IgE interacting with human mast cells, we performed additional experiments to completely rule out the possibility that murine mast cells might be mediating the human IgE effect. To this end we looked for human and murine mast cells in the huNSG mice both by histology and by flow cytometric analysis of human IgE and FcεRI staining. Human IgE does not bind to murine FcεRI, and the absence of γc signaling in cells of murine origin in these chimeric mice would be expected to greatly reduce the viability and function of murine FcεRI-bearing effector cells26–29. Histological analysis by chloroacetate esterase (CAE) staining, which detects mast cell granules by chymotryptic protease activity, revealed a complete absence of mast cells in the small intestine of mice that did not receive human stem cells (Fig E1). In contrast, some murine mast cells were observed in skin. Following HSC engraftment, unsensitized huNSG mice exhibited elevated numbers of mast cells in the stomach, small intestine and skin but not the lungs (Table E2). We further quantified human and murine mast cells by flow cytometry in the spleen and jejunum, finding that human mast cells outnumbered murine FcεRI+ cells by at least 100-fold in the spleen and 60-fold in the small intestine (Fig E3). No human IgE was detected on the surface of murine FcεRI+ cells. Consistent with the absence of murine B and T cells in NSG mice, murine IgE was undetectable in serum. We monitored the possibility of murine mast cell activation by quantification of murine mast cell protease (MMCP)-1, which, similarly to tryptase in human anaphylaxis, is readily detected in serum following mast cell degranulation in murine food allergy and anaphylaxis models8, 13, 14. MMCP-1 was undetectable in NSG mice, irrespective of engraftment or PN-induced anaphylaxis (Fig E3).
We further tested whether human IgE could elicit anaphylaxis or mast cell activation in mice by passive sensitization with human IgE and systemic challenge with antigen. For these experiments we used IgE-deficient (Igh7−/−) mice, which are immunocompetent and have an intact mast cell compartment. Because of their lack of endogenously produced IgE, these mice are highly sensitive to passive systemic anaphylaxis mediated by injected IgE and antigen. While mice passively sensitized with murine IgE exhibited a robust loss of core body temperature and elevated MMCP-1 levels upon antigen challenge as expected, no response was observed in mice that received human IgE (Fig E3). In contrast, antigen challenge of a stem cell-engrafted huNSG mouse sensitized with human IgE did result in loss of core body temperature (Fig E3). This confirms that human IgE cannot elicit responses by murine mast cells and, taken together, these findings strongly implicate engrafted human mast cells triggered by human IgE antibodies produced by engrafted B cells as the sole inducers of anaphylactic responses to PN exhibited by the humanized mice.
IgE neutralization blocks anaphylaxis in PN-fed huNSG mice
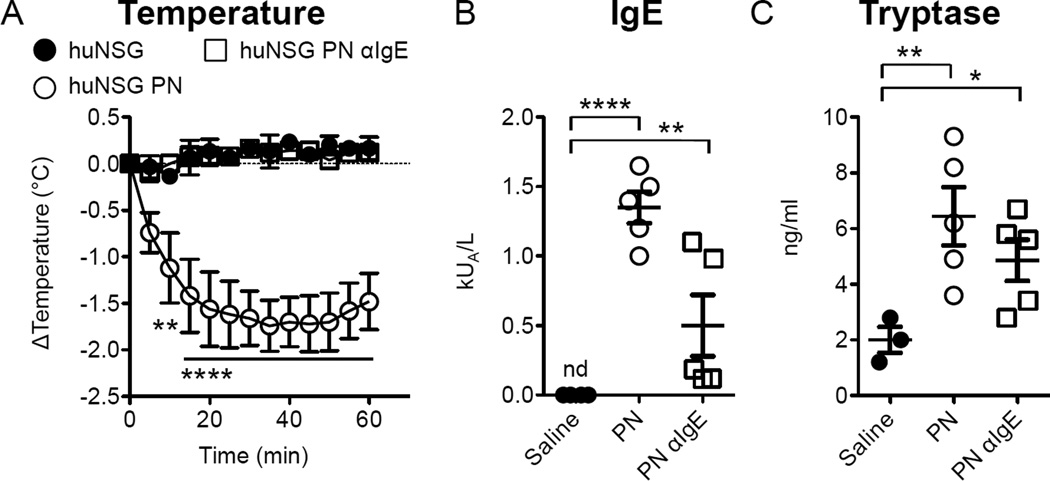
Leung and colleagues have previously reported that PN anaphylaxis can be blocked by treatment with anti-IgE30 and we have found that murine PN anaphylaxis is fully dependent on the presence of IgE and on signaling via FcεRI6, 8, 11. We directly assessed the requirement for human IgE in PN-induced anaphylaxis in huNSG mice by neutralization of IgE with omalizumab (αIgE). While omalizumab does not interact with receptor-bound IgE, it can reduce the amount of soluble IgE that is available to bind to cellular receptors31, 32. Mice receiving standard PN sensitization developed specific IgE responses and exhibited anaphylaxis upon gavage challenge (Fig 4A). In contrast, animals receiving omalizumab 48 hours prior to each of the sensitizing PN were completely protected from the loss of core body temperature observed in response to PN challenge (Fig 4A). Omalizumab treatment resulted in a significant increase in serum levels of total IgE (Fig E4A), an effect that is commonly observed in human subjects treated with the antibody that is attributable to the persistence of stable circulating omalizumab:IgE complexes33. However, we observed variable effects on the production of PN-specific IgE with omalizumab treatment. We suspect that reductions in detection might be related to interference with the anti-IgE capture antibody in our PN-specific IgE ELISA (Fig 4B). Although there was a trend towards lower tryptase levels in the omalizumab-treated animals, following PN challenge this did not achieve statistical significance. Overall the tryptase responses were low in this experiment, likely due to variations in efficiency of reconstitution.
FIG 4.
Impact of omalizumab on PN-induced anaphylaxis. Mice were sensitized as in Fig 2. Omalizumab (αIgE)was injected i.p. weekly 48h prior to sensitization dosing at 120mg/kg. A. Core body temperature loss after enteral PN challenge in sensitized animals (n=3–5), P=0.0004 by repeated measures 2-way ANOVA for an effect of αIgE. B. Serum PN-specific IgE levels. C. Serum levels of human tryptase. *P<0.05, **P<0.01, ***P<0.0001 by Bonferroni post-test on ANOVA of log-transformed values. Data are representative of two independent experiments.
Antigen-specific immune responses in huNSG mice are induced by the huMHC-restricted interaction of donor-derived human antigen presenting cells (APC) and T cells
One issue requiring consideration with humanized mouse models is the possibility that developing human T cells might receive their training and selection upon murine major histocompatibility (MHC) molecules in the murine host’s thymus in addition to—or instead of— on human MHC expressed on donor APC. In characterizing models such as the huNSG one under study here, it is important to understand whether T cells emerging following antigen exposure exhibit human MHC-restricted recognition, which is a requirement for effective T cell help in the cognate T:B cell interactions leading both to specific antibody production and to IgE isotype switching. We therefore investigated the presence of murine and human dendritic cells in huNSG mice and their contributions to specific immune responses.
The vast majority of murine leukocytes present in huNSG mice expressed the dendritic cell marker CD11c, and most also expressed the NOD-specific I-Ag7 MHCII molecule (Fig E5). CD11c+ leukocytes in the human CD45+ compartment almost universally expressed the human MHCII molecule HLA-DR in both the spleen and small intestine. Modest upregulation of MHCII was observed in human dendritic cells from PN-sensitized mice in the spleen and small intestine, as well in murine intestinal dendritic cells, an effect consistent with the stimulatory effect of IgE and mast cell-derived cytokines on dendritic cell activation34, 35.
In order to assess the competence of the murine and human dendritic cells coexisting in huNSG mice to present antigen to human T cells, we examined recall proliferative responses to PN and tetanus toxoid in CD4+ T cells. When cultured with isolated, antigen-pulsed human CD11c+ dendritic cells from huNSG mice, CellTrace Violet-labeled CD4+ T cells from immunized huNSG mice exhibited dye dilution (indicative of proliferation) (Fig E6). In contrast only minimal cell division was evident in the same CD4+ T cells cultured with murine CD11c+ cells, or when the T cells were isolated from naïve huNSG mice. No cross reactivity between the human and murine anti-CD11c antibodies used for isolation was observed. While not definitive, these results strongly suggest that in the long-term (6 month) engraftment used in these experiments, CD4+ T cells in huNSG mice respond to presentation of antigens by human MHCII. The consistent presence of low levels of all human immunoglobulins except IgA, as well as the production of PN-specific IgG antibodies in PN-fed mice (Fig E4), is consistent with previous reports that antibody responses are enhanced by increased engraftment times36, 37.
DISCUSSION
Humanized mouse models have proven useful in a number of settings including the study of human-specific pathogens such as HIV and research on mechanisms of cancer and autoimmunity19, 38. They have provided invaluable tools both for the dissection of disease mechanisms and for preclinical assessments of novel therapies. In this report we describe a strategy for the investigation of PN allergy, using humanized NSG mice engrafted with human CD34+ stem cells. The mice exhibited robust reconstitution of the T and B cell compartments of the adaptive immune response and developed PN-specific huIgE responses and anaphylactic sensitivity to ingested PN following sensitization. Like humans undergoing anaphylaxis, their plasma tryptase levels were markedly elevated, consistent with a human mast cell-mediated reaction. Anti-IgE treatment with Omalizumab blunted their anaphylactic reactions as expected. In each of its features, this model nicely recapitulates the typical features of human food allergy.
The use of animals transgenic for the obligate mast cell growth factor, SCF, in this model ensured the population of their tissues by human mast cells. Un-reconstituted NSG mice had no intestinal tissue mast cells whereas engrafted animals had jejunal mast cell densities of about 1–2 times those reported in normal human biopsies. Murine mast cell protease was undetectable in huNSG mice undergoing PN-induced anaphylaxis, and administration of human IgE to mice without human immune systems failed to elicit any discernable reaction upon antigen challenge. Thus, our data are consistent with a human IgE-mediated anaphylactic reaction produced by human mast cells, without any involvement of murine effector cells.
In this study we found evidence that PN allergens (as well as tetanus toxoid) can be presented to human CD4+ T cells by engrafted human APCs in huNSG mice. In contrast, resident host APCs, expressing murine MHC antigens were not efficient inducers of recall responses in T cells from the humanized mice, consistent with a dominant role for human MHC restriction. Current understanding concerning the education of developing T cells holds that selection occurs in the thymus on MHC molecules expressed by stromal cells of nonhematopoietic origin39, 40 While human hematopoietic cells bearing human MHC molecules might enter the mouse thymus in our huNSG mice, some published studies have predicted that these cells would not efficiently select developing human T cells19, 39, 40. Consistent with this dogma, initial reports describing the development of human leukocytes from hematopoietic stem cells in NSG mice concluded that the human T cells were restricted to murine MHC and dysfunctional, failing to respond to anti-CD3 stimulation41. These past conclusions contrast strongly with our findings that human MHC-restricted T cell development and HLA-restricted antigen presentation are functional in these mice. Consistent with our findings, several studies have demonstrated HLA-restriction of cytotoxicity of CD8 T cells42 and delayed type hypersensitivity reactions43. Most tellingly, direct testing for human HLA restriction of human T cells in humanized mice by mixed leukocyte reaction demonstrated strong responses to allogeneic human DCs, but weak reactions to mismatched murine MHC44, consistent with human MHC restriction.
The discordance in findings regarding human vs. murine MHC restriction in the immune responses of huNSG mice suggests the existence of as yet unresolved complexities. It also points to potential strategies for the further improvement of such humanized mouse models. The surgical implantation of human organoids (bone, liver and thymus) or transgenic expression of human HLA alleles has been shown to enhance human T cell development and function19, 38, 45, 46, and it is likely that the PN allergy model described here could be improved by either of these approaches.
Normally, allergen ingestion by humans or mice results in tolerance. Thus the default tendency of PN-fed huNSG mice to exhibit allergy rather than tolerance came as a bit of a surprise. We speculate that several factors might contribute towards the allergic predisposition of these animals. These include the mismatch between thymic stromal murine MHC and human APC MHC described above, as well as the relative lymphopenia of these animals; a condition associated with loss of tolerance perhaps either because of low Treg numbers or repertoire mismatch between Teff and Treg47–49. The generally naïve T and B cell repertoire related to short-term immune reconstitution could additionally be contributing to this dysregulation50, 51. We speculate that altered colonization might also be affecting the immune response of these mice. The prolonged antibiotic treatment necessary to maintain immunodeficient NSG mice in our facility could skew their intestinal microbiota in a pro-allergic direction. We and others have observed that the food allergic phenotype of mice is linked to the complexity and community structures of their intestinal microbiota and it has been observed that germ-free animals have a tendency towards allergy52–55.
Finally we have considered the possibility that both the high density of tissue mast cells (higher than reported in normal human tissues) and a potentially activated phenotype related to the constant provision of SCF (both a driver of mast cell differentiation and a stimulus for activation) might together give rise to an expanded and very sensitive intestinal mast cell compartment. Such densely packed and sensitive mast cells might mediate hypersensitivity reactions even in the context of modest IgE levels or antigenic stimuli. In this way the mice might behave similarly to humans with mast cell activation disorders who can sometimes present with frequent and intense allergic reactions56.
The abundant and relatively activated mast cells likely also create an environment conducive to allergic sensitization. Using a mouse model of PN allergy, we have shown that the presence of mast cells and their activation by antigen-specific IgE antibodies are both conducive to the induction of Th2 responses and suppress the emergence of Treg-mediated tolerance. Thus a combination of inherent immune dysregulation, altered microbiota and amplified mast cell responses might render the huNSG mice particularly allergy prone. The high frequencies of skin-resident mast cells in these huNSG mice might also render them susceptible to allergic sensitization through cutaneous or epicutaneous exposure, routes that are effective in standard mouse models and have been considered to be physiologic routes of sensitization for human patients14, 20, 35, 57, 58.
The efficient adaptive immune reconstitution of the huNSG mice described in this report along with their inherent predisposition to allergic sensitization and mast cell-mediated anaphylactic responses render them ideally suited for the study of cellular and molecular interactions in the human food allergy response. The experiments presented here establish that both the processes of sensitization (antigen presentation and T cell activation) and of effector responses (mast cell activation) are mediated exclusively by cells of human origin and without significant contributions by murine APCs or mast cells. The results of blocking studies with omalizumab clearly demonstrate that human IgE mediates the observed responses. We anticipate that this novel system will provide a powerful platform for future efforts to identify key checkpoints in allergic sensitization and tolerance to food allergens as well for testing innovative strategies for manipulating these responses.
Supplementary Material
Acknowledgments
We acknowledge Jessica Harris for technical assistance in performing the tryptase assays. This work was funded by the Bunning Food Allergy Foundation and the NIAID grants number 1R01AI119918-01 and 5T32AI007512-28. Dr. Oliver Burton is currently funded by an NIDDK K01 career development grant, number 1K01DK106303-01.
Except LBS receives royalties from Virginia Commonwealth University that are collected from ThermoFisher for their tryptase assay.
Abbreviations
- NOD
non-obese diabetic
- SCID
severe combined immunodeficiency
- NSG
NOD SCID common gamma chain-deficient
- SCF
stem cell factor
- IL-3
interleukin-3
- HSC
hematopoietic stem cell
- PN
peanut
- CAE
chloroacetate esterase
- LAMP-1
lysosomal-associated membrane protein-1
- Th
T helper
- Treg
regulatory T cell
- Teff
effector T cell
- huNSG
humanized NSG SCF mouse
- MMCP-1
murine mast cell protease-1
- OIT
oral immunotherapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest
REFERENCES
- 1.Burton OT, Oettgen HC. Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol Rev. 2011;242:128–143. doi: 10.1111/j.1600-065X.2011.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oettgen HC, Burton OT. IgE receptor signaling in food allergy pathogenesis. Curr Opin Immunol. 2015;36:109–114. doi: 10.1016/j.coi.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 4.Nimmerjahn F, Ravetch JV. FcgammaRs in health and disease. Curr Top Microbiol Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Kepley CL, Morel PA, Okumoto LM, Fukuoka Y, Schwartz LB. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. J Immunol. 2006;177:694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42:512–523. doi: 10.1016/j.immuni.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, et al. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013;6:740–750. doi: 10.1038/mi.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, et al. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol. 2011;127:795–805. e1–e6. doi: 10.1016/j.jaci.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 10.FDA C. US Department of Health and Human Services. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2005. Guidance for Industry–Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. [Google Scholar]
- 11.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, et al. Immunoglobulin E Signal Inhibition during Allergen Ingestion Leads to Reversal of Established Food Allergy and Induction of Regulatory T Cells. Immunity. 2014;41:141–151. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenzel S, Irani AM, Sanders JM, Bradford TR, Schwartz LB. Immunoassay of tryptase from human mast cells. J Immunol Methods. 1986;86:139–142. doi: 10.1016/0022-1759(86)90277-2. [DOI] [PubMed] [Google Scholar]
- 13.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartnikas LM, Gurish MF, Burton OT, Leisten S, Janssen E, Oettgen HC, et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol. 2013;131:451–460. e1–e6. doi: 10.1016/j.jaci.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kweon MN, Yamamoto M, Kajiki M, Takahashi I, Kiyono H. Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest. 2000;106:199–206. doi: 10.1172/JCI8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009;123:231–238. e4. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–158. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 18.Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, et al. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. J Allergy Clin Immunol. 2013;132:881–888. e1–e11. doi: 10.1016/j.jaci.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito R, Takahashi T, Katano I, Kawai K, Kamisako T, Ogura T, et al. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J Immunol. 2013;191:2890–2899. doi: 10.4049/jimmunol.1203543. [DOI] [PubMed] [Google Scholar]
- 21.Takagi S, Saito Y, Hijikata A, Tanaka S, Watanabe T, Hasegawa T, et al. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood. 2012;119:2768–2777. doi: 10.1182/blood-2011-05-353201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigmann B, Schughart N, Wiebe C, Sudowe S, Lehr HA, Jonuleit H, et al. Allergen-induced IgE-dependent gut inflammation in a human PBMC-engrafted murine model of allergy. J Allergy Clin Immunol. 2012;129:1126–1135. doi: 10.1016/j.jaci.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Kenney L, Bryce P, Falahati R, Bebbington C, Tomasevic N, Shultz L, et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis (HYP4P.302) The Journal of Immunology. 2015;194:123.1. doi: 10.1016/j.jaci.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brehm MA, Racki WJ, Leif J, Burzenski L, Hosur V, Wetmore A, et al. Engraftment of human HSCs in nonirradiated newborn NOD-scid IL2rgamma null mice is enhanced by transgenic expression of membrane-bound human SCF. Blood. 2012;119:2778–2788. doi: 10.1182/blood-2011-05-353243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26:451–463. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Nakajima H, Watanabe N, Kagami S, Suto A, Saito Y, et al. Role of common cytokine receptor gamma chain (gamma(c))- and Jak3-dependent signaling in the proliferation and survival of murine mast cells. Blood. 2000;96:2172–2180. [PubMed] [Google Scholar]
- 27.Helm BA, Sayers I, Higginbottom A, Machado DC, Ling Y, Ahmad K, et al. Identification of the high affinity receptor binding region in human immunoglobulin E. J Biol Chem. 1996;271:7494–7500. doi: 10.1074/jbc.271.13.7494. [DOI] [PubMed] [Google Scholar]
- 28.Conrad DH, Wingard JR, Ishizaka T. The interaction of human and rodent IgE with the human basophil IgE receptor. J Immunol. 1983;130:327–333. [PubMed] [Google Scholar]
- 29.Fung-Leung WP, De Sousa-Hitzler J, Ishaque A, Zhou L, Pang J, Ngo K, et al. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. J Exp Med. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung DY, Sampson HA, Yunginger JW, Burks AW, Jr, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 31.MacGlashan D. Loss of receptors and IgE in vivo during treatment with anti-IgE antibody. J Allergy Clin Immunol. 2004;114:1472–1474. doi: 10.1016/j.jaci.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 32.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton RG, Marcotte GV, Saini SS. Immunological methods for quantifying free and total serum IgE levels in allergy patients receiving omalizumab (Xolair) therapy. J Immunol Methods. 2005;303:81–91. doi: 10.1016/j.jim.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 35.Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 36.Lang J, Kelly M, Freed BM, McCarter MD, Kedl RM, Torres RM, et al. Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. J Immunol. 2013;190:2090–2101. doi: 10.4049/jimmunol.1202810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seung E, Tager AM. Humoral immunity in humanized mice: a work in progress. J Infect Dis. 2013;208(Suppl 2):S155–S159. doi: 10.1093/infdis/jit448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goettel JA, Biswas S, Lexmond WS, Yeste A, Passerini L, Patel B, et al. Fatal autoimmunity in mice reconstituted with human hematopoietic stem cells encoding defective FOXP3. Blood. 2015;125:3886–3895. doi: 10.1182/blood-2014-12-618363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bix M, Raulet D. Inefficient positive selection of T cells directed by haematopoietic cells. Nature. 1992;359:330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 40.Markowitz JS, Auchincloss H, Jr, Grusby MJ, Glimcher LH. Class II-positive hematopoietic cells cannot mediate positive selection of CD4+ T lymphocytes in class II-deficient mice. Proc Natl Acad Sci U S A. 1993;90:2779–2783. doi: 10.1073/pnas.90.7.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–858. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajesh D, Zhou Y, Jankowska-Gan E, Roenneburg DA, Dart ML, Torrealba J, et al. Th1 and Th17 immunocompetence in humanized NOD/SCID/IL2rgammanull mice. Hum Immunol. 2010;71:551–559. doi: 10.1016/j.humimm.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chicha L, Tussiwand R, Traggiai E, Mazzucchelli L, Bronz L, Piffaretti JC, et al. Human adaptive immune system Rag2−/−gamma(c)−/− mice. Ann N Y Acad Sci. 2005;1044:236–243. doi: 10.1196/annals.1349.029. [DOI] [PubMed] [Google Scholar]
- 45.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 47.Calzascia T, Pellegrini M, Lin A, Garza KM, Elford AR, Shahinian A, et al. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci U S A. 2008;105:2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer HJ, Witte AK, Walter L, Grone HJ, van den Brandt J, Reichardt HM. Distinct roles of T-cell lymphopenia and the microbial flora for gastrointestinal and CNS autoimmunity. FASEB J. 2016 doi: 10.1096/fj.15-277384. [DOI] [PubMed] [Google Scholar]
- 49.Milner JD, Ward JM, Keane-Myers A, Paul WE. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc Natl Acad Sci U S A. 2007;104:576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Protonotariou E, Malamitsi-Puchner A, Rizos D, Papagianni B, Moira E, Sarandakou A, et al. Age-related differentiations of Th1/Th2 cytokines in newborn infants. Mediators Inflamm. 2004;13:89–92. doi: 10.1080/09629350410001688468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 52.Madan JC, Farzan SF, Hibberd PL, Karagas MR. Normal neonatal microbiome variation in relation to environmental factors, infection and allergy. Curr Opin Pediatr. 2012;24:753–759. doi: 10.1097/MOP.0b013e32835a1ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A. 2014;111:13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131:201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molloy J, Allen K, Collier F, Tang ML, Ward AC, Vuillermin P. The potential link between gut microbiota and IgE-mediated food allergy in early life. Int J Environ Res Public Health. 2013;10:7235–7256. doi: 10.3390/ijerph10127235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez-Twose I, Zanotti R, Gonzalez-de-Olano D, Bonadonna P, Vega A, Matito A, et al. Nonaggressive systemic mastocytosis (SM) without skin lesions associated with insect-induced anaphylaxis shows unique features versus other indolent SM. J Allergy Clin Immunol. 2014;133:520–528. doi: 10.1016/j.jaci.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 57.Flohr C, Perkin M, Logan K, Marrs T, Radulovic S, Campbell LE, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345–350. doi: 10.1038/jid.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.