Abstract
Background
A multidisciplinary task force, designated Target Zero (TZ), has developed protocols for prevention of surgical site infection (SSI) for spine surgery at our institution. The purpose of this study was to evaluate how compliance with an antibiotic bundle impacts infection incidences in pediatric spine surgery.
Methods
After institutional review board approval, a consecutive series of 511 patients (517 procedures) who underwent primary spine procedures from 2008-2012 were retrospectively reviewed to identify patients who developed SSI. Patients were followed for a minimum of 90 days postoperatively. Compliance data was collected prospectively in 511 consecutive patients and a total of 517 procedures. Three criteria were required for antibiotic bundle compliance: appropriate antibiotics completely administered within one hour prior to incision, antibiotics appropriately re-dosed intraoperatively for blood loss and time, and antibiotics discontinued within 24 hours postoperatively. A multivariable logistic regression analysis was used to test the association between compliance and the development of an infection.
Results
Overall antibiotic bundle compliance rate was 85%. After adjusting for risk category, estimated blood loss, and study year, the likelihood of an infection was increased in the non-compliant group compared to the compliant group [adjusted odds ratio: 3.0, 95% CI: 0.96 to 9.47, p = 0.0587]. When expressed as the Number Needed to Treat (NNT), strict adherence to antibiotic bundle compliance prevented one SSI within 90 days of surgery for every 26 patients treated with the antibiotic bundle. Reasons for non-compliance included failure to infuse preoperative antibiotics one hour prior to incision (10.3%), failure to re-dose antibiotics intraoperatively based on time or blood loss (5.5%), and failure to discontinue antibiotics within 24 hours postoperatively (1.9%).
Conclusions
Compliance with a comprehensive antibiotic protocol can lead to meaningful reductions in surgical site infection (SSI) incidences in pediatric spine surgery. Institutions should focus on improving compliance with prophylactic antibiotic protocols to decrease SSI in pediatric spine surgery.
Level of Evidence
Retrospective cohort study, Level III
Introduction
Surgical site infections (SSI) are commonly reported complications of pediatric spinal deformity surgery. Recent studies document postoperative and delayed-onset SSI rates associated with elective pediatric spinal surgery ranging from 3.7% to 8.5%.[1-6] Patients with adolescent idiopathic scoliosis (AIS) are at lower risk than patients with neuromuscular scoliosis (NMS), with infection rates ranging from 0.1% to 1.3% in AIS and from 8.4% to 17.8% in NMS.[6]
The development of a postoperative SSI causes tremendous burden on the health and well-being of affected children and their families. The management of infections involving instrumented fusions often requires complex treatment strategies.[7, 8] The average economic burden of treating an implant-associated spinal SSI has been reported to be $154,537, ranging from $26,977 to $961,722. Infection related treatment has been reported to add 29 hospital admission days to a patient's care.[9]
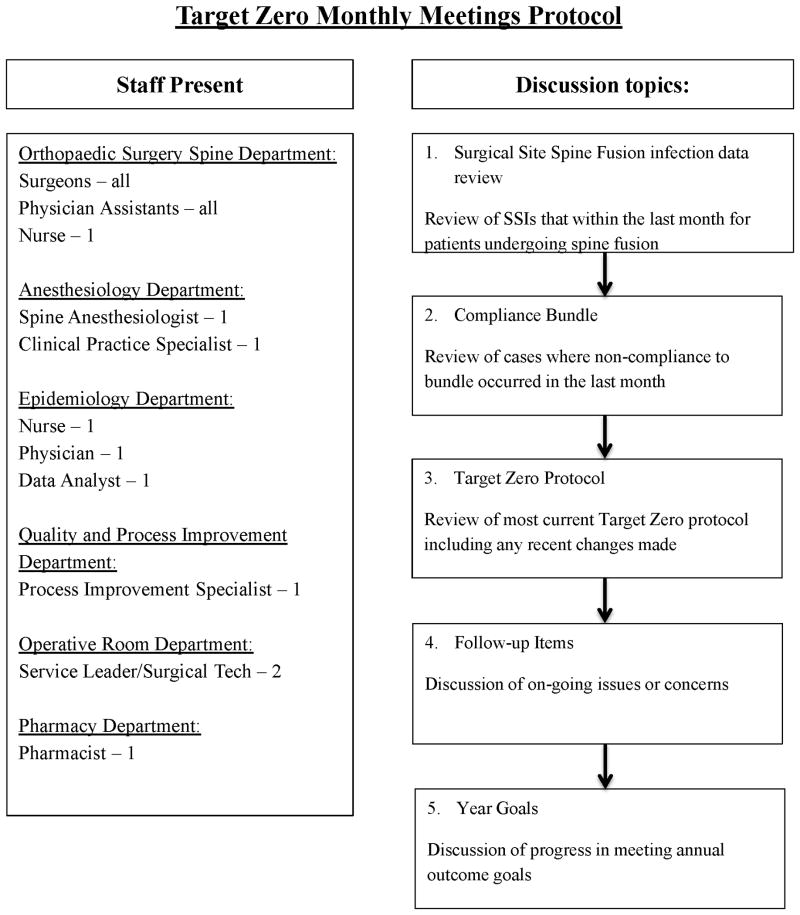
Our institution implemented a multidisciplinary infection task force in July 2007 to address these issues. Designated Target Zero (TZ), this task force consists of representatives from the departments of orthopedic surgery, infectious disease, epidemiology, pharmacy, anesthesiology, quality improvement, and the operating room. Protocols include preoperative, intraoperative, and postoperative initiatives to address potentially modifiable risk factors for SSI.[2, 3, 5, 10-12] Methods of surveillance and formative evaluation have included monthly multidisciplinary meetings to improve compliance (Appendix A).
Previous investigations from our institution have demonstrated that the TZ protocols result in a clinically meaningful reduction in infection rates.[13] The purpose of this study was to evaluate how compliance with the TZ perioperative antibiotic protocol impacted infection rates in both low risk (LR) and high risk (HR) patients undergoing spinal surgery at a tertiary pediatric hospital. We hypothesized that compliance with the antibiotic protocol would lead to reduced SSI in pediatric patients undergoing spine surgery.
Materials and Methods
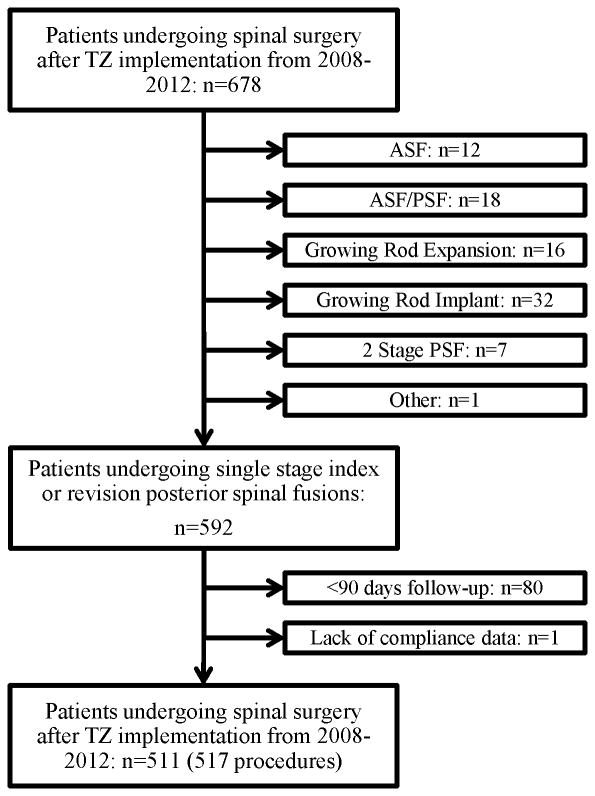
This study was performed with institutional review board approval. A retrospective chart review was used to collect demographic and clinical data on a consecutive series of 678 patients that underwent spine surgery at a single tertiary pediatric hospital between January 1, 2008 and June 30, 2012. Included in the study were patients undergoing index or revision posterior spine fusion (PSF) with a minimum 90 day follow-up. Expansion of non-fusion constructs for early onset scoliosis, implant removals, anterior spinal fusions, growing rod implants, and any other spinal procedures were excluded from the data analysis. 511 patients were included based on the criteria above. Six patients underwent surgery twice within this time frame, making the total procedures 517. One patient was excluded due to lack of available compliance data (Figure 1). A power analysis was not performed prior to data collection.
The following variables were collected and assessed: demographics, diagnosis, risk category (HR or LR), type of surgery, length of fusion, leading surgeon, operative time, incidence and amount of intraoperative and postoperative transfusions, compliance with the institution's infection protocol guidelines, infection within 90 days of surgery, infection type (deep or superficial), time to infection, return to operating room for incision and drainage, infecting organism, antibiotic treatment, and total follow-up from surgery to last clinical visit. The definitions of HR and LR in this study were based on recently published best practice guidelines.[7] LR was defined as a primary fusion in a patient with adolescent idiopathic scoliosis (AIS) without significant comorbidities, while all other patients were classified as HR. The development of a deep SSI was the primary outcome variable of interest.
The Center for Disease Control (CDC)-National Healthcare Safety Network (NHSN)'s definition of a deep SSI was used; which is any infection that appears to be related to surgery, involves tissues and spaces at or beneath the facial layer, and exhibits at least one of the following: purulent drainage from the site, wound dehiscence, or evidence of infection found by direct examination or by histopathologic or radiographic examination.[14-16] All patients were monitored for SSI for 90 days postoperatively based on the most recent CDC definition of a deep surgical site infection.
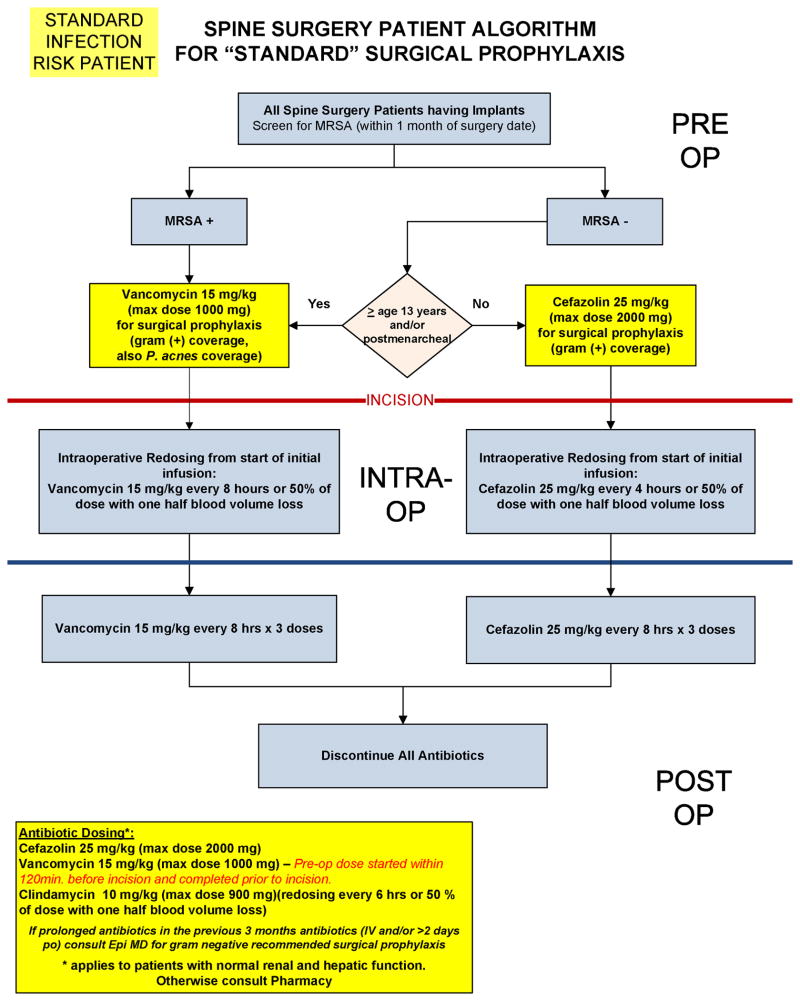
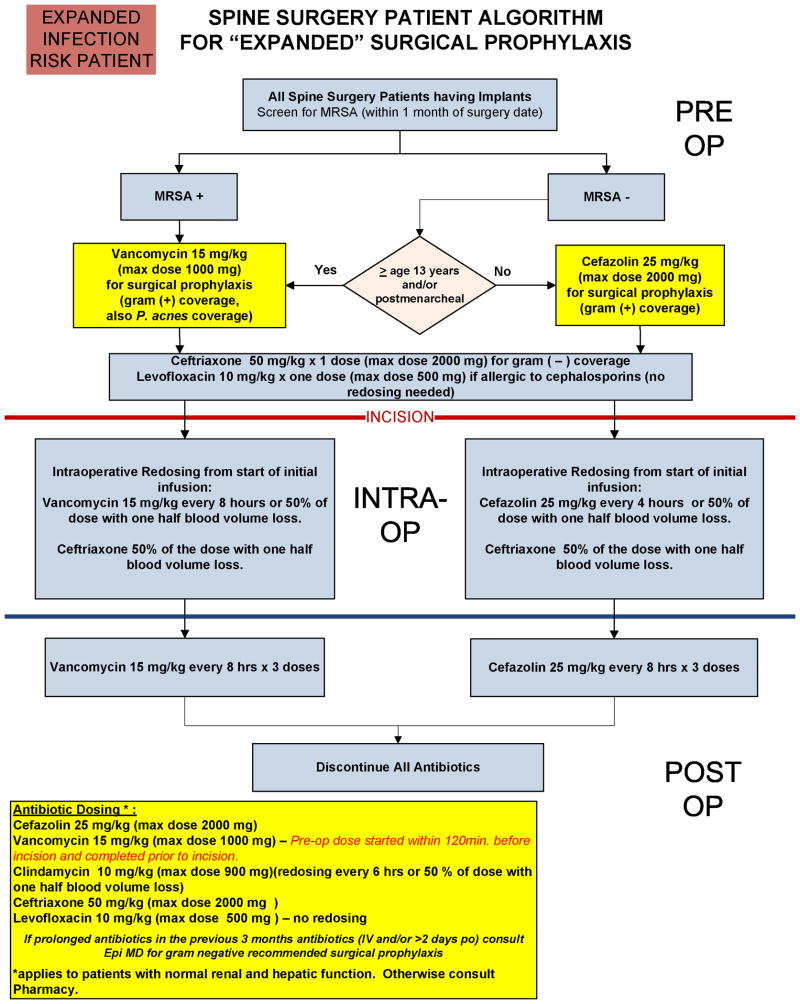
With implementation of TZ, standardized algorithms for surgical antibiotic prophylaxis have been developed for both LR and HR patient groups (Appendices B and C). These algorithms guide practitioners in selecting the appropriate perioperative antibiotics based on patient age, risk category, and presence or absence of methicillin-resistant Staphylococcus aureus on preoperative testing by nasal swab, while also guiding dosing regimens both intraoperatively and postoperatively. Our institution's antibiotic dosing regimens are largely based on American Society of Health-System Pharmacist recommendations.[17]
A perioperative antibiotic bundle was created by a multidisciplinary team of specialists at our institution, and compliance with this TZ antibiotic bundle has been measured through prospective data collection. Successful antibiotic bundle compliance required all three of the following: 1. appropriate IV antibiotics completely administered within one hour prior to incision, 2. IV antibiotics appropriately re-dosed intraoperatively for blood loss and surgical time, and 3. IV antibiotics discontinued within 24 hours postoperatively.
Statistical Methods
Descriptive statistics were used to summarize the demographics and clinical characteristics of all subjects included in the two cohorts. Chi-square, Student's t-test or Wilcoxon rank sum tests, when appropriate, were used to compare the demographics and clinical characteristics in the two groups. Based on this analysis and previous studies, study year, risk status, levels fused, operative time, and estimated blood loss were considered as potential confounding variables. A degree of correlation was noted between the intra-operative variables of levels fused, operative time, and estimated blood loss. From this group of related variables, we elected to include estimated blood loss in subsequent statistical analyses as this variable was clearly related to the compliance protocol (re-dosing after intra-operative transfusion was included in the antibiotic protocol) and is known to increase risk of infections (outcome variable). A multi-variable logistic regression analysis was used to test the null hypothesis of no difference in the odds of an infection in the compliant group compared to the non-compliant group. The final model included study year, risk status, estimated blood loss, and the primary exposure variable of interest, protocol compliance. Based on the logistic regression analysis, adjusted estimates of the absolute risk reduction and number needed to treat were obtained using the methods described by Austin.[18] Bootstrap samples (100 iterations) were used to estimate the 95% confidence intervals for the absolute risk reduction.
Results
Complete data were available for 517 procedures (511 patients) with at least 90 days of follow up. The two groups were similar with respect to surgery year, study quarter, gender, surgical intervention, diagnosis, and BMI. There was a significant difference in the distribution of protocol compliance with respect to study year, levels fused, operative time, and estimated blood loss (Table 1). The overall TZ antibiotic bundle compliance rate was 84.9% (439/517). The occurrence of non-compliance was 10.3% (53/517) for bundle component 1 (failure to infuse preoperative antibiotics one hour prior to incision), 5.5% (23/517) for bundle component 2 (failure to re-dose antibiotics intraoperatively based on time or blood loss), and 1.9% (10/517) for bundle component 3 (failure to discontinue antibiotics within 24 hours postoperatively). Among the non-compliant subjects, 8 were non-compliant with respect to multiple bundle components (Table 2).
Table 1. Demographics and Clinical Characteristics in the Compliant and Non-Compliant Groups.
| Non-Compliant | Compliant | Pvalue | |||
|---|---|---|---|---|---|
| N = 78 | N = 439 | ||||
| Quarter, N (%) | |||||
| Q1 | 12 | 10.7% | 100 | 89.3% | 0.3439 |
| Q2 | 31 | 18.3% | 138 | 81.7% | |
| Q3 | 15 | 13.6% | 95 | 86.4% | |
| Q4 | 20 | 16.0% | 105 | 84.0% | |
| Study Year, N (%) | |||||
| 2008 | 14 | 12.0% | 103 | 88.0% | 0.0219 |
| 2009 | 11 | 9.8% | 101 | 90.2% | |
| 2010 | 18 | 14.9% | 103 | 85.1% | |
| 2011 | 28 | 24.6% | 86 | 75.4% | |
| 2012 | 7 | 13.2% | 46 | 86.8% | |
| Gender, N (%) | |||||
| Female | 53 | 15.0% | 301 | 85.0% | 0.914 |
| Male | 25 | 15.3% | 138 | 84.7% | |
| Surgery, N (%) | |||||
| PSF Only | 76 | 15.5% | 415 | 84.5% | 0.4023 |
| Other | 2 | 7.7% | 24 | 92.3% | |
| Risk, N (%) | |||||
| High | 45 | 16.7% | 225 | 83.3% | 0.2941 |
| Low | 33 | 13.4% | 214 | 86.6% | |
| Diagnosis, N (%) | |||||
| Idiopathic | 33 | 13.4% | 214 | 86.6% | 0.1441 |
| Neuromuscular/Syndromic | 34 | 19.3% | 142 | 80.7% | |
| Other* | 11 | 11.7% | 83 | 88.3% | |
| BMI, Mean (Stdev) | 20.3 | 5.6 | 20.3 | 5.0 | 0.9986 |
| Levels Fused, Mean (Stdev) | 12.2 | 3.8 | 11.3 | 3.4 | 0.0345 |
| Operative Time [minutes], Median (Range) | 248 | 133 to 518 | 234 | 84 to 576 | 0.0096 |
| Estimated Blood Loss [mL], Median (Range) | 1200 | 250 to 9000 | 1000 | 150 | 0.0238 |
Includes congenital deformities, curve progression, implant failure, kyphosis/kyphoscoliosis, pseudoarthrosis, spondylolisthesis/spondylolysis, trauma, and tumor
Table 2. Reason(s) for Antibiotic Bundle Non-Compliance.
| N | % | |
|---|---|---|
| Compliant | 439 | 84.9% |
| Non-Compliant with Bundle Component 1 only* | 45 | 8.7% |
| Non-Compliant with Bundle Component 2 only † | 17 | 3.3% |
| Non-Compliant with Bundle Components 3 only ‡ | 8 | 1.5% |
| Non-Compliant with Bundle Components 1 and 2 | 6 | 1.7% |
| Non-Compliant with Bundle Components 1 and 3 | 1 | 0.2% |
| Non-Compliant with Bundle Components 2 and 3 | 1 | 0.2% |
1 = appropriate IV antibiotics completely administered within one hour prior to incision
2 = IV antibiotics appropriately re-dosed intraoperatively for blood loss and surgical time
3 = IV antibiotics discontinued within 24 hours postoperatively
The cumulative incidence of SSI in the first 90 days after surgery was 3.1% [95% CI: 1.6 to 5%]. To estimate the independent effect of protocol compliance on the likelihood of compliance, we adjusted for estimated blood loss [adjusted odds of infection for every 1 mL increase in blood loss: 1.0, 95% CI: 0.9 to 1.0, p = 0.8247], risk category [odds of infection high risk vs. low risk: 2.8, 95% CI: 0.9 to 9.3, p = 0.0849], and surgical year [odds of infection [odds of infection first study year after implementation of the compliance bundle vs. last full year of study period: 0.6, 95% CI: 0.1 to 3.93, p = 0.2242] in the multi-variable logistic regression analysis. Compliance with the antibiotic bundle was associated with reduced likelihood of developing SSI. The odds of SSI in the non-compliant group were 3.0 times [95% CI: 0.96 to 9.47, p = 0.0587] the odds of infection in the compliant group. We also tested the interaction between compliance and risk category. The odds of infection among compliant versus non-compliant subjects did not significantly depend on risk status [p = 0.2474]. Considered in terms of the absolute risk reduction, the adjusted difference in the incidence of infection between the compliant and non-compliant groups was 3.9% [95% CI: -1.1 to 10.0%]. When expressed as the number needed to treat (NNT), strict adherence to antibiotic bundle compliance prevented one SSI within 90 days of surgery for every 26 patients treated with the antibiotic bundle [NNT (benefit) = 25.6, 95% CI: NNT (harm) 84.4 to ∞ and NNT (benefit) 10 to ∞].
A secondary analysis was used to describe the distribution of organisms on the basis of compliance status. MSSA was the most common organism identified in both the compliant (4/11) and non-compliant groups (4/5). To account for a potential different distribution of organisms among high and low risk patients, the distribution of organism type was stratified by risk status (See Table 3).
Table 3. Distribution of Organisms by Compliance and Risk Status.
| High Risk | Low Risk | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Compliant | Compliant | Non-Compliant | Compliant | |||||
| N | % | N | % | N | % | N | % | |
| MRSA | 0 | 0% | 2 | 22% | 0 | 0% | 0 | 0% |
| MSSA | 2 | 67% | 3 | 33% | 2 | 100% | 1 | 50% |
| Polymicrobial | 1 | 33% | 1 | 11% | 0 | 0% | 0 | 0% |
| Propionibacterium/Actin O Group | 0 | 0% | 1 | 11% | 0 | 0% | 0 | 0% |
| Staphylococcus Aureus | 0 | 0% | 1 | 11% | 0 | 0% | 0 | 0% |
| Staphylococcus Epidermidids | 0 | 0% | 1 | 11% | 0 | 0% | 1 | 50% |
Discussion
Our hypothesis that compliance with the TZ perioperative antibiotic protocol would lead to decreased infection incidences in pediatric spinal surgery was confirmed. It is widely recognized that the risk of SSI can be reduced with the coordinated efforts of a multidisciplinary team.[13, 15, 19] Compliance with an institution's perioperative antibiotic prophylaxis bundle is one modifiable risk factor that can minimize the risk for SSI. Previous reports have detailed the challenges associated with antimicrobial SSI prophylaxis, including selection of the appropriate antibiotic, timely preoperative dosing, intraoperative re-dosing, and postoperative antibiotic administration.[14, 20] A related retrospective study analyzing 5309 pediatric patients in multiple subspecialty areas of pediatric surgery found a significant reduction in SSI from 4.3% to 3.0% when complete compliance with antibiotic prophylaxis was performed, resulting in a 30% decreased risk of SSI.[21] Larger studies have also shown significantly lower SSI rates across multiple surgical specialties with appropriate antibiotic selection and when antibiotic timing was improved.[22-24] Our study is the first, to our knowledge, to specifically assess the relationship between perioperative antibiotic bundle compliance and SSI in pediatric spinal surgery using prospectively collected compliance data.
The NNT in this study was 26, or simply, for every 26 patients who were treated with compliance to the antibiotic bundle, one SSI was prevented. This NNT value can be put into context with reference to published studies utilizing this analysis tool. A NNT of 16 has been found for prevention of early infection after open fracture when antibiotics were used vs. no antibiotics or placebo.[25]
We used an estimate of cumulative incidence to compare effectiveness of the compliance bundle at decreasing infection risk. Cumulative incidence, defined as the number of new cases of disease (infection) over a specific time period (first 90 days post-surgery), was compared between compliant versus non-compliant subjects.
The determination of confounding variables is challenging. It is important to remember that a variable must be related to both the outcome and the exposure, which is compliance status in this study, to be a confounding variable. We felt that it was reasonable to assume that certain demographic and clinical characteristics were not related to compliance. In order for demographic variables to be related to compliance, the providers would have to consciously or unconsciously focus on a particular demographic factor when implementing the compliance bundle. The program was inclusive, and thus we were comfortable with the assumption that all patients were treated equally. Yet, estimates were adjusted for potential confounding variables. Based on the comparison of demographic and clinical characteristics in the compliant versus non-compliant group, we included risk category, surgical year, and estimated blood. It should be noted that levels fused, operative time, and estimated blood loss were all significantly different between groups. In order to keep the model as parsimonious as possible, we only included estimated blood loss from this group of related variables. We chose estimated blood loss based on the fact it is related to the exposure (compliance) and outcome (infection). Increased blood loss is recognized as a risk factor for development of a SSI in pediatric spine surgery.[26] Estimated blood loss is also clearly related to compliance status, as one component of the compliance bundle was re-dosing after blood transfusion. Levels fused and operative time cause blood loss which are likely to lead to non-compliance, and more specifically, failure to re-dose after transfusion. Further, we included estimated blood loss in the logistic regression model, and after adjusting for surgery year, compliance, and risk category, there was no association between blood loss and the likelihood of an infection [p = 0.8247].
The underlying reason for non-compliance in 8.7% out of the 15% total non-compliant subjects was that appropriate IV antibiotics were not completely administered within one hour prior to incision. Table 2 further delineates the reasons for non-compliance. The number of different combinations of compliance factors makes it too challenging to estimate the independent effect of each compliance factor on the overall incidence of SSI in a meaningful way. More importantly, we believe that compliance with all 3 components of compliance bundle is a surrogate variable for higher level of care. Providers that are more likely to be attentive to all aspects of the compliance protocol are also going to more likely to be attentive to other relevant areas of care. In this way, we felt it was best to test compliance as a composite variable.
Constant feedback and multidisciplinary oversight have been shown to be essential in optimizing the TZ protocol and minimizing the risks for SSI.[27] With the creation of these feedback strategies, all non-compliant cases are reviewed at monthly meetings in order to address the error and encourage future compliance (Appendix A). When this study's time frame was broken down into quarters, no significant differences were noted with respect to compliance and study quarter, suggesting that although active efforts to improve compliance over time were made, notable changes were not shown.
There are several limitations to this study. Compliance to the perioperative antibiotic protocol was defined to require all three components listed, including discontinuation of antibiotics within 24 hours postoperatively. While multiple published reports have demonstrated the importance of appropriate antibiotic choice and timing of administration in preventing SSI, the continuation of antibiotics beyond 24 hours postop has not been shown to increase rates of SSI.[28] While discontinuation of antibiotics may not alter infection incidences, adherence to this aspect of the protocol demonstrates an attention to detail that underscores the emphasis placed on compliance to the defined protocol and appropriately managing a patient's infection risk. Since the perioperative antibiotic compliance criteria had been established by the TZ task force prior to development of this study, using this original definition of compliance throughout the prospective data collection was most consistent. Moreover, of the non-compliant patients, only 10% were due to continuation of antibiotics beyond 24 hours postoperatively, and when antibiotics were continued for an appropriate clinical indication such as a remaining chest tube, these instances were not recorded as non-compliant.
Another limitation in the interpretation of our results is that we changed our institutional definition for HR and LR in the beginning of 2013. As mentioned, the new definitions for HR and LR were then retroactively applied to all patients in the study, and many patients were analyzed as HR, despite not being treated at the time of surgery through our Care Pathway for HR patients. HR patients typically participated in the Care Pathway for Spinal Surgery (CAPSS) which was established by our institution as a means for facilitating a multidisciplinary approach to children with significant comorbidities, and therefore underwent significant evaluations preoperatively.[13, 19] Given that the LR group included only AIS patients without significant comorbidities, and the HR group included all others, it should be recognized that the HR group in this study likely represents a population of patients with disparate risk factors for infection, and the infection rate for HR patients with known risk factors for infection may be underreported in this study.
Through a multidisciplinary approach and previously described feedback mechanism, the evolution of TZ during the study period has included such measures as developing standardized order sets in the electronic medical record to comply with the TZ protocol, standardizing preoperative skin preparation, limiting OR traffic, and prohibiting family members from entering the operating suite during anesthesia induction. Without isolating these changing variables it is difficult to know the degree to which the factors impact SSI incidences. Also, comorbidities such as obesity, skin acne, and implant material were not controlled for in this study. No significant differences were found with either compliance rate or SSI between different surgeons, but other potential confounding factors that were not recorded may have been present such as variability in the operating room surgical team or differences in surgical techniques. Additionally, it is difficult to demonstrate a direct causal relationship between adherence to the antibiotic bundle and reduction in SSI since the study was not a randomized controlled design. Moreover, the TZ protocol is constantly evolving in an effort to optimize the reduction in SSI. An illustration of the iterative process that exemplifies the TZ task force came in the summer of 2012, when topical antibiotics placed into the bone graft became part of the standardized intraoperative protocol for all patients undergoing spine surgery. As such, isolating individual variables and their correlation with SSI is challenging. This study, however, attempts to prospectively study the impact of antibiotic compliance on infection rates within the context of balancing the real-time clinical dilemmas of making continual changes that will optimize patient care.
Postoperative infections secondary to spinal surgery are economically burdensome, demand an excessive allocation of resources, and can have significant negative effects on patient outcomes while increasing hospital LOS, patient morbidity, and mortality.[9, 29] Our data demonstrated a significant decreased incidence of SSI when compliance to the TZ perioperative antibiotic protocol was met. Antibiotic compliance is merely one component of an effective infection prevention program, and this study underscores the importance of consistently adhering to all of the individual components of an institution's infection control program. This study shows that compliance with a comprehensive antibiotic protocol can lead to meaningful reductions in surgical site infection (SSI) incidences in pediatric spine surgery. Standardization of preoperative, intraoperative, and postoperative measures with a mechanism for reviewing adherence to these measures and optimizing protocols on a regular basis and in a multidisciplinary fashion is critical in the delivery of predictable, quality, and value-based medical care.
Figure 1. Flow diagram representing patient cohort.
Acknowledgments
The authors thank Elise Benefield, Mary Wintz, Suzanne Evans, and Nicole Michael for their contributions to this study through rigorous and detailed prospective antibiotic compliance data collection.
Source of Funding: There was no external source of funding.
Appendix A
A summary of our intuition's monthly Target Zero meetings for patients undergoing spinal fusion.
Appendix B
Our Institution's perioperative antibiotic clinical care guidelines for spinal fusion procedures in low risk pediatric patients.
Appendix C
Our Institution's perioperative antibiotic clinical care guidelines for spinal fusion procedures in high risk pediatric patients.
Footnotes
Conflicts of Interest: Sumeet Garg has been paid a one-time consultant fee by Medtronic for teaching a course for surgeons in October 2014, a one-time consultant fee by DePuy Synthes Spine for teaching a course for surgeons in November 2013, payment for testifying in trial for a patient, and receives royalties from Decision Support in Medicine. Dr. Erickson is on the Board of Directors for POSNA and received payment from Biomet for lectures. Patrick Carry received a grant towards his institution from NIH/NCATS Colorado CTSA Grant Number UL1 TR001082. Curt Vandenberg, Cameron Niswander, Nikki Bloch and Zhaoxing Pan have no conflicts of interest.
References
- 1.Cahill PJ, et al. Infection after spinal fusion for pediatric spinal deformity: thirty years of experience at a single institution. Spine (Phila Pa 1976) 2010;35(12):1211–7. doi: 10.1097/BRS.0b013e3181c212d1. [DOI] [PubMed] [Google Scholar]
- 2.Ho C, et al. Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine (Phila Pa 1976) 2007;32(24):2739–44. doi: 10.1097/BRS.0b013e31815a5a86. [DOI] [PubMed] [Google Scholar]
- 3.Ho C, Sucato DJ, Richards BS. Risk factors for the development of delayed infections following posterior spinal fusion and instrumentation in adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976) 2007;32(20):2272–7. doi: 10.1097/BRS.0b013e31814b1c0b. [DOI] [PubMed] [Google Scholar]
- 4.Labbe AC, et al. Surgical-site infection following spinal fusion: a case-control study in a children's hospital. Infect Control Hosp Epidemiol. 2003;24(8):591–5. doi: 10.1086/502259. [DOI] [PubMed] [Google Scholar]
- 5.Linam WM, et al. Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect Control Hosp Epidemiol. 2009;30(2):109–16. doi: 10.1086/593952. [DOI] [PubMed] [Google Scholar]
- 6.Mackenzie WG, et al. Surgical site infection following spinal instrumentation for scoliosis: a multicenter analysis of rates, risk factors, and pathogens. J Bone Joint Surg Am. 2013;95(9):800–6. S1–2. doi: 10.2106/JBJS.L.00010. [DOI] [PubMed] [Google Scholar]
- 7.Glotzbecker MP, et al. What's the evidence? Systematic literature review of risk factors and preventive strategies for surgical site infection following pediatric spine surgery. J Pediatr Orthop. 2013;33(5):479–87. doi: 10.1097/BPO.0b013e318285c507. [DOI] [PubMed] [Google Scholar]
- 8.Glassman SD, et al. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine (Phila Pa 1976) 1996;21(18):2163–9. doi: 10.1097/00007632-199609150-00021. [DOI] [PubMed] [Google Scholar]
- 9.Hedequist D, et al. Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine (Phila Pa 1976) 2009;34(1):60–4. doi: 10.1097/BRS.0b013e31818ed75e. [DOI] [PubMed] [Google Scholar]
- 10.Milstone AM, et al. Timing of preoperative antibiotic prophylaxis: a modifiable risk factor for deep surgical site infections after pediatric spinal fusion. Pediatr Infect Dis J. 2008;27(8):704–8. doi: 10.1097/INF.0b013e31816fca72. [DOI] [PubMed] [Google Scholar]
- 11.Murphy NA, et al. Spinal surgery in children with idiopathic and neuromuscular scoliosis. What's the difference? J Pediatr Orthop. 2006;26(2):216–20. doi: 10.1097/01.bpo.0000206516.61706.6e. [DOI] [PubMed] [Google Scholar]
- 12.Vitale MG, et al. Building consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high-risk pediatric spine surgery. J Pediatr Orthop. 2013;33(5):471–8. doi: 10.1097/BPO.0b013e3182840de2. [DOI] [PubMed] [Google Scholar]
- 13.Ballard MR, et al. A multidisciplinary approach improves infection rates in pediatric spine surgery. J Pediatr Orthop. 2012;32(3):266–70. doi: 10.1097/BPO.0b013e31824b29c1. [DOI] [PubMed] [Google Scholar]
- 14.Goede WJ, et al. Assessment of prophylactic antibiotic use in patients with surgical site infections. Hosp Pharm. 2013;48(7):560–7. doi: 10.1310/hpj4807-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tillman M, et al. Surgical care improvement project and surgical site infections: can integration in the surgical safety checklist improve quality performance and clinical outcomes? J Surg Res. 2013;184(1):150–6. doi: 10.1016/j.jss.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Surgical Site Infection (SSI) Event. National Healthcare Safety Network, Editor 2016. Atlanta, GA: Available at http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf. [Google Scholar]
- 17.Bratzler DW, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J Clin Epidemiol. 2010;63(1):2–6. doi: 10.1016/j.jclinepi.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Alexander JW, Solomkin JS, Edwards MJ. Updated recommendations for control of surgical site infections. Ann Surg. 2011;253(6):1082–93. doi: 10.1097/SLA.0b013e31821175f8. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins RB, et al. Beyond surgical care improvement program compliance: antibiotic prophylaxis implementation gaps. Am J Surg. 2013;206(4):451–6. doi: 10.1016/j.amjsurg.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Khoshbin A, et al. Antibiotic Prophylaxis to Prevent Surgical Site Infections in Children: A Prospective Cohort Study. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000938. [DOI] [PubMed] [Google Scholar]
- 22.Cataife G, et al. The effect of Surgical Care Improvement Project (SCIP) compliance on surgical site infections (SSI) Med Care. 2014;52(2 Suppl 1):S66–73. doi: 10.1097/MLR.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 23.Leaper DJ, et al. Surgical site infection: poor compliance with guidelines and care bundles. Int Wound J. 2014 doi: 10.1111/iwj.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Slegt J, et al. Implementation of a bundle of care to reduce surgical site infections in patients undergoing vascular surgery. PLoS One. 2013;8(8):e71566. doi: 10.1371/journal.pone.0071566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosselin RA, Roberts I, Gillespie WJ. Antibiotics for preventing infection in open limb fractures. Cochrane Database Syst Rev. 2004;(1):CD003764. doi: 10.1002/14651858.CD003764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croft LD, et al. Risk factors for surgical site infections after pediatric spine operations. Spine (Phila Pa 1976) 2015;40(2):E112–9. doi: 10.1097/BRS.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 27.Winston KR, Dolan SA. Multidisciplinary approach to cerebrospinal fluid shunt infection with an appeal for attention to details in assessment and standardization in reporting. J Neurosurg Pediatr. 2011;7(5):452–61. doi: 10.3171/2011.2.PEDS10304. [DOI] [PubMed] [Google Scholar]
- 28.Subramanyam R, et al. Systematic review of risk factors for surgical site infection in pediatric scoliosis surgery. Spine J. 2015 doi: 10.1016/j.spinee.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Kirkland KB, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20(11):725–30. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]