Abstract
Objective
To develop an observed-to-expected ratio (O/E) for adherence to National Comprehensive Cancer Network (NCCN) ovarian cancer treatment guidelines as a risk-adjusted hospital measure of quality care correlated with disease-specific survival.
Methods
Consecutive patients with stages I–IV epithelial ovarian cancer were identified from the California Cancer Registry (1/1/96–12/31/06). Using a fit logistic regression model, O/E for guideline adherence was calculated for each hospital and distributed into quartiles stratified by hospital annual case volume: lowest O/E quartile or annual hospital case volume <5, middle two O/E quartiles and volume ≥5, and highest O/E quartile and volume ≥5. A multivariable logistic regression model was used to characterize the independent effect of hospital O/E on ovarian cancer-specific survival.
Results
Overall, 18,491 patients were treated at 405 hospitals; 37.3% received guideline adherent care. Lowest O/E hospitals (n = 285) treated 4661 patients (25.2%), mean O/E = 0.77 ± 0.55 and median survival 38.9 months (95%CI = 36.2–42.0 months). Intermediate O/E hospitals (n = 85) treated 8715 patients (47.1%), mean O/E = 0.87 ± 0.17 and median survival of 50.5 months (95% CI = 48.4–52.8 months). Highest O/E hospitals (n = 35) treated 5115 patients (27.7%), mean O/E = 1.34 ± 0.14 and median survival of 53.8 months (95% CI = 50.2–58.2 months). After controlling for other variables, treatment at highest O/E hospitals was associated with independent and statistically significant improvement in ovarian cancer-specific survival compared to intermediate O/E (HR = 1.06, 95% CI = 1.01–1.11) and lowest O/E (1.16, 95% CI = 1.10–1.23) hospitals.
Conclusions
Calculation of hospital-specific O/E for NCCN treatment guideline adherence, combined with minimum case volume criterion, as a measure of ovarian cancer quality of care is feasible and is an independent predictor of survival.
Keywords: Humans, Ovarian cancer, Hospitals, Risk assessment, Quality measurement
1. Introduction
Ovarian cancer is the leading cause of gynecologic cancer related mortality in the United States; therefore, improving the quality of epithelial ovarian cancer care remains of the utmost importance. In 2014 nearly 22,000 new cases were diagnosed with over 14,000 disease related deaths [1]. The National Comprehensive Cancer Network (NCCN) guidelines for treatment of epithelial ovarian cancer established by an expert panel are the standard for quality cancer care [2]. Population based studies from both national and institutional databases show improved survival in ovarian cancer patients who received care adherent to NCCN guidelines [3–4]. Furthermore, recent studies have shown that treatment by low-volume hospitals and low-volume physicians is associated with decreased disease-specific survival after adjusting for NCCN adherent care, suggesting that centralization of ovarian cancer care may be associated with optimized patient outcomes [3,5–13]. Additional studies have examined the effects of institutional and provider surgical volumes and have concluded that patients treated at high volume referral centers receive more comprehensive surgical care [4,14–18].
One long-standing paradigm for evaluating the quality of care and health services is the Donabedian model [19]. This model uses three categories—structure, process and outcomes in its assessment [19–20]. The Donabedian model’s linear framework is structured as a sequential progression from structure to process to outcome and fails to incorporate key characteristics such as patient population and environmental factors limiting the correlation between quality measures and survival [21–22]. Unlike the Donabedian paradigm, the National Surgical Quality Improvement Program (NSQIP) model compares risk-adjusted surgical outcomes to national averages as a measure of quality of care. NSQIP is an established outcomes-based assessment for the quality of surgical treatment and can be used to improve the quality of comprehensive ovarian cancer care [23].
Focusing on process measures such as increased adherence to NCCN guidelines and centralization of care may improve ovarian cancer treatment. Although there are several studies favoring the centralization of care for epithelial ovarian cancer patients, these studies have used survival, surgical, and hospital volume as isolated measures for quality of care. Outcomes-based measures take a long time to acquire, are expensive to gather, and are not representative of granularity of quality care. The current report represents a pilot study to evaluate the feasibility of developing an observed-to-expected ratio (O/E) for adherence to National Comprehensive Cancer Network (NCCN) treatment guidelines as a risk-adjusted hospital measure of ovarian cancer quality care correlated with disease-specific survival.
2. Methods
The study design was a retrospective population-based case-only study of invasive epithelial ovarian cancer reported to California Cancer Registry (CCR) and received exempt status by the Institutional Review Board of the University of California, Irvine (HS#2011–8317). The CCR is California’s statewide population-based cancer surveillance system that has collected information about tumor characteristics, patient characteristics, tumor diagnosis, and treatment for all cancers diagnosed in California since 1988. Standardized data collection and quality control procedures have been in place since that time [24–25]. Case reporting is estimated to be 99% for the entire state of California, with follow-up completion rates exceeding 95% [26]. International Classification of Disease Codes for Oncology (ICD-O) based on the World Health Organization’s criteria was used for tumor location and histology. Cases were identified using ovarian Surveillance, Epidemiology, and End Results (SEER) primary site code (C569).
The study population included women who were older than 18 and diagnosed with first or only invasive epithelial ovarian cancer. 20,943 incident ovarian cancer cases were identified during the time period from January 1996 to December 2006 in CCR with follow-up through January 2008. After sequentially excluding 165 non-epithelial histological sub-types, 246 cases had missing ICD-O-2 morphology code, 742 cases were identified from autopsy or death certificate only, and 1299 have incomplete clinical information. A total of 18,491 patients who were treated in 405 California hospitals ended up in our final study population.
A multivariate logistic model was used to identify independent predictors of adherence to NCCN treatment guidelines, which is considered a measure of the quality of cancer care and the therapeutic standard that the majority of ovarian cancer patients should be provided. Adherence to treatment guidelines for ovarian cancer was based on NCCN recommendations for surgery and chemotherapy according to the time period of diagnosis (1997–2005) [27–31]. For stages I–IIIB, surgical treatment was considered adherent to NCCN guidelines if it included a minimum of oophorectomy (± hysterectomy), pelvic and/or para-aortic lymph node biopsy, and omentectomy. A minimum of oophorectomy (± hysterectomy) and omentectomy was considered adherent surgical care for stages IIIC–IV disease. For cases of stages IA–IB, grade 1–2 disease, no adjuvant treatment was considered adherent to NCCN guidelines. Administration of multi-agent chemotherapy was considered adherent for cases of Stages IC–IV or grade 3 disease. Surgery must have preceded chemotherapy for Stages I–IIIB to be considered adherent to NCCN guidelines, while for stages IIIC–IV either initial surgery or chemotherapy was characterized as adherent care.
Explanatory variables included patient and tumor characteristics: patient’s age at diagnosis, race/ethnicity (White, Black, Hispanic, and Asian/Pacific Islander), insurance type (Managed care, Medicaid, Medicare, or Other insurance), socioeconomic status (SES) quintile of Yost score (lowest SES, low-middle SES, middle SES, high-middle SES and highest SES) [32], tumor stage, grade, histology and size. Univariate analyses were conducted to examine the relation between each predictor variable and overall treatment adherence using Chi square test for categorical variable and t-test for continuous variable. Model selection was based on our clinical knowledge and univariate analyses. Predictors in final logistic model included patient’s age at diagnosis (continuous variable), tumor stage (I, II, III or IV), grade (I, II, III, IV or unknown), histology (Serous, Mucinous, Endometrioid, Clear cell, or others) and size (≤5 cm, 5–10 cm, >10 cm or unknown). The c-statistic was used to measure the predictive accuracy of the model and Hosmer–Lemeshow test was used to test the calibration of the model.
The final multivariate logistic model was used to estimate the probability of adherence to NCCN overall treatment plan for each patient. The expected adherence rate for each hospital was calculated by summing up the probabilities of adherence for all patients that were treated in that hospital. Observed adherence for each hospital was calculated as the number of patients that received adherent care in the hospital. The ratio of observed to expected adherence (O/E) was calculated for each hospital. An O/E ratio >1.0 indicated that the hospital was more adherent to the guidelines than would be expected according to its patient mix. An O/E ratio <1.0 indicated that the hospital was less adherent to the guidelines than would be expected based on its patient mix. The hospital O/E ratio was then classified into three categories based on O/E quartiles: lowest quartile, middle two quartiles and highest quartile. Hospitals with very low case volume tend to have too few cases to calculate stable O/E ratio; sensitivity analysis showed hospitals with 5 or more cases had reasonable standard deviation of O/E ratio. Thus, we grouped O/E ratio into three categories: lowest quartile of O/E or annual case volume <5, middle two quartiles of O/E and annual case volume ≥5, highest quartile of O/E and annual case volume ≥5.
Cause of death was recorded according to International Classification of Disease (ICD) criteria in effect at the time of death [33]. Ovarian cancer-specific mortality was defined as death caused by ovarian cancer alone. Patients that died from other causes were treated as censored events at the time of the event.
Descriptive statistics for demographic, clinical and treatment characteristics were analyzed with χ2 test or Fisher’s Exact test for categorical variables. Survival analysis was performed using the Kaplan–Meier estimate of survival probability and log rank tests. After verifying the proportionality assumption, a Cox-proportional hazards model was fitted to evaluate the independent effect on survival of each predictor. Possible interaction terms of main effects were tested by comparing nested models that included the interaction term to a model with no interaction term. Adjusted hazard ratios and 95% confidence intervals (CI) were generated. All statistical analyses were performed on SAS 9.2.
3. Results
A total of 18,491 patients with complete clinical and pathologic information were identified from 405 hospitals. The majority of patients were White (69.8%), while 15.7% were Hispanic, 10.1% were Asian/Pacific Islander, and 4.4% were Black. Managed care and Medicare were the most common insurance payers, and 69.6% of patients had Stage III/IV disease. Serous histology was found in 40.6% (n = 7504). Combined, 47.5% of patients were higher-middle to highest socioeconomic status (SES), while only 12.5% were classified as lowest SES. Overall, 37.3% of patients received NCCN guideline adherent care (combined surgical and chemotherapeutic treatment), 52.8% of patients underwent guideline adherent surgical management, and 62.7% received guideline adherent chemotherapy. The demographic and clinical characteristics of the patient study population in each O/E quartile are shown in Table 1.
Table 1.
Study population characteristics in each O/E* category.
| Characteristic | Low O/E or volume <5 cases/year n = 4661 n (%) |
Middle O/E and volume ≥5 cases/year n = 8715 n (%) |
High O/E and volume ≥5 cases/year n = 5115 n (%) |
|||
|---|---|---|---|---|---|---|
| Age at diagnosis (Mean ± Standard deviation) | 62.6 ± 15.6 | 61.0 ± 15.0 | 59.9 ± 14.4 | |||
| Race | ||||||
| White | 3058 | (65.6) | 6016 | (69.0) | 3828 | (74.8) |
| African American | 260 | (5.6) | 367 | (4.2) | 191 | (3.7) |
| Hispanic | 891 | (19.1) | 1458 | (16.7) | 560 | (10.9) |
| Asian/Pacific Islander | 452 | (9.7) | 874 | (10.0) | 536 | (10.5) |
| Insurance | ||||||
| Managed Care | 1716 | (36.8) | 4262 | (48.9) | 2744 | (53.6) |
| Medicare | 1642 | (35.2) | 2450 | (28.1) | 1122 | (21.9) |
| Medicaid | 542 | (11.6) | 535 | (6.1) | 394 | (7.7) |
| Other ins | 761 | (16.3) | 1468 | (16.8) | 855 | (16.7) |
| SES | ||||||
| Lowest SES | 921 | (19.8) | 1085 | (12.4) | 370 | (7.2) |
| Lower-middle SES | 1058 | (22.7) | 1619 | (18.6) | 701 | (13.7) |
| Middle SES | 1069 | (22.9) | 1821 | (20.9) | 1059 | (20.7) |
| Higher-middle SES | 945 | (20.3) | 1970 | (22.6) | 1380 | (27.0) |
| Highest SES | 668 | (14.3) | 2220 | (25.5) | 1605 | (31.4) |
| Stage | ||||||
| I | 960 | (20.6) | 2010 | (23.1) | 1113 | (21.8) |
| II | 395 | (8.5) | 726 | (8.3) | 422 | (8.3) |
| III | 1933 | (41.5) | 3820 | (43.8) | 2471 | (48.3) |
| IV | 1373 | (29.5) | 2159 | (24.8) | 1109 | (21.7) |
| Grade | ||||||
| I | 337 | (7.2) | 723 | (8.3) | 408 | (8.0) |
| II | 774 | (16.6) | 1564 | (17.9) | 866 | (16.9) |
| III | 1529 | (32.8) | 3278 | (37.6) | 1923 | (37.6) |
| IV | 365 | (7.8) | 794 | (9.1) | 571 | (11.2) |
| Unknown | 1656 | (35.5) | 2356 | (27.0) | 1347 | (26.3) |
| Histology | ||||||
| Serous | 1718 | (36.9) | 3580 | (41.1) | 2206 | (43.1) |
| Adenocarcinoma,NOSa | 347 | (7.4) | 617 | (7.1) | 357 | (7.0) |
| Clear cell | 501 | (10.7) | 1026 | (11.8) | 565 | (11.0) |
| Endometrioid | 160 | (3.4) | 536 | (6.2) | 302 | (5.9) |
| Mucinous | 769 | (16.5) | 1039 | (11.9) | 558 | (10.9) |
| Other | 1166 | (25.0) | 1917 | (22.0) | 1127 | (22.0) |
| Tumor size | ||||||
| <5 cm | 546 | (11.7) | 1101 | (12.6) | 586 | (11.5) |
| 5–10 cm | 757 | (16.2) | 1743 | (20.0) | 968 | (18.9) |
| >10 cm | 877 | (18.8) | 2114 | (24.3) | 1208 | (23.6) |
| Unknown | 2481 | (53.2) | 3757 | (43.1) | 2353 | (46.0) |
| Surgery adherence | ||||||
| Yes | 2033 | (43.6) | 4413 | (50.6) | 3316 | (64.3) |
| Chemotherapy adherence | ||||||
| Yes | 2440 | (52.4) | 5351 | (61.4) | 3804 | (74.4) |
| Overall treatment adherence | ||||||
| Yes | 1278 | (27.4) | 2914 | (33.4) | 2703 | (52.8) |
Observed-to-expected ratio.
Not Otherwise Specified (NOS).
When stratified by distribution of hospital annual case volume, 148 facilities performed less than 2 cases per year (SD = 0.67), 134 performed 2–5 cases per year (SD = 0.38), and 123 hospitals performed more than 5 cases annually (SD = 0.27–0.29) (Table 2). Notably, the standard deviation of the O/E ratio for low volume hospitals (<5 cases annually) was higher while a stability of variance for both O/E and standard deviation was noted in high volume hospitals (>5 cases) leading to the decision to combine the lower categories and use 5 as the minimum volume requirement.
Table 2.
Statistics and O/E* by hospital case volume.
| Hospital annual case volume | n | % | Mean O/E | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|---|---|
| ≤2 cases per year | 148 | 36.54 | 0.73 | 0.67 | 0.00 | 2.97 |
| 2.001–5 cases per year | 134 | 33.09 | 0.84 | 0.38 | 0.00 | 2.44 |
| 5.001–9 cases per year | 54 | 13.33 | 0.93 | 0.29 | 0.33 | 1.65 |
| 9.001–19.999 cases per | 57 | 14.07 | 1.00 | 0.27 | 0.38 | 1.55 |
| ≥20 cases per year | 12 | 2.96 | 1.22 | 0.27 | 0.80 | 1.77 |
Observed-to-expected ratio.
The 405 California hospitals that treated ovarian cancer patients were grouped into low O/E adherence (<5 cases per year and O/E ratio <1.0), intermediate O/E (≥5 cases per year and O/E ratio <1.0) and high O/E (≥5 cases per year and O/E ratio >1.0). Over 70% of the hospitals were classified into the low O/E category (n = 285); 4661 (25.2%) patients were treated at these facilities. The mean O/E for this group was 0.77 ± 0.55 with median survival of 38.9 months (95% CI = 36.2–42.0 months). Eighty five hospitals met criteria for intermediate O/E treating 47.1% (n = 8, 715) patients with a mean O/E = 0.87 ± 0.17 and a median survival of 50.5 months (95% CI = 48.4–52.8 months). Hospitals with the highest O/E (n = 35) treated 5115 patients (27.7%) with a mean O/E = 1.34 ± 0.14 and were also found to have the highest median survival at median survival at 53.8 months (95% CI = 50.2–58.2 months) (Table 3). Improved survival probability is seen with middle to high O/E and a minimum hospital case volume of 5 (Fig. 1).
Table 3.
Hospital, volume and O/E* distribution in each O/E quartile.
| O/E Quartile | # of Hospitals | # of Cases | % of Cases | O/E (mean ± standard deviation) | Median survival (months) and 95% C.I. |
|---|---|---|---|---|---|
| Lowest O/E and hospital volume <5 cases/year | 285 | 4661 | 25.2 | 0.77 ± 0.55 | 38.9 (36.2–42.0) |
| Middle O/E and hospital volume ≥ 5 cases/year | 85 | 8715 | 47.1 | 0.87 ± 0.17 | 50.5 (48.4–52.8) |
| Highest O/E and hospital volume ≥ 5 cases/year | 35 | 5115 | 27.7 | 1.34 ± 0.14 | 53.8 (50.2–58.2) |
Observed-to-expected ratio.
Fig. 1.
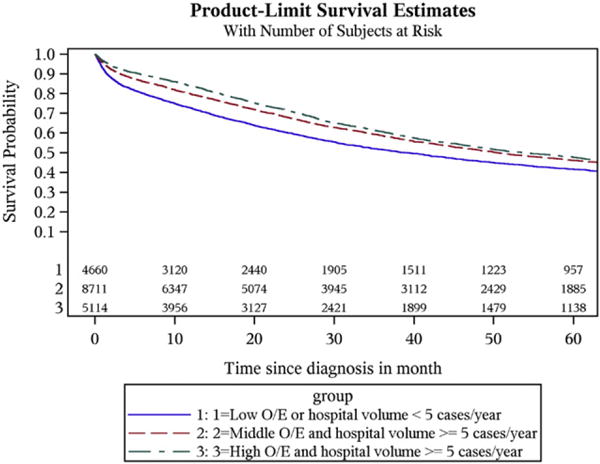
Survival graph by O/E* category. Improved survival probability is noted when a minimum hospital case volume of 5 is combined with middle to high O/E. * O/E: observed-to-expected ratio.
In the multivariable survival model, advancing age, increasing stage, higher tumor grade, and atypical histological subtype were associated with worse survival. After controlling for age and disease-related characteristics, care at a high O/E hospitals (HR = 1.00) was associated with a statistically significant and independent improvement in ovarian cancer-specific survival compared to intermediate O/E hospitals (HR = 1.06, 95% CI = 1.01–1.11) and low O/E hospitals (HR = 1.16, 95% CI = 1.10–1.23) (Table 4).
Table 4.
Multivariable survival model.
| Characteristic | Hazard ratio | 95% Confidence interval |
|---|---|---|
| Age | 1.02 | 1.02–1.03 |
| Year of diagnosis | 1.00 | 0.99–1.01 |
| Stage of Disease | ||
| I | 1.00 | |
| II | 2.62 | 2.29–2.99 |
| III | 6.36 | 5.74–7.05 |
| IV | 9.94 | 9.93–11.05 |
| Tumor Grade | ||
| 1 | 1.00 | |
| 2 | 2.62 | 1.27–1.67 |
| 3 | 1.46 | 1.42–1.85 |
| 4 | 1.67 | 1.45–1.94 |
| Unknown | 2.15 | 1.88–2.47 |
| Histology | ||
| Serous | 1.00 | |
| Endometrioid | 0.85 | 0.78–0.94 |
| Mucinous | 1.48 | 1.33–1.65 |
| Clear Cell | 1.35 | 1.20–1.52 |
| Adenocarcinoma NOSb | 1.52 | 1.43–1.62 |
| Other | 1.31 | 1.24–1.39 |
| Tumor Size | ||
| <5 cm | 1.00 | |
| 5–10 cm | 0.99 | 0.91–1.07 |
| >10 cm | 0.93 | 0.86–1.01 |
| Unknown | 1.14 | 1.06–1.22 |
| Hospital Adherence Metric (O/Ea + volume) | ||
| High O/E | 1.00 | |
| Intermediate O/E | 1.06 | 1.01–1.11 |
| Low E/E | 1.16 | 1.10–1.23 |
Observed-to-expected ratio.
NOS: not otherwise specified.
4. Discussion
The purpose of this study was to develop a risk-adjusted hospital measure of quality care correlated with disease specific survival. We demonstrate that the use of NCCN guideline adherence combined with a minimum case volume criterion as a measure of ovarian cancer quality of care is feasible. While there is a continued trend for research in the field of ovarian cancer care quality improvement, previous research has limited applicability to large scale implementation [34–38].
Verleye et al. reported that ovarian cancer process measures include performance of comprehensive staging, maximal surgical cytoreduction, and administration of recommended chemotherapy [39]. A study by Bristow and colleagues showed a direct correlation between NCCN adherent ovarian cancer care and improved survival [3]. Additional findings of this study revealed that both low-volume hospitals and low-volume physicians were associated with decreased disease-specific survival after adjusting for NCCN adherent care. These results have been demonstrated in several population-based studies that support superior ovarian cancer care from high-volume surgeons and high-volume centers [5–15].
While there is extensive data to support administration of care at high-volume centers, a recent study by Phippen et al. demonstrated that NCCN adherent quality cancer care can be administered at low volume hospitals. The results of this retrospective study showed 73% of patients achieved optimal cytoreduction and 85.4% of the 48 patients included in the analysis received NCCN adherent care. These findings are similar to those reported nationally and argue against centralization of care based on volume alone [40].
Our study findings also support the rationale for moving beyond the crude structural measure of annual case volume as the main criterion for identifying high performing centers, as evidenced by the large standard deviation among low-volume hospitals due to the high O/E ratio of some of those institutions. Furthermore, data from the National Cancer Data Base suggests that the majority of cancer surgery is being performed by low-volume institutions [17]. Strengths of the current study include a large study population, the proven reliability of the California Cancer Registry, and incorporation of NCCN ovarian cancer treatment guidelines—a validated quality process measure.
Importantly, there are several limitations that must be considered when interpreting the current data. First, this was a retrospective study design using a population based data set that is subject to reporting and selection bias. Second, we were unable to control for variables such as physician and surgeon specialty, provider volume, medical comorbidities, extent of residual disease, and participation in clinical trials. These unreported variables could influence both administration of NCCN recommended standard of care for ovarian cancer and survival outcome. The current model is imperfect, as evidenced by the narrow discrimination between intermediate and high O/E hospitals.
Future studies should adjust for variation attributable to differences in medical comorbidities, amount of residual tumor, and route of chemotherapy administration to further develop our risk-adjusted model. Once fully developed, implementation of this quality of care measure would identify highest O/E hospitals and emphasize centralization of care based on O/E score. One way to encourage patients to seek care at high O/E hospitals would be for insurance payers to support payments to high O/E hospitals and limit payment to low O/E hospitals. Ultimately, this would also encourage physicians to provide NCCN adherent care.
Despite the above limitations, the current study suggests that treatment at a high O/E institution was associated with an independent and statistically significant improvement in ovarian cancer-specific survival compared to intermediate and low O/E hospitals. Perhaps more importantly, these data demonstrate that development of a quality metric that combines a minimum case volume requirement with a validated quality process measure is feasible and is an independent predictor of survival and facilitates standardized evaluation of hospitals irrespective of volume. Moving beyond isolated structural measures will lead to more comprehensive assessment of quality ovarian cancer treatment. Finally, the current analysis supports previous findings that only one in three women with ovarian cancer in California received NCCN adherent care further emphasizing the need for quality improvement and centralizing care to high performing facilities and providers [3].
HIGHLIGHTS.
Adherence to NCCN guidelines is correlated with improved survival and is a useful measure of quality cancer care.
Treatment at high O/E hospitals is associated with significant improvement in ovarian cancer-specific survival.
Development of a quality metric combining case volume with a quality process measure is feasible and predicts survival.
Acknowledgments
Dr. Bristow was supported in part by an unrestricted research grant from the Queen of Hearts Foundation and supported by National Institutes of Health T-32 training grant (Ruth L. Kirschstein National Research Service Award Institutional Training Research Grant, 2 T32 CA-060396-11). Funding sources had no involvement with the study design, data collection, analysis and interpretation of data, and in the writing of this manuscript or decision to submit this paper for publication.
Footnotes
The authors report no conflicts of interest or disclosures. Presented at the Society of Gynecologic Oncology 46th annual meeting on Women’s Cancer, Chicago, IL, March 27–31, 2015.
The authors declare that there are no conflicts of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Morgan RJ, Jr, Alvarez RD, Armstrong DK, Burger RA, Chen LM, et al. Ovarian cancer, version 2.2013. J Natl Compr Cancer Netw. 2013 Oct;11(10):1199–1209. doi: 10.6004/jnccn.2013.0142. [DOI] [PubMed] [Google Scholar]
- 3.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121:1226–1234. doi: 10.1097/AOG.0b013e3182922a17. [DOI] [PubMed] [Google Scholar]
- 4.Erickson BK, Martin YJ, Shah MM, Straughn JM, Leath CA. Reasons for failure to delivery national comprehensive cancer network (NCCN)-adherent care in the treatment of epithelial ovarian cancer at an NCCN cancer center. Gynecol Oncol. 2014;133:142–146. doi: 10.1016/j.ygyno.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume of specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000;18:2327–2340. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 6.Carney ME, Lancaster JM, Ford C, Tsodikov A, Wiggins CL. A population-based study of patterns of care for ovarian cancer: who is seen by a gynecologic oncologist and who is not? Gynecol Oncol. 2002;84:36–42. doi: 10.1006/gyno.2001.6460. [DOI] [PubMed] [Google Scholar]
- 7.duBois A, Rochon J, Pfisterer J, Hoskins WJ. Variations in institutional infrastructure, physician specialization and experience, and outcome on ovarian cancer: a systematic review. Gynecol Oncol. 2009;112:422–436. doi: 10.1016/j.ygyno.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Kumpulainen S, Grenman S, Kyyronen P, Pukkula E, Sankila R. Evidence of benefit from centralized treatment of ovarian cancer: a nationwide population-based survival analysis in Finland. Int J Cancer. 2002;102:541–544. doi: 10.1002/ijc.10754. [DOI] [PubMed] [Google Scholar]
- 9.Oberainger W, Stuhlinger W. Influence of department volume on cancer survival for gynaecological cancers—a population based study in Tyeol, Austria. Gynecol Oncol. 2006;103:527–534. doi: 10.1016/j.ygyno.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Ioka A, Tsukuma H, Ajiki W, Oshima A. Influence of hospital procedure volume on ovarian cancer survival in Japan, a country with a low incidence of ovarian cancer. Cancer Sci. 2004;95:233–237. doi: 10.1111/j.1349-7006.2004.tb02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goff BA, Matthews BJ, Wynn M, Muntz HG, Lishner DM, Baldwin LM. Ovarian cancer: patterns of surgical care across the United States. Gynecol Oncol. 2006;103:383–390. doi: 10.1016/j.ygyno.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Marth C, Hieble S, Oberainger W, Winter R, Leodolter S, Sevelda P. Influence of department volume on survival for ovarian cancer: results from a prospective quality assurance program of the Austrian association for gynecologic oncology. Int J Gynecol Cancer. 2009;19:94–102. doi: 10.1111/IGC.0b013e31819915cb. [DOI] [PubMed] [Google Scholar]
- 13.Tingulstad S, Skjeldestad FE, Hagen B. The effect of centralization of primary surgery on survival in ovarian cancer patients. Obstet Gynecol. 2003;102:499–505. doi: 10.1016/s0029-7844(03)00579-9. [DOI] [PubMed] [Google Scholar]
- 14.Mercado C, Zingmond D, Karlan BY, et al. Quality of care in advanced ovarian cancer: the importance of provider specialty. Gynecol Oncol. 2010 Apr;117(1):18–22. doi: 10.1016/j.ygyno.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Goff BA, Matthews BJ, Larson EH, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer. 2007;109:2031–2042. doi: 10.1002/cncr.22604. [DOI] [PubMed] [Google Scholar]
- 16.Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol. 2009;115:334–338. doi: 10.1016/j.ygyno.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Bristow RE, Palis BE, Chi DS, Cliby WA. The national cancer database on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010;118:262–267. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Bristow RE, Puri I, Diaz-Montes TP, Guintoli RL, Armstrong DK. Analysis of contemporary trends in access to high-volume ovarian cancer surgical care. Ann Surg Oncol. 2009 Dec;16(12):3422–3430. doi: 10.1245/s10434-009-0680-5. [DOI] [PubMed] [Google Scholar]
- 19.Donabedian A. The quality of care: How can it be assessed? JAMA. 1988;121(11):1145–1150. [PubMed] [Google Scholar]
- 20.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44:166–206. [PubMed] [Google Scholar]
- 21.Mitchell PH, Ferketich S, Jennings BM. Quality health outcomes model. Image J Nurs Sch. 1998;30(1):43–46. doi: 10.1111/j.1547-5069.1998.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 22.Carayon P, Schoofs Hundt A, Karsh BT, et al. Work system design for patient safety: the SEIPS model. Qual Saf Health Care. 2006;15(Suppl1):i50–i58. doi: 10.1136/qshc.2005.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khuri SF, Daley J, Henderson W, et al. Department of Veteran’s Affairs NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA surgical quality improvement program. Ann Surg. 1998;228:491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.California Cancer Reporting system Standards Vol I–Vol III. California Department of Health Services, Cancer Surveillance Section; Sacramento, CA: 1997. Cancer Reporting in California: Standards for Automated Reporting. [Google Scholar]
- 25.California Cancer Reporting system Standards Vol IV. California Department of Health Services, Cancer Surveillance Section; 1998. Cancer Reporting in California: Standards for Automated Reporting. [Google Scholar]
- 26.California Cancer Registry. How complete are California Cancer Registry data. Available at: http://www.ccrcal.org/Inside_CCR/FAQ.shtml. Accessed June 2014.
- 27.Morgan RJ, Copeland L, Gershenson D, et al. Update of the NCCN ovarian cancer practice guidelines. Oncology. 1997;11:95–105. [PubMed] [Google Scholar]
- 28.Morgan R, Alvarez RD, Armstrong DK, et al. NCCN practice guidelines for ovarian cancer. Version 2000. J Natl Compr Cancer Netw. 2000 [Google Scholar]
- 29.Morgan R, Alvarez RD, Armstrong DK, et al. Ovarian cancer guideline. Version 1.2002. National comprehensive cancer, Network; 2002. [Google Scholar]
- 30.Morgan R, Alvarez RD, Armstrong DK, et al. Ovarian cancer. Version 1.2003. National comprehensive cancer, Network; 2003. [Google Scholar]
- 31.Morgan R, Alvarez RD, Armstrong DK, et al. Ovarian cancer. Version 1.2005. National comprehensive cancer, Network; 2005. [Google Scholar]
- 32.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 33.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. third. World Health Organization; Geneva: 2000. [Google Scholar]
- 34.Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98:172–180. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 35.Schrag D, Earle C, Xu F, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst. 2006;98:163–171. doi: 10.1093/jnci/djj018. [DOI] [PubMed] [Google Scholar]
- 36.Aletti GD, Dowdy SC, Gostout BS, et al. Quality improvement in the surgical approach to advanced ovarian cancer: the mayo clinic experience. J Am Coll Surg. 2009;208:614–620. doi: 10.1016/j.jamcollsurg.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Harter P, Muallem ZM, Buhrmann C, et al. Impact of a structured quality management program on surgical outcome in primary advanced ovarian cancer. Gynecol Oncol. 2011;121:615–619. doi: 10.1016/j.ygyno.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Verley L, Ottevanger PB, van der Graff W, Reed NS, Vergote I. EORTC-GCG process quality indicators for ovarian cancer surgery. Eur J Cancer. 2009;45:517–526. doi: 10.1016/j.ejca.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 40.Phippen NT, Barnett JC, Lowery WJ, Miller CR, Leath CA. Surgical outcomes and national comprehensive cancer network compliance in advanced ovarian cancer surgery in a low volume military treatment facility. Gynecol Oncol. 2013;131:158–162. doi: 10.1016/j.ygyno.2013.07.001. [DOI] [PubMed] [Google Scholar]


