Abstract
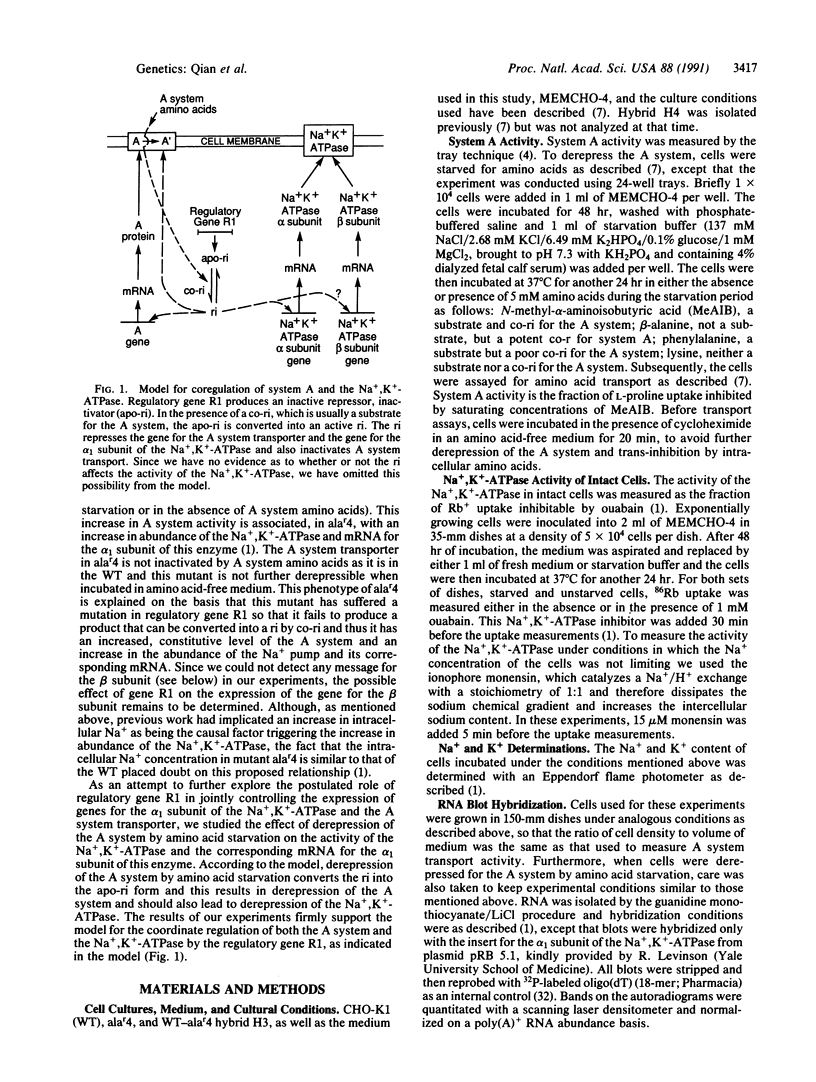
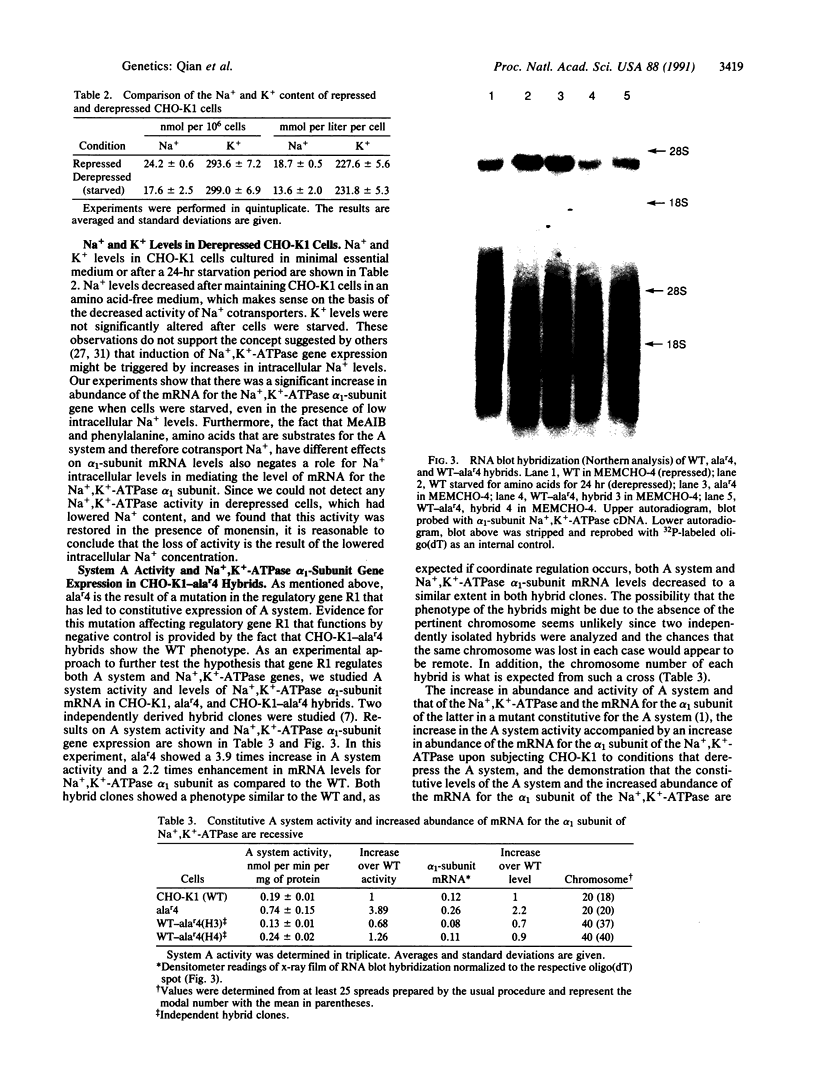
Previous work suggested that the structural gene for the A system transporter and the mRNA for the alpha subunit of the Na+,K(+)-ATPase in Chinese hamster ovary cells CHO-K1 [wild type (WT)] are coordinately controlled by regulatory gene R1. This conclusion was based on analysis of a mutant for the A system, alar4. This mutant had a constitutive level of A system transport activity equal to the level found in derepressed WT cells and a 4 times increase in abundance of the alpha 1 subunit of Na+,K(+)-ATPase mRNA over that found in repressed WT. The level of Na+ per cell in alar4 was not significantly greater than that found in the WT. To further characterize the likely coregulation of both genes, we have studied the A system activity and Na+,K(+)-ATPase mRNA alpha 1-subunit levels in cells grown under various conditions that result in repression or derepression of the A system in the WT. System A activity increased up to 2-3 times the basal transport rate (repressed state) and Na+,K(+)-ATPase mRNA alpha 1-subunit levels showed a 3-fold increase after amino acid starvation (derepressed state). These changes occurred along with a decrease in intracellular Na+ levels. N-Methyl-alpha-aminoisobutyric acid and beta-alanine, previously shown to be corepressors for the A system, prevented to a similar extent A system derepression and Na+,K(+)-ATPase mRNA alpha 1-subunit accumulation. On the other hand, phenylalanine and lysine, amino acids that are not corepressors of the A system, failed to significantly prevent derepression of both genes. Hybrids between the WT and alar4 have the phenotype of the WT when grown under repressed conditions. These results give further support to the proposition that both the A system transporter and mRNA for the alpha 1 subunit of the Na+,K(+)-ATPase are coordinately controlled by regulatory gene R1 and elevated Na+ concentrations are not involved. No Na+,K(+)-ATPase activity was detected in derepressed cells. Activity was restored by the addition of monensin. However, this activity was no greater than that obtained in repressed cells. Indications are that the reduced Na+ content in derepressed cells inhibits Na+,K(+)-ATPase activity and that conditions that favored derepression do not allow for de novo synthesis of the Na+,K(+)-ATPase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiton J. F., Lamb J. F. Effect of the serum concentration of the growth medium on the sodium pump site density of cultured HeLa cells. Q J Exp Physiol. 1984 Jan;69(1):97–115. doi: 10.1113/expphysiol.1984.sp002799. [DOI] [PubMed] [Google Scholar]
- Boardman L., Huett M., Lamb J. F., Newton J. P., Polson J. M. Evidence for the genetic control of the sodium pump density in HeLa cells. J Physiol. 1974 Sep;241(3):771–794. doi: 10.1113/jphysiol.1974.sp010684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdel G., Forestier M., Gouhot B. Na(+)-dependent transport of alanine and serine by liver plasma-membrane vesicles from rats fed a low-protein or a high-protein diet. Biochim Biophys Acta. 1990 Jul 9;1026(1):1–12. doi: 10.1016/0005-2736(90)90325-i. [DOI] [PubMed] [Google Scholar]
- Bowen J. W., McDonough A. Pretranslational regulation of Na-K-ATPase in cultured canine kidney cells by low K+. Am J Physiol. 1987 Feb;252(2 Pt 1):C179–C189. doi: 10.1152/ajpcell.1987.252.2.C179. [DOI] [PubMed] [Google Scholar]
- Brodie C., Sampson S. R. Regulation of the sodium-potassium pump in cultured rat skeletal myotubes by intracellular sodium ions. J Cell Physiol. 1989 Jul;140(1):131–137. doi: 10.1002/jcp.1041400116. [DOI] [PubMed] [Google Scholar]
- Collarini E. J., Oxender D. L. Mechanisms of transport of amino acids across membranes. Annu Rev Nutr. 1987;7:75–90. doi: 10.1146/annurev.nu.07.070187.000451. [DOI] [PubMed] [Google Scholar]
- Emanuel J. R., Garetz S., Stone L., Levenson R. Differential expression of Na+,K+-ATPase alpha- and beta-subunit mRNAs in rat tissues and cell lines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9030–9034. doi: 10.1073/pnas.84.24.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Moffett J. A genetic approach to the study of neutral amino acid transport in mammalian cells in culture. J Membr Biol. 1986;91(3):199–212. doi: 10.1007/BF01868814. [DOI] [PubMed] [Google Scholar]
- Graves J. S., Wheeler D. D. Increase in K+ and alpha-AIB active transport in CHO cells after low [K+] treatment. Am J Physiol. 1982 Sep;243(3):C124–C132. doi: 10.1152/ajpcell.1982.243.3.C124. [DOI] [PubMed] [Google Scholar]
- Hubert J. J., Schenk D. B., Skelly H., Leffert H. L. Rat hepatic (Na+, K+)-ATPase: alpha-subunit isolation by immunoaffinity chromatography and structural analysis by peptide mapping. Biochemistry. 1986 Jul 15;25(14):4156–4163. doi: 10.1021/bi00362a025. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F., Pressley T. A., Haber R. S., Gick G. G., Loeb J. N., Edelman I. S. Kinetic analysis of Na,K-activated adenosine triphosphatase induced by low external K+ in a rat liver cell line. J Biol Chem. 1988 Jun 15;263(17):8162–8167. [PubMed] [Google Scholar]
- Kim D., Marsh J. D., Barry W. H., Smith T. W. Effects of growth in low potassium medium or ouabain on membrane Na,K-ATPase, cation transport, and contractility in cultured chick heart cells. Circ Res. 1984 Jul;55(1):39–48. doi: 10.1161/01.res.55.1.39. [DOI] [PubMed] [Google Scholar]
- Leister K. J., Schenerman M. A., Racker E. Altered sensitivity of system A amino acid transport to ouabain in normal and transformed C3H-10T1/2 cells during the cell cycle. Proc Natl Acad Sci U S A. 1989 Feb;86(3):783–786. doi: 10.1073/pnas.86.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister K. J., Schenerman M. A., Racker E. Energetic mechanism of system A amino acid transport in normal and transformed mouse fibroblasts. J Cell Physiol. 1988 May;135(2):163–168. doi: 10.1002/jcp.1041350203. [DOI] [PubMed] [Google Scholar]
- Lytton J., Lin J. C., Guidotti G. Identification of two molecular forms of (Na+,K+)-ATPase in rat adipocytes. Relation to insulin stimulation of the enzyme. J Biol Chem. 1985 Jan 25;260(2):1177–1184. [PubMed] [Google Scholar]
- Mendiaz E., Mamounas M., Moffett J., Englesberg E. A defined medium for and the effect of insulin on the growth, amino acid transport, and morphology of Chinese hamster ovary cells, CHO-K1 (CCL 61) and the isolation of insulin "independent" mutants. In Vitro Cell Dev Biol. 1986 Feb;22(2):66–74. doi: 10.1007/BF02623535. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Wigglesworth N. M., Pohjanpelto P., Rozengurt E. Na entry and Na-K pump activity in murine, hamster, and human cells--effect of monensin, serum, platelet extract, and viral transformation. J Cell Physiol. 1980 Apr;103(1):17–27. doi: 10.1002/jcp.1041030104. [DOI] [PubMed] [Google Scholar]
- Moffett J., Curriden S., Ertsey R., Mendiaz E., Englesberg E. Alanine-resistant mutants of Chinese hamster ovary cells, CHO-K1, producing increases in velocity of proline transport through the A, ASC, and P systems. Somatic Cell Genet. 1983 Mar;9(2):189–213. doi: 10.1007/BF01543177. [DOI] [PubMed] [Google Scholar]
- Moffett J., Englesberg E. Recessive constitutive mutant Chinese hamster ovary cells (CHO-K1) with an altered A system for amino acid transport and the mechanism of gene regulation of the A system. Mol Cell Biol. 1984 Apr;4(4):799–808. doi: 10.1128/mcb.4.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett J., Englesberg E. Regulation of the A system of amino acid transport in Chinese hamster ovary cells, CHO-K1: the difference in specificity between the apo-repressor inactivator (apo-ri) and the transporter and the characterization of the proposed apo-ri. J Cell Physiol. 1986 Mar;126(3):421–429. doi: 10.1002/jcp.1041260313. [DOI] [PubMed] [Google Scholar]
- Moffett J., Jones M., Englesberg E. Amino acid transport in membrane vesicles from CHO-K1 and alanine-resistant transport mutants. Biochemistry. 1987 May 5;26(9):2487–2494. doi: 10.1021/bi00383a013. [DOI] [PubMed] [Google Scholar]
- Moffett J., Mendiaz E., Jones M., Englesberg E. Two membrane-bound proteins associated with alanine resistance and increased A-system amino acid transport in mutants of CHO-K1. Somat Cell Mol Genet. 1988 Jan;14(1):1–12. doi: 10.1007/BF01535044. [DOI] [PubMed] [Google Scholar]
- Moffett J., Périer F., Jones M., Englesberg E. Control of A-system amino acid transport by a second regulatory gene R2 in Chinese hamster ovary cells CHO-K1 and the possible connection of this gene with insulin activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8040–8043. doi: 10.1073/pnas.84.22.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressley T. A., Haber R. S., Loeb J. N., Edelman I. S., Ismail-Beigi F. Stimulation of Na,K-activated adenosine triphosphatase and active transport by low external K+ in a rat liver cell line. J Gen Physiol. 1986 Apr;87(4):591–606. doi: 10.1085/jgp.87.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian N. X., Jones M., McDonough A., Englesberg E. alar4, a constitutive mutant of the A system for amino acid transport, has increased abundance of the Na+,K+-ATPase and mRNA for alpha 1 subunit of this enzyme. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7984–7988. doi: 10.1073/pnas.86.20.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosić N. K., Standaert M. L., Pollet R. J. The mechanism of insulin stimulation of (Na+,K+)-ATPase transport activity in muscle. J Biol Chem. 1985 May 25;260(10):6206–6212. [PubMed] [Google Scholar]
- Schenerman M. A., Leister K. J., Trachtenberg D. K., Racker E. Induction of system A amino acid transport through long-term treatment with ouabain: correlation with increased (Na+/K+)-ATPase activity. J Cell Physiol. 1988 May;135(2):157–162. doi: 10.1002/jcp.1041350202. [DOI] [PubMed] [Google Scholar]
- Schmitt C. A., McDonough A. A. Developmental and thyroid hormone regulation of two molecular forms of Na+-K+-ATPase in brain. J Biol Chem. 1986 Aug 5;261(22):10439–10444. [PubMed] [Google Scholar]
- Van Dyke R. W., Scharschmidt B. F. (Na,K)-ATPase-mediated cation pumping in cultured rat hepatocytes. Rapid modulation by alanine and taurocholate transport and characterization of its relationship to intracellular sodium concentration. J Biol Chem. 1983 Nov 10;258(21):12912–12919. [PubMed] [Google Scholar]
- Wolitzky B. A., Fambrough D. M. Regulation of the (Na+ + K+)-ATPase in cultured chick skeletal muscle. Modulation of expression by the demand for ion transport. J Biol Chem. 1986 Jul 25;261(21):9990–9999. [PubMed] [Google Scholar]
- Zibirre R., Poronnik P., Koch G. Na+-dependent amino acid transport is a major factor determining the rate of (Na+,K+)-ATPase mediated cation transport in intact HeLa cells. J Cell Physiol. 1986 Oct;129(1):85–93. doi: 10.1002/jcp.1041290113. [DOI] [PubMed] [Google Scholar]