Summary
Small RNAs (sRNAs) associated with the RNA chaperon protein Hfq are key posttranscriptional regulators of gene expression in bacteria. Deciphering the sRNA-target interactome is an essential step toward understanding the roles of sRNAs in the cellular networks. We developed a broadly applicable methodology termed RIL-seq (RNA interaction by ligation and sequencing), which integrates experimental and computational tools for in vivo transcriptome-wide identification of interactions involving Hfq-associated sRNAs. By applying this methodology to Escherichia coli we discovered an extensive network of interactions involving RNA pairs showing sequence complementarity. We expand the ensemble of targets for known sRNAs, uncover additional Hfq-bound sRNAs encoded in various genomic regions along with their trans encoded targets, and provide insights into binding and possible cycling of RNAs on Hfq. Comparison of the sRNA interactome under various conditions has revealed changes in the sRNA repertoire as well as substantial re-wiring of the network between conditions.
Graphical Abstract

Highlights
-
•
A widely applicable method for in vivo global mapping of small RNA interactome
-
•
Substantial re-wiring of the network upon changes in cellular conditions
-
•
Regulatory circuits involving two regulators derived from the same transcript
-
•
sRNAs acting in trans are encoded within almost every possible genomic element
Melamed et al. describe RIL-seq, an approach that can identify Hfq-bound pairs of small RNAs (sRNAs) and their targets. They apply RIL-seq to E. coli grown in different conditions, identifying new sRNAs and describing sRNA interactome changes upon change in conditions.
Introduction
Posttranscriptional control of gene expression by small RNAs (sRNAs) is a major layer of regulation in bacteria. Most known sRNAs exert their regulatory function by base-pairing with their target RNAs, usually affecting the target translation, stability, and/or processing (Storz et al., 2011, Wagner and Romby, 2015). Base-pairing sRNAs are commonly divided into cis- and trans-encoded. While the former are transcribed antisense to their targets, the trans sRNAs and their targets are encoded in different genomic locations, usually exhibiting partial sequence complementarity. Base pairing between trans-encoded sRNAs and their targets is often mediated by the RNA chaperon protein Hfq, a ring-shaped hexamer that is abundant in Gram-negative bacteria (Wagner and Romby, 2015). A major challenge is to identify the complete network of Hfq-mediated sRNA-target interactions and to study its structure and dynamics. Yet, no methodology for large-scale direct capturing of these interactions has been published.
Previous large-scale approaches identified RNA transcripts bound on Hfq by co-immunoprecipitation (coIP) followed by microarray (Zhang et al., 2003) or RNA-seq analysis (Bilusic et al., 2014, Chao et al., 2012, Sittka et al., 2008) and were further revised by including Hfq-RNA UV crosslinking (Holmqvist et al., 2016, Tree et al., 2014). These experiments provided a global view of the RNA molecules bound to Hfq, but specific sRNA-target pairs could only be indirectly deduced by additional sequence-dependent predictive schemes.
To overcome this limitation, and inspired by the CLASH and iPAR-CLIP methodologies that were applied to detect protein-mediated miRNA-target interactions in eukaryotes (Grosswendt et al., 2014, Helwak et al., 2013), we developed RIL-seq (RNA interaction by ligation and sequencing), an experimental-computational methodology for detecting Hfq-bound sRNA-target pairs in bacteria. We applied RIL-seq to Escherichia coli grown under three different growth conditions and identified ∼2,800 Hfq-bound RNA pairs, dominated by mRNA-sRNA pairs exhibiting sequence complementarity. Our re-discovery of known sRNA targets, as well as the computational and experimental assessments that we provide, strongly support the reliability of RIL-seq data and the applicability of this approach for capturing the sRNA interaction network. The network we uncovered includes additional targets of established sRNAs as well as additional sRNAs transcribed from various genomic regions, along with their targets. Comparison of the interactions discovered under different conditions revealed substantial re-wiring of the network, implying the role of sRNAs in regulation of global cellular processes.
Results
RIL-seq Protocol Optimization
The major steps of RIL-seq are described in Figure 1 and the Experimental Procedures. The experiments were performed in E. coli carrying a functional tagged Hfq (hfq-Flag) (Morita et al., 2005) and involved culturing of bacteria in desired conditions, in vivo protein-RNA crosslinking, cell lysis, coIP of Hfq and bound RNAs (Figure S1), RNA ligation, RNA isolation, paired-end sequencing, and mapping of the sequenced fragments to E. coli genome. Each sequenced fragment was represented by two sequences at its ends (pair mates), which were mapped individually to the genome. This mapping revealed two types of fragments: (1) “single” fragments, where the pair mates were mapped to the same genomic location, most likely originating from a single transcript and (2) “chimeric” fragments, where the pair mates were mapped to two distinct genomic locations, supporting a putative interaction between RNAs derived from these regions. To avoid spurious interactions (e.g., resulting from the ligation of two random adjacent RNAs), we selected RNA pairs that were statistically significantly over-represented in chimeric fragments. We assessed the statistical significance by Fisher’s exact test with Bonferroni correction and computed the odds ratio (considering the numbers of chimeric fragments involving the two RNAs, other fragments they are involved in, and all other fragments) (Figure 1). Only statistically significant RNA pairs supported by at least 10 chimeric fragments were regarded as putatively interacting (hereinafter, S-chimeras). While the odds ratio corresponds to the over-representation of the pair above random expectation, it does not convey the functional impact of the interaction on the target. To estimate the latter, we computed an additional measure based on the assumption that the higher the enrichment of the RNAs on Hfq, the larger the effect the interaction may have. This measure, termed normalized odds ratio, considers both the odds ratio and the enrichment of the two interacting RNAs on Hfq (Supplemental Experimental Procedures).
Figure 1.
Overview of RIL-seq Experimental and Computational Procedures
See also Figures S1 and S2 and Table S1.
We tested various versions of the protocol in log-phase cells (Supplemental Experimental Procedures), six of which yielded consistent sequencing results for both the single and chimeric fragments (Table S1 and Figures S2A–S2D) and hence were considered repetitions of the same experiment. In all successive experiments, including three in stationary phase and three under iron limitation, one protocol (highlighted in Table S1) was used.
We assessed the quality of RIL-seq protocol in several ways (Supplemental Experimental Procedures). (1) By applying RIL-seq to a wild-type (WT) E. coli strain, where Hfq was not tagged (hfq-WT), we demonstrated that in all three conditions the counts of chimeric fragments as well as fragments supporting S-chimeras were substantially smaller in hfq-WT compared to hfq-Flag libraries (Table S1). (2) By mixing the E. coli lysate with a Saccharomyces cerevisiae lysate before the coIP and applying the protocol to the mixture, we verified that the vast majority of RIL-seq interactions originated in vivo (Table S1). (3) By computational analysis of short chimeric fragments where the ligation point could be located, we verified that potential biases stemming from possible tendency of the ligase to fuse specific sequences or structures were minimal (Figures S2E–S2G). Still, there might be some bias that we did not identify, mainly because only 0.02% of the sequenced chimeric fragments were short enough to enable the above analysis.
A major difference between RIL-seq and iPAR-CLIP or CLASH protocols is that RIL-seq does not employ stringent conditions to remove RNAs that were not crosslinked to the protein after the coIP, in order to ensure the Hfq hexamer integrity. Therefore, especially as the crosslinking efficiency is estimated at 1%–5% (Darnell, 2010), it is conceivable that our libraries contain RNAs that were and were not crosslinked to Hfq, questioning the necessity of crosslinking. Thus, we re-applied the protocol without crosslinking (Table S1). Since the experiment with mixed E. coli and yeast lysates yielded a higher fraction of in vitro interactions in the protocol without crosslinking (3.2%–3.8%) compared to the one with crosslinking (<1%) (Table S1), we concluded that the crosslinking allows better capturing of in vivo Hfq-mediated RNA interactions. Hence, all the results described below are based on the protocol with crosslinking.
RIL-seq Results Are Reproducible
We evaluated the reproducibility of the results within same-condition libraries (log phase, stationary phase, and iron limitation) (Supplemental Experimental Procedures). For both single and chimeric fragments, there was correlation in the numbers of mapped sequenced fragments in corresponding genomic windows between same-condition libraries (Figures S2A–S2C). In addition, about half of the S-chimeras were detected in at least two same-condition libraries (Figure S2D), compared to only one chimera in the hfq-WT libraries. Given the reproducibility of the results and to gain better statistical power, we assembled the mapped single and chimeric sequenced fragments of all libraries in each condition to a unified dataset, resulting in three datasets, to each of which we applied the statistical test. This has allowed many interactions that did not pass the threshold of 10 sequenced chimeric fragments or the statistical test in the individual libraries to be revealed, while only a relatively small number of interactions that passed these criteria in the individual libraries were lost. All further analyses were applied to the interactions in the unified datasets (Table S2).
Corroborating the Interactions Revealed by RIL-seq
RIL-seq Data Are Enriched with sRNA-mRNA Pairs
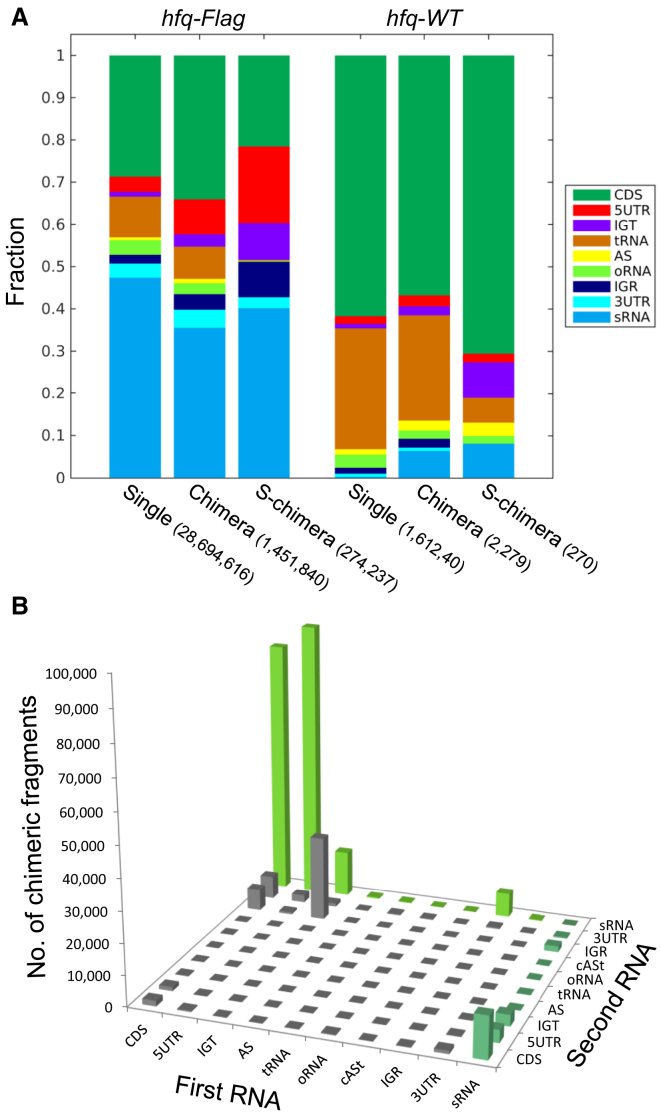
Classification of all mapped fragments by their genomic annotation revealed high fractions of single and chimeric fragments mapped to sRNAs in the hfq-Flag libraries, which were substantially smaller in the hfq-WT control libraries (Figures 2A, S3A, and S3B). Furthermore, the distribution of genomic element pairs in S-chimeras was dominated by sRNAs paired with RNAs derived from CDS and/or 5′ UTR regions (Figures 2B, S3C, and S3D).
Figure 2.
RIL-seq Data Are Enriched with sRNAs and mRNAs
(A) Distribution of RNAs derived from various genomic elements. Single (Single), chimeric (Chimera), and statistically significant chimeric (S-chimera) sequenced fragments from the log phase hfq-Flag and hfq-WT libraries were classified into nine major categories: 5UTR (5′ UTR), CDS, 3UTR (3′ UTR), tRNA, sRNA, oRNA (other non-coding RNAs), AS (antisense), IGR (intergenic region), and IGT (intergenic within transcript). rRNA-derived fragments were excluded. Fractions are shown (total counts are denoted in parentheses).
(B) Total number of S-chimera fragments for each combination of genomic elements. Mapped fragments were classified as in (A) with an additional sub-division of the AS category to cASt (cis antisense with putative trans target). Bars representing fragments with sRNAs as the first/second RNA in the chimera are colored in dark/light green, respectively.
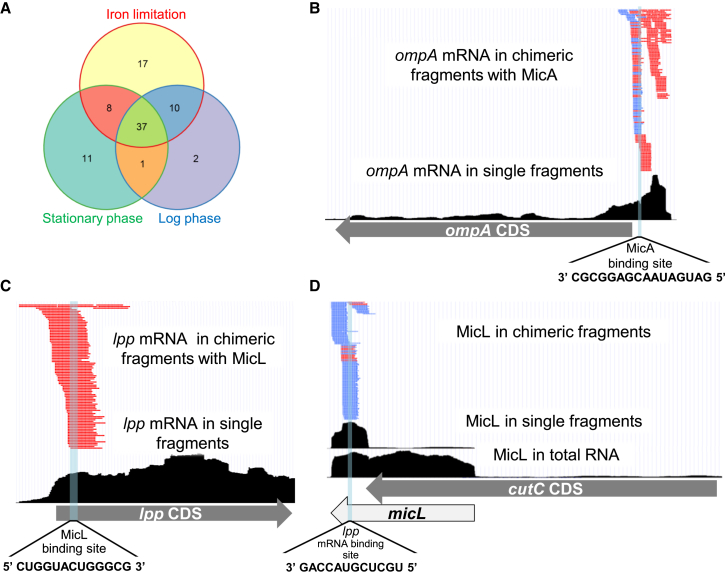
We compiled a set of 154 experimentally verified Hfq-mediated sRNA-target interactions (hereinafter “known” interactions), involving 26 sRNAs (Table S3), out of which 25 were revealed by RIL-seq (Table S2). Compatible with the transcriptome study of Thomason et al. (2015), we detected most of the 25 sRNAs in all three conditions (Figure S3E), although their relative fractions could differ between conditions, consistent with the observations of Chao et al. (2012). Out of the 154 known interactions, 117 were previously determined under the growth conditions studied here, and we discovered 63 of them. While the remaining 37 interactions were originally determined under other specific stress conditions, we detected 23 of them, resulting in the identification of a total of 86 known interactions (Figure 3A and Table S3). Examples of re-discovered known interactions are illustrated in Figures 3B–3D for ompA-MicA interaction (Udekwu et al., 2005) and lpp-MicL interaction (Guo et al., 2014).
Figure 3.
Known Targets Are Recovered by RIL-seq
(A) 86 out of 154 known sRNA-target pairs (∼56%) were collectively identified by RIL-seq under the three tested conditions (not drawn to scale).
(B–D) Examples of identified interactions in log-phase library. (B) ompA-MicA interaction. Coverage of ompA, a known target of MicA is shown. Bottom: in single fragments (black). Top: in S-chimeras with MicA (broken lines in red or blue for ompA mRNA as first or second RNA in the chimera, respectively). MicA reported binding site (Udekwu et al., 2005) is highlighted. (C and D) lpp-MicL interaction. (C) Coverage of lpp, the major target of MicL, in RIL-seq library is shown. Bottom: in single fragments (black). Top: in S-chimeras with MicL (red broken lines). Colors are as in (B). The reported MicL binding site (Guo et al., 2014) is highlighted. (D) Coverage of MicL, a sRNA processed from a transcript transcribed from within the cutC gene, is shown. Bottom: in a total RNA library (black). Middle: in single fragments in RIL-seq library (black), showing that Hfq binds mainly the MicL processed fragment. Top: in S-chimeras (colors are as in B). The reported lpp mRNA binding site (Guo et al., 2014) is highlighted. Data are illustrated with UCSC genome browser (Kent et al., 2002).
See also Table S3.
Objective Re-discovery of Known Binding Sites Supports the Interacting Partners
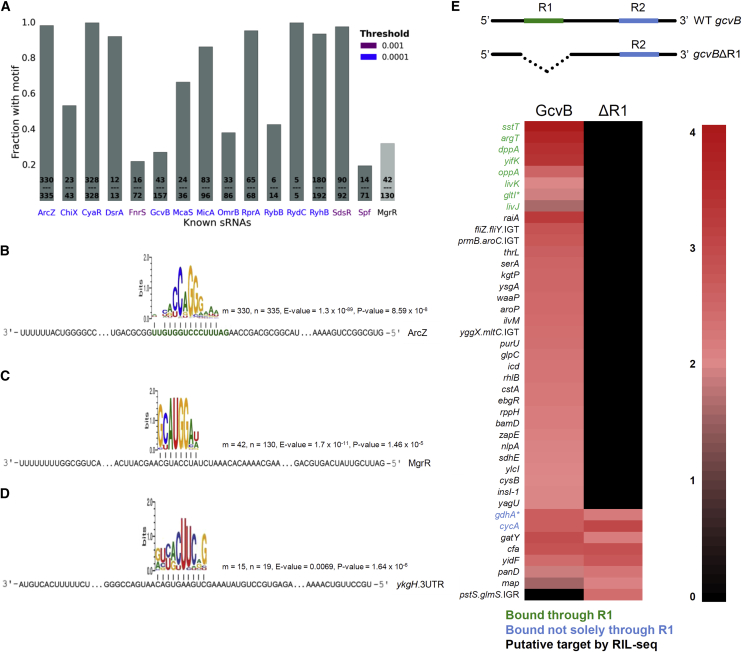
Since a given sRNA often binds different targets through the same binding site, objective identification of a common sequence motif in its set of RIL-seq putative targets, which is complementary to the sRNA known binding site, would support the identified targets. 20 of the known sRNAs had both at least four RIL-seq targets and a previously experimentally determined binding site (Peer and Margalit, 2011, Peer and Margalit, 2014). We applied MEME (Bailey et al., 2009) to search for a common motif in each target sequence set of these sRNAs. For 18 sets we identified a common motif complementary to the sRNA. For 15 sRNAs the best common motif complemented the known sRNA binding site (Figures 4A and 4B and Table S4). Intriguingly, for some sRNAs such a motif could be identified in greater than 90% of the putative target sequences (Figure 4A and Table S4). Additionally, for MgrR, which lacks a known binding site, we identified a common motif in the target sequences that complements it, hinting at its binding site (Figures 4A and 4C). This suggests that such an analysis can identify the binding sites of newly discovered sRNAs (Figure 4D and Table S4). These results strongly support the validity of the targets identified by RIL-seq.
Figure 4.
Computational and Experimental Analyses Support the Validity of the Interactions Revealed by RIL-seq
(A–D) Common sequence motifs in putative target sets are complementary to binding sites on the sRNA. (A) Bars represent the fractions of targets sharing a common motif that complements the known binding site of a sRNA. Numbers on the bars indicate the number of targets that shared the motif out of all RIL-seq targets of that sRNA. The color of the sRNA name presents the MAST p value threshold in which the motif matched the known binding site, when applicable. MgrR (p ≤ 5.55 × 10−8) has no known binding site. (B–D) Common sequence motifs identified in RIL-seq target sets shown for: (B) ArcZ, (the known binding site is marked in green), (C) MgrR, showing a putative binding site, and (D) ykgH 3′ UTR, a putative 3′ UTR-derived sRNA, for which a putative binding site was identified. Indicated are the number of target sequences that shared the common motif (m), the number of sequences in the target set (n), the E value of MEME, and the p value of MAST. See also Table S4.
(E) RIL-seq captures true sRNA-target interactions. Upper part: a schematic representation of the plasmids expressing WT gcvB and gcvBΔR1 used in the experiment. Lower part: a heatmap presenting RIL-seq results of GcvB interactions identified in a ΔgcvB strain carrying WT gcvB or gcvBΔR1 plasmid. For clarity, only known GcvB-target interactions and interactions supported by ≥100 sequenced fragments are shown. For each GcvB-target chimera, the normalized count of sequenced fragments in the respective library multiplied by 105 is presented (log scale). Targets of GcvB, shown in Salmonella to interact through R1 (green), are revealed only with the WT gcvB, while targets shown in Salmonella to interact not solely through R1 (blue) are revealed in both WT gcvB and gcvBΔR1, implying RIL-seq captures true interactions. ∗Known targets in Salmonella not included in our compilation (Table S3).
See also Table S5.
RIL-seq Captures True sRNA-Target Interactions
GcvB is a predominant sRNA in E. coli, which is highly conserved in evolution and regulates a large number of target genes, many of which encode amino acid transporters (Miyakoshi et al., 2015, Sharma et al., 2011). In Salmonella, GcvB base pairs with most of its targets through two binding sites, R1 and R2 (conserved also in E. coli), R1 being the dominant one (Sharma et al., 2011). We evaluated the capability of RIL-seq to capture true interactions, by applying it to a ΔgcvB strain carrying plasmids expressing WT gcvB or a gcvB where R1 was deleted, gcvBΔR1. As demonstrated in Figure 4E and detailed in Table S5, targets of GcvB known to interact through R1 were revealed only with the WT gcvB plasmid, while targets known to interact not solely through R1 were revealed in both WT gcvB and gcvBΔR1, indicating that the chimeras captured by RIL-seq represent true interactions. In this experiment the false negative rate was 20%, as we identified eight out of ten known GcvB interactions reported in Table S3. Following the pattern observed for known GcvB targets, we can deduce whether additional RIL-seq GcvB targets are bound mainly via R1 or via an alternative binding site (Figure 4E). This experiment not only supports the reliability of RIL-seq protocol and the revealed interactions, it also suggests that targets that share a binding site for a specific sRNA can be revealed by a “comparative RIL-seq.”
Effect of the sRNA on the Expression Level of Its Bound Partners
The previous experimental and computational analyses strongly suggest that RIL-seq captures sRNA-target duplexes bound on Hfq, providing a snapshot of the sRNA interactome under the studied condition. Since functional sRNA interactions are believed to impact the transcript and/or protein expression level of the target, we tested whether the expression levels of RIL-seq bound partners are affected by the sRNA. To this end, we first used data from seven published transcriptome experiments that followed the change in gene expression upon overexpression of the sRNA (Supplemental Experimental Procedures). Indeed, for six of the seven sRNAs, the overall change in expression of the set of their bound partners was larger compared to the rest of the genes (p ≤ 0.002 by Kolmogorov-Smirnov test; Figure S4A, right panel for each sRNA). Next, we conducted a similar analysis to test the sRNA effect on the translation of RIL-seq targets, using ribosome profiling data upon change in sRNA expression, available for RyhB (Wang et al., 2015). Indeed, there was a greater change in the ribosome occupancy of RyhB bound partners identified by RIL-seq compared to background genes (p ≤ 1.16 × 10−8 by Kolmogorov-Smirnov test; Figure S4B, right panel).
A close inspection of the datasets of the transcriptome and ribosome profiling experiments suggested that each of the statistically significant results corresponded to a subset of the targets, while other targets showed relatively little changes in their transcript levels or ribosome occupancy (Figure S4, left panel for each sRNA). While it is possible that RIL-seq data include false-positive targets, the identified common motifs described above, which are complementary to the known binding sites of the sRNAs, suggest that many of these transcripts indeed form duplexes with the sRNA on Hfq. However, the impact of these interactions is not evident at the mRNA or translation level of the bound partners under the studied conditions.
To identify for each sRNA the subset of targets that not only bind but are also affected at their expression level, we computed the normalized odds ratio (see above). Using the ribosome profiling data, we verified that the normalized odds ratio values (Table S2, iron limitation data) of highly affected targets (fold change of ribosome profiling measure ≥ 2) were higher than the values of all other RyhB targets (p ≤ 8.42 × 10−6 by Wilcoxon test). Thus, sRNA targets with relatively high normalized odds ratio values are more likely to be affected by the sRNA at their expression level compared to targets of the same sRNA with low values of this measure (Table S2).
Biological Insights from RIL-seq Data
RNA Order in the Chimeras Is Consistent with the Hfq Binding Mode of sRNAs
In the majority of chimeras involving sRNAs, the sRNA was the second RNA (RNA2) (i.e., at the 3′ end of the chimera), while the target was the first RNA (RNA1) (Figures 2B, 5A, S3C, S3D, S5A, and S5B). These findings are consistent with recent crystallographic results showing that the 3′ end of the sRNA is bound within the Hfq hexamer (Dimastrogiovanni et al., 2014), rendering it less accessible to the ligase. Furthermore, a search for a common motif in all RNA2 sequences identified a GC-rich motif followed by poly-U (Figure 5B), matching the Rho-independent transcription terminator. Consistently, small-scale experiments showed that a poly-U tail at the end of sRNAs is critical for their binding to Hfq (Morita et al., 2015, Otaka et al., 2011, Sauer and Weichenrieder, 2011). Interestingly, we found that the poly-U tails of Rho-independent terminators of sRNAs are longer than those of mRNAs (p ≤ 2.7 × 10−7 by Wilcoxon test; Figure S5C). A search for a common motif in RNA1 sequences of the chimeras did not yield such a motif. Notably, non-random order of RNAs in chimeras was also observed in miRNA-target chimeras on Argonaute (Moore et al., 2015). Altogether, it seems that RIL-seq data hold valuable information on RNA binding to other RNAs as well as to Hfq itself.
Figure 5.
sRNAs Tend to be Second in the Chimeric Fragments
(A) Distribution of RNA locations as first (red) and second (blue) in S-chimera fragments for RNAs derived from various genomic elements. Mapped fragments were classified as in Figure 2A. See also Figures S5A and S5B and Table S6.
(B) The Rho-independent transcription terminator motif was revealed as a common motif for the second RNAs in S-chimeras.
See also Figure S5C. The results presented in (A) and (B) are based on S-chimera fragments in the log-phase dataset. n and m are as in Figure 4.
sRNA Candidates Are Encoded within Various Genomic Elements
About one-third of RIL-seq interactions do not involve well-established sRNAs (Figure 2B and Table S2). We performed a rigorous analysis examining if the RNAs involved in these interactions show features of known sRNAs. These features included the location of sRNAs as second RNA in the chimeric fragment, a sequence compatible with Rho-independent terminator with a relatively long poly-U tail, as well as involvement in multiple interactions with CDSs or 5′ UTRs (Table S6). We found that RNAs encoded in various genomic elements could be characterized by their location in the chimeric fragment (Figures 5A, S5A, and S5B). tRNAs and RNAs derived from 5′ UTR, CDS, intergenic regions within transcripts and antisense regions tended to appear first in the chimeras, as observed for targets of sRNA. RNAs derived from 3′ UTR and from intergenic regions between transcripts tended to be second in the chimeras, supporting their annotation as putative sRNAs. In addition, the poly-U tails at the 3′ ends of these sRNA candidates are on average longer than those of other mRNAs (p ≤ 0.012 by Wilcoxon test; Figure S5C). In further support of this classification, the sequences of interactors for 10 of these sRNA candidates show common sequence motifs that are complementary to the respective sRNA sequence (Figure 4D and Table S4). While most sRNA candidates originated from 3′ UTRs and intergenic regions, there were exceptions. For example, fragments overlapping the CDS of aceK and the 5′ UTR of motA were preferentially located as second in their chimeras, exhibited a long U-tail, and had relatively high numbers of interactions, mostly with CDS-derived fragments, suggesting that they may be classified as sRNAs.
cis-Antisense RNAs Can Act as trans Regulators
Whereas most antisense regions identified in chimeras exhibited characteristics of targets, two antisense RNAs, ArrS and GadY, which were previously documented to act as cis-antisense RNA regulators in the acid response pathway, exhibited characteristics of sRNAs (cASt in Figure 5A). GadY was shown to bind Hfq (Opdyke et al., 2004), supporting its classification by RIL-seq data as an Hfq-dependent sRNA. RIL-seq identified bound partners for GadY and ArrS, enriched with genes involved in acid stress response (p ≤ 0.004 and p ≤ 6.4 × 10−7, respectively, by Fisher’s exact test), some of which were further supported by reporter experiments using GFP translational fusions (Corcoran et al., 2012) (Supplemental Experimental Procedures, Figure 6A, and Table S7). These findings support a model by which some sRNAs can act as both cis and trans regulators (Thomason and Storz, 2010, Mandin and Gottesman, 2010, Jäger et al., 2012, Sayed et al., 2012). Furthermore, our data suggest that the same RNA can act within a pathway as both cis and trans regulator. Interestingly, no sense-antisense S-chimeras were revealed in RIL-seq data, probably because this type of interaction does not require the involvement of Hfq.
Figure 6.
Functional sRNAs
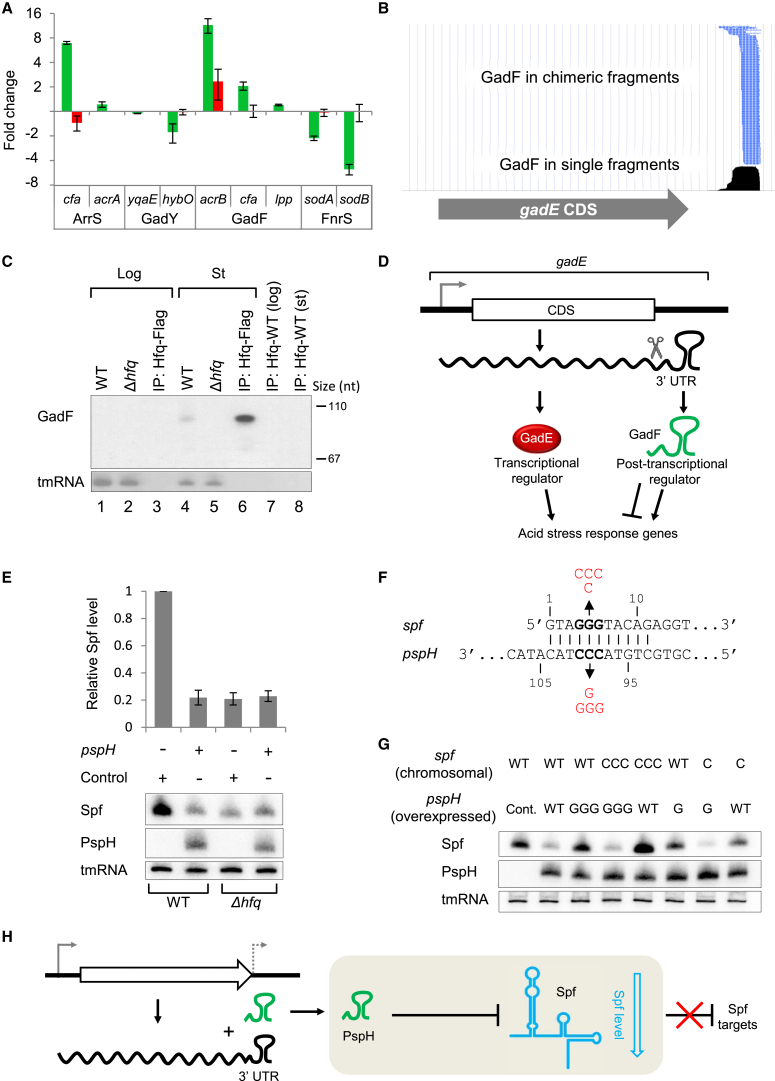
(A) Effect of ArrS, GadY and GadF on the GFP intensity of GFP translational fusions of targets identified by RIL-seq. The effect of FnrS on its GFP fused known targets, sodA and sodB, is shown as a positive control. The fold change between GFP fluorescence in the presence of a sRNA overexpressing plasmid and of a control plasmid is presented (WT cells, green bars; Δhfq mutant, red bars). To maintain a unified scale, negative values represent the fold change of control/sRNA overexpression (reduction in GFP intensity) and positive values represent the fold change of sRNA overexpression/control (increase in GFP intensity). Error bars indicate 95% confidence intervals based on at least three independent repeats. The predicted binding sites for each pair are shown in Table S7.
(B) The coverage of GadF, a sRNA encoded in the gadE 3′ UTR, in RIL-seq stationary-phase library. Bottom: single fragments (black). Top: S-chimeras (broken lines in blue, indicating that GadF is found always as second RNA in the chimera).
(C) Northern blot of GadF. Total RNA from cells grown to log phase (OD600 = 0.3) (lanes 1, 2) and to stationary phase (6 hr) (lanes 4, 5); RNA coimmunoprecipitated from lysates of hfq-Flag cells (lanes 3, 6) or hfq-WT cells (lanes 7, 8) grown to log or stationary phase.
(D) Schematic representation of a regulatory circuit involving a transcription factor (GadE) and a sRNA (GadF), generated from the same transcript and regulating genes in the acid stress response pathway at the transcriptional and posttranscriptional level, respectively.
(E) The effect of overexpressing PspH on its target, Spf. Northern blot analysis of total RNA from WT or Δhfq cells carrying either a pJV300 plasmid (Control) or a pspH overexpressing plasmid (pspH). The relative Spf level was determined from three independent replicates; Error bars are as in (A).
(F) Spf-PspH base-pairing region. Compensatory mutations in chromosomal spf and in plasmid-borne pspH, of either one base change (G or C) or three base changes (GGG or CCC), are indicated in red.
(G) The effect of compensatory mutations in spf and pspH on Spf levels. WT cells or cells carrying mutations in chromosomal spf were transformed with a PspH (WT or mutated) overexpressing plasmid or with a pJV300 plasmid as control (Cont.). Cells were grown to OD600 = 0.3, and total RNA was extracted and subjected to northern blot.
(H) Schematic description of Spf regulation by PspH. PspH acts as a sponge of Spf, leading to a decrease in Spf level and to abolishment of the negative regulation of Spf targets. In all northern blot experiments, tmRNA served as a loading control.
See also Figure S6.
A Regulatory Circuit Involving a Transcription Factor and a sRNA Encoded in the Same Transcript
E. coli often encounters acidic environments and hence has evolved sophisticated regulatory systems to survive under these conditions. A central transcription factor that regulates genes involved in acid stress response is GadE, which is highly expressed in stationary phase and under acidic stress (Hommais et al., 2004, Masuda and Church, 2003). We identified a putative sRNA in the 3′ UTR region of gadE (annotated originally in our data as gadE.mdtE.IGT and termed here GadF), which was highly abundant among both single and chimeric fragments of stationary-phase libraries (Table S2 and Figure 6B). We further verified that GadF is an autonomous RNA by northern blot analysis (Figure 6C). Apparently, GadF is processed from gadE transcript, as gadE full-length transcripts were recognized by a probe complementary to GadF (Figure S6A), and GadF level substantially declined in RNA-seq libraries treated with 5′ phosphate-dependent exonuclease (Thomason et al., 2015). GadF also appears to be involved in the response to acidic stress, as its RIL-seq targets were enriched with genes involved in acid response (p ≤ 2.7 × 10−5 by Fisher’s exact test). Our results suggest an intriguing structure of a regulatory circuit in which a transcription factor (GadE) and a sRNA (GadF) are encoded in the same transcript and co-regulate genes in the same pathway (Figure 6D).
Two of GadF RIL-seq targets, acrB and cfa, were upregulated upon GadF overexpression, as indicated by GFP reporter assays (Figure 6A). cfa was also upregulated by ArrS (Figure 6A). Cfa protein plays an important role in membrane modification, conferring resistance to acidic stress (Chang and Cronan, 1999). Recently, another sRNA, RydC in Salmonella, was shown to upregulate cfa by sequestration of RNase E binding site at its 5′ UTR (Fröhlich et al., 2013). The binding sites of RydC and cfa are both conserved in E. coli, and indeed the cfa-RydC interaction was detected by RIL-seq. Interestingly, ArrS and GadF probably use the same activation mechanism as RydC, as their putative binding sites on cfa (Table S7) match the RydC binding site reported in Salmonella. Finally, ArrS is also known to stabilize the mRNA of the transcription factor GadE by binding to it in cis. It seems that there is an intricate network of sRNAs involved in regulating the acid stress response in cis and trans.
A 3′ UTR-Derived sRNA Acts as a Sponge of Spf
RIL-seq identified an interaction between a putative sRNA originating from the 3′ UTR region of pspG (annotated originally in our data as pspG.qor.IGR and termed here PspH) and its RIL-seq-identified sole partner, the sRNA Spf. Data from previous studies (Romero et al., 2014, Thomason et al., 2015) suggest that this sRNA could be independently transcribed from its own promoter. PspH overexpression from a plasmid led to almost 5-fold reduction in Spf level in WT E. coli, while in Δhfq mutant the level of Spf was rather low already without PspH, and its overexpression had no effect (Figure 6E). In a complementary assay, Spf overexpression had a small effect on PspH level (Figure S6B). However, since Spf level was high at the tested condition even without plasmid expression, we cannot conclude whether the Spf-PspH interaction also affects the level of PspH. In addition, we showed by compensatory mutations that PspH directly base pairs with Spf through the known binding site of Spf (Beisel and Storz, 2011) (Figures 6F and 6G). Finally, overexpression of PspH led to upregulation of Spf targets sthA and gltA in WT cells, while no change in their expression was observed in Δspf cells (Figure S6C). Our results support a role for PspH as a sponge of Spf (Figure 6H).
Discussion
RIL-seq Pros and Cons
RIL-seq protocol has several prominent advantages: first, by ligating the Hfq-bound RNAs it directly captures the interacting partners without any additional computational sequence matching. Second, RIL-seq does not require manipulations of the cellular RNAs, such as over- or under-expression, and can be applied to cells without interfering with the in vivo cellular environment under study. Third, it simultaneously captures the Hfq-mediated interactions of all sRNAs expressed under a given condition. This is crucial for obtaining a realistic picture of the sRNA interactome, as due to the nature of sRNA-target interaction, targets that are co-regulated by the same RNA affect each other’s binding to the sRNA and sRNAs that share targets affect each other’s binding to the target (Figueroa-Bossi et al., 2009, Lalaouna et al., 2015, Miyakoshi et al., 2015, Overgaard et al., 2009). RIL-seq inherently takes these mutual effects into account. Taken together, these properties of RIL-seq allow it to provide an in vivo snapshot of the Hfq-mediated sRNA interactome under any desired naturally occurring or artificially manipulated condition.
Since targeting by sRNAs is achieved by base pairing, there have been attempts to harness computational approaches employing binding free energy considerations to develop target prediction algorithms, such as CopraRNA (Wright et al., 2013), which also takes into account evolutionary considerations. While RIL-seq is slightly better than CopraRNA at identifying known targets in E. coli (53.4% versus 52.7% sensitivity and 4.7% versus 2.7% precision; see Supplemental Experimental Procedures), it has several important advantages over current computational approaches. (1) It provides a specific sRNA-target network per condition, capturing the effect of competition between RNAs and allowing network comparison between conditions, while the network established by predictions is generic, ignoring the in vivo considerations. (2) It does not require prior knowledge of the RNA sequences, which is essential for computational predictions. (3) RIL-seq is not limited to reported sRNAs and enables the detection of novel sRNAs along with their interacting partners. In fact, RIL-seq can be potentially applied to any bacteria with a sequenced genome, without previous knowledge of its sRNA repertoire, given the interactions are protein mediated. Especially, it can be a powerful tool to reveal sRNA functions in bacterial pathogenesis. Many pathogenic bacteria express Hfq-dependent sRNAs from horizontally acquired virulence regions. These sRNAs as well as their binding sites are usually less conserved, and thus it is more difficult to predict their targets computationally (Padalon-Brauch et al., 2008, Westermann et al., 2016). RIL-seq can overcome this obstacle since it captures interacting RNAs without any prior knowledge of their binding sites.
Like other large-scale approaches, RIL-seq is prone to potential technical biases that can affect the final set of reported interactions. Two major bias types can be noted. (1) Over- or under-representation of true biological interactions due to the characteristics of the involved RNA molecules. These may include, for example, distinct sequences and/or structures that may affect the ligation potential. However, our analysis revealed a negligible ligation bias (Figures S2E–S2G). (2) Capture of non-biological interactions, resulting from non-specific binding to the antibody or ligation of RNAs transiently sharing the same Hfq hexamer. There are weak traces of such interactions in the data, such as those detected for the hfq-WT control, or from in vitro ligation of unrelated RNAs, as observed in the mixed yeast-E. coli library. However, the number of such interactions is rather small and is dramatically reduced with the application of the statistical filter. This emphasizes the importance of the statistical filtering step, which substantially reduces the number of spurious interactions and enriches RIL-seq final set with true interactions. Evidently our stringent criteria filter out known interactions, implying that the network we provide is incomplete. However, this is a price worth paying for increasing the reliability of the reported interactions, which is demonstrated by the GcvB experiment and the common motif analysis (Figure 4 and Table S4). Our assessments strongly imply that the S-chimeras represent duplexes that take place on Hfq and that there is an extensive network of base-pairing RNAs on Hfq.
Different Consequences of sRNA-Target Interaction
The whole spectrum of RNA pairs in RIL-seq data can be divided into two subsets: those that were identified but did not pass the statistical filter and those that passed the statistical filter and are included in the RIL-seq final set (Table S2). Most of the RNA pairs in the first subset (including 59 known interactions) probably represent transient interactions that stem from low expression of the RNA participants under the studied conditions, or weak binding. In support of that we found for the three studied conditions a correlation of about 0.5 (Spearman correlation, p ≤ 2.2 × 10−31) between the expression levels of interacting transcripts and their involvement in chimeric fragments, which was increased to ∼0.75 (p ≤ 2.9 × 10−4) when only the known sRNAs themselves were regarded. Also, the RNA duplexes that did not pass the statistical filter were predicted to be less stable than the other duplexes (median of ∼−4.4 versus ∼−8.2 kcal/mol for computed hybridization free energy (Mückstein et al., 2006); p ≤ 1.0 × 10−10 by Wilcoxon test). Thus, the RNA pairs in the RIL-seq final dataset form relatively stable duplexes on Hfq, as also supported by the motif analysis (Figures 4A–4D). However, while these interactions are relatively stable, their impact on the target’s transcript level or translation was not obvious. Some targets were affected at their transcript or translation level, while others were not (Figure S4). A similar observation was reported recently for miRNA targets revealed by the CLASH methodology (Helwak et al., 2013, Agarwal et al., 2015).
The potential effect of the interaction depends on many factors, which are not necessarily directly represented by RIL-seq data, such as the enrichment of a target mRNA on Hfq. The normalized odds ratio measure, calculated within the computational pipeline, may assist in distinguishing functional targets of a given sRNA from its binders per se under the studied condition (Table S2). The above conjectures about Hfq-mediated sRNA-target interaction and regulation are consistent with current models that suggest continuous recycling of transcripts on Hfq (Fender et al., 2010), and kinetic differences between the interactions and regulatory outcomes for different targets of a sRNA (Fei et al., 2015).
RIL-seq Substantially Expands the sRNA and Target Repertoires
We discovered a total of ∼2,800 putative interactions mediated via Hfq, expanding the current sRNA interactome by an order of magnitude (Table S2). RIL-seq re-discovered ∼56% of previously reported sRNA-target interactions (Table S3) and also identified recently reported less-conventional interactions, such as an interaction between two sRNAs, GcvB and SroC (Miyakoshi et al., 2015).
While the sRNAs detected in the first genome-wide surveys were predominantly independent transcripts encoded in intergenic regions (Argaman et al., 2001, Rivas et al., 2001, Wassarman et al., 2001), recent studies revealed expression of candidate sRNAs originating from various genomic regions, including antisense to coding regions (Thomason et al., 2015), 3′ UTR (Chao et al., 2012, Chao and Vogel, 2016), 5′ UTR, and even coding regions (Bilusic et al., 2014). Consistently, ∼2/3 of RIL-seq interactions involved 25 of the 26 well-characterized sRNAs (Figure S3E), while 1/3 involved other RNAs, including additional sRNAs derived from various genomic regions along with their bound partners. Whereas many of these transcripts were identified before, in transcriptome or Hfq coIP studies, their classification as sRNAs was indefinite, and their capturing with their targets by RIL-seq supports this classification and provides clues into their cellular roles. In turn, finding these putative sRNAs in previous studies supports our conclusions. Table S6 includes additional information about all RNAs in RIL-seq data, including the support of sRNA candidates by previous studies.
Some of the discovered 3′ UTR-derived sRNAs determine intriguing regulatory circuits, where two layers of regulation are encoded within the same transcript. One such regulatory module, encoded in cpxP transcript and defined by the protein CpxP and the sRNA CpxQ, both involved in response to cell envelope stress, was recently reported (Chao and Vogel, 2016, Grabowicz et al., 2016) and identified here as well. Another circuit discovered in our study, which seems to play a role in acid stress response (Figure 6A), is determined by the transcription factor GadE and the sRNA GadF, both encoded in gadE transcript (Figure 6D). In such circuits there is ultimate coordination between the two regulators.
Dynamic Changes in the Hfq-Mediated sRNA Interactome upon Change in Cellular Conditions
RIL-seq data suggest that there is substantial re-wiring of the sRNA-target interaction network between conditions, reflecting the changing transcriptome and the different functional roles of the sRNAs (Figure 7). Changes in interactions between conditions can be achieved either by change in the cellular levels of sRNAs or target mRNAs or by the expression of additional targets that attract the regulator (competition effects). Indeed, we observed both scenarios in our data. Consistent with previous coIP experiments (Chao et al., 2012), we observed a dynamic change in the Hfq-bound sRNA repertoire upon change in cellular conditions (Figure S3E). For example, under iron limitation the relative level of RyhB increased and the number of interactions involving RyhB increased accordingly (Figures 7B–7D). An example of the second scenario is provided by GcvB, for which the distribution of interactions changed dramatically between log and stationary phase (Table S2). Our data indicate that in stationary phase 70% of GcvB’s interactions were with its sponge, SroC (Miyakoshi et al., 2015), lowering the number of interactions with other targets and suggesting a relief of the repression of these targets. Indeed, we observed that the expression levels of most GcvB known targets were higher in stationary phase than in log phase (p ≤ 3.38 × 10−2 by one-sided Wilcoxon test; Table S2). Sponging interactions were also reported for ChiX by chbBC intergenic region (Figueroa-Bossi et al., 2009, Overgaard et al., 2009) and for RyhB and RybB by the 3′ processed region of leuZ tRNA (Lalaouna et al., 2015), all also identified by RIL-seq. Another sponge we identified is PspH, a sRNA overlapping the 3′ UTR of pspG, which interacted with Spf and led to upregulation of its targets (Figures 6E–6H). In summary, increasing and decreasing the activity of a sRNA can be achieved by changing its cellular level or by the binding of a sponge, and RIL-seq data provide hints at these mechanisms.
Figure 7.
Substantial Re-wiring of the Posttranscriptional Regulatory Network between Conditions
(A) Numbers of unique interactions of known sRNAs with targets detected by RIL-seq under the three tested conditions. 1,813 unique interactions were collectively identified for these 25 sRNAs.
(B–D) Re-wiring of the sRNA regulatory network between conditions. RIL-seq interactions are represented on E. coli chromosome. Edges connect between the genomic locations of interacting RNAs, where the thickness of an edge is proportional to the normalized number of chimeras. Interactions involving known sRNAs are marked in black, and all other interactions are in pink. Re-wiring is observed for many sRNAs (note, for example, the increase in RyhB interactions under iron limitation). Network views were drawn by circos software (http://circos.ca/).
Outlook
The examples of sRNA-target interactions discussed here are only the tip of the iceberg of RIL-seq data. Given that ∼95% of the 2,817 unique interactions revealed in our data were not reported before, the potential outcome of investigating them, as well as interactions that might be found in other growth conditions, is exciting. As RIL-seq is not restricted to a certain type of RNA, a specific RNA-binding protein, or a specific organism, it can be widely applied for finding RNA interacting pairs in general and sRNA-target interactions in particular. In many bacteria, including pathogenic or biotechnologically relevant bacteria, sRNA research is in its infancy. Applying RIL-seq to these organisms will provide a precious view of their posttranscriptional regulatory network, leading to further insights into the regulation of their physiology and/or pathogenesis.
Experimental Procedures
Strains and Media
The E. coli MG1655 or TOP10 strains served as wild-type. An E. coli MG1655 strain carrying hfq-Flag (HM34) was used in the RIL-seq protocol. All other bacterial and yeast strains used in this study are listed in Table S8. Bacterial strains were routinely grown at 37°C in rich medium. Antibiotics or 2,2′-Dipyridyl (200 μM) were added where appropriate. All oligonucleotides and plasmids used in this study are listed in Table S8.
RIL-seq Protocol
The RIL-seq protocol is described here in brief and detailed in the Supplemental Experimental Procedures.
Experimental Procedure
hfq-Flag strain or hfq-WT strain (control) were grown to log phase, with or without the addition of the iron chelator 2,2′-Dipyridyl, or to stationary phase, and exposed to 800 mJ of 254 nm UV irradiation in order to in vivo crosslink the proteins and RNA molecules. Following mechanical lysis of the cells, Hfq with its bound RNAs was coimmunoprecipitated using anti-Flag antibody. Exposed regions of the RNAs were trimmed by RNases, the 5′ and 3′ ends of the RNAs were treated with T4 PNK, neighboring RNAs were ligated, the Hfq protein was digested, and RNA was then isolated. Sequencing libraries (RIL-seq and total RNA libraries) were constructed by RNAtag-seq protocol (Shishkin et al., 2015), with a few modifications that prevented loss of sRNA fragments. The libraries were sequenced by paired-end sequencing using Nextseq500 Sequencer (Illumina).
Computational Procedure
Raw reads were processed to remove adaptor sequences, low quality ends, and low-complexity reads. The first 25 nucleotides of each read were mapped to the genome of E. coli K12 MG1655 (GenBank: NC_000913.2), sorting the fragments into “single” and “chimeric” fragments.
Next, for the detection of over-represented chimeras, we divided the genome into non-overlapping 100 nt-long windows and for each window pair counted the number of mapped chimeric sequenced fragments. We applied Fisher’s exact test to identify the chimeric fragments that appeared in the experiment statistically significantly more than expected at random based on the number of single and chimeric fragments mapped to each region (p ≤ 0.05 after Bonferroni correction). The computational pipeline is implemented in Python and is available as a package called RILseq on pypi (https://pypi.python.org/pypi/RILseq). Annotation of inferred interacting RNAs was based on EcoCyc (http://ecocyc.org) version 19.0 (Keseler et al., 2013). Representation of RIL-seq single and chimeric fragments in UCSC genome browser (Kent et al., 2002) can be found in the following links: RIL-seq single fragments (http://microbes.ucsc.edu/cgi-bin/hgTracks?db=eschColi_K12&hgt.customText=http://www.cs.huji.ac.il/~asafp5/RILseq/RILseq_coverage.wig.gz), RIL-seq chimeric fragments (http://microbes.ucsc.edu/cgi-bin/hgTracks?db=eschColi_K12&hgt.customText=http://www.cs.huji.ac.il/~asafp5/RILseq/RILseq_interactions.bed.gz). Note that chimeras with ≥5 sequenced fragments are presented, but only chimeras with ≥10 sequenced fragments were included in all other analyses in the paper; chimera tracks are hidden by default.
GcvB Comparative RIL-seq
RIL-seq experiments were performed in log phase using E. coli ΔgcvB strain (HM54) transformed with a WT gcvB or a gcvBΔR1 plasmid, each repeated three times. Mapping of the sequenced reads was done to the chromosome and to the relevant plasmid. Presented interactions in Figure 4E and Table S5 are those identified in both the unified log-phase libraries and in this experiment.
Classification of RNAs Revealed by RIL-seq
RNAs were classified based on their genomic annotation (see legend of Figure 2 and Supplemental Experimental Procedures). Importantly, only sRNAs with at least one validated trans target were regarded as sRNAs. Genomic regions encoding other annotated small RNAs were all termed other-RNA (oRNA). The fraction among the mapped single, chimeric, and S-chimera fragments was computed for each genomic element. For the assessment of RIL-seq results, we compiled an updated dataset of 154 experimentally determined sRNA-target interactions (Table S3) based on our previous compilations (Peer and Margalit, 2011, Peer and Margalit, 2014) and information in EcoCyc (http://ecocyc.org) version 19.0 (Keseler et al., 2013).
Recognition of Binding Motifs
The target sequences of each sRNA that had at least four targets (in each condition or summed over all tested conditions) were extracted and searched for a common motif by MEME (Bailey et al., 2009). To verify that an identified motif matched a complementary binding site on the respective sRNA, we searched for it in the sRNA reverse-complement sequence using MAST (Bailey and Gribskov, 1998). For more detail, see Supplemental Experimental Procedures.
Author Contributions
H.M. initiated and supervised the study; Methodology, S.M. and A.P.; Experimental Investigation, S.M., R.F.-R., and L.A.; Data Curation, A.P., Y.A., and R.F.-R.; Software Development, A.P.; Computational Analysis, A.P., Y.A., R.F.-R., Y.E.G., N.R., and A.B.; Writing – Original Draft, Review & Editing, S.M., A.P., Y.A., L.A., R.F.-R., and H.M.
Acknowledgments
This study was supported by the European Research Council Advanced Grant #322920, I-CORE Programs of the Planning and Budgeting Committee and The Israel Science Foundation (grants 1796/12 and 41/1), and the Israel Science Foundation administered by the Israeli Academy for Sciences and Humanities (grant 1411/13). We thank T. Kaplan for his helpful advice, R. Rehani for her contribution to the experiments, H. Aiba for the hfq-Flag strain, O. Pines for the Saccharomyces cerevisiae strain, S. Pearl-Mizrahi, O. Shechter, and M. Nitzan for technical assistance, G. Storz for work conducted by S.M. in her lab and for very helpful advice, S. Altuvia, E. Holmqvist, I. Rosenshine, and S. Ben-Yehuda for their very useful comments, and K. McDowall and J. Wade for data sharing.
Published: September 1, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and eight tables and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2016.07.026.
Contributor Information
Yael Altuvia, Email: yaelal@md.huji.ac.il.
Liron Argaman, Email: liron.argaman@mail.huji.ac.il.
Hanah Margalit, Email: hanahm@ekmd.huji.ac.il.
Accession Numbers
The accession number for the sequencing data reported in this paper is ArrayExpress: E-MTAB-3910.
Supplemental Information
References
- Agarwal V., Bell G.W., Nam J.W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaman L., Hershberg R., Vogel J., Bejerano G., Wagner E.G., Margalit H., Altuvia S. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr. Biol. 2001;11:941–950. doi: 10.1016/s0960-9822(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Bailey T.L., Gribskov M. Methods and statistics for combining motif match scores. J. Comput. Biol. 1998;5:211–221. doi: 10.1089/cmb.1998.5.211. [DOI] [PubMed] [Google Scholar]
- Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel C.L., Storz G. The base-pairing RNA spot 42 participates in a multioutput feedforward loop to help enact catabolite repression in Escherichia coli. Mol. Cell. 2011;41:286–297. doi: 10.1016/j.molcel.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilusic I., Popitsch N., Rescheneder P., Schroeder R., Lybecker M. Revisiting the coding potential of the E. coli genome through Hfq co-immunoprecipitation. RNA Biol. 2014;11:641–654. doi: 10.4161/rna.29299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.Y., Cronan J.E., Jr. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 1999;33:249–259. doi: 10.1046/j.1365-2958.1999.01456.x. [DOI] [PubMed] [Google Scholar]
- Chao Y., Vogel J. A 3′ UTR-derived small RNA provides the regulatory noncoding arm of the inner membrane stress response. Mol. Cell. 2016;61:352–363. doi: 10.1016/j.molcel.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Chao Y., Papenfort K., Reinhardt R., Sharma C.M., Vogel J. An atlas of Hfq-bound transcripts reveals 3′ UTRs as a genomic reservoir of regulatory small RNAs. EMBO J. 2012;31:4005–4019. doi: 10.1038/emboj.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C.P., Podkaminski D., Papenfort K., Urban J.H., Hinton J.C., Vogel J. Superfolder GFP reporters validate diverse new mRNA targets of the classic porin regulator, MicF RNA. Mol. Microbiol. 2012;84:428–445. doi: 10.1111/j.1365-2958.2012.08031.x. [DOI] [PubMed] [Google Scholar]
- Darnell R.B. HITS-CLIP: panoramic views of protein-RNA regulation in living cells. Wiley Interdiscip. Rev. RNA. 2010;1:266–286. doi: 10.1002/wrna.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimastrogiovanni D., Fröhlich K.S., Bandyra K.J., Bruce H.A., Hohensee S., Vogel J., Luisi B.F. Recognition of the small regulatory RNA RydC by the bacterial Hfq protein. eLife. 2014;3:e05375. doi: 10.7554/eLife.05375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei J., Singh D., Zhang Q., Park S., Balasubramanian D., Golding I., Vanderpool C.K., Ha T. RNA biochemistry. Determination of in vivo target search kinetics of regulatory noncoding RNA. Science. 2015;347:1371–1374. doi: 10.1126/science.1258849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fender A., Elf J., Hampel K., Zimmermann B., Wagner E.G. RNAs actively cycle on the Sm-like protein Hfq. Genes Dev. 2010;24:2621–2626. doi: 10.1101/gad.591310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Bossi N., Valentini M., Malleret L., Fiorini F., Bossi L. Caught at its own game: regulatory small RNA inactivated by an inducible transcript mimicking its target. Genes Dev. 2009;23:2004–2015. doi: 10.1101/gad.541609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich K.S., Papenfort K., Fekete A., Vogel J. A small RNA activates CFA synthase by isoform-specific mRNA stabilization. EMBO J. 2013;32:2963–2979. doi: 10.1038/emboj.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowicz M., Koren D., Silhavy T.J. The CpxQ sRNA negatively regulates Skp to prevent mistargeting of β-barrel outer membrane proteins into the cytoplasmic membrane. MBio. 2016;7:e00312–e00316. doi: 10.1128/mBio.00312-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosswendt S., Filipchyk A., Manzano M., Klironomos F., Schilling M., Herzog M., Gottwein E., Rajewsky N. Unambiguous identification of miRNA:target site interactions by different types of ligation reactions. Mol. Cell. 2014;54:1042–1054. doi: 10.1016/j.molcel.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M.S., Updegrove T.B., Gogol E.B., Shabalina S.A., Gross C.A., Storz G. MicL, a new σE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev. 2014;28:1620–1634. doi: 10.1101/gad.243485.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwak A., Kudla G., Dudnakova T., Tollervey D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell. 2013;153:654–665. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist E., Wright P.R., Li L., Bischler T., Barquist L., Reinhardt R., Backofen R., Vogel J. Global RNA recognition patterns of post-transcriptional regulators Hfq and CsrA revealed by UV crosslinking in vivo. EMBO J. 2016;35:991–1011. doi: 10.15252/embj.201593360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommais F., Krin E., Coppée J.Y., Lacroix C., Yeramian E., Danchin A., Bertin P. GadE (YhiE): a novel activator involved in the response to acid environment in Escherichia coli. Microbiology. 2004;150:61–72. doi: 10.1099/mic.0.26659-0. [DOI] [PubMed] [Google Scholar]
- Jäger D., Pernitzsch S.R., Richter A.S., Backofen R., Sharma C.M., Schmitz R.A. An archaeal sRNA targeting cis- and trans-encoded mRNAs via two distinct domains. Nucleic Acids Res. 2012;40:10964–10979. doi: 10.1093/nar/gks847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent W.J., Sugnet C.W., Furey T.S., Roskin K.M., Pringle T.H., Zahler A.M., Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keseler I.M., Mackie A., Peralta-Gil M., Santos-Zavaleta A., Gama-Castro S., Bonavides-Martínez C., Fulcher C., Huerta A.M., Kothari A., Krummenacker M. EcoCyc: fusing model organism databases with systems biology. Nucleic Acids Res. 2013;41:D605–D612. doi: 10.1093/nar/gks1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalaouna D., Carrier M.C., Semsey S., Brouard J.S., Wang J., Wade J.T., Massé E. A 3′ external transcribed spacer in a tRNA transcript acts as a sponge for small RNAs to prevent transcriptional noise. Mol. Cell. 2015;58:393–405. doi: 10.1016/j.molcel.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Mandin P., Gottesman S. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 2010;29:3094–3107. doi: 10.1038/emboj.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Church G.M. Regulatory network of acid resistance genes in Escherichia coli. Mol. Microbiol. 2003;48:699–712. doi: 10.1046/j.1365-2958.2003.03477.x. [DOI] [PubMed] [Google Scholar]
- Miyakoshi M., Chao Y., Vogel J. Cross talk between ABC transporter mRNAs via a target mRNA-derived sponge of the GcvB small RNA. EMBO J. 2015;34:1478–1492. doi: 10.15252/embj.201490546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M.J., Scheel T.K.H., Luna J.M., Park C.Y., Fak J.J., Nishiuchi E., Rice C.M., Darnell R.B. miRNA-target chimeras reveal miRNA 3′-end pairing as a major determinant of Argonaute target specificity. Nat. Commun. 2015;6:8864. doi: 10.1038/ncomms9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Maki K., Aiba H. RNase E-based ribonucleoprotein complexes: mechanical basis of mRNA destabilization mediated by bacterial noncoding RNAs. Genes Dev. 2005;19:2176–2186. doi: 10.1101/gad.1330405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Ueda M., Kubo K., Aiba H. Insights into transcription termination of Hfq-binding sRNAs of Escherichia coli and characterization of readthrough products. RNA. 2015;21:1490–1501. doi: 10.1261/rna.051870.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mückstein U., Tafer H., Hackermüller J., Bernhart S.H., Stadler P.F., Hofacker I.L. Thermodynamics of RNA-RNA binding. Bioinformatics. 2006;22:1177–1182. doi: 10.1093/bioinformatics/btl024. [DOI] [PubMed] [Google Scholar]
- Opdyke J.A., Kang J.G., Storz G. GadY, a small-RNA regulator of acid response genes in Escherichia coli. J. Bacteriol. 2004;186:6698–6705. doi: 10.1128/JB.186.20.6698-6705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaka H., Ishikawa H., Morita T., Aiba H. PolyU tail of rho-independent terminator of bacterial small RNAs is essential for Hfq action. Proc. Natl. Acad. Sci. USA. 2011;108:13059–13064. doi: 10.1073/pnas.1107050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard M., Johansen J., Møller-Jensen J., Valentin-Hansen P. Switching off small RNA regulation with trap-mRNA. Mol. Microbiol. 2009;73:790–800. doi: 10.1111/j.1365-2958.2009.06807.x. [DOI] [PubMed] [Google Scholar]
- Padalon-Brauch G., Hershberg R., Elgrably-Weiss M., Baruch K., Rosenshine I., Margalit H., Altuvia S. Small RNAs encoded within genetic islands of Salmonella typhimurium show host-induced expression and role in virulence. Nucleic Acids Res. 2008;36:1913–1927. doi: 10.1093/nar/gkn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer A., Margalit H. Accessibility and evolutionary conservation mark bacterial small-rna target-binding regions. J. Bacteriol. 2011;193:1690–1701. doi: 10.1128/JB.01419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer A., Margalit H. Evolutionary patterns of Escherichia coli small RNAs and their regulatory interactions. RNA. 2014;20:994–1003. doi: 10.1261/rna.043133.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas E., Klein R.J., Jones T.A., Eddy S.R. Computational identification of noncoding RNAs in E. coli by comparative genomics. Curr. Biol. 2001;11:1369–1373. doi: 10.1016/s0960-9822(01)00401-8. [DOI] [PubMed] [Google Scholar]
- Romero D.A., Hasan A.H., Lin Y.F., Kime L., Ruiz-Larrabeiti O., Urem M., Bucca G., Mamanova L., Laing E.E., van Wezel G.P. A comparison of key aspects of gene regulation in Streptomyces coelicolor and Escherichia coli using nucleotide-resolution transcription maps produced in parallel by global and differential RNA sequencing. Mol. Microbiol. 2014;94:963–987. doi: 10.1111/mmi.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer E., Weichenrieder O. Structural basis for RNA 3′-end recognition by Hfq. Proc. Natl. Acad. Sci. USA. 2011;108:13065–13070. doi: 10.1073/pnas.1103420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed N., Jousselin A., Felden B. A cis-antisense RNA acts in trans in Staphylococcus aureus to control translation of a human cytolytic peptide. Nat. Struct. Mol. Biol. 2012;19:105–112. doi: 10.1038/nsmb.2193. [DOI] [PubMed] [Google Scholar]
- Sharma C.M., Papenfort K., Pernitzsch S.R., Mollenkopf H.J., Hinton J.C., Vogel J. Pervasive post-transcriptional control of genes involved in amino acid metabolism by the Hfq-dependent GcvB small RNA. Mol. Microbiol. 2011;81:1144–1165. doi: 10.1111/j.1365-2958.2011.07751.x. [DOI] [PubMed] [Google Scholar]
- Shishkin A.A., Giannoukos G., Kucukural A., Ciulla D., Busby M., Surka C., Chen J., Bhattacharyya R.P., Rudy R.F., Patel M.M. Simultaneous generation of many RNA-seq libraries in a single reaction. Nat. Methods. 2015;12:323–325. doi: 10.1038/nmeth.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittka A., Lucchini S., Papenfort K., Sharma C.M., Rolle K., Binnewies T.T., Hinton J.C., Vogel J. Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. PLoS Genet. 2008;4:e1000163. doi: 10.1371/journal.pgen.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Vogel J., Wassarman K.M. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell. 2011;43:880–891. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.K., Storz G. Bacterial antisense RNAs: how many are there, and what are they doing? Annu. Rev. Genet. 2010;44:167–188. doi: 10.1146/annurev-genet-102209-163523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.K., Bischler T., Eisenbart S.K., Förstner K.U., Zhang A., Herbig A., Nieselt K., Sharma C.M., Storz G. Global transcriptional start site mapping using differential RNA sequencing reveals novel antisense RNAs in Escherichia coli. J. Bacteriol. 2015;197:18–28. doi: 10.1128/JB.02096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree J.J., Granneman S., McAteer S.P., Tollervey D., Gally D.L. Identification of bacteriophage-encoded anti-sRNAs in pathogenic Escherichia coli. Mol. Cell. 2014;55:199–213. doi: 10.1016/j.molcel.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udekwu K.I., Darfeuille F., Vogel J., Reimegård J., Holmqvist E., Wagner E.G. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes Dev. 2005;19:2355–2366. doi: 10.1101/gad.354405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E.G., Romby P. Small RNAs in bacteria and archaea: who they are, what they do, and how they do it. Adv. Genet. 2015;90:133–208. doi: 10.1016/bs.adgen.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Wang J., Rennie W., Liu C., Carmack C.S., Prévost K., Caron M.P., Massé E., Ding Y., Wade J.T. Identification of bacterial sRNA regulatory targets using ribosome profiling. Nucleic Acids Res. 2015;43:10308–10320. doi: 10.1093/nar/gkv1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman K.M., Repoila F., Rosenow C., Storz G., Gottesman S. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 2001;15:1637–1651. doi: 10.1101/gad.901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann A.J., Förstner K.U., Amman F., Barquist L., Chao Y., Schulte L.N., Müller L., Reinhardt R., Stadler P.F., Vogel J. Dual RNA-seq unveils noncoding RNA functions in host-pathogen interactions. Nature. 2016;529:496–501. doi: 10.1038/nature16547. [DOI] [PubMed] [Google Scholar]
- Wright P.R., Richter A.S., Papenfort K., Mann M., Vogel J., Hess W.R., Backofen R., Georg J. Comparative genomics boosts target prediction for bacterial small RNAs. Proc. Natl. Acad. Sci. USA. 2013;110:E3487–E3496. doi: 10.1073/pnas.1303248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Wassarman K.M., Rosenow C., Tjaden B.C., Storz G., Gottesman S. Global analysis of small RNA and mRNA targets of Hfq. Mol. Microbiol. 2003;50:1111–1124. doi: 10.1046/j.1365-2958.2003.03734.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.