Abstract
Peroxidases (PRXs) are widely existed in various organisms and could be divided into different types according to their structures and functions. Specifically, the Class III Peroxidase, a plant-specific multi-gene family, involves in many physiological processes, such as the metabolism of auxin, the extension and thickening of cell wall, as well as the formation of lignin. By searching the pear genome database, 94 non-redundant PRXs from Pyrus bretschneideri (PbPRXs) were identified. Subsequently, analysis of phylogenetic relationships, gene structures, conserved motifs, and microsynteny was performed. These PbPRXs were unevenly distributed among 17 chromosomes of pear. In addition, 26 segmental duplication events but only one tandem duplication were occurred in these PbPRXs, implying segmental duplication was the main contributor to the expansion of the PbPRX family. By the Ka/Ks analysis, 26 out of 27 duplicated PbPRXs has experienced purifying selection. Twenty motifs were identified in PbPRXs based on the MEME analysis, 11 of which were enriched in pear. A total of 41 expressed genes were identified from ESTs of pear fruit. According to qRT-PCR, the expression trends of five PbPRXs in subgroup C were consistent with the change of lignin content during pear fruit development. So we inferred that the five PbPRXs were candidate genes involved in the lignin synthesis pathway. These results provided useful information for further researches of PRX genes in pear.
Keywords: pear, class III peroxidase, gene duplication, microsynteny, expression
Introduction
Pathogenesis related protein (PR) is a type of stress-responsive protein whose expression could be induced by pathogen invasion. According to their amino acid sequences, structures and functions, the PRs could be classified into 17 subfamilies, including Class III Peroxidase (PRX) subfamily (Van Loon et al., 2006; Almagro et al., 2009). As we know, PRXs are widely existed in all kinds of animals, plants and microorganisms. The previous study showed that plants have two kinds of peroxidases, i.e., Class I PRX and Class III PRX (Cosio and Dunand, 2010). Class I PRX contains ascorbate peroxidase (APx) and glutathione peroxidase (GPx), while Class III PRX is a large gene family, including a plurality of members. These Class III Peroxidases members are involved in two possible catalytic cycles, and maintaining both peroxyl radicals and H2O2 at a stable level in plants. Moreover, they could be extensively involved in many physiological processes of plants, such as resistance to pathogen infection, formation of lignin, auxin decomposition metabolism, seed germination and aging, etc. (Passardi et al., 2004; Almagro et al., 2009; Cosio and Dunand, 2009; Kwon et al., 2015). The numbers of Class III PRX family in Arabidopsis thaliana, Zea mays, and Populus are 73, 107, and 93, respectively (Almagro et al., 2009; Ren et al., 2014; Wang et al., 2015b). Current research showed that 44 Class III PRX genes in Arabidopsis thaliana might be involved in one or more specific mechanisms. For example, AtPrx21, AtPrx34, AtPrx37, AtPrx52, AtPrx59, AtPrx69, and AtPrx71, could resist to the infection of Pseudomonas syringae (Mohr and Cahill, 2007). And 17 Class III PRX genes appeared to decline in A. thaliana under microgravity condition, 12 genes of which accumulated in root hairs to participate in the biosynthesis of root hair cell wall, oxidative stress and root development process (Bruex et al., 2012; Petricka et al., 2012; Lan et al., 2013; Kwon et al., 2015). In addition, Ehlting et al. (2005) found that 8 PRX genes (At2g37130, At5g42180, At4g37530, At4g37520, At1g30870, At2g43480, At3g28200, and At5g05340) were involved in the biosynthesis of lignin in A. thaliana.
The PRX gene family has been well studied in several model plants, such as Arabidopsis thaliana (Tognolli et al., 2002; Welinder et al., 2002), Populus trichocarpa (Ren et al., 2014), and Zea mays (Wang et al., 2015b). However, to date, no previous systematic study of this gene family has been conducted in Chinese pear. In this study, a genome-wide analysis of PRX gene family from Chinese pear (Pyrus bretschneideri) was conducted via genomic sequence, including PbPRX gene models, phylogenetic relationship, genomic structure, chromosome location and other structural features. Furthermore, the expression patterns of 41 PbPRX genes during fruit development of pear were also analyzed based on the quantitative real-time PCR (qRT-PCR). The obtained results will establish a solid foundation for further research of the functional roles of PRX genes in Chinese pear.
Materials and methods
Identification and collection of PRX proteins
In present study, the pear genome database was downloaded from the Pear Genome Project (http://peargenome.njau.edu.cn/), peach was obtained from the Phytozome database (https://phytozome.jgi.doe.gov/pz/portal.html), yang mei was downloaded from the GigaDB database (http://gigadb.org/site/index), and strawberry was obtained from the GDR database (http://www.rosaceae.org/). Subsequently, to identify and annotate PRX proteins in Rosaceae (pear, peach, yang mei, and strawberry), the following approaches were performed. (i) The Hidden Markov Model (HMM) profile of PRX (PF00141) was obtained from the Pfam database (http://pfam.sanger.ac.uk/search) (Punta et al., 2011). (ii) The HMM profile was used to survey all pear proteins by DNAtools software. (iii) To confirm the reliability of searched results, all candidate protein sequences were checked in SMART (Letunic et al., 2012) and Pfam (Punta et al., 2011). (iiii) By sequence alignment and chromosomal location analysis, all non-redundant PRX proteins were identified for further analysis.
Phylogenetic analysis
For the phylogenetic tree of PbPRX, 94 full-length PRX proteins were aligned using ClustalX2.1 (http://clustalx.software.informer.com/2.1/). By the alignments, an unrooted neighbor-joining (N-J) tree was constructed by MEGA 7.0 software (Kumar et al., 2016) with bootstrap analysis (1000 replicates). For the composite phylogenetic tree, PRX proteins from peach, yang mei, strawberry, and Chinese pear were similarly aligned with ClustalX 2.1. Phylogenetic trees were generated using the neighbor-joining (N-J) method with MEGA 7.0 software (Kumar et al., 2016).
Gene structural and conserved motif analysis
Genomic sequences of Chinese pear v.1.0 annotation were obtained from GigaDB database (http://gigadb.org/site/index). CDS sequences were aligned to DNA sequences and schematics generated using Gene Structure Display Server (http://gsds.cbi.pku.edu.cn) (Guo et al., 2007). Sequences of the 94 PbPRX proteins were analyzed using online MEME website (http://meme-suite.org/tools/meme) (Bailey et al., 2015) with the following parameters: minimum motif width, 6; maximum motif width, 200; maximum number of motifs, 20. The conserved motifs were annotated using the SMART database (Letunic et al., 2012) and Pfam database(Punta et al., 2011). HMMs were constructed from the MEME alignment of each motif using DNAtools software. PRX protein sequences of Arabidopsis thaliana, Zea mays, Populus, and Vitis vinifera retrieved from the TAIR database (http://www.arabidopsis.org/) and Phytozome database (https://phytozome.jgi.doe.gov/pz/portal.html) were searched with the HMMs using DNAtools software.
Chromosomal localization and test for selection neutrality
Chromosomal linkage visualization was proformed by MapInspect software (http://mapinspect.software.informer.com/). Synonymous (Ks) and nonsynonymous (Ka) substitution rates were calculated by DnasP v5.0 (Librado and Rozas, 2009). The selection modes of PbPRX paralogs were determined by analyzing the Ka/Ks ratios. Subsequently, a sliding window analysis of nonsynonymous substitutions per nonsynonymous site Ka/Ks ratios was conducted with the following parameters: window size, 150 bp; step size, 9 bp.
Interspecies microsynteny analysis
To detect the syntenic regions among peach, yang mei, strawberry and Chinese pear, the MCScanX software (Wang et al., 2012) was used. Subsequently, the duplicated PRXs were identified in these syntenic regions, as well as the relationships of PRX orthologous pairs among peach, yang mei, strawberry and Chinese pear were plotted using Circos software (Krzywinski et al., 2009).
Expression analysis by ESTs
Chinese white pear EST sequences were downloaded from Pear Genome Project (http://peargenome.njau.edu.cn/). PbPRX genes were used as query to blast against Chinese pear ESTs using BLASTN and the parameters were set as follows: E-value < 10e−10, length > or = 200 bp, and maximum identity >95%.
Plant material
The Chinese pears, picked from 40-year-old pear trees grown on a pear orchard (Dangshan, Anhui, China). Ten robust and healthy trees that were managed in a consistent manner were selected. In April 2015 (i.e., when the trees were at the bud stage), short branches bearing buds of similar developmental stages and sizes were selected from the mid-crown area on the south side of each tree, and then labeled. Only two fruits were kept for each short branch. Fruits were picked every 8 days from 15 days after flowering. Forty uniformly sized fruits were collected at each time, refrigerated, and transferred to the laboratory for further experiment.
RNA extraction and qRT-PCR
In present study, eight fruit samples at 15days after flowering (DAF), 39 DAF, 47 DAF, 55 DAF, 63 DAF, 79 DAF, 102 DAF, and 145 DAF were collected for quantitative real-time PCR (qRT-PCR) analysis. We used the RNAprep pure Plant Kit (Tiangen, Beijing) to extract total RNA from all the samples based on the manufacturer's instructions. Subsequently, about 1 μg of total RNA was used for reverse transcription using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Japan). We designed gene-specific primers (Table S1) to amplify 41 PbPRX genes using Beacon Designer 7 software. qRT-PCR analysis was carried out Bio-rad CFX96 Touch™ Deep Well Real-Time PCR Detection System using SYBR® Premix Ex Taq™ II (Takara, Japan) based on the manufacturer's protocol. Pyrus tubulin gene (Wu et al., 2012) was used as internal control gene and the relative expression levels of these PbPRXs were estimated by the 2−ΔΔCT method (Livak and Schmittgen, 2001). The statistical analyses were performed using SPSS20.0 software. The data were presented as mean value ± standard error. Student's t-test was used to illuminate the statistical analysis of significant differences between controls (15 DAF) and the treatments.
Determination of the lignin content of pear
According to Syros et al. (2004), 0.02 g dry powder of pear stone cell was transferred into the 10 mL grinding mouth tube, and then 2 mL 25% acetyl bromide-glacial acetic acid (w/w) and 0.1 mL of perchloric acid were add, followed by the water bath for 30 min at 70°C (oscillation every 10 min). After the dry powder dissolved, 3 mL 2 mol/L NaOH was added and the mixture was transferred to the centrifuge tube, and the supernatant was fixed volume to 50 mL with ice acetic. The absorbance value (ABS) was measured at 280 nm, 3 repeats.
Results
Identification of PRX genes in chinese pear
To identify members of PRX family, HMM searches were performed against the pear genome database with the BLASTP program. A total of 101 candidate PRX proteins were identified in Chinese pear. To identify the members of PRX proteins family, the proteins were checked for the presence of PRX domain by Pfam database (Punta et al., 2011) and SMART database (Letunic et al., 2012). Four of 101 candidate PRX proteins were discarded in this study because of the lacking of PRX domain or complete PRX domain. In addition, 3 redundant PRX proteins were excluded based on the sequences similarity analysis. All other 94 non-redundant PbPRXs were named as PbPRX1-PbPRX94, and used for further analysis. Their detailed information, including chromosome location, gene name and gene identifier, was listed in Table S2.
Phylogenetic analysis of the PRX genes in four species from rosaceae
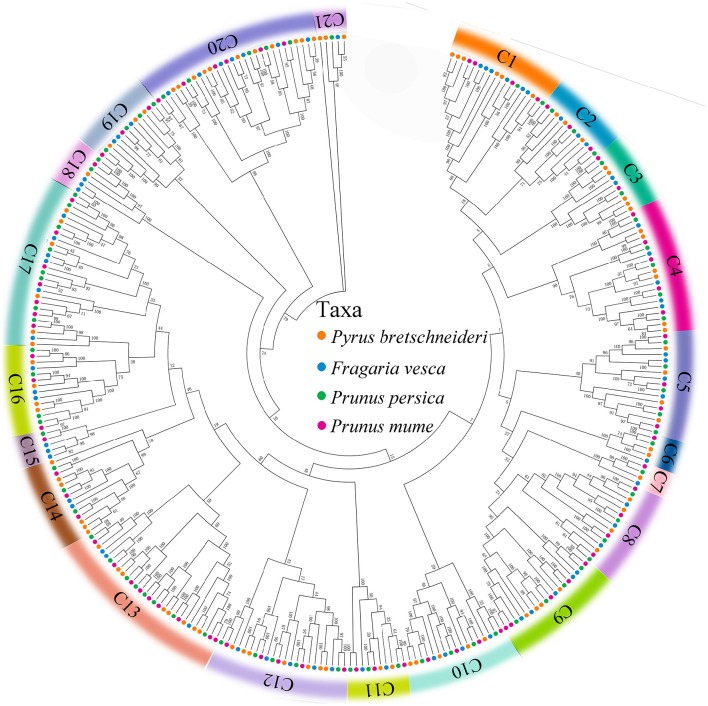
To investigate the evolutionary relationships of the PRX gene family in peach, yang mei, strawberry, and Chinese pear, we used all of the PRX genes from peach (74), yang mei (76), strawberry (73), and Chinese pear (94) to construct an Neighbour-Joining (N-J) phylogenetic tree by MEGA 7.0 software (Kumar et al., 2016). Bootstrapping tests were performed on this tree. Based on the bootstrap values and the topology of the tree, all of the PRX proteins were divided into 21 clades (Figure 1 and Figure S1), designated as clade 1 to clade 21, with strong bootstrap values. The results showed that there was not equal representation of peach, yang mei, strawberry, and Chinese pear PRX genes within given clades (C1-C21). For instance, the C7 subfamily includes four peach PRX genes, five yang mei PRX genes, five strawberry PRX genes, and includes six Chinese pear PRX genes (Figure 1). In contrast, phylogeny subfamily C15 includes three strawberry PRX genes, one peach PRX gene, and one yang mei PRX genes, but not one pear PRX gene. Remarkably, we found that PRX genes from peach and yang mei show close pairwise relationships based on genetic distance, compared with other proteins from different species (Figure 1 and Figure S1).
Figure 1.
Phylogenetic relationships and subfamily designations in PRX proteins from Pyrus bretschneideri Rehd, Prunus mume, Prunus persica, and Fragaria vesca. Theneighbor-joining tree includes 94 PRX proteins from Pyrus bretschneideri Rehd, 76 from Prunus mume, 74 from Prunus persica and 73 from Fragaria vesca. These proteins were divided into 21 Clades (C1–C21), and were represented by different colors, respectively.
Interestingly, we found that each of the four Rosaceae species (peach, yang mei, strawberry, and Chinese pear) contributed at least one PRX gene to each clade by a strong bootstrap support, except clade 15. This result implied that rapid duplication of PRX genes occurred before these dicotyledon species diverged (Figure 1 and Figure S1).
Phylogenetic, structural, and conserved motifs analyses of PRX proteins in pear
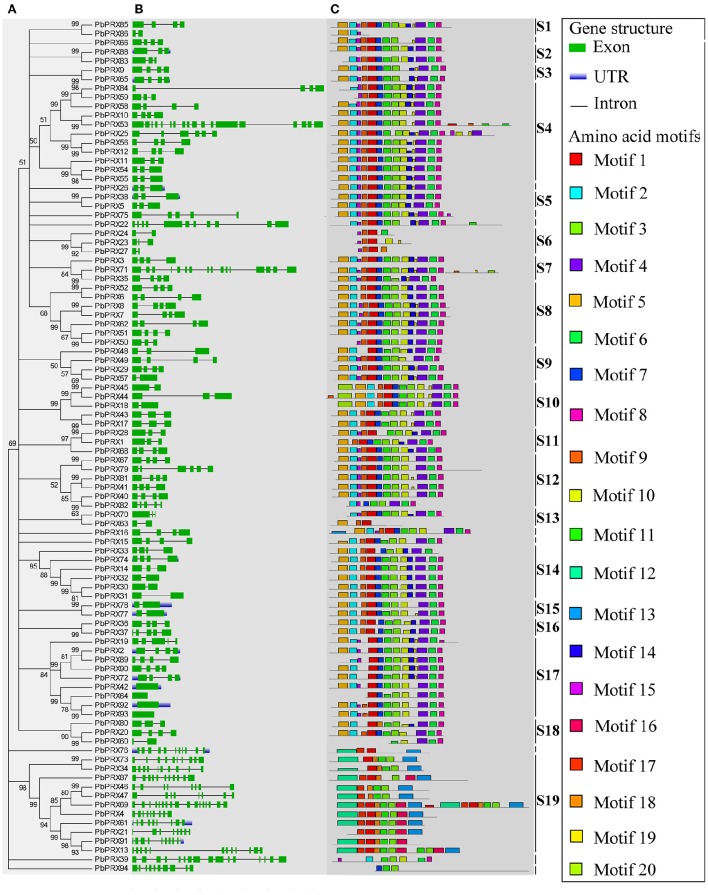
A phylogenetic tree of 94 PbPRX proteins in pear was constructed using Neighbour-Joining method. By phylogenetic analysis, these PbPRXs were further divided into 19 subfamilies (Figure 2A), designated as S1 to S19, with strong bootstrap values. The subfamily S4 and S19 are of the largest two groups with 11 members. On the contrary, subfamily S1, S2, S3, S5, S13, S15, and S16 only had two members (Figure 2A).
Figure 2.
Predicted Pyrus bretschneideri Rehd PRX (PbPRX) protein phylogeny, conserved motifs and exon–intron structure. (A) Unrooted Neighbour-Joining phylogeny of PbPRXs, with bootstrap values>50. (B) Exon/intron organization of PbPRX gene models. (C) Distribution of amino acid motifs in pear PRX proteins. Exons, introns, and untranslated regions (UTRs) are indicated by green boxes, gray lines and blue lines, respectively. Relative protein or gene lengths can be estimated by gray bars.
To understand their functional regions, conserved motifs analyses of PbPRX proteins were performed. Twenty conserved motifs (Table S3) with 6-200 residues in the 94 PbPRX proteins were identified using the MEME tool (Bailey et al., 2015). Motif composition and arrangement were in good agreement with the phylogenetic tree (Figure 2C). The arrangement and composition of the motifs were basically consistent with the results of the phylogenetic tree (Figure 2). Using Hidden Markov Models (HMMs) describing subdomains A-F of the PRX protein based on the previous approaches (Ooka et al., 2003; Hussey et al., 2015), we assigned Motif 1, Motif 2, Motif 3, and Motif 4 to subdomain A, Motif 5, Motif 6, Motif 7, Motif 8, and Motif 9 to subdomain B, subdomain C, subdomain D, subdomain E, and subdomain F, respectively. In addition, we found that these motifs occurred in the N-terminal half of PbPRX proteins (Figures 2, 3). Due to their distribution in most (>65%) of the PbPRX proteins, these motifs were called “general motifs.” The remaining Motif 10–20 were classified as “specific motifs” that were restricted to 3–47 PbPRX proteins (Figure 3). Specific motifs were occurred in the diverse C-terminal region with unknown function (outside the PRX domain), based on both annotation of SMART (Letunic et al., 2012), and Pfam databases (Punta et al., 2011).
Figure 3.
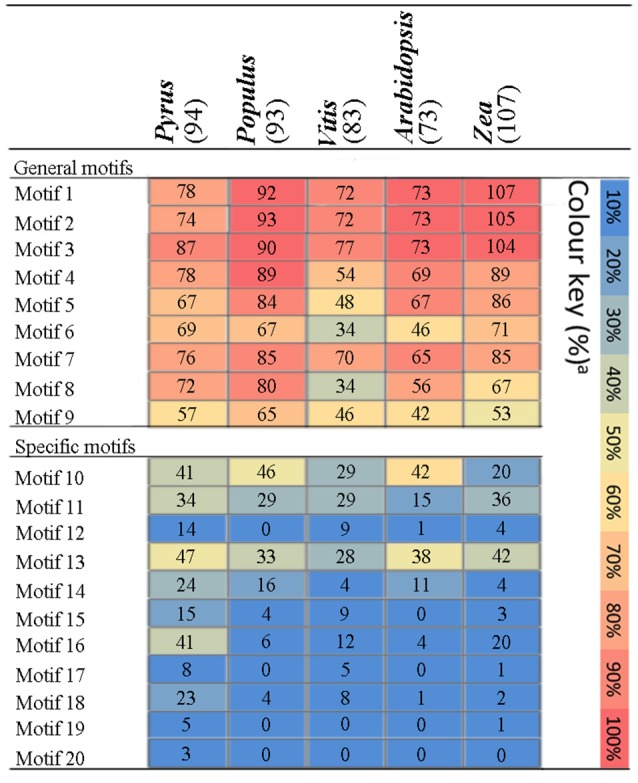
Distribution of amino acid motifs in PRX proteins from Pyrus bretschneideri Rehd, Populus trichocarpa, Arabidopsis thaliana, Vitis vinifera, and Zea mays. Colure key: The percentage of PRX sequences in each genome containing a particular motif was represented by a heat map.
The distribution of these motifs in other plant genomes was further evaluated by HMMs that were obtained from the pear alignments of each motif (Table S3). The matching motifs were identified from the PRX pronteins of Populus (Ren et al., 2014), A. thaliana (Livak and Schmittgen, 2001), Vitis vinifera, and Zea mays (Wang et al., 2015b), using pear as a positive control. The general motifs were enriched in PRX proteins from the genomes tested (Figure 3). By contrast, compared with other genomes, the specific motifs of the PRX protein were detected at high frequencies in pear, with the exception of Motif 10 and Motif 11. Motif 17 only exist in pear, Vitis vinifera and Zea mays (Figure 3), implying that it might be related to the maturation or quality of fruits. Interestingly, Motif 20 was exclusively found in PbPRX proteins and may thus represent motifs unique to pear (Figure 3). Compared to other genomes, there was no bias observed in the cumulative frequency of general motifs identified in pear, so pear-specific motifs were an artifact of HMMs built on pear alignments is impossible.
Then, exon-intron analysis was performed in PbPRX genes (Figure 2B). The results revealed that, there were no introns in PbPRX42, PbPRX64, PbPRX92, and PbPRX93, whereas in the others the intron number was ranged from 1 to 17 (Figure 2B). Additionally, both numbers of intron and exon were well conserved between closely related genes. These results were consistent with the previously reported for maize PRX genes (Wang et al., 2015b).
Interspecies microsynteny analysis
To understand the evolutionary relationship of the PRX gene family among peach, yang mei, strawberry, and Chinese pear, interspecies microsynteny analysis was conducted to identify orthologous PRX genes (Figure 4 and Table S4). Our results show that pear and yang mei shared the most orthologous pairs, of up to 46 pairs of orthologous PRXs, followed by pear and peach (41). However, we only identified 39 pair of orthologous PRXs between pear and strawberry (Figure 4 and Table S4). These results may reflect the closer relationship between pear and peach/yang mei vs. pear and strawberry. Additionally, we found some orthologous gene pairs between pear and peach/yang mei, and were not found between pear and strawberry (Figure 4 and Table S4), such as PbPRX29/ppa023401m/Pm019665, PbPRX33/ppa008667m/Pm004069, PbPRX36/ppa025451m/Pm005657, indicating these orthologous pairs appeared after strawberry diverged from the common ancestor of pear and peach/yang mei. Interestingly, we found a series of two or more matched one PRX gene between pear and peach/yang mei/strawberry. We speculated that these genes were paralogous gene pairs and played a key role in the expansion of the PRX gene family in the process of evolution. For instance, Pm026922 and Pm020853 are orthologous genes to PbPRX4, ppa023088m, and ppa008516m are orthologous genes to PbPRX41, as well as mrna18099 and mrna25391 are orthologous genes to PbPRX13 (Figure 4 and Table S4).
Figure 4.
Microsynteny of PRX regions across pear, yang mei, peach, and strawberry. The pear, yang mei, peach and strawberry chromosomes are shown in different color boxes, as well as labeled Pb, Pm, Pp, and Fv, respectively. Syntenic relationships between PRX regions are represented by gray lines.
Physical distribution of pear PRX genes
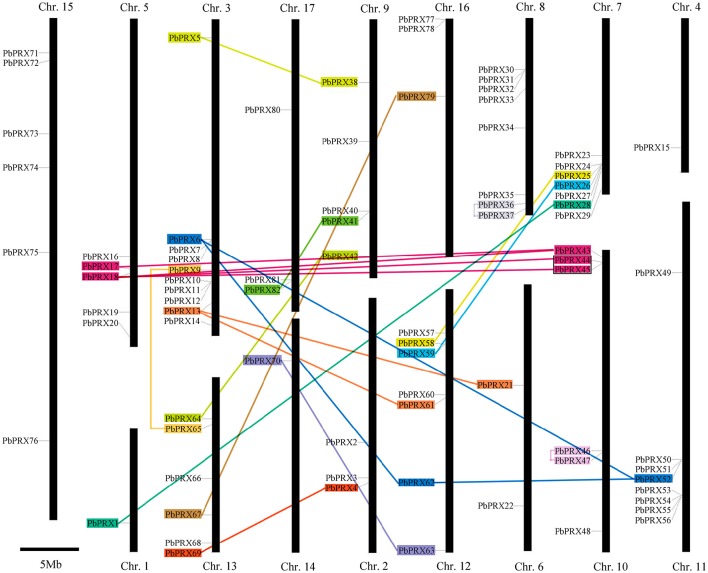
In our research, 94 PbPRXs were distributed among 17 chromosomes or scaffolds, based on their positions in the pear genome. Chromosome 3 contained highest numbers of PbPRXs (10), followed by Chromosome 8 (8) and Chromosome 11 (8). By contrast, there presented only one PbPRX on both chromosome 1 and chromosome 4 (Figure 5). In addition, higher density of PbPRXs was found on some regions of chromosomes, such as the top of chromosome 10, and the bottoms of chromosomes 3, 7, 11, respectively. The PbPRX family has undergone through many processes, including tandem duplication, segmental duplication, or whole-genome duplication. To further understand how PbPRX genes were evolved, gene duplication events were investigated in pear. In present study, 26 gene pairs were arranged in blocks of segmental duplicate and only one gene pairs (PbPRX44/PbPRX45) were arranged in blocks of tandem duplicate (Figure 5). These results strongly implied that segmental duplication events were the major contributors to the expansion of the pear PRX family, but tandem duplication events. However, in previous studies, segmental duplication (16) and tandem duplication (12) were identified in maize PRX family (Wang et al., 2015b). There was a significant difference with the expansion pattern of the PRX genes in maize and pear, which strongly implied that PRX family members of different species have different expansion pattern. It might be the reason why the PRX family members (94) in pear were less than those in the maize (119) (Wang et al., 2015b).
Figure 5.
Chromosomal locations of Pyrus bretschneideri Rehd PRX (PbPRX) genes. Segmental duplicates represented by color boxes and connected by color lines, and tandem duplicates are marked by black sides.
Strong purifying selection for PRX genes in pear
The above results indicate that the gene duplication events were the main contributor to the expansion of the PRX gene family in pear. To explore the selection pressures acting on this gene family, Ka, Ks, and Ka/Ks ratios were calculated for the 27 gene pairs. Generally, Ka/Ks < 1 indicates purifying or negative selection, Ka/Ks = 1 stood for neutral selection and Ka/Ks > 1 stood for positive selection. In this study, all the Ka/Ks values from the 26 pairs of pear PRX gene were less than 0.6, with the exception ofPbPRX36/PbPRX37 (Figure 6 and Table S5). So we proposed that the PRX gene family had mainly undergone strong purifying selection, with the slowly evolving at the protein level of this gene family. Subsequently, the dates of the duplication events were estimated according to estimations for Ks. As a result, the duplicated events in pear were calculated to have occurred at about 8 to 84.3 million years ago (Table S5).
Figure 6.

Scatter plots of the Ka/Ks ratios of duplicated PRX genes in pear. X-axis represents the synonymous distance, and y-axis the Ka/Ks ratio for each pair, respectively.
In the process of positive selection, some codon sites might be masked by the overall strong negative selection. Therefore, a sliding-window analysis of Ka/Ks ratios were performed between each pair of the duplicated PbPRX. As expected, numerous sites/regions were under neutral to strong purifying selection based on the sliding window analysis of Ka/Ks values (Figure S2). Meanwhile we also clearly found that most of Ka/Ks ratios across coding regions were far less than 1, except for one or several distinct peaks (Ka/Ks > 1). Interestingly, the conserved domains of PbPRX have undergone strong purifying selections (Ka/Ks < 1). Eight exception (PbPRX6/PbPRX52, PbPRX9/PbPRX65, PbPRX17/PbPRX43, PbPRX42/PbPRX84, PbPRX36/PbPRX37, PbPRX44/PbPRX45, PbPRX59/PbPRX84, and PbPRX67/PbPRX79) with Ka/Ks ratios >1 in sites of their PRX domains (Figure S2), suggested a positive selection in this region, indicating these genes undergone slightly different selection pressure. Moreover, positive selection could result in a higher ratio of Ka/Ks. However, it could not guarantee that the average Ka/Ks ratio of the gene would be more than one. These results strongly suggested that purifying selection might play a key role in the evolution of the PRX gene family in pear.
Expression characteristics of pear PRX genes
To further explore PRX gene functions in pear, expression of PbPRX genes during fruit development were investigated. First, the PbPRX genes were searched for in the pear expressed sequence tags (ESTs) database. 41 out of 94 PbPRX genes were found to have EST hits (Table S6). A total of 96 EST hits were found for all PbPRX genes, and PbPRX20 contained highest number of EST hits (44), followed by PbPRX59 (4). However, 53 PbPRX genes were not searched any EST hits in the EST database, revealing the functions of these genes need to be further studied at other time points or other tissues.
Then, qRT–PCR was conducted using fruit samples (15 DAF, 39 DAF, 47 DAF, 55 DAF, 63 DAF, 79 DAF, 102 DAF, and 145 DAF). 33 out of 41 PbPRX genes showed a variety of expression patterns during different developmental stages of pear fruit (Figure 7). According to the expression profiles, the PbPRXs can be divided into six subgroups (A–F). This result revealed that most subgroups have similar expression patterns (Figure 7). Three genes from subgroup A (PbPRX61, PbPRX73, and PbPRX81) showed the highest transcript level at 145 DAF; four from subgroup B (PbPRX3, PbPRX26, PbPRX49, and PbPRX78) at 39 DAF; three from subgroup D (PbPRX20, PbPRX25, and PbPRX94) at 79 DAF; eighteen from subgroup E (PbPRX48, PbPRX56, PbPRX68, and PbPRX88 and subgroup F: PbPRX4, PbPRX7, PbPRX8, PbPRX19, PbPRX36, PbPRX41, PbPRX42, PbPRX53, PbPRX59, PbPRX69, PbPRX74, PbPRX83, and PbPRX92) at 15 DAF, indicating that these genes might play important roles in the early stages, or middle stages, or mature stage of pear fruit development.
Figure 7.

Heat map representation of PbPRX genes the eight stages of pear fruit development, 15 days after flowering (DAF), 39 DAF, 47 DAF, 55 DAF, 63 DAF, 79 DAF, 102 DAF, and 145 DAF. These expression profile data were obtained using qRT-PCR, and relative expression was log2 transformed, thereby values −2, −1, 0, 1, and 2 represent low, intermediate and high expression, respectively.
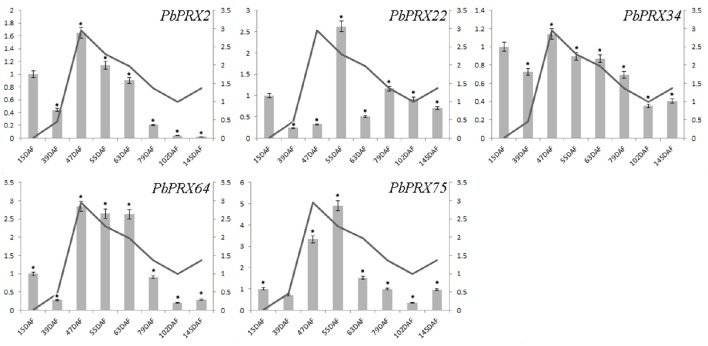
The content of stone cells was an important factor to affect the quality of pear fruit. As one of main components in stone cell walls, lignin synthesis had a direct impact on the formation of stone cells which are enriched in pear fruit (Cai et al., 2010; Jin et al., 2013). In addition, the change of lignin content was also correlated with that of stone cell content (Cai et al., 2010; Jin et al., 2013). In this study, we found the lignin content was relatively low at the beginning of pear fruit development, then reached a peak at the middle stage, and gradually decreased at mature stage (Figure 8). Interestingly, five genes from subgroup C (PbPRX2, PbPRX22, PbPRX34, PbPRX64, and PbPRX75) showed the tendency with the highest transcription accumulation at 47, 55, or 63 DAF (that is at the middle stage), which were consistent with physiological indicators of lignin in pear fruit. Therefore, it was postulated that these genes should be considered as putative candidate genes involved in regulation of the lignin synthesis pathway for pear fruit.
Figure 8.
Comparison of relative expression levels of five PbPRXs involved in lignin synthesis and content of stone cells during fruit development in Pyrus bretschneideri. Y- axes on the left indicates the relative gene expression levels:15, 39, 47, 55, 63, 79, 102, 145 DAF (X-axis) by bar charts, and the Y- axes on right showed the content of stone cells during fruit development with line charts. Each histogram represents the mean value and the bar ± standard error of three biological replicates. *significant difference at P < 0.05.
Discussion
Members of Class III Peroxidases gene family are involved in the regulation of a variety of processes (Tognolli et al., 2002; Ren et al., 2014; Wang et al., 2015b). Systematic and comprehensive analyses of PRX gene families have been published for Populus trichocarpa (Ren et al., 2014), Zea mays (Wang et al., 2015b), Arabidopsis thaliana (Tognolli et al., 2002), and Oryza sativa (Passardi et al., 2004). As the Chinese Pear Genome Project (http://peargenome.njau.edu.cn/) was completed in 2012 (Wu et al., 2013), the genome data of pear (Pyrus bretschneideri Rehd.) provides a useful tool for analysis of the pear PRX gene family. In the present study, 94 non-redundant PRX proteins were identified in pear, which consist of one of the largest PRX families known in woody plants. Subsequently, gene structure, chromosomal locations, phylogenic relationships, gene duplication events, and interspecies microsynteny analysis were performed.
To explore the evolutionary relationships of PRX gene family in Rosaceae, phylogenetic tree was constructed with 94 non-redundant PbPRXs, plus74 PpPRXs in peach, 76 PmPRXs in yang mei, and 73 FvPRXs in strawberry by MEGA software (Kumar et al., 2016) using Neighbour-Joining method, which divided into 21 clades. These results revealed that most of clades contained different numbers of PRX genes from pear, peach, yang mei, and strawberry, displaying a relatively conserved evolution in the Rosaceae species tested. In addition, certain divergences have also been found among these species. For example, the PRXs genes from pear and yang mei/peach appear to be more closely related to each other than to those from strawberry. This conclusion was supported by the analysis of interspecific microsynteny in pear, peach, yang mei, and strawberry (Figure 4), such as the number of orthologous genes between pear and yang mei (46), pear and peach (41) was higher than that between pear and strawberry (39).
In the process of genome evolution, tandem duplication and segmental duplication were the main factors that led to the expansion of gene family (Cao et al., 2016b). Like A. thaliana, maize, and Populus, the genome of the Chinese pear has undergone several genome-wide duplication events in early evolution (Wu et al., 2013). A total of 26 PbPRX gene pairs were identified as segmental duplication in this study, which accounted for 35% of the total genes. However, only PbPRX46 and PbPRX47 were involved in tandem duplication, indicating segmental duplication was the main way to expand PRX genes in Chinese pear. In Populus, about 38% of the PRX genes were involved in segmental duplication, and there were no obvious gene clusters and tandem duplications in the Populus genome (Ren et al., 2014). On the other hand, about 26% of the PRX genes were involved in the segmental duplication, and 20% of the PRX genes were involved in tandem duplication in maize (Wang et al., 2015b). These results showed that segmental duplication might be more significant in both PRX genes of poplar (38%) and Chinese pear (35%) than those of maize (26%). These results suggested that the segmental duplication is likely the main reason for the expansion of PRX gene family in Chinese pear and poplar. However, in maize, the segmental duplication and tandem duplication almost identically contributed to the PRX gene family expansion. These also explain why the number of PRX genes in Chinese pear (94) and Populus (93) were less than those in maize (119).
Notably, the conserved motif 19 and motif 20 was identified as the pear-specific protein motifs in the C-termini of PbPRXs. They were restricted to specific clades of PbPRX proteins, and although the function remains to be further studied, might play a key role during fruit development of pear. Additionally, gene structures were also investigated in PbPRX genes. We found that 94 PbPRX genes contained different numbers of exons or introns, implying a great diversity in PRX gene family of pear. Many studies have shown that structural diversification of genes plays an important role in the evolution of multi-gene families (Muthamilarasan et al., 2014; Cao et al., 2016a,b,c; Han et al., 2016). In our research, we found that there presented different characteristics of PRXs from different subfamilies, indicating PbPRXs have functionally diversified. In addition, previous studies have reported that introns could be specifically inserted into the plants, as well as were retained in the plant genome during evolution (Rogozin et al., 2003; Wang et al., 2015a). So we inferred that loss or gain of introns may be caused by specific approach. In the present study, we also found this phenomenon, which might explain the functional differences and diversity of closely related PbPRX genes, such as PbPRX7 and PbPRX8, PbPRX72, and PbPRX90 (Figure 2).
In this study, expression patterns of 41 PbPRXs during fruit development of pear were tested using qRT-PCR. Among them, the expression levels of five genes (subgroup C: PbPRX2, PbPRX22, PbPRX34, PbPRX64, and PbPRX75) were lower at early stages, and gradually increased from the early to the middle stage, then decreased during pear fruit ripening, which were significantly correlated to lignin content during fruit development. In addition, previous research have demonstrated that PRX could directly affect the synthesis of lignin via regulation of the last reaction in lignin metabolism pathway (Davin et al., 2008). Therefore, it was suggested that these five genes were participated in regulation of the lignin synthesis in pear fruit. Remarkably, two pairs of segmental duplication genes (PbPRX4 and PbPRX69, PbPRX26, and PbPRX59), showed similarly expression levels at eight time points after flowering. Although their expression levels peaked at different time points, both of PbPRX26 and PbPRX59 showed the similar tendency throughout pear fruit development.
Conclusion
In short, a total of 94 full-length PRX genes were identified in pear and divided into 19 subfamilies, as supported by intron-exon structures, conserved motifs and phylogeny. Chromosomal mapping and microsynteny analysis suggested that these PbPRXs were unevenly distributed in all pear chromosomes. Segmental duplication and tandem duplication were identified as the main patterns contributors to the expansion of PRX gene expansion in pear. In addition, a sliding-window analysis of Ka/Ks ratios showed some amino acid site under positive selection. Finally, expressed sequence tags analysis revealed that 41 PbPRX genes are expressed during fruit development. Furthermore, qRT-PCR analysis showed that five PbPRX genes from subgroup C might be involved in the regulation of lignin synthesis in pear fruit. This present study increases our understanding of PRX genes in pear, as well as lays the foundation for further clarify of the biological functions of these PRX proteins in other plants.
Author contributions
YCao and YCai conceived and designed the experiments; YCao performed the experiments; YCao, YH, DM, DL, and QJ analyzed the data; YCao, YH, YL, and YCai contributed reagents/materials/analysis tools; YCao and YH wrote the paper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by The National Natural Science Foundation of China (grant 30771483, 31171944 and 31640068).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01874/full#supplementary-material
Phylogenetic tree reconstruction of PRX proteins. The PRX proteins from the pear, yang mei, peach and strawberry were aligned using Clustalw software, and the neighbor-joining tree was constructed by MEGA7.0 software, with1000 bootstrap replicates.
Sliding window plots of duplicated PRX genes in pear. The window size is 150 bp, and the step size is 9 bp. The positions of the PRX domain were represented by gray blocks, respectively.
Primers in this study.
The detailed information of PRX genes in pear.
All MEME motif sequences in pear PRX proteins.
Orthologous analyses of PRX genes between pear, Populus and Arabidopsis thaliana.
Estimates of the divergence time for pear duplicated PRX paralogs.
The ESTs of putative PbPRX genes.
References
- Almagro L., Gómez Ros L. V., Belchi-Navarro S., Bru R., Ros Barceló A., Pedreno M. A. (2009). Class III peroxidases in plant defence reactions. J. Exp. Bot. 60, 377–390. 10.1093/jxb/ern277 [DOI] [PubMed] [Google Scholar]
- Bailey T. L., James J., Grant C. E., Noble W. S. (2015). The MEME Suite. Nucleic Acids Res. 37, W202–W208. 10.1093/nar/gkv416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruex A., Kainkaryam R. M., Wieckowski Y., Kang Y. H., Bernhardt C., Xia Y., et al. (2012). A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PLoS Genet. 8:e1002446. 10.1371/journal.pgen.1002446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Li G., Nie J., Lin Y., Nie F., Zhang J., et al. (2010). Study of the structure and biosynthetic pathway of lignin in stone cells of pear. Sci. Hortic. 125, 374–379. 10.1016/j.scienta.2010.04.029 [DOI] [Google Scholar]
- Cao Y., Han Y., Li D., Lin Y., Cai Y. (2016a). Systematic analysis of the 4-coumarate:coenzyme a ligase (4cl) related genes and expression profiling during fruit development in the chinese pear. Genes 7:89. 10.3390/genes7100089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. P., Han Y., Jin Q., Lin Y., Cai Y. (2016b). Comparative genomic analysis of the GRF genes in Chinese pear (Pyrus bretschneideri Rehd), poplar (Populous), grape (Vitis vinifera), Arabidopsis and rice (Oryza sativa). Front. Plant Sci. 7:1750 10.3389/fpls.2016.01750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. P., Han Y., Li D., Lin Y., Cai Y. (2016c). MYB Transcription Factors in Chinese Pear (Pyrus bretschneideri Rehd.): genome-wide identification, classification and expression profiling during fruit development. Front. Plant. Sci. 7:577. 10.3389/fpls.2016.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio C., Dunand C. (2009). Specific functions of individual class III peroxidase genes. J. Exp. Bot. 60, 391–408. 10.1093/jxb/ern318 [DOI] [PubMed] [Google Scholar]
- Cosio C., Dunand C. (2010). Transcriptome analysis of various flower and silique development stages indicates a set of class III peroxidase genes potentially involved in pod shattering in Arabidopsis thaliana. BMC Genomics 11:528. 10.1186/1471-2164-11-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davin L. B., Jourdes M., Patten A. M., Kim K.-W., Vassao D. G., Lewis N. G. (2008). Dissection of lignin macromolecular configuration and assembly: comparison to related biochemical processes in allyl/propenyl phenol and lignan biosynthesis. Nat. Prod. Rep. 25, 1015–1090. 10.1039/b510386j [DOI] [PubMed] [Google Scholar]
- Ehlting J., Mattheus N., Aeschliman D. S., Li E., Hamberger B., Cullis I. F., et al. (2005). Global transcript profiling of primary stems from Arabidopsis thaliana identifies candidate genes for missing links in lignin biosynthesis and transcriptional regulators of fiber differentiation. Plant J. 42, 618–640. 10.1111/j.1365-313X.2005.02403.x [DOI] [PubMed] [Google Scholar]
- Guo A., Zhu Q., Chen X., Luo J. (2007). [GSDS: a gene structure display server]. Yi Chuan 29, 1023–1026. 10.1360/yc-007-1023 [DOI] [PubMed] [Google Scholar]
- Han Y., Ding T., Su B., Jiang H. (2016). Genome-Wide Identification, characterization and expression analysis of the chalcone synthase family in maize. Int. J. Mol. Sci. 17, 161. 10.3390/ijms17020161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey S. G., Saïdi M. N., Hefer C. A., Myburg A. A., Grima-Pettenati J. (2015). Structural, evolutionary and functional analysis of the NAC domain protein family in eucalyptus. New Phytol. 206, 1337–1350. 10.1111/nph.13139 [DOI] [PubMed] [Google Scholar]
- Jin Q., Yan C., Qiu J., Zhang N., Lin Y., Cai Y. (2013). Structural characterization and deposition of stone cell lignin in Dangshan Su pear. Sci. Hortic. 155, 123–130. 10.1016/j.scienta.2013.03.020 [DOI] [Google Scholar]
- Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., et al. (2009). Circos: an information aesthetic for comparative genomics. Genome Res. 19, 1639–1645. 10.1101/gr.092759.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T., Sparks J. A., Nakashima J., Allen S. N., Tang Y., Blancaflor E. B. (2015). Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development. Am. J. Bot. 102, 21–35. 10.3732/ajb.1400458 [DOI] [PubMed] [Google Scholar]
- Lan P., Li W., Lin W.-D., Santi S., Schmidt W. (2013). Mapping gene activity of Arabidopsis root hairs. Genome Biol. 14:R67. 10.1186/gb-2013-14-6-r67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Doerks T., Bork P. (2012). SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40, D302–D305. 10.1093/nar/gkr931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P., Rozas J. (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Mohr P. G., Cahill D. M. (2007). Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. Tomato. Funct. Integr. Genomics 7, 181–191. 10.1007/s10142-006-0041-4 [DOI] [PubMed] [Google Scholar]
- Muthamilarasan M., Khandelwal R., Yadav C. B., Bonthala V. S., Khan Y., Prasad M. (2014). Identification and molecular characterization of MYB transcription factor superfamily in C4 model plant foxtail millet (Setaria italica L.). PLoS ONE 9:e109920. 10.1371/journal.pone.0109920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka H., Satoh K., Doi K., Nagata T., Otomo Y., Murakami K., et al. (2003). Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 10, 239–247. 10.1093/dnares/10.6.239 [DOI] [PubMed] [Google Scholar]
- Passardi F., Longet D., Penel C., Dunand C. (2004). The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 65, 1879–1893. 10.1016/j.phytochem.2004.06.023 [DOI] [PubMed] [Google Scholar]
- Petricka J. J., Schauer M. A., Megraw M., Breakfield N. W., Thompson J. W., Georgiev S., et al. (2012). The protein expression landscape of the Arabidopsis root. Proc. Natl. Acad. Sci. U.S.A. 109, 6811–6818. 10.1073/pnas.1202546109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punta M., Coggill P. C., Eberhardt R. Y., Mistry J., Tate J., Boursnell C., et al. (2011). The Pfam protein families database. Nucleic Acids Res. 40, D290–D301. 10.1093/nar/gkr1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L.-L., Liu Y.-J., Liu H.-J., Qian T.-T., Qi L.-W., Wang X.-R., et al. (2014). Subcellular relocalization and positive selection play key roles in the retention of duplicate genes of populus class III peroxidase family. Plant Cell 26, 2404–2419. 10.1105/tpc.114.124750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogozin I. B., Wolf Y. I., Sorokin A. V., Mirkin B. G., Koonin E. V. (2003). Remarkable interkingdom conservation of intron positions and massive, lineage-specific intron loss and gain in eukaryotic evolution. Curr. Biol. 13, 1512–1517. 10.1016/S0960-9822(03)00558-X [DOI] [PubMed] [Google Scholar]
- Syros T., Yupsanis T., Zafiriadis H., Economou A. (2004). Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J. Plant Physiol. 161, 69–77. 10.1078/0176-1617-00938 [DOI] [PubMed] [Google Scholar]
- Tognolli M., Penel C., Greppin H., Simon P. (2002). Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 288, 129–138. 10.1016/S0378-1119(02)00465-1 [DOI] [PubMed] [Google Scholar]
- Van Loon L. C., Rep M., Pieterse C. M. (2006). Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162. 10.1146/annurev.phyto.44.070505.143425 [DOI] [PubMed] [Google Scholar]
- Wang Y., Feng L., Zhu Y., Li Y., Yan H., Xiang Y. (2015a). Comparative genomic analysis of the WRKY III gene family in populus, grape, Arabidopsis and rice. Biol. Direct 10, 48. 10.1186/s13062-015-007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tang H., Debarry J. D., Tan X., Li J., Wang X., et al. (2012). MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40:e49. 10.1093/nar/gkr1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Q., Zhao Y., Han G., Zhu S. (2015b). Systematic analysis of maize class III peroxidase gene family reveals a conserved subfamily involved in abiotic stress response. Gene 566, 95–108. 10.1016/j.gene.2015.04.041 [DOI] [PubMed] [Google Scholar]
- Welinder K. G., Justesen A. F., Kjærsgård I. V., Jensen R. B., Rasmussen S. K., Jespersen H. M., et al. (2002). Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. Eur. J. Biochem. 269, 6063–6081. 10.1046/j.1432-1033.2002.03311.x [DOI] [PubMed] [Google Scholar]
- Wu J., Wang Z., Shi Z., Zhang S., Ming R., Zhu S., et al. (2013). The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 23, 396–408. 10.1101/gr.144311.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Zhang R., Gu C., Wu J., Wan H., Zhang S., et al. (2012). Evaluation of candidate reference genes for real time quantitative PCR normalization in pear fruit. Afr. J. Agric. Res. 7, 3701–3704. 10.5897/AJAR11.1842 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree reconstruction of PRX proteins. The PRX proteins from the pear, yang mei, peach and strawberry were aligned using Clustalw software, and the neighbor-joining tree was constructed by MEGA7.0 software, with1000 bootstrap replicates.
Sliding window plots of duplicated PRX genes in pear. The window size is 150 bp, and the step size is 9 bp. The positions of the PRX domain were represented by gray blocks, respectively.
Primers in this study.
The detailed information of PRX genes in pear.
All MEME motif sequences in pear PRX proteins.
Orthologous analyses of PRX genes between pear, Populus and Arabidopsis thaliana.
Estimates of the divergence time for pear duplicated PRX paralogs.
The ESTs of putative PbPRX genes.